Evaluation of the Therapeutic Potential of Synthetic Growth Hormone-Releasing Hormone Antagonist MIA-690 as a Cognitive Modulator in a Mouse Model of Gulf War Illness
Abstract
1. Introduction
2. Results
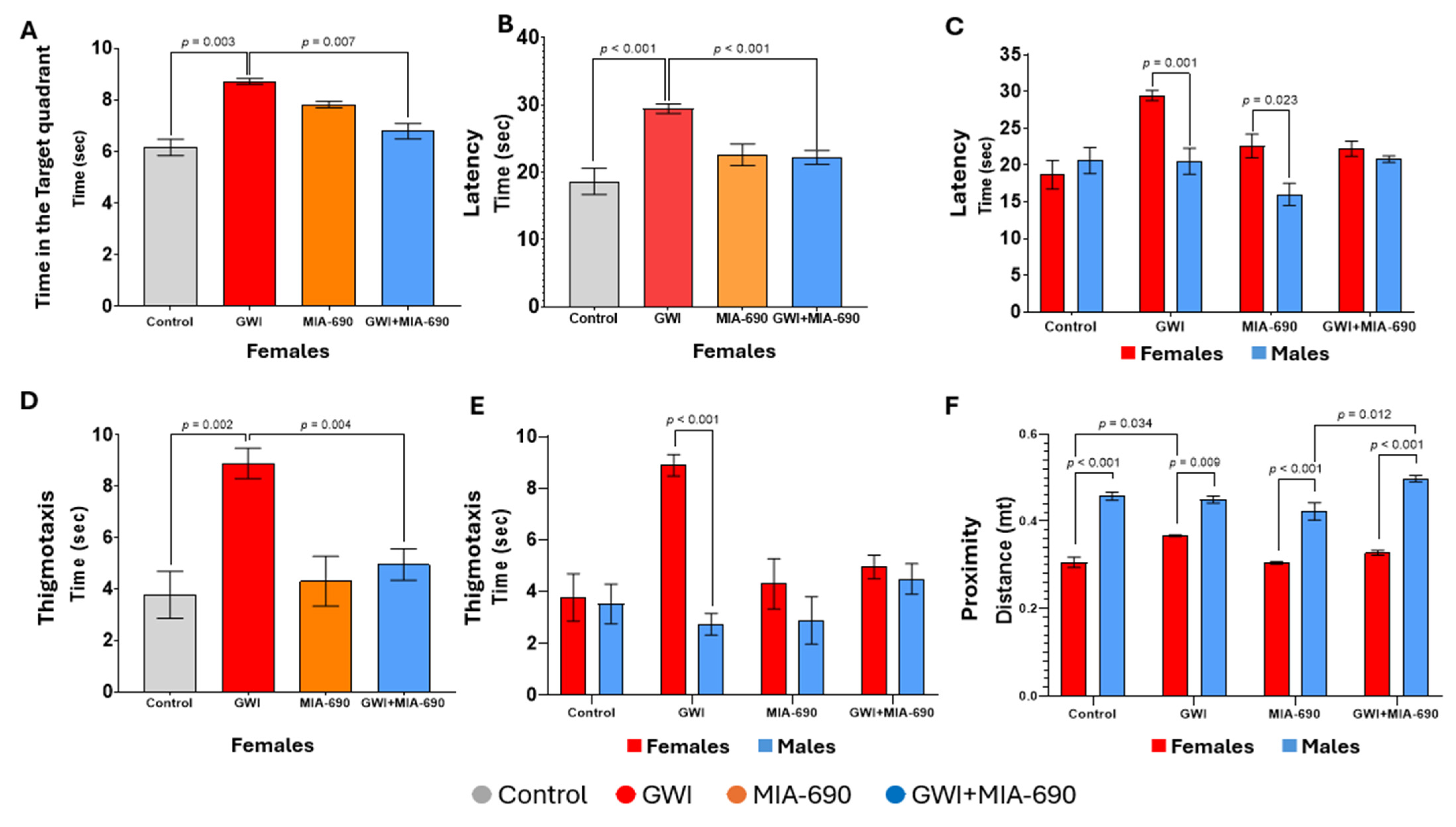
2.1. Morris Water Maze
Learning
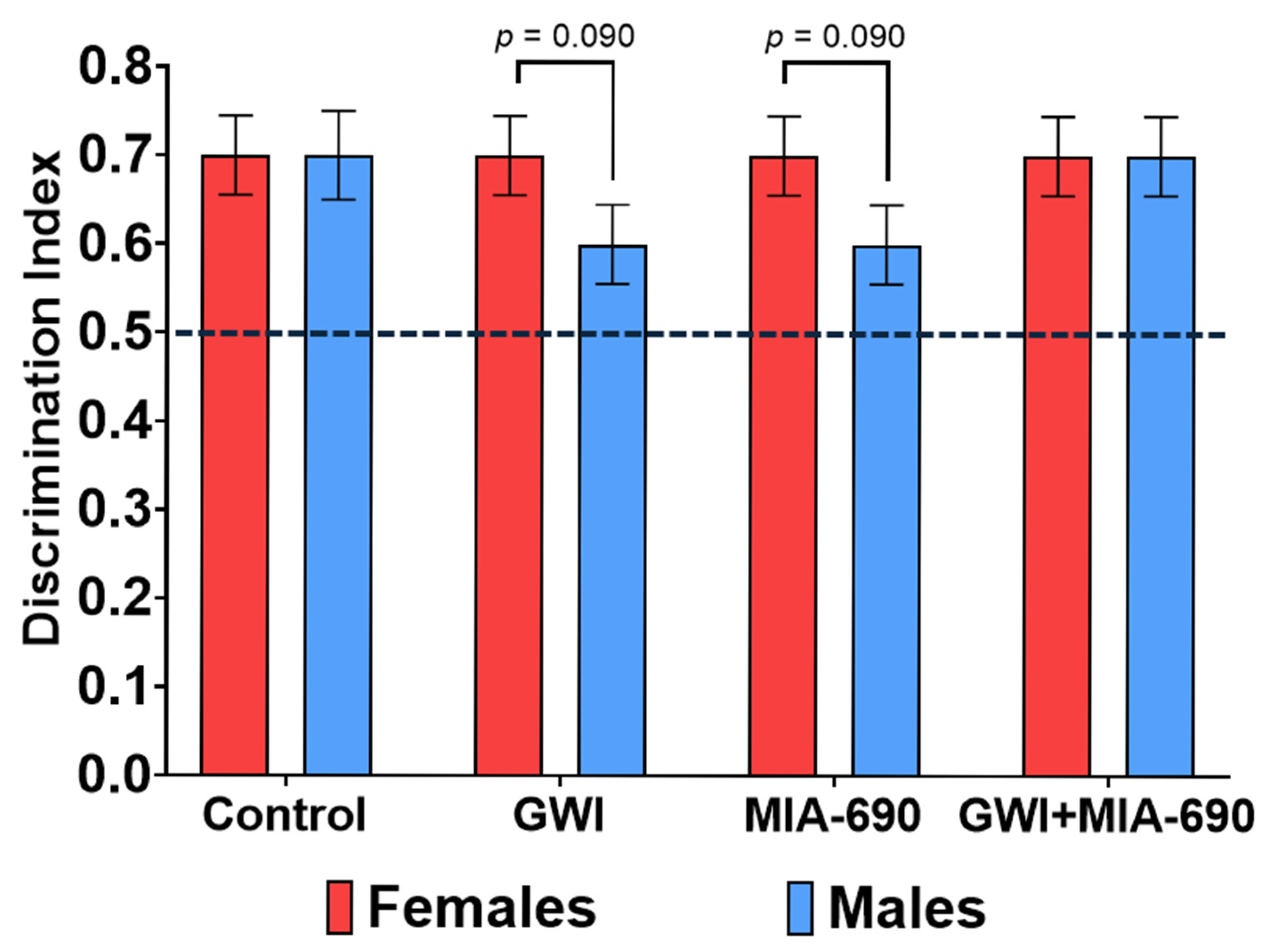
2.2. Novel Object Recognition
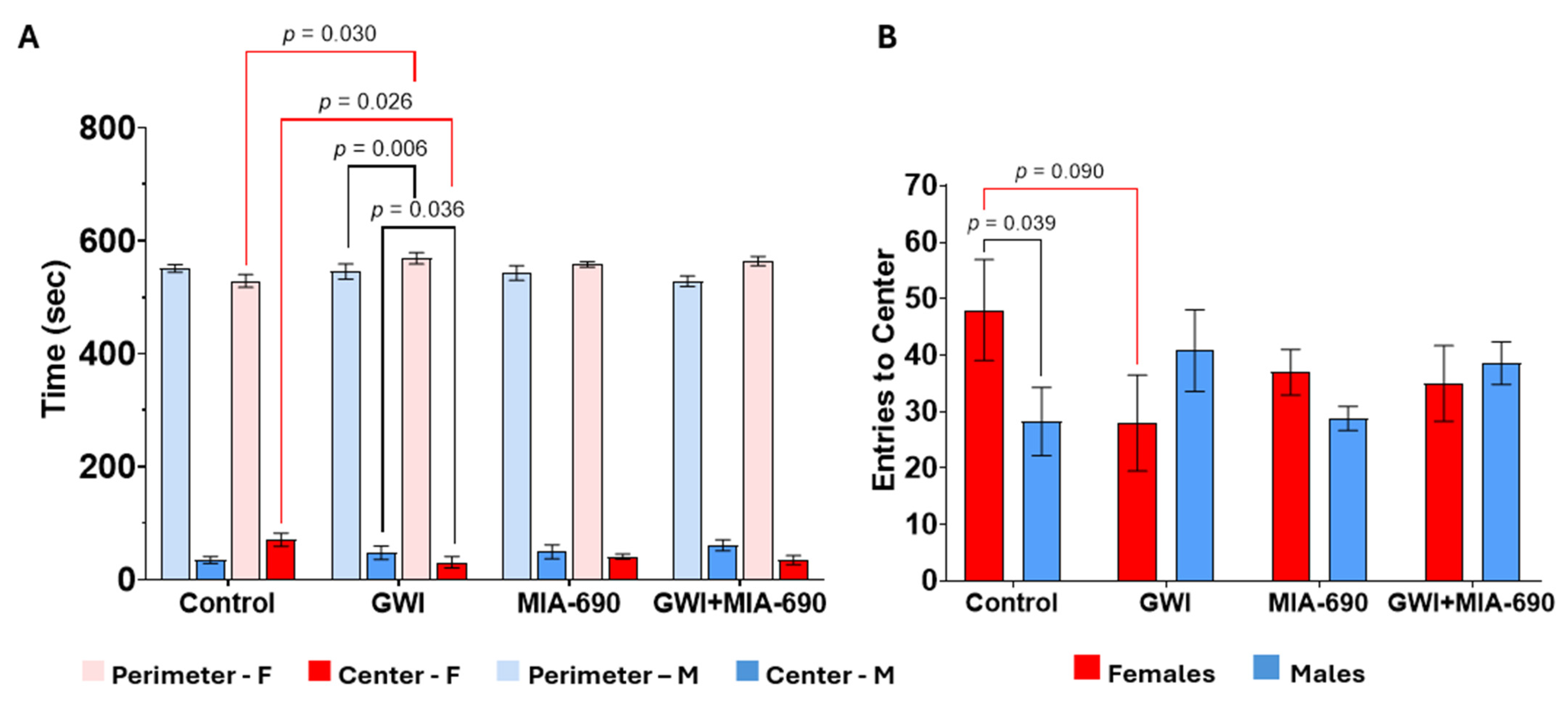
2.3. Open Field Test
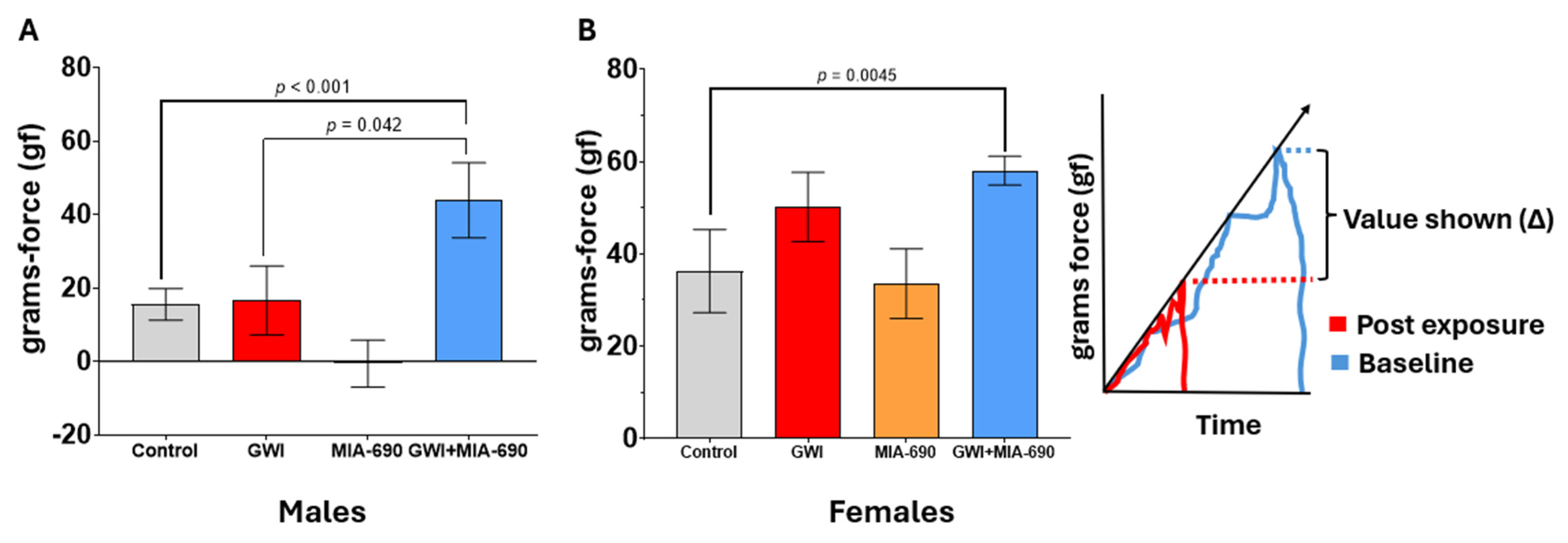
2.4. Grip Strength Test Results
3. Discussion
4. Materials and Methods
4.1. Peptide
4.2. Chemicals
4.3. Animals
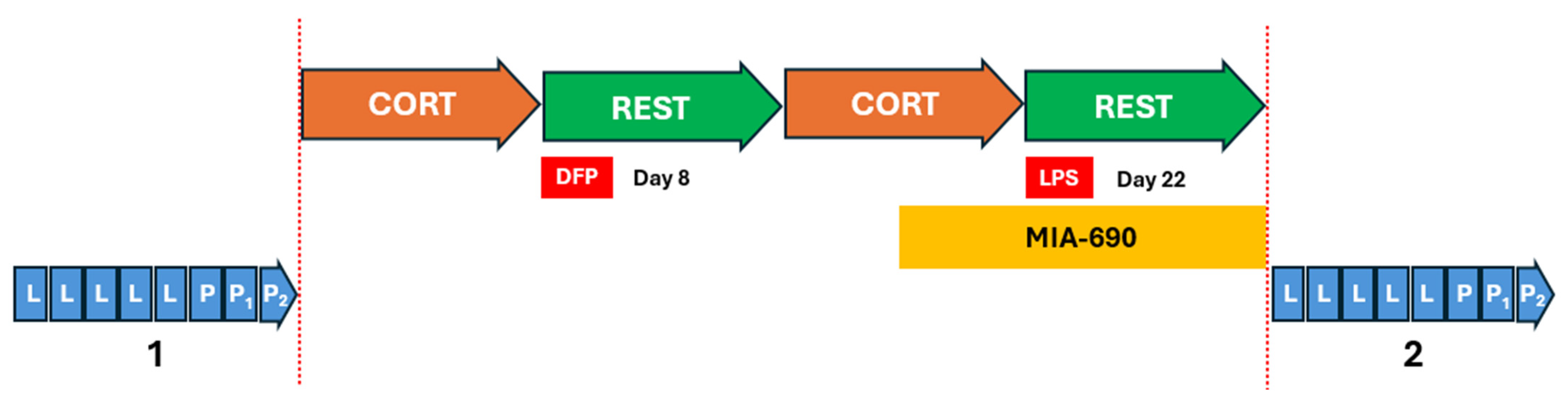
4.4. Experimental GWI Exposure (CORT/DFP-CORT/LPS)
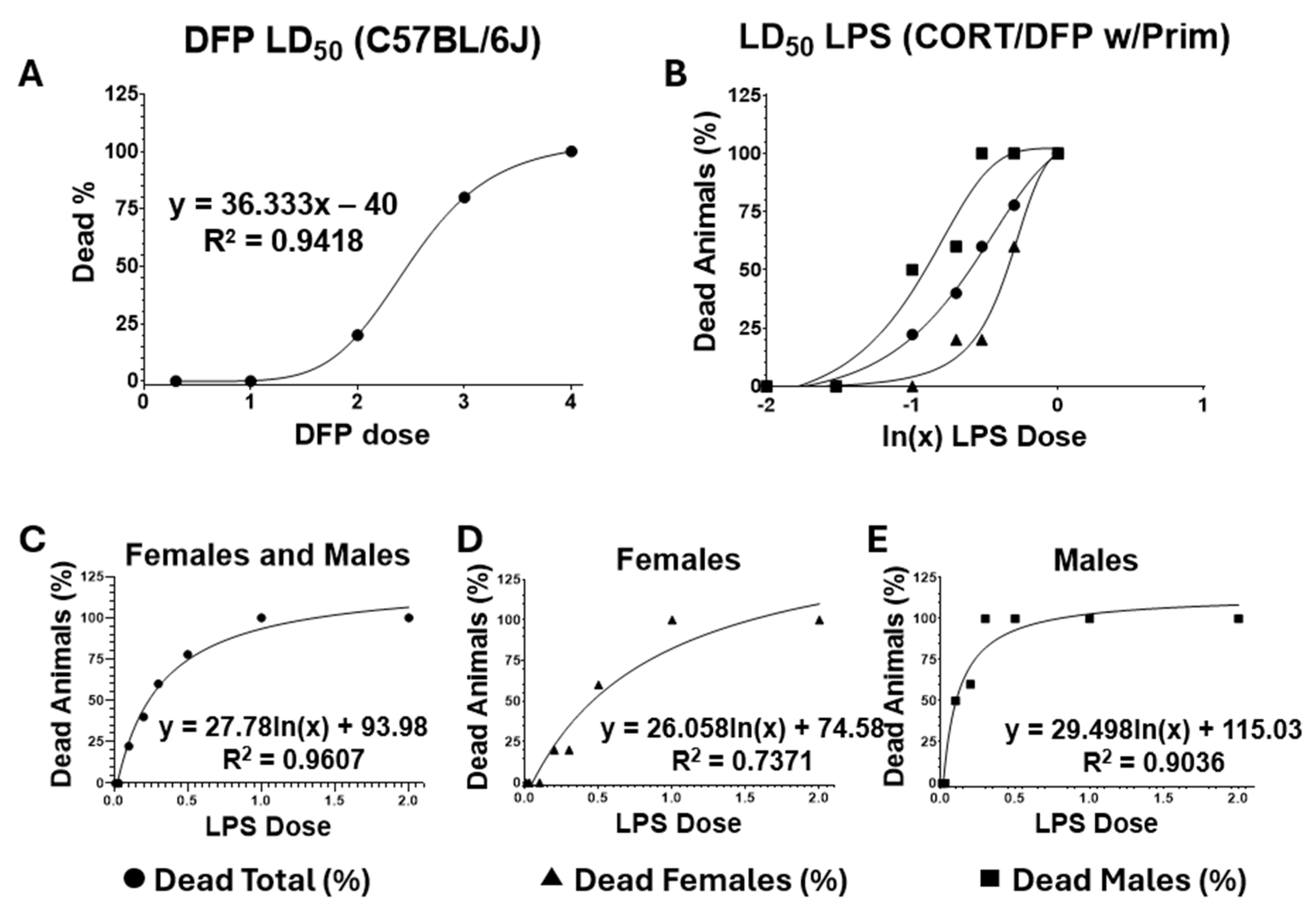
4.5. Titration of Lethal Doses 50 (LD50)
4.6. Exposure to the Toxicants
4.7. Supportive Care
4.8. Behavioral Tasks
4.8.1. Morris Water Maze (Spatial Memory Evaluation)
4.8.2. Novel Object Recognition Test (Recognition Memory Task)
4.8.3. Grip Strength Test
4.8.4. Adaptive Stress Responses
Open Field Test (Exploratory Behavior)
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, R.F.; Steele, L.; O’CAllaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C.; et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef]
- Kang, H.K.; Mahan, C.M.; Lee, K.Y.; Magee, C.A.; Murphy, F.M. Illnesses among united states veterans of the gulf war: A population-based survey of 30,000 veterans. J. Occup. Environ. Med. 2000, 42, 491–501. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; Engdahl, B.E.; James, L.M.; Miller, R.D.; Leuthold, A.C.; Lewis, S.M.; Carpenter, A.F. Brain function in Gulf War Illness (GWI) and associated mental health comorbidities. J. Neurol. Neuromed. 2018, 3, 24–34. [Google Scholar] [CrossRef]
- Burzynski, H.E.; Reagan, L.P. Exposing the latent phenotype of Gulf War Illness: Examination of the mechanistic mediators of cognitive dysfunction. Front. Immunol. 2024, 15, 1403574. [Google Scholar] [CrossRef] [PubMed]
- Elhaj, R.; Reynolds, J.M. Chemical exposures and suspected impact on Gulf War Veterans. Mil. Med Res. 2023, 10, 11. [Google Scholar] [CrossRef]
- Dickey, B.; Madhu, L.N.; Shetty, A.K. Gulf War illness: Mechanisms underlying brain dysfunction and promising therapeutic strategies. Pharmacol. Ther. 2021, 220, 107716. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.; Jamieson, J.D.; Malone, P.; Rayhan, R.U.; Washington, S.; VanMeter, J.W.; Baraniuk, J.N. Connectivity differences between Gulf War Illness (GWI) phenotypes during a test of attention. PLoS ONE 2019, 14, e0226481. [Google Scholar] [CrossRef]
- Toomey, R.; Kang, H.K.; Karlinsky, J.; Baker, D.G.; Vasterling, J.J.; Alpern, R.; Reda, D.J.; Henderson, W.G.; Murphy, F.M.; Eisen, S.A. Mental health of US Gulf War veterans 10 years after the war. Br. J. Psychiatry 2007, 190, 385–393. [Google Scholar] [CrossRef]
- Jeffrey, M.; Collado, F.; Kibler, J.; DeLucia, C.; Messer, S.; Klimas, N.; Craddock, T.J.A. Post-traumatic stress impact on health outcomes in Gulf War Illness. BMC Psychol. 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Zhang, X.; Wang, H.; Cui, T.; Halmos, G.; Sha, W.; He, J.; Popovics, P.; Vidaurre, I.; Zhang, C.; et al. Synthesis of potent antagonists of receptors for growth hormone-releasing hormone with antitumor and anti-inflammatory activity. Peptides 2022, 150, 170716. [Google Scholar] [CrossRef]
- Granata, R.; Leone, S.; Zhang, X.; Gesmundo, I.; Steenblock, C.; Cai, R.; Sha, W.; Ghigo, E.; Hare, J.M.; Bornstein, S.R.; et al. Growth hormone-releasing hormone and its analogues in health and disease. Nat. Rev. Endocrinol. 2025, 21, 180–195, Correction in Nat. Rev. Endocrinol. 2025, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Zarandi, M.; Cai, R.; Kovacs, M.; Popovics, P.; Szalontay, L.; Cui, T.; Sha, W.; Jaszberenyi, M.; Varga, J.; Zhang, X.; et al. Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides 2017, 89, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Siejka, A.; Lawnicka, H.; Melen-Mucha, G.; Motylewska, E.; Komorowski, J.; Stepien, H. Antineoplastic action of growth hormone-releasing hormone (GHRH) antagonists. In Topics in Anti-Cancer Research; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; Volume 7, pp. 530–551. [Google Scholar]
- Barabutis, N.; Schally, A.V.; Siejka, A. P53, GHRH, inflammation and cancer. EBioMedicine 2018, 37, 557–562. [Google Scholar] [CrossRef]
- Schally, A.V.; Varga, J.L.; Engel, J.B. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 33–43. [Google Scholar] [CrossRef]
- Schally, A.V.; Perez, R.; Block, N.L.; Rick, F.G. Potentiating effects of GHRH analogs on the response to chemotherapy. Cell Cycle 2015, 14, 699–704. [Google Scholar] [CrossRef]
- Leone, S.; Chiavaroli, A.; Recinella, L.; Di Valerio, V.; Veschi, S.; Gasparo, I.; Bitto, A.; Ferrante, C.; Orlando, G.; Salvatori, R.; et al. Growth hormone-releasing hormone (GHRH) deficiency promotes inflammation-associated carcinogenesis. Pharmacol. Res. 2020, 152, 104614. [Google Scholar] [CrossRef]
- Leone, S.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gasparo, I.; Bitto, A.; Salvatori, R.; Brunetti, L. Increased pain and inflammatory sensitivity in growth hormone-releasing hormone (GHRH) knockout mice. Prostaglandins Other Lipid Mediat. 2019, 144, 106362. [Google Scholar] [CrossRef]
- Siejka, A.; Barabutis, N. Growth hormone–Releasing hormone in the context of inflammation and redox biology. Front. Immunol. 2024, 15, 1403124. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; et al. Antinflammatory, antioxidant, and behavioral effects induced by administration of growth hormone-releasing hormone analogs in mice. Sci. Rep. 2020, 10, 732, Correction in Sci. Rep. 2020, 10, 4850. [Google Scholar] [CrossRef]
- Kanashiro-Takeuchi, R.M.; Takeuchi, L.M.; Rick, F.G.; Dulce, R.; Treuer, A.V.; Florea, V.; Rodrigues, C.O.; Paulino, E.C.; Hatzistergos, K.E.; Selem, S.M.; et al. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI). Proc. Natl. Acad. Sci. USA 2011, 109, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, H. Effects of GHRH and its analogues on the Vascular System. Rev. Endocr. Metab. Disord. 2024, 26, 493–505. [Google Scholar] [CrossRef]
- Muccioli, G.; Broglio, F.; Valetto, M.R.; Ghè, C.; Catapano, F.; Graziani, A.; Papotti, M.; Bisi, G.; Deghenghi, R.; Ghigo, E. Growth hormone-releasing peptides and the cardiovascular system. Ann. Endocrinol. 2000, 61, 27–31. [Google Scholar]
- Cai, R.; Schally, A.V.; Cui, T.; Szalontay, L.; Halmos, G.; Sha, W.; Kovacs, M.; Jaszberenyi, M.; He, J.; Rick, F.G.; et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides 2014, 52, 104–112. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Jia, H.; Yang, K.; Ren, F.; Zhou, M.-S. GHRH and its analogues in central nervous system diseases. Rev. Endocr. Metab. Disord. 2024, 26, 427–442. [Google Scholar] [CrossRef]
- Martínez-Moreno, C.G.; Calderón-Vallejo, D.; Harvey, S.; Arámburo, C.; Quintanar, J.L. Growth hormone (GH) and gonadotropin-releasing hormone (GnRH) in the central nervous system: A potential neurological combinatory therapy? Int. J. Mol. Sci. 2018, 19, 375. [Google Scholar] [CrossRef]
- Wilber, J.; Montoya, E.; Plotnikoff, N.; White, W.; Gendrich, R.; Renaud, L.; Martin, J. Gonadotropin-releasing hormone and thyrotropin-releasing hormone: Distribution and effects in the central nervous system. In Proceedings of the 1975 Laurentian Hormone Conference; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Jászberényi, M.; Rick, F.G.; Szalontay, L.; Block, N.L.; Zarandi, M.; Cai, R.-Z.; Schally, A.V. Beneficial effects of novel antagonists of GHRH in different models of Alzheimer’s disease. Aging 2012, 4, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, S.; Sikorska, H.; Wiecek, A.; Zukowska-Szczechowska, E.; Załecka, K.; Gorczyńska, J.; Kubik, M.; Czerwieńska, B.; Gosek, K.; Veldhuis, J.D.; et al. A super-agonist of growth hormone–releasing hormone causes rapid improvement of nutritional status in patients with chronic kidney disease. Kidney Int. 2010, 77, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.C.; Bagno, L.L.; Salerno, A.; Florea, V.; Rodriguez, J.; Rosado, M.; Turner, D.; Dulce, R.A.; Takeuchi, L.M.; Kanashiro-Takeuchi, R.M.; et al. Growth hormone-releasing hormone agonists ameliorate chronic kidney disease-induced heart failure with preserved ejection fraction. Proc. Natl. Acad. Sci. USA 2021, 118, e2019835118. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, P.; Wang, N.-G.; Sundaram, K.; Rivier, J.; Vale, W.; Bardin, C.W. The antiandrogenic action of gonadotropin-releasing hormone and its agonists on the mouse kidney. Endocrinology 1982, 110, 1–6. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, R.; Lazerson, A.; Delcroix, G.; Wangpaichitr, M.; Mirsaeidi, M.; Griswold, A.J.; Schally, A.V.; Jackson, R.M. Growth hormone-releasing hormone receptor antagonist modulates lung inflammation and fibrosis due to bleomycin. Lung 2019, 197, 541–549. [Google Scholar] [CrossRef]
- Uddin, M.A.; Akhter, M.S.; Singh, S.S.; Kubra, K.-T.; Schally, A.V.; Jois, S.; Barabutis, N. GHRH antagonists support lung endothelial barrier function. Tissue Barriers 2019, 7, 1669989. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Sridhar, S.; Rick, F.G.; Gorshkov, B.; Umapathy, N.S.; Yang, G.; Oseghale, A.; Verin, A.D.; Chakraborty, T.; Matthay, M.A.; et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci. USA 2012, 109, 2084–2089. [Google Scholar] [CrossRef]
- Barabutis, N.; Schally, A.V. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle 2010, 9, 4110–4116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on the brain function in mice. Neuropeptides 2009, 43, 229–234. [Google Scholar] [CrossRef]
- Meijer, O.C.; Topic, B.; Steenbergen, P.J.; Jocham, G.; Huston, J.P.; Oitzl, M.S. Correlations between hypothalamus-pituitary-adrenal axis parameters depend on age and learning capacity. Endocrinology 2005, 146, 1372–1381. [Google Scholar] [CrossRef][Green Version]
- Telegdy, G.; Schally, A.V. Involvement of neurotransmitters in the action of growth hormone-releasing hormone antagonist on passive avoidance learning. Behav. Brain Res. 2012, 233, 326–330. [Google Scholar] [CrossRef]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behav. Brain Res. 2011, 224, 155–158. [Google Scholar] [CrossRef]
- Jaeger, L.B.; Banks, W.A.; Varga, J.L.; Schally, A.V. Antagonists of growth hormone-releasing hormone cross the blood-brain barrier: A potential applicability to treatment of brain tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 12495–12500. [Google Scholar] [CrossRef]
- Kovács, M.; Schally, A.V.; Zarándi, M.; Groot, K. Inhibition of GH release in rats by new potent antagonists of growth hormone-releasing hormone (GH-RH). Peptides 1997, 18, 431–438. [Google Scholar] [CrossRef]
- Varga, J.L.; Schally, A.V.; Horvath, J.E.; Kovacs, M.; Halmos, G.; Groot, K.; Toller, G.L.; Rekasi, Z.; Zarandi, M. Increased activity of antagonists of growth hormone-releasing hormone substituted at positions 8, 9, and 10. Proc. Natl. Acad. Sci. USA 2004, 101, 1708–1713. [Google Scholar] [CrossRef]
- Kovacs, M.; Schally, A.V.; Varga, J.L.; Zarandi, M. Endocrine and antineoplastic actions of growth hormone-releasing hormone antagonists. Curr. Med. Chem. 2008, 15, 314–321. [Google Scholar] [CrossRef]
- Kovács, M.; Schally, A.V.; Hohla, F.; Rick, F.G.; Pozsgai, É.; Szalontay, L.; Varga, J.L.; Zarándi, M. A correlation of endocrine and anticancer effects of some antagonists of GHRH. Peptides 2010, 31, 1839–1846. [Google Scholar] [CrossRef]
- Kiaris, H.; Chatzistamou, I.; Papavassiliou, A.G.; Schally, A.V. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol. Metab. 2011, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, M.D.; Samson, W.K.; McCANN, S.M. Effects of intraventricular growth hormone-releasing factor on growth hormone release: Further evidence for ultrashort loop feedback. Endocrinology 1985, 116, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Wilhelm, I.; Michel, C.; Perras, B.; Born, J.; Goel, N. A role for central nervous growth hormone-releasing hormone signaling in the consolidation of declarative memories. PLoS ONE 2011, 6, e23435. [Google Scholar] [CrossRef] [PubMed]
- Merriam, G.R.; Schwartz, R.S.; Vitiello, M.V. Growth hormone-releasing hormone and growth hormone secretagogues in normal aging. Endocrine 2003, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, M.; Moe, K.; Merriam, G.; Mazzoni, G.; Buchner, D.; Schwartz, R. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol. Aging 2006, 27, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Barsness, S.M.; Borson, S.; Merriam, G.R.; Friedman, S.D.; Craft, S.; Vitiello, M.V. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch. Neurol. 2012, 69, 1420–1429. [Google Scholar] [CrossRef]
- Kerr, K.J. Gulf War illness: An overview of events, most prevalent health outcomes, exposures, and clues as to pathogenesis. Rev. Environ. Health 2015, 30, 273–286. [Google Scholar] [CrossRef]
- Bradburne, C.; Lewis, J.A. The US Military and the Exposome. In Unraveling the Exposome: A Practical View; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–85. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Gulf War and Health, Volume 11: Generational Health Effects of Serving in the Gulf War. Moving Forward. In Gulf War and Health: Volume 11: Generational Health Effects of Serving in the Gulf War; National Academies Press: Washington, DC, USA, 2018.
- Hodgson, M.J.; Kipen, H.M. Gulf War illnesses: Causation and treatment. J. Occup. Environ. Med. 1999, 41, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Zavestoski, S.; Linder, M.; McCormick, S.; Mayerv, B. Chemicals and casualties: The search for causes of Gulf War Illnesses. In Synthetic Planet; Routledge: London, UK, 2013; pp. 213–236. [Google Scholar]
- Jones, B.C. Genetic Basis of Individual Differences in Susceptibility to Gulf War Illness; University of Tennessee Health Science Center: Memphis, TN, USA, 2020. [Google Scholar]
- Haddad, J.J.; Saadé, N.E.; Safieh-Garabedian, B. Cytokines and neuro–immune–endocrine interactions: A role for the hypothalamic–pituitary–adrenal revolving axis. J. Neuroimmunol. 2002, 133, 1–19. [Google Scholar] [CrossRef]
- Rice, M.A., Jr.; Craddock, T.J.; Folcik, V.A.; del Rosario, R.M.; Barnes, Z.M.; Klimas, N.G.; Fletcher, M.A.; Zysman, J.; Broderick, G. Gulf War Illness: Is there lasting damage to the endocrine-immune circuitry? Syst. Biomed. 2014, 2, 80–89. [Google Scholar] [CrossRef][Green Version]
- McGrath, R.; Vincent, B.M.; Hackney, K.J.; Robinson-Lane, S.G.; Downer, B.; Clark, B.C. The Longitudinal Associations of Handgrip Strength and Cognitive Function in Aging Americans. J. Am. Med Dir. Assoc. 2020, 21, 634–639.e1. [Google Scholar] [CrossRef]
- Ahrenfeldt, L.J.; Scheel-Hincke, L.L.; Kjærgaard, S.; Möller, S.; Christensen, K.; Lindahl-Jacobsen, R. Gender differences in cognitive function and grip strength: A cross-national comparison of four European regions. Eur. J. Public Health 2018, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Grip strength: An indispensable biomarker for older adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.-K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Franklin, J.; Farris, A.; Ryu, S. Handedness, grip strength, and memory function: Considerations by biological sex. Medicina 2019, 55, 444. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip strength and the risk of cognitive decline and dementia: A systematic review and meta-analysis of longitudinal cohort studies. Front. Aging Neurosci. 2021, 13, 625551. [Google Scholar] [CrossRef]
- Schally, A.V. Endocrine approaches to treatment of Alzheimer’s disease and other neurological conditions: Part I: Some recollections of my association with Dr. Abba Kastin: A tale of successful collaboration. Peptides 2015, 72, 154–163. [Google Scholar]
- Jászberényi, M.; Thurzó, B.; Jayakumar, A.R.; Schally, A.V. The Aggravating Role of Failing Neuropeptide Networks in the Development of Sporadic Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 13086. [Google Scholar] [CrossRef]
- Barabutis, N.; Schally, A.V. Antioxidant activity of growth hormone-releasing hormone antagonists in LNCaP human prostate cancer line. Proc. Natl. Acad. Sci. USA 2008, 105, 20470–20475. [Google Scholar] [CrossRef]
- Stangelberger, A.; Schally, A.V.; Rick, F.G.; Varga, J.L.; Baker, B.; Zarandi, M.; Halmos, G. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate 2011, 72, 555–565. [Google Scholar] [CrossRef]
- Popovics, P.; Schally, A.V.; Salgueiro, L.; Kovacs, K.; Rick, F.G. Antagonists of growth hormone-releasing hormone inhibit proliferation induced by inflammation in prostatic epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1359–1364. [Google Scholar] [CrossRef]
- Schally, A.V.; Salgueiro, L.M. Part III: Experimental studies on antagonists of LH-RH and GH-RH in animal models of Alzheimer’s disease: Projections for treatment of other neurological conditions. Peptides 2015, 72, 158–163. [Google Scholar]
- Craddock, T.J.; Fritsch, P.; Rice, M.A., Jr.; del Rosario, R.M.; Miller, D.B.; Fletcher, M.A.; Klimas, N.G.; Broderick, G. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome. PLoS ONE 2014, 9, e84839, Correction in PLoS ONE 2014, 9, e100355. [Google Scholar] [CrossRef]
- O’CAllaghan, J.P.; Kelly, K.A.; Locker, A.R.; Miller, D.B.; Lasley, S.M. Corticosterone primes the neuroinflammatory response to DFP in mice: Potential animal model of Gulf War Illness. J. Neurochem. 2015, 133, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Locker, A.R.; Michalovicz, L.T.; Kelly, K.A.; Miller, J.V.; Miller, D.B.; O’CAllaghan, J.P. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 2017, 142, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.V.; LeBouf, R.F.; A Kelly, K.; Michalovicz, L.T.; Ranpara, A.; Locker, A.R.; Miller, D.B.; O’cAllaghan, J.P. The Neuroinflammatory Phenotype in a Mouse Model of Gulf War Illness is Unrelated to Brain Regional Levels of Acetylcholine as Measured by Quantitative HILIC-UPLC-MS/MS. Toxicol. Sci. 2018, 165, 302–313. [Google Scholar] [CrossRef]
- Michalovicz, L.T.; Locker, A.R.; Kelly, K.A.; Miller, J.V.; Barnes, Z.; Fletcher, M.A.; Miller, D.B.; Klimas, N.G.; Morris, M.; Lasley, S.M.; et al. Corticosterone and pyridostigmine/DEET exposure attenuate peripheral cytokine expression: Supporting a dominant role for neuroinflammation in a mouse model of Gulf War Illness. NeuroToxicology 2019, 70, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K. Brain Immune Interactions as the Basis of Gulf War Illness: Gulf War Illness Consortium (GWIC); Boston University Medical Campus: Boston, MA, USA, 2020. [Google Scholar]
- Whistler, T.; Fletcher, M.A.; Lonergan, W.; Zeng, X.-R.; Lin, J.-M.; LaPerriere, A.; Vernon, S.D.; Klimas, N.G. Impaired immune function in Gulf War Illness. BMC Med. Genom. 2009, 2, 12. [Google Scholar] [CrossRef]
- Craddock, T.J.A.; Del Rosario, R.R.; Rice, M.; Zysman, J.P.; Fletcher, M.A.; Klimas, N.G.; Broderick, G.; Chao, L. Achieving remission in gulf war illness: A simulation-based approach to treatment design. PLoS ONE 2015, 10, e0132774. [Google Scholar] [CrossRef]
- Ashbrook, D.G.; Hing, B.; Michalovicz, L.T.; Kelly, K.A.; Miller, J.V.; de Vega, W.C.; Miller, D.B.; Broderick, G.; O’cAllaghan, J.P.; McGowan, P.O. Epigenetic impacts of stress priming of the neuroinflammatory response to sarin surrogate in mice: A model of Gulf War illness. J. Neuroinflamm. 2018, 15, 86. [Google Scholar] [CrossRef]
- Naviaux, R.K. Metabolic features and regulation of the healing cycle—A new model for chronic disease pathogenesis and treatment. Mitochondrion 2019, 46, 278–297. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Wang, L.; Monk, J.M.; Bright, A.T.; Koslik, H.J.; Ritchie, J.B.; Golomb, B.A.; Chao, L. Metabolic features of Gulf War illness. PLoS ONE 2019, 14, e0219531. [Google Scholar] [CrossRef] [PubMed]
- Coller, J.K.; Tuke, J.; Wain, T.J.; Quinn, E.; Steele, L.; Abreu, M.; Aenlle, K.; Klimas, N.; Sullivan, K. Associations of immune genetic variability with gulf war illness in 1990–1991 gulf war veterans from the gulf war illness consortium (gwic) multisite case-control study. Brain Sci. 2021, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Tehrani, L.; Gamer, J.; Van Booven, D.J.; Ballarin, S.; Rossman, R.; Edelstein, A.; Uppalati, S.; Reuthebuck, A.; Collado, F.; et al. Gulf War Illness Induced Sex-Specific Transcriptional Differences Under Stressful Conditions. Int. J. Mol. Sci. 2025, 26, 3610. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.; Hauser, E.R.; Polimanti, R.; Helmer, D.A.; Provenzale, D.; McNeil, R.B.; Maffucci, A.; Quaden, R.; Zhao, H.; Whitbourne, S.B.; et al. Genomics of Gulf War Illness in U.S. veterans who served during the 1990–1991 persian Gulf War: Methods and rationale for Veterans Affairs Cooperative Study #2006. Brain Sci. 2021, 11, 845. [Google Scholar] [CrossRef]
- Yates, P.L.; Patil, A.; Sun, X.; Niceforo, A.; Gill, R.; Callahan, P.; Beck, W.; Piermarini, E.; Terry, A.V.; Sullivan, K.A.; et al. A cellular approach to understanding and treating Gulf War Illness. Cell. Mol. Life Sci. 2021, 78, 6941–6961. [Google Scholar] [CrossRef]
- Broderick, G.; Kreitz, A.; Fuite, J.; Fletcher, M.A.; Vernon, S.D.; Klimas, N. A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain Behav. Immun. 2011, 25, 302–313. [Google Scholar] [CrossRef]
- Terry, A.V., Jr.; Beck, W.D.; Zona, V.; Itokazu, Y.; Tripathi, A.; Madeshiya, A.K.; Pillai, A. Acute exposure to diisopropylfluorophosphate in mice results in persistent cognitive deficits and alterations in senescence markers in the brain. Front. Neurosci. 2024, 18, 1498350. [Google Scholar] [CrossRef]
- Jiang, R.; Westwater, M.L.; Noble, S.; Rosenblatt, M.; Dai, W.; Qi, S.; Sui, J.; Calhoun, V.D. Associations between grip strength, brain structure, and mental health in> 40,000 participants from the UK Biobank. BMC Med. 2022, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.E.; O’DOnnell, A.; Samra, J.; Gonzales, M.M.; Satizabal, C.; Pase, M.P.; Murabito, J.M.; Beiser, A.; Seshadri, S. Grip strength, gait speed and plasma markers of neurodegeneration in asymptomatic middle-aged and older adults. J. Frailty Aging 2022, 11, 291–298. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Veschi, S.; Cama, A.; Marconi, G.D.; Diomede, F.; Gesmundo, I.; Granata, R.; et al. Effects of growth hormone-releasing hormone receptor antagonist MIA-602 in mice with emotional disorders: A potential treatment for PTSD. Mol. Psychiatry 2021, 26, 7465–7474. [Google Scholar] [CrossRef]
- Schally, A.V.; Zhang, X.; Cai, R.; Hare, J.M.; Granata, R.; Bartoli, M. Actions and potential therapeutic applications of growth hormone–releasing hormone agonists. Endocrinology 2019, 160, 1600–1612. [Google Scholar] [CrossRef]
- Halmos, G.; Dobos, N.; Juhasz, E.; Szabo, Z.; Schally, A.V. Hypothalamic Releasing Hormones. In Hormonal Signaling in Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 43–68. [Google Scholar]
- Halmos, G.; Halmos, G.; Szabo, Z.; Juhasz, E.; Schally, A.V. Signaling mechanism of growth hormone-releasing hormone receptor. Vitam. Horm. 2023, 123, 1–26. [Google Scholar]
- Harada, C.N.; Love, M.C.N.; Triebel, K. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737. [Google Scholar] [CrossRef]
- Jorge, R. Growth Hormone Replacement Therapy in Veterans with Gulf War Illness and GH Deficiency; Baylor College of Medicine: Houston, TX, USA, 2022. [Google Scholar]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Spindler, M.; Palombo, M.; Zhang, H.; Thiel, C.M. Dysfunction of the hypothalamic-pituitary adrenal axis and its influence on aging: The role of the hypothalamus. Sci. Rep. 2023, 13, 6866. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Morley, J.E.; Farr, S.A.; Price, T.O.; Ercal, N.; Vidaurre, I.; Schally, A.V. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 22272–22277. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Bioletto, F.; Varaldo, E.; Gasco, V.; Maccario, M.; Arvat, E.; Ghigo, E.; Grottoli, S. Central and peripheral regulation of the GH/IGF-1 axis: GHRH and beyond. Rev. Endocr. Metab. Disord. 2024, 26, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rang, Y.; Liu, C. Effects of Caloric Restriction and Intermittent Fasting and Their Combined Exercise on Cognitive Functioning: A Review. Curr. Nutr. Rep. 2024, 13, 691–700. [Google Scholar] [CrossRef]
- O’lEary, J.; Georgeaux-Healy, C.; Serpell, L. The impact of continuous calorie restriction and fasting on cognition in adults without eating disorders. Nutr. Rev. 2024, 83, 146–159. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001, 933, 265–277. [Google Scholar] [CrossRef]
- Bobba-Alves, N.; Juster, R.-P.; Picard, M. The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology 2022, 146, 105951. [Google Scholar] [CrossRef]
- Bobba-Alves, N.; Sturm, G.; Lin, J.; Ware, S.A.; Karan, K.R.; Monzel, A.S.; Bris, C.; Procaccio, V.; Lenaers, G.; Higgins-Chen, A.; et al. Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging. Psychoneuroendocrinology 2023, 155, 106322. [Google Scholar] [CrossRef]
- Vaidya, N.; Marquand, A.F.; Nees, F.; Siehl, S.; Schumann, G. The impact of psychosocial adversity on brain and behaviour: An overview of existing knowledge and directions for future research. Mol. Psychiatry 2024, 29, 3245–3267. [Google Scholar] [CrossRef]
- Yadav, S.S.; Singh, M.K.; Yadav, R.S. Organophosphates induced Alzheimer’s disease: An epigenetic aspect. J. Clin. Epigenet. 2016, 2. [Google Scholar] [CrossRef]
- Jones, B.C.; Miller, D.B.; Lu, L.; Zhao, W.; Ashbrook, D.G.; Xu, F.; Mulligan, M.K.; Williams, R.W.; Zhuang, D.; Torres-Rojas, C.; et al. Modeling the genetic basis of individual differences in susceptibility to Gulf War Illness. Brain Sci. 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ashbrook, D.G.; Gao, J.; Starlard-Davenport, A.; Zhao, W.; Miller, D.B.; O’CAllaghan, J.P.; Williams, R.W.; Jones, B.C.; Lu, L. Genome-wide transcriptome architecture in a mouse model of Gulf War Illness. Brain Behav. Immun. 2020, 89, 209–223. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Rao, K.V.R.; Norenberg, M.D. Neuroinflammation in hepatic encephalopathy: Mechanistic aspects. J. Clin. Exp. Hepatol. 2015, 5, S21–S28. [Google Scholar] [CrossRef]
- Zou, J.; Li, J.; Wang, X.; Tang, D.; Chen, R. Neuroimmune modulation in liver pathophysiology. J. Neuroinflamm. 2024, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.; Villalon, A.; Li, R.; Elammari, M.; Price, A.; Steele, L.; Garcia, J.M.; Marcelli, M.; Jorge, R. Hormonal changes in veterans with Gulf War Illness. Life Sci. 2023, 328, 121908. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.M.; Sweatt, J.D. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005, 6, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. The stress of Gulf War syndrome. Nature 1998, 393, 308–309. [Google Scholar] [CrossRef]
- Spradling, K.D.; A Lumley, L.; Robison, C.L.; Meyerhoff, J.L.; Dillman, J.F. Transcriptional responses of the nerve agent-sensitive brain regions amygdala, hippocampus, piriform cortex, septum, and thalamus following exposure to the organophosphonate anticholinesterase sarin. J. Neuroinflamm. 2011, 8, 84. [Google Scholar] [CrossRef]
- Kelly, K.A.; Miller, D.B.; Bowyer, J.F.; O’cAllaghan, J.P. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. J. Neurochem. 2012, 122, 995–1009. [Google Scholar] [CrossRef]
- O’CAllaghan, J.P.; Michalovicz, L.T.; Kelly, K.A. Supporting a neuroimmune basis of Gulf War Illness. EBioMedicine 2016, 13, 5–6. [Google Scholar] [CrossRef]
- Sullivan, K.; O’callaghan, J.P. Advancing the Role of Neuroimmunity and Genetic Susceptibility in Gulf War Illness. Brain Sci. 2022, 12, 1068. [Google Scholar] [CrossRef]
- Gallagher, M.; Burwell, R.; Burchinal, M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav. Neurosci. 2015, 107, 618–626. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. JoVE 2017, 126, 55718. [Google Scholar]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. In Pre-Clinical Models: Techniques and Protocols; Humana: New York, NY, USA, 2019; Volume 1916, pp. 99–103. [Google Scholar]
| Peptides | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position of Amino Acid Residues | ||||||||||||||||
| 0 | 1 | 2 | 6 | 8 | 9 | 10 | 11 | 12 | 15 | 20 | 21 | 27 | 28 | 29 | 30 | |
| hGHGH * | H | Tyr | Ala | Phe | Asn | Ser | Tyr | Arg | Lys | Gly | Arg | Lys | Met | Ser | Arg | NH2 |
| MIA-690 ** | PhAc-Ada | Tyr | D-Arg | Cpa | Ala | Har | Fpa5 | His | Orn | Abu | His | Orn | Nle | D-Arg | Har | NH2 |
| MWM and GST | NORT and OFT | |
|---|---|---|
| Vehicle control | 10 (5♂ + 5♀) | 9 (4♂ + 5♀) |
| MIA-690 control | 14 (10♂ + 4♀) | 10 (5♂ + 5♀) |
| GWI | 10 (5♂ + 5♀) | 10 (5♂ + 5♀) |
| GWI + MIA-690 | 17 (8♂ + 9♀) | 10 (5♂ + 5♀) |
| Total number | 51 (22♂ + 29♀) | 39 (19♂ + 20♀) |
| Toxicant | DFP | LPS |
|---|---|---|
| Administration route | i.p. | s.c. |
| LD50 | 2.5 mg/kg (Males and Females) | 0.389 μg/kg (Females), 0.110 μg/kg (Males) 0.205 μg/kg (combined). |
| * Dose used | Starting: 2.0 mg/kg Maximal: 4.0 mg/kg | 0.05 (Males)–0.1 μg/kg (Females) |
| Dosage regime | Escalated injections 10% increments every 10 min | Single injection |
| Monitored signs of toxicity | Non-lethal signs: Mild dyspnea, general distress, mild lethargy (hypoactivity, hunched posture), moderate trembling, loss of hiding behavior, moderate colic, and gastrointestinal upset (arched tail, tenesmus, hyper-defecation). Severe signs of lethality: Polyuria, severe dyspnea, severe tremors (muscle fasciculations), ataxia, seizures, severe lethargy (sluggishness, stupor, prostration). | Mainly septic shock |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgueiro-Tosta, L.M.; Jayakumar, A.R.; Kochen, W.; Cai, R.; Sha, W.; Johnson, E.; O’Callaghan, J.; Jászberényi, M.; Schally, A.V.; Klimas, N. Evaluation of the Therapeutic Potential of Synthetic Growth Hormone-Releasing Hormone Antagonist MIA-690 as a Cognitive Modulator in a Mouse Model of Gulf War Illness. Int. J. Mol. Sci. 2025, 26, 8516. https://doi.org/10.3390/ijms26178516
Salgueiro-Tosta LM, Jayakumar AR, Kochen W, Cai R, Sha W, Johnson E, O’Callaghan J, Jászberényi M, Schally AV, Klimas N. Evaluation of the Therapeutic Potential of Synthetic Growth Hormone-Releasing Hormone Antagonist MIA-690 as a Cognitive Modulator in a Mouse Model of Gulf War Illness. International Journal of Molecular Sciences. 2025; 26(17):8516. https://doi.org/10.3390/ijms26178516
Chicago/Turabian StyleSalgueiro-Tosta, Luis Manuel, Arumugam Radhakrishnan Jayakumar, William Kochen, Renzhi Cai, Wei Sha, Erik Johnson, James O’Callaghan, Miklós Jászberényi, Andrew Victor Schally, and Nancy Klimas. 2025. "Evaluation of the Therapeutic Potential of Synthetic Growth Hormone-Releasing Hormone Antagonist MIA-690 as a Cognitive Modulator in a Mouse Model of Gulf War Illness" International Journal of Molecular Sciences 26, no. 17: 8516. https://doi.org/10.3390/ijms26178516
APA StyleSalgueiro-Tosta, L. M., Jayakumar, A. R., Kochen, W., Cai, R., Sha, W., Johnson, E., O’Callaghan, J., Jászberényi, M., Schally, A. V., & Klimas, N. (2025). Evaluation of the Therapeutic Potential of Synthetic Growth Hormone-Releasing Hormone Antagonist MIA-690 as a Cognitive Modulator in a Mouse Model of Gulf War Illness. International Journal of Molecular Sciences, 26(17), 8516. https://doi.org/10.3390/ijms26178516





