Latest Nanoparticles to Modulate Hypoxic Microenvironment in Photodynamic Therapy of Cervical Cancer: A Review of In Vivo Studies
Abstract
1. Introduction
2. H2O2 Decomposition
2.1. Manganese (II) Oxide
2.2. Metallic Nanozymes
2.3. FeOOH
3. Oxygen Generator
Endoperoxides
4. Inhibition of Mitochondrial Respiration
4.1. Nitrogen Monoxide (II)
4.2. Atovaquone
5. Conclusions
6. Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203, Correction in Lancet Glob. Health 2022, 10, e41. [Google Scholar] [CrossRef]
- Cervical Cancer Overview: Prevalence and Differences—ClinicalKey. Available online: https://www.Clinicalkey.com/#!/Content/playContent/1-S2.0-S0027968420300432?scrollTo=%23bib1 (accessed on 25 June 2025).
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie Und Nachsorge Der Patientin Mit Zervixkarzinom, Langversion, 2.2. 2022; AWMF-Registernummer: 032/033OL. Available online: https://www.Leitlinienprogramm-Onkologie.de (accessed on 17 August 2022).
- Ibeanu, O.A. Molecular Pathogenesis of Cervical Cancer. Cancer Biol. Ther. 2011, 11, 295–306. [Google Scholar] [CrossRef]
- Schubert, M.; Bauerschlag, D.O.; Muallem, M.Z.; Maass, N.; Alkatout, I. Challenges in the Diagnosis and Individualized Treatment of Cervical Cancer. Medicina 2023, 59, 925. [Google Scholar] [CrossRef]
- Yadav, G.; Srinivasan, G.; Jain, A. Cervical Cancer: Novel Treatment Strategies Offer Renewed Optimism. Pathol.-Res. Pract. 2024, 254, 155136. [Google Scholar] [CrossRef]
- Kashyap, N.; Krishnan, N.; Kaur, S.; Ghai, S. Risk Factors of Cervical Cancer: A Case-Control Study. Asia Pac. J. Oncol. Nurs. 2019, 6, 308–314. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key Changes to the World Health Organization (WHO) Classification of Female Genital Tumours Introduced in the 5th Edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Lax, S.F.; McCluggage, W.G. The Central Role of the Pathologist in the Management of Patients with Cervical Cancer: ESGO/ESTRO/ESP Guidelines. Virchows Arch. 2018, 473, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Chu, T.; Lin, S.; Wu, P.; Zhi, W.; Peng, T.; Ding, W.; Luo, D.; Wu, P. Clinicopathological Characteristics and Prognosis of Cervical Cancer with Different Histological Types: A Population-Based Cohort Study. Gynecol. Oncol. 2021, 163, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wen, H.; Feng, Z.; Han, X.; Wu, X. Distinctive Clinicopathologic Characteristics and Prognosis for Different Histologic Subtypes of Early Cervical Cancer. Int. J. Gynecol. Cancer 2019, 29, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines for Cervical Cancer n.d. Available online: https://www.Nccn.org/Professionals/Physician_gls/Pdf/Cervical.Pdf (accessed on 18 June 2023).
- Kokka, F.; Bryant, A.; Brockbank, E.; Jeyarajah, A. Surgical Treatment of Stage IA2 Cervical Cancer. Cochrane Database Syst. Rev. 2014, 2014, CD010870. [Google Scholar] [CrossRef]
- Dicu-Andreescu, I.-G.; Marincaș, A.-M.; Ungureanu, V.-G.; Ionescu, S.-O.; Prunoiu, V.-M.; Brătucu, E.; Simion, L. Current Therapeutic Approaches in Cervical Cancer Based on the Stage of the Disease: Is There Room for Improvement? Medicina 2023, 59, 1229. [Google Scholar] [CrossRef] [PubMed]
- Usami, T.; Takahashi, A.; Matoda, M.; Okamoto, S.; Kondo, E.; Kanao, H.; Umayahara, K.; Takeshima, N. Review of Treatment and Prognosis of Stage IVB Cervical Carcinoma. Int. J. Gynecol. Cancer 2016, 26, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Mahantshetty, U.; Engineer, R.; Chopra, S.; Hawaldar, R.; Hande, V.; Kerkar, R.A.; Maheshwari, A.; Shylasree, T.S.; Ghosh, J.; et al. Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Okuma, K.; Kawana, K.; Nakagawa, S.; Oda, K.; Yano, T.; Kobayashi, S.; Wakui, R.; Ohtomo, K.; Nakagawa, K. Comparison between Conventional Surgery plus Postoperative Adjuvant Radiotherapy and Concurrent Chemoradiation for FIGO Stage IIB Cervical Carcinoma: A Retrospective Study. Am. J. Clin. Oncol. 2010, 33, 583–586. [Google Scholar] [CrossRef]
- Poddar, P.; Maheshwari, A. Surgery for Cervical Cancer: Consensus & Controversies. Indian. J. Med. Res. 2021, 154, 284–292. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The current status of photodynamic therapy in cancer treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Myśliwiec, A.; Dynarowicz, K.; Krupka-Olek, M.; Bożek, A.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Current photodynamic therapy for glioma treatment: An update. Biomedicines 2024, 12, 375. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Liu, B.; Tan, Y.N. Emerging strategies in enhancing singlet oxygen generation of nano-photosensitizers toward advanced phototherapy. Nano-Micro Lett. 2022, 14, 123. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagnosis Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Austin, E.; Wang, J.Y.; Ozog, D.M.; Zeitouni, N.; Lim, H.W.; Jagdeo, J. Photodynamic therapy: Overview and mechanism of action. J. Am. Acad. Dermatol. 2025; in press. [Google Scholar] [CrossRef]
- De Silva, P.; Saad, M.A.; Thomsen, H.C.; Bano, S.; Ashraf, S.; Hasan, T. Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization—A Thomas Dougherty Award for Excellence in PDT paper. J. Porphyr. Phthalocyanines 2020, 24, 1320–1360. [Google Scholar] [CrossRef]
- Du, Y.; Han, J.; Jin, F.; Du, Y. Recent Strategies to Address Hypoxic Tumor Environments in Photodynamic Therapy. Pharmaceutics 2022, 14, 1763. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Peng, X.; Kim, J.S. From low to no O2-dependent hypoxia photodynamic therapy (hPDT): A new perspective. Acc. Chem. Res. 2022, 55, 3253–3264. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent Advances in Strategies for Overcoming Hypoxia in Photodynamic Therapy of Cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef]
- Hong, L.; Li, J.; Luo, Y.; Guo, T.; Zhang, C.; Ou, S.; Long, Y.; Hu, Z. Recent Advances in Strategies for Addressing Hypoxia in Tumor Photodynamic Therapy. Biomolecules 2022, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Pliss, A.M.; Zhan, Y.; Zheng, W.; Xia, J.; Liu, L.; Qu, J.; Prasad, P.N. Perfluoropolyether Nanoemulsion Encapsulating Chlorin E6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor. Nanomaterials 2020, 10, 2058. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, D.; Wang, G.; Wang, Y.; Cao, L.; Sun, J.; Jiang, Q.; He, Z. Recent Progress of Hypoxia-Modulated Multifunctional Nanomedicines to Enhance Photodynamic Therapy: Opportunities, Challenges, and Future Development. Acta Pharm. Sin. B 2020, 10, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.E.; Rockwell, S. Tumor Hypoxia: Its Impact on Cancer Therapy. Cancer Metastasis Rev. 1987, 5, 313–341. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; de la Guardia, M.; Ahmadi, D.; Yousefi, B. Modulating Tumor Hypoxia by Nanomedicine for Effective Cancer Therapy. J. Cell Physiol. 2018, 233, 2019–2031. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef]
- Du, J.; Shi, T.; Long, S.; Chen, P.; Sun, W.; Fan, J.; Peng, X. Enhanced Photodynamic Therapy for Overcoming Tumor Hypoxia: From Microenvironment Regulation to Photosensitizer Innovation. Coord. Chem. Rev. 2021, 427, 213604. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative Strategies for Photodynamic Therapy against Hypoxic Tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal miR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, Y.; Yu, P.; Wang, H.; Zhang, Y.; Xu, H.; Ye, Q.; Yuan, A.; Hu, Y.; Wu, J. Perfluorocarbon Regulates the Intratumoural Environment to Enhance Hypoxia-Based Agent Efficacy. Nat. Commun. 2019, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, W.; Wang, P.; Zhao, M.; Li, X.; Zhang, F. Near-Infrared Upconversion Mesoporous Cerium Oxide Hollow Biophotocatalyst for Concurrent pH-/H2 O2 -Responsive O2 -Evolving Synergetic Cancer Therapy. Adv. Mater. 2018, 30, 1704833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon Nanoparticles Enhance Reactive Oxygen Levels and Tumour Growth Inhibition in Photodynamic Therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef]
- Song, X.; Feng, L.; Liang, C.; Gao, M.; Song, G.; Liu, Z. Liposomes Co-Loaded with Metformin and Chlorin E6 Modulate Tumor Hypoxia during Enhanced Photodynamic Therapy. Nano Res. 2017, 10, 1200–1212. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor Acidity Activating Multifunctional Nanoplatform for NIR-Mediated Multiple Enhanced Photodynamic and Photothermal Tumor Therapy. Biomaterials 2018, 157, 107–124. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Z.; Song, R.; Lv, B.; Tang, Z.; Meng, X.; Chen, X.; Zheng, X.; Zhang, J.; Yao, Z.; et al. SnWO4-Based Nanohybrids with Full Energy Transfer for Largely Enhanced Photodynamic Therapy and Radiotherapy. Biomaterials 2018, 155, 135–144. [Google Scholar] [CrossRef]
- Zhou, T.-J.; Xing, L.; Fan, Y.-T.; Cui, P.-F.; Jiang, H.-L. Light Triggered Oxygen-Affording Engines for Repeated Hypoxia-Resistant Photodynamic Therapy. J. Control. Release 2019, 307, 44–54. [Google Scholar] [CrossRef]
- Zhou, R.; Zeng, X.; Zhao, H.; Chen, Q.; Wu, P. Combating the Hypoxia Limit of Photodynamic Therapy through Reversing the Survival-Related Pathways of Cancer Cells. Coord. Chem. Rev. 2022, 452, 214306. [Google Scholar] [CrossRef]
- Yan, W.; Lang, T.; Zhu, R.; Zhu, X.; Li, Y.; Wu, T.; Yin, Q.; Li, Y. Anti-Hypoxia Nanosized Drug Delivery Systems Improving Cancer Therapy. Nano Today 2022, 42, 101376. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. HIF-1α Inhibition Reverses Multidrug Resistance in Colon Cancer Cells via Downregulation of MDR1/P-Glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Annilo, T. Evolution of the ATP-Binding Cassette (ABC) Transporter Superfamily in Vertebrates. Annu. Rev. Genom. Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef]
- Cerrada, I.; Ruiz-Saurí, A.; Carrero, R.; Trigueros, C.; Dorronsoro, A.; Sanchez-Puelles, J.M.; Diez-Juan, A.; Montero, J.A.; Sepúlveda, P. Hypoxia-Inducible Factor 1 Alpha Contributes to Cardiac Healing in Mesenchymal Stem Cells-Mediated Cardiac Repair. Stem Cells Dev. 2013, 22, 501–511. [Google Scholar] [CrossRef]
- Gui, L.; Liu, B.; Lv, G. Hypoxia Induces Autophagy in Cardiomyocytes via a Hypoxia-Inducible Factor 1-Dependent Mechanism. Exp. Ther. Med. 2016, 11, 2233–2239. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Yao, W.; Alsiddig, M.C.; Huo, L.; Liu, H.; Miao, Y.-L. Hypoxia-Inducible Factor-1α-Dependent Autophagy Plays a Role in Glycolysis Switch in Mouse Granulosa Cells. Biol. Reprod. 2018, 99, 308–318. [Google Scholar] [CrossRef]
- Abdul Rahim, S.A.; Dirkse, A.; Oudin, A.; Schuster, A.; Bohler, J.; Barthelemy, V.; Muller, A.; Vallar, L.; Janji, B.; Golebiewska, A.; et al. Regulation of Hypoxia-Induced Autophagy in Glioblastoma Involves ATG9A. Br. J. Cancer 2017, 117, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of Hypoxia in Cancer Therapy by Regulating the Tumor Microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex That Signals to the Cell Growth Machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Ro, S.-H.; Cao, J.; Otto, N.M.; Kim, D.-H. mTOR Regulation of Autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Rosin, F.C.P.; Teixeira, M.G.; Pelissari, C.; Corrêa, L. Resistance of Oral Cancer Cells to 5-ALA-Mediated Photodynamic Therapy. J. Cell Biochem. 2018, 119, 3554–3562. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, R.; Xiao, D.; Shi, S.; Peng, S.; Wu, S.; Wu, P.; Lin, Y. Polypeptide Uploaded Efficient Nanophotosensitizers to Overcome Photodynamic Resistance for Enhanced Anticancer Therapy. Chem. Eng. J. 2021, 403, 126344. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health Effects—Pros and Cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells1. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Liu, C.-P.; Wu, T.-H.; Liu, C.-Y.; Chen, K.-C.; Chen, Y.-X.; Chen, G.-S.; Lin, S.-Y. Self-Supplying O2 through the Catalase-Like Activity of Gold Nanoclusters for Photodynamic Therapy against Hypoxic Cancer Cells. Small 2017, 13, 1700278. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nishikawa, M.; Hyoudou, K.; Yamashita, F.; Hashida, M. Hydrogen Peroxide-mediated Nuclear Factor κB Activation in Both Liver and Tumor Cells during Initial Stages of Hepatic Metastasis. Cancer Sci. 2008, 99, 1546–1552. [Google Scholar] [CrossRef]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1alpha Promoter via a Functional NFkappaB Site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jia, X.; Bai, J.; Ruan, Y.; Wang, C.; Li, J.; Zhang, M.; Jiang, X. MnO2 Gatekeeper: An Intelligent and O2-Evolving Shell for Preventing Premature Release of High Cargo Payload Core, Overcoming Tumor Hypoxia, and Acidic H2O2-Sensitive MRI. Adv. Funct. Mater. 2017, 27, 1604258. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Bai, Z.; Cui, Z.; Liang, W.; Zhang, J.; Li, K.; Shi, M.; Liu, Z.; Wang, J.; et al. Progressive Enhanced Photodynamic Therapy and Enhanced Chemotherapy Fighting against Malignant Tumors with Sequential Drug Release. Biomed. Mater. 2024, 19, 045004. [Google Scholar] [CrossRef]
- Cai, J.; Yang, Y.; Zhang, J.; Bai, Z.; Zhang, X.; Li, K.; Shi, M.; Liu, Z.; Gao, L.; Wang, J.; et al. Multilayer Nanodrug Delivery System with Spatiotemporal Drug Release Improves Tumor Microenvironment for Synergistic Anticancer Therapy. Biofabrication 2024, 16, 025012. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor Cell-Targeting and Tumor Microenvironment–Responsive Nanoplatforms for the Multimodal Imaging-Guided Photodynamic/Photothermal/Chemodynamic Treatment of Cervical Cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.-J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct Evidence for Catalase and Peroxidase Activities of Ferritin–Platinum Nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Byun, S.W.; Shin, H.; Ju, Y.; Min, H.; Kim, Y.J.; Heo, I.; Hazlett, M.J.; Kim, M.; et al. Impact of Pd:Pt Ratio of Pd/Pt Bimetallic Catalyst on CH4 Oxidation. Appl. Catal. B Environ. 2022, 316, 121623. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Li, J. X-Ray Triggered Scintillator Versatile Nanocatalytic Platform for Synergistic Photodynamic/Chemodynamic Therapy. Talanta 2025, 281, 126886. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, Y.-D.; Sun, Y.-Q.; Wang, Y.; Yin, X.-B. “Three-in-One” Nanozyme Composite for Augmented Cascade Catalytic Tumor Therapy. Adv. Mater. 2024, 36, 2308033. [Google Scholar] [CrossRef]
- Qhattal, H.S.S.; Liu, X. Characterization of CD44-Mediated Cancer Cell Uptake and Intracellular Distribution of Hyaluronan-Grafted Liposomes. Mol. Pharm. 2011, 8, 1233–1246. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome Maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, Y.; Jiang, Y.; Luo, S.; Li, M.; Cao, Y.; Ma, Y.; Tang, B. A Novel Multi-Carboxyl Functionalized MOF Platform for Effective Photodynamic Therapy with Hypoxia Modulation Based on Prominent Self-Oxygen Generation. Inorg. Chem. Front. 2024, 11, 1186–1197. [Google Scholar] [CrossRef]
- Benz, S.; Nötzli, S.; Siegel, J.S.; Eberli, D.; Jessen, H.J. Controlled Oxygen Release from Pyridone Endoperoxides Promotes Cell Survival under Anoxic Conditions. J. Med. Chem. 2013, 56, 10171–10182. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V. Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules 2021, 26, 686. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.B.; Hu, L. Natural Endoperoxides as Drug Lead Compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef]
- Zhu, P.; Tong, B.M.; Wang, R.; Chen, J.P.; Foo, S.; Chong, H.C.; Wang, X.L.; Ang, G.Y.; Chiba, S.; Tan, N.S. Nox4-Dependent ROS Modulation by Amino Endoperoxides to Induce Apoptosis in Cancer Cells. Cell Death Dis. 2013, 4, e552. [Google Scholar] [CrossRef]
- Berdelle, N.; Nikolova, T.; Quiros, S.; Efferth, T.; Kaina, B. Artesunate Induces Oxidative DNA Damage, Sustained DNA Double-Strand Breaks, and the ATM/ATR Damage Response in Cancer Cells. Mol. Cancer Ther. 2011, 10, 2224–2233. [Google Scholar] [CrossRef]
- Zhou, R.; Lv, W.; Li, B.; Yu, B.; Zhang, S.; Zhou, Y.; Liu, S.; Zhao, Q. Reversibly Sensitizing-Storing-Releasing 1O2 within a Single Platinum(II)-Acetylide-Based Metallacycle Molecule via Laser Power Modulation. Sci. China Chem. 2024, 67, 604–611. [Google Scholar] [CrossRef]
- Bettink, M.A.W.; Arbous, M.S.; Raat, N.J.H.; Mik, E.G. Mind the Mitochondria! J. Emerg. Crit. Care Med. 2019, 3, 45. [Google Scholar] [CrossRef]
- Zheng, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Zhao, Y.; Lv, B.; Xue, G.; Sun, Y.; Cao, J. Smart Nanosystem-Mediated Inhibition of Mitochondrial Respiration for Enhanced Phototherapy-Induced Antitumor Immunity. Int. J. Nanomed. 2023, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Chen, X.; Liu, Y.; Yu, B.; Yan, M.; Yang, N.; Kong, R.; Li, S.; Ti, H.; Cheng, H. Drug Induced Mitochondria Dysfunction to Enhance Photodynamic Therapy of Hypoxic Tumors. J. Control. Release 2023, 358, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Ridnour, L.A.; McGinity, C.L.; Bhattacharyya, D.; Wink, D.A. Nitric Oxide and Cancer: When to Give and When to Take Away? Inorg. Chem. 2021, 60, 15941–15947. [Google Scholar] [CrossRef] [PubMed]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric Oxide and Its Derivatives in the Cancer Battlefield. Nitric Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; McStay, G.P. Functions of Cytochrome c Oxidase Assembly Factors. Int. J. Mol. Sci. 2020, 21, 7254. [Google Scholar] [CrossRef]
- Fry, N.L.; Mascharak, P.K. Photoactive Ruthenium Nitrosyls as NO Donors: How to Sensitize Them toward Visible Light. Acc. Chem. Res. 2011, 44, 289–298. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Chen, H.; Wu, Y.; Liu, J.; Che, H.; Zhang, Y.; Zhu, X. Motor-Cargo Structured Nanotractors for Augmented NIR Phototherapy via Gas-Boosted Tumor Penetration and Respiration-Impaired Mitochondrial Dysfunction. Adv. Healthc. Mater. 2024, 13, e2402063. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, T.; Zhong, X. NIR-Triggered NO Production Combined with Photodynamic Therapy for Tumor Treatment. Photodiagnosis Photodyn. Ther. 2024, 49, 104241. [Google Scholar] [CrossRef]
- Janczyk, A.; Wolnicka-Glubisz, A.; Chmura, A.; Elas, M.; Matuszak, Z.; Stochel, G.; Urbanska, K. NO-Dependent Phototoxicity of Roussin’s Black Salt against Cancer Cells. Nitric Oxide 2004, 10, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-S.; Zeng, J.-Y.; Cheng, H.; Zhang, X.-Z. ROS-Induced NO Generation for Gas Therapy and Sensitizing Photodynamic Therapy of Tumor. Biomaterials 2018, 185, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, M.; Cheng, J.; Yin, J.; Huang, C.; Cui, H.; Zhang, X.; Zhao, G. Acidity-Triggered Tumor-Targeted Nanosystem for Synergistic Therapy via a Cascade of ROS Generation and NO Release. ACS Appl. Mater. Interfaces 2020, 12, 28975–28984. [Google Scholar] [CrossRef]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial Pharmacology and Therapeutics of Atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The Anti-Malarial Atovaquone Increases Radiosensitivity by Alleviating Tumour Hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef]
- Li, M.; Shao, Y.; Kim, J.H.; Pu, Z.; Zhao, X.; Huang, H.; Xiong, T.; Kang, Y.; Li, G.; Shao, K.; et al. Unimolecular Photodynamic O2-Economizer To Overcome Hypoxia Resistance in Phototherapeutics. J. Am. Chem. Soc. 2020, 142, 5380–5388. [Google Scholar] [CrossRef]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Pan, Z.; Xu, C.; Zhang, X.; Li, M.; Wang, W.; Jia, F.; Wu, Y. OXPHOS-Targeted Nanoparticles for Boosting Photodynamic Therapy against Hypoxia Tumor. Int. J. Pharm. 2024, 654, 123943. [Google Scholar] [CrossRef]
| Inclusion Criteria |
|---|
| Articles describing photodynamic therapy |
| Articles describing cancer therapy |
| Articles describing nanoparticles |
| Articles published in 2024 and by March 2025 |
| Exclusion Criteria |
| Articles describing nanoparticles without a strategy for overcoming hypoxia |
| Articles describing cancers other than cervical cancer |
| Articles other than original research papers |
| Articles in which the results of therapy were described only in vitro |
| Articles in a language other than English and Polish |
| Nanoparticle | Construction | Hypoxia Reversal Method | Effectiveness of Hypoxia Reversal | Therapeutic Efficacy | Reference |
|---|---|---|---|---|---|
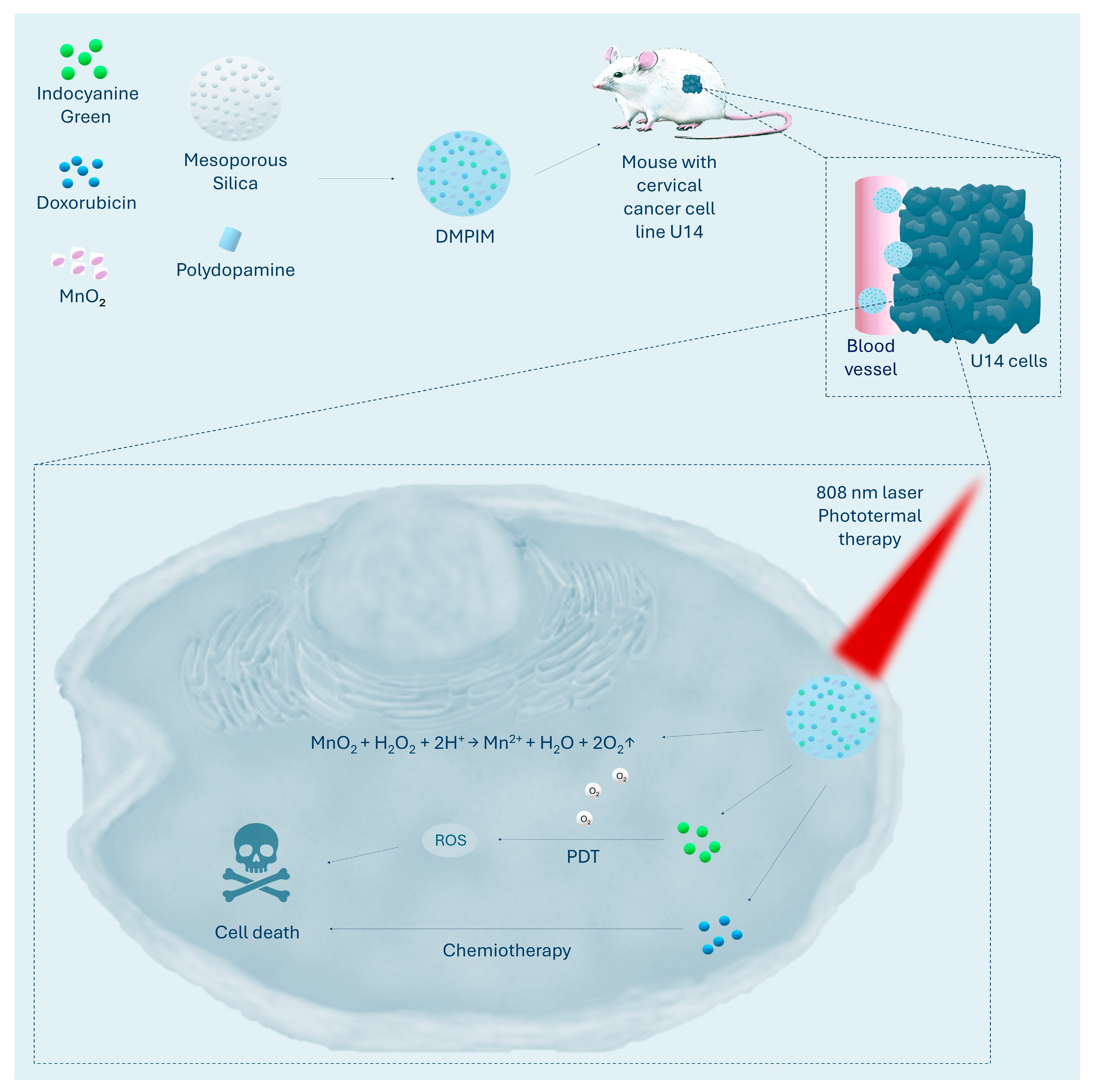
| DMPIM | Mesoporous silica + MnO2 + green indocyanine + doxorubicin + polydopamine | Decomposition of H2O2 to O2 via MnO2 | Increase in ROS production relative to NP without MnO2 | 92.91% inhibition of tumour growth in a mouse model of U14 | [68] |
| DPIMGC | Doxorubicin + polidopamine + green indocyanine + MnO2 + gelatin with celecoxib | Decomposition of H2O2 to O2 via MnO2 | Increased ROS production relative to NP without MnO2, decreased HIF-1α in musim model U14 | 91.47% inhibition of tumour growth in a mouse model of U14 | [67] |
| M-HMnO2@ICG | MnO2 core + poly(allylamine hydrochloride) + green indocyanine + HeLa cell membrane | Distribution of H2O2 to O2 via MnO2 | Downregulation of HIF-1α in a mouse model of HeLa | 88% inhibition of tumour growth in a mouse model of HeLa | [70] |
| LRZAPH | LuAG:Tb/Ce + Rose Bengal + ZIF-8 + Au2Pt + hyaluronic acid | Catalase/peroxidase activity Au2Pt | Increase in ROS production relative to NP without Au2Pt 1 | 93% inhibition of tumour growth in a mouse model of HeLa | [73] |
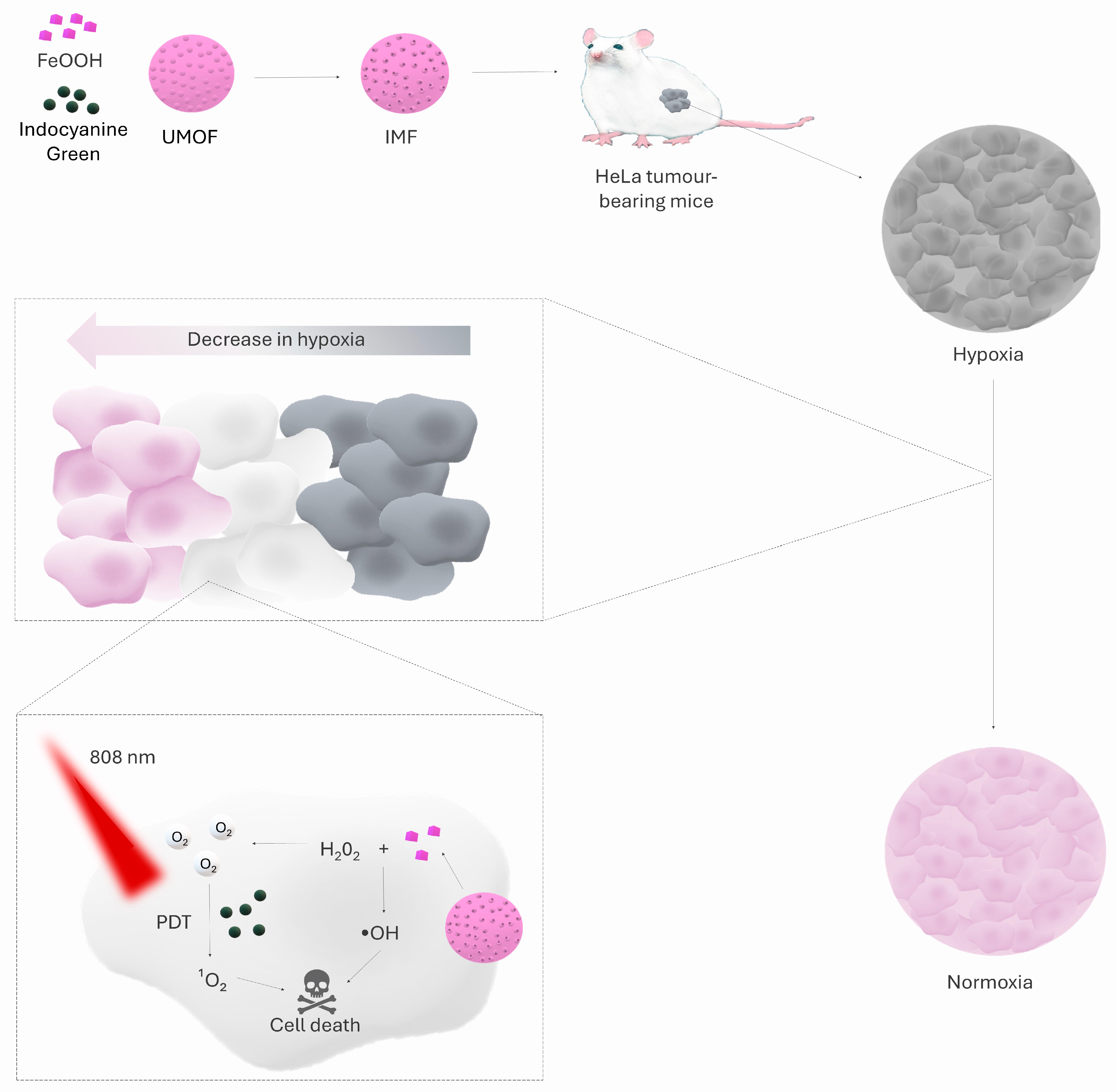
| IMF (ICG ⊂ UMOF@FeOOH) | UMOF + FeOOH + ICG coating | Decomposition of H2O2 to O2 via FeOOH | Downregulation of HIF-1α in normoxia and hypoxia | Complete regression of 2/3 of tumours in a mouse model of HeLa | [78] |
| M1-EPO-NPs | Metallacycle + aza-BODIPY + endoperoxides + DSPE-PEG2000 | Release of O2 from endoperoxides | Generation of singlet oxygen in both normoxia and hypoxia by M1-EPO-NPs relative to NPs without endoperoxides, in which 1O2 production occurred exclusively in normoxia | Complete tumour regression in a mouse model of HeLa | [84] |
| UM-RZ | UCNP + mesoporous silica + ZnPc + Roussin’s Black Salt | NO-mediated inhibition of mitochondrial respiration | Activation of ZnPc with 808 nm light led to a decrease in oxygen levels to ≈20%, while pre-exposure to 980 nm (UM-RZ activation and NO release) maintained oxygen levels at ≈70%. | 86% tumour inhibition in a mouse model of HeLa | [93] |
| UCN@mSiO2@ZnPc@L-Arg | UCNP + mesoporous silica + ZnPc + L-arginine | NO-mediated inhibition of mitochondrial respiration | No clear assessment of hypoxia | 78.5% inhibition of tumour growth in a mouse model of HeLa | [94] |
| TNPs/IA | mPEG-PLGA + PLGA-b-PEG + IR780 + atovaquone + TPP+ | OXPHOS inhibition (atovaquone) | Increased ROS production under hypoxia relative to NP without atovaquone | Highest in vivo efficacy in a mouse model of HeLa relative to control groups | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Saad, M.A.; Przygórzewska, A.; Woźnicki, P.; Aebisher, D. Latest Nanoparticles to Modulate Hypoxic Microenvironment in Photodynamic Therapy of Cervical Cancer: A Review of In Vivo Studies. Int. J. Mol. Sci. 2025, 26, 8503. https://doi.org/10.3390/ijms26178503
Bartusik-Aebisher D, Saad MA, Przygórzewska A, Woźnicki P, Aebisher D. Latest Nanoparticles to Modulate Hypoxic Microenvironment in Photodynamic Therapy of Cervical Cancer: A Review of In Vivo Studies. International Journal of Molecular Sciences. 2025; 26(17):8503. https://doi.org/10.3390/ijms26178503
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Mohammad A. Saad, Agnieszka Przygórzewska, Paweł Woźnicki, and David Aebisher. 2025. "Latest Nanoparticles to Modulate Hypoxic Microenvironment in Photodynamic Therapy of Cervical Cancer: A Review of In Vivo Studies" International Journal of Molecular Sciences 26, no. 17: 8503. https://doi.org/10.3390/ijms26178503
APA StyleBartusik-Aebisher, D., Saad, M. A., Przygórzewska, A., Woźnicki, P., & Aebisher, D. (2025). Latest Nanoparticles to Modulate Hypoxic Microenvironment in Photodynamic Therapy of Cervical Cancer: A Review of In Vivo Studies. International Journal of Molecular Sciences, 26(17), 8503. https://doi.org/10.3390/ijms26178503








