Prunus mume Extract Inhibits SARS-CoV-2 and Influenza Virus Infection In Vitro by Directly Targeting Viral Particles
Abstract
1. Introduction
2. Results
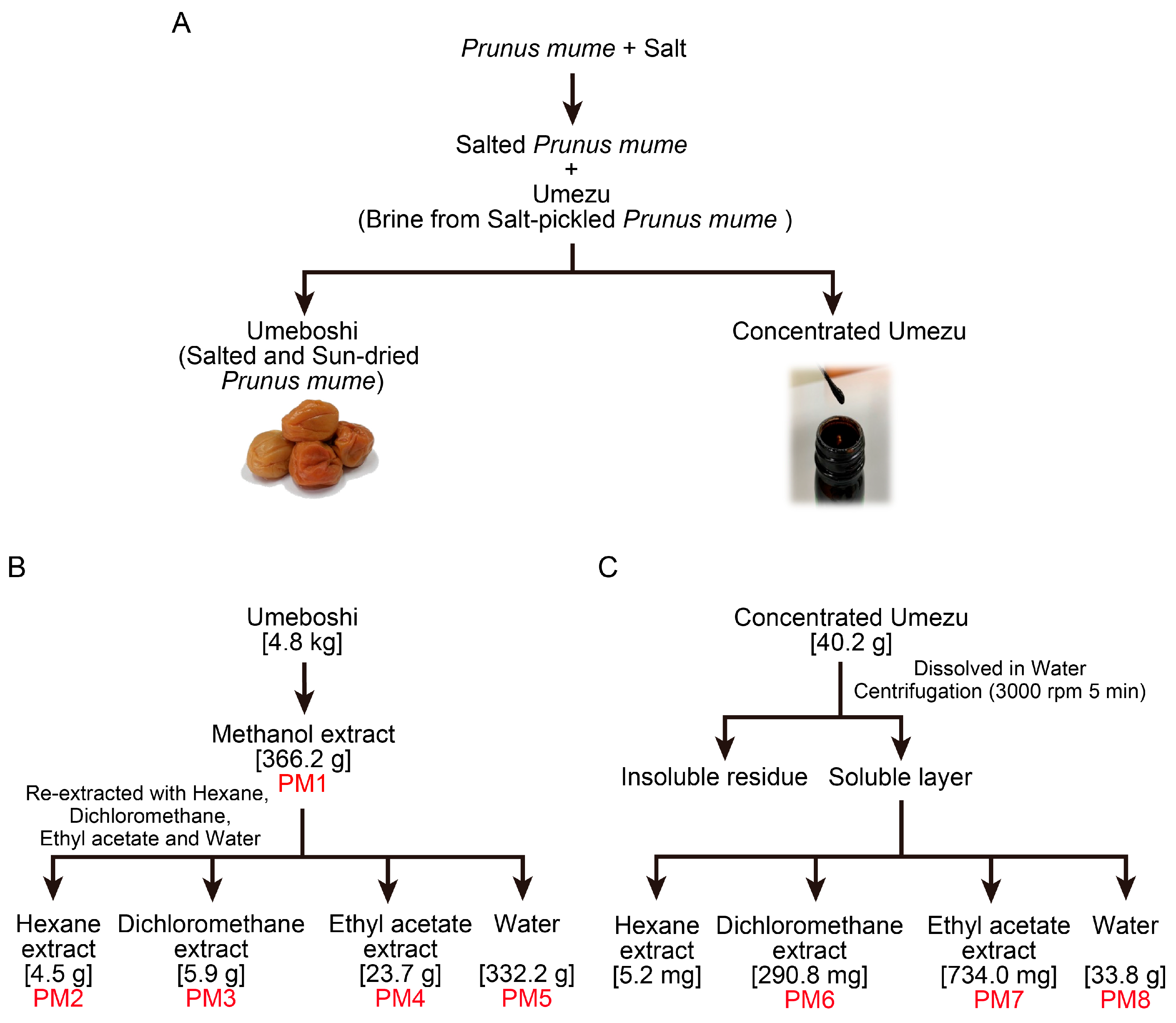
2.1. Preparation of P. mume Extracts for Antiviral Evaluation
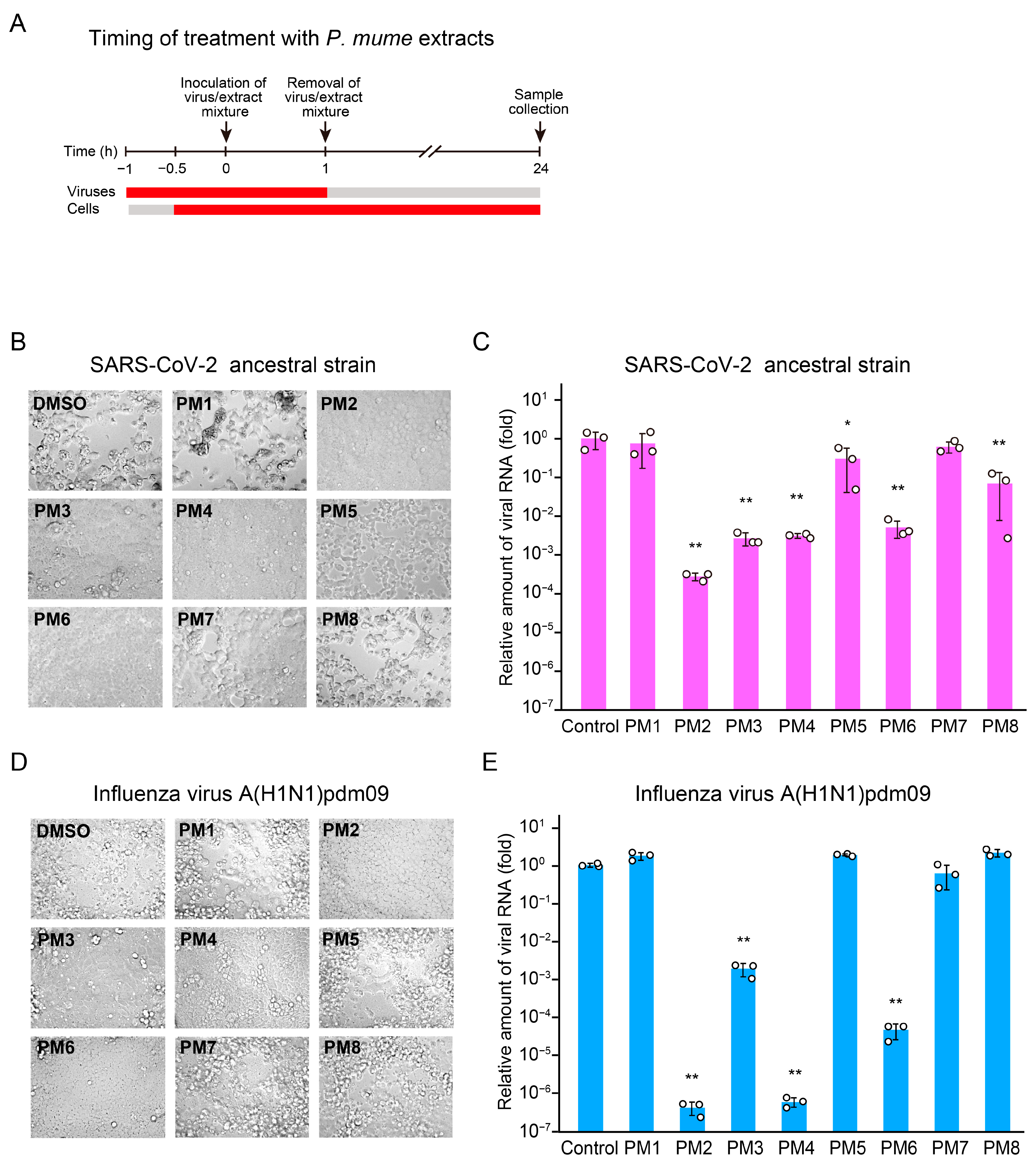
2.2. PM2, PM3, PM4, and PM6 Inhibit Both SARS-CoV-2 and Influenza Virus Replication upon Treatment of Both Viruses and Host Cells
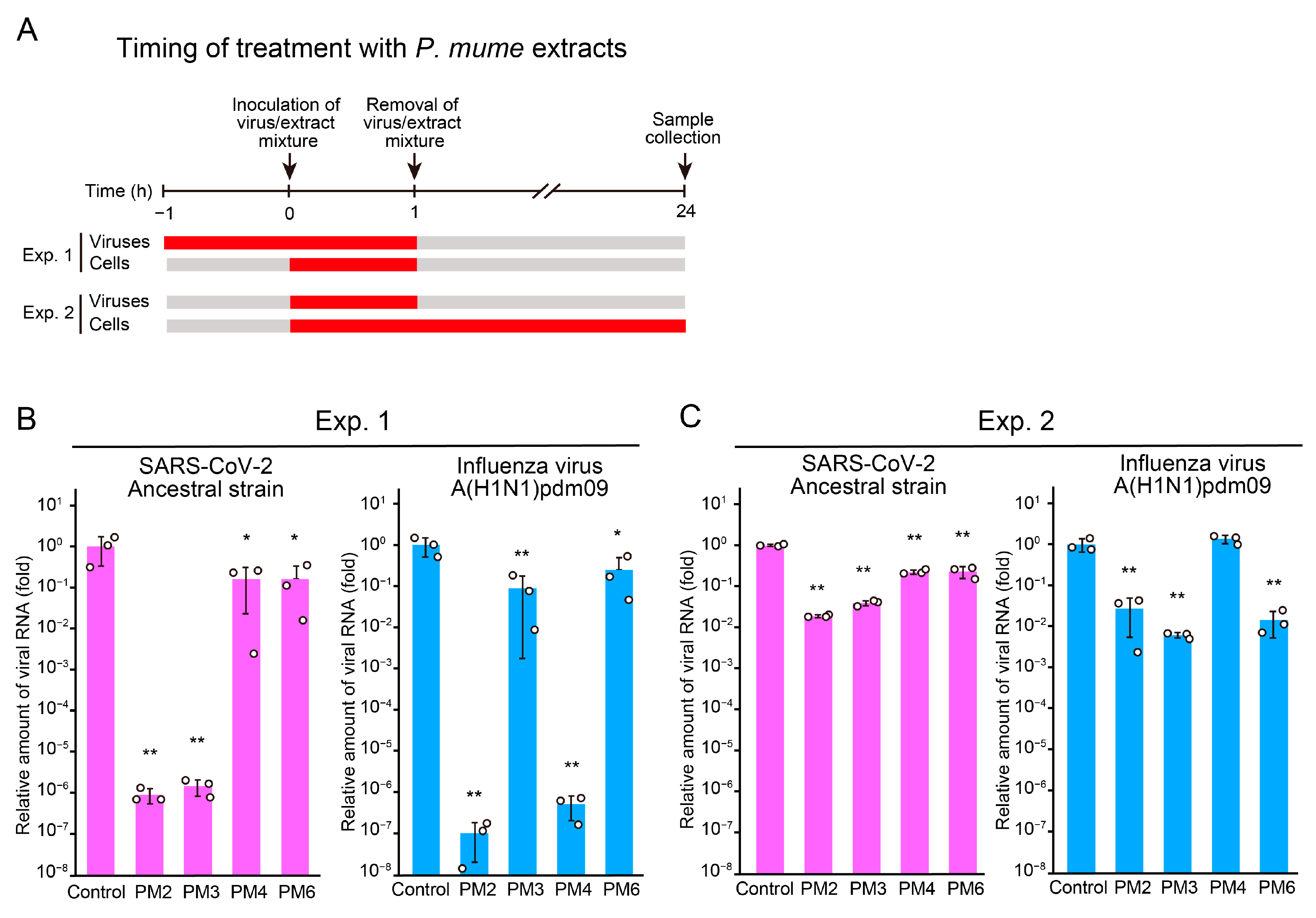
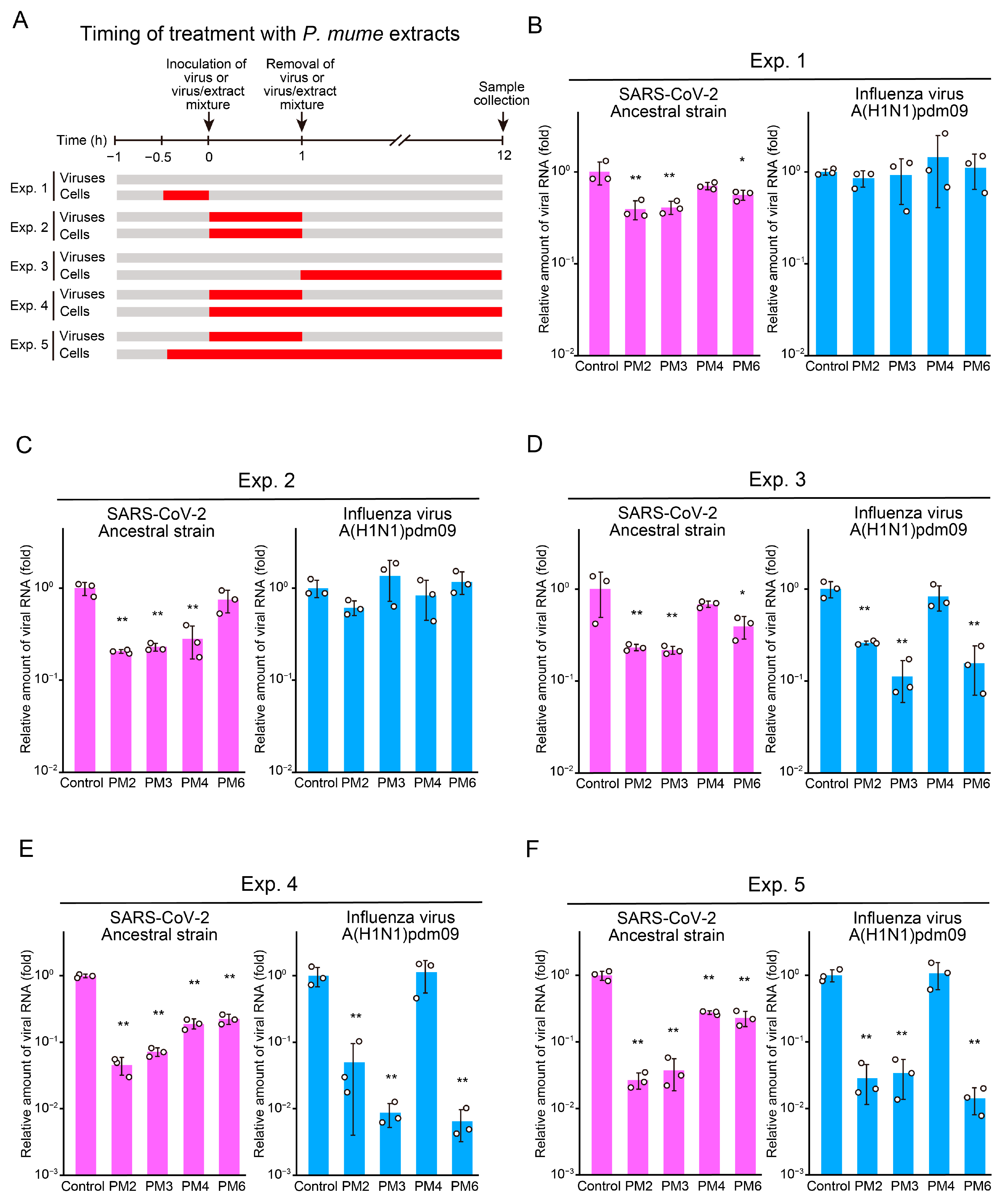
2.3. Time-of-Addition Assays to Identify Targets and Stages of Action of Active P. mume Extracts
2.4. PM2 and PM3 Exhibit Direct Antiviral Activity Against SARS-CoV-2 Virions
2.5. PM2 and PM4 Exhibit Direct Antiviral Activity Against Influenza Virus
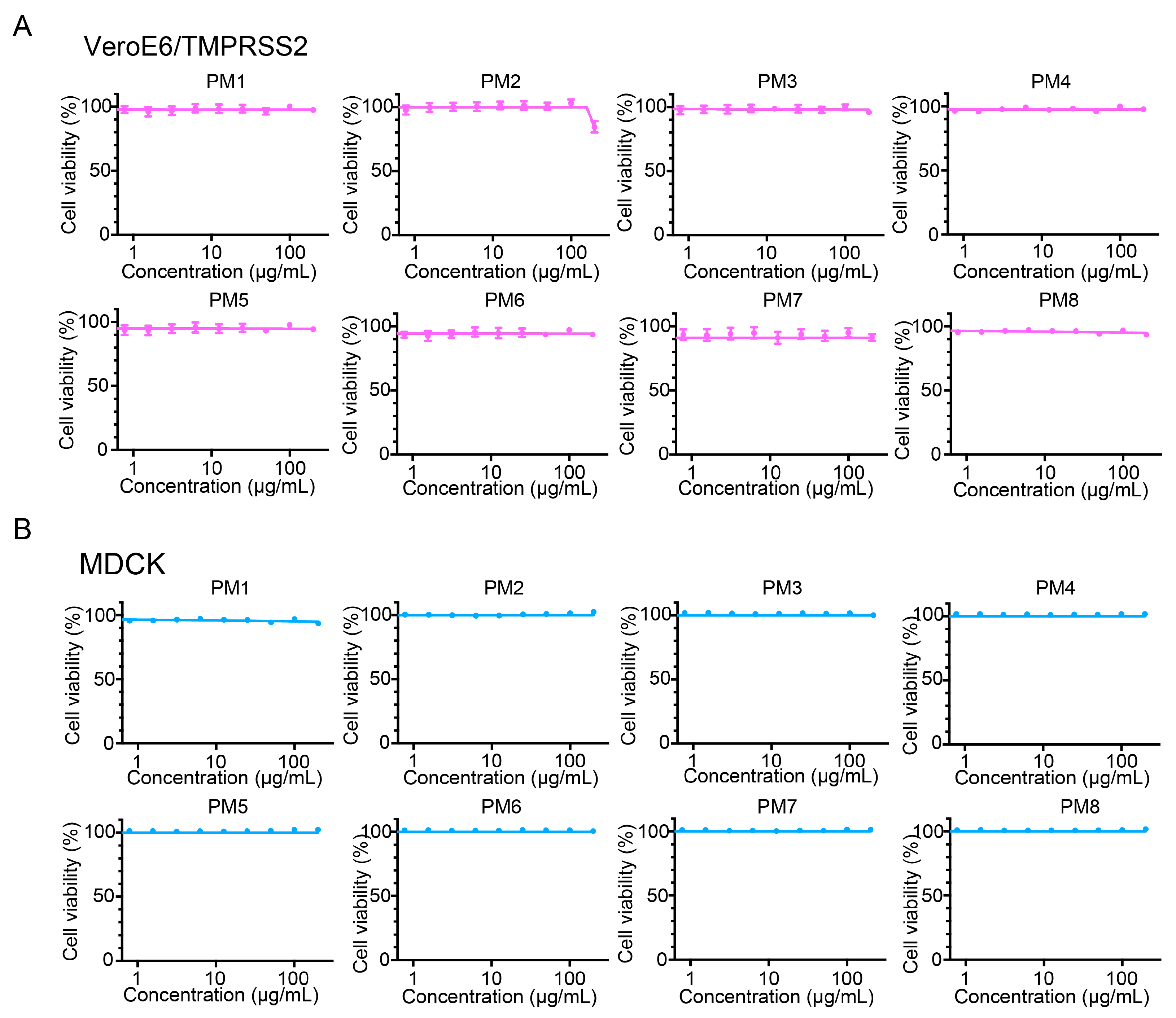
2.6. P. mume Extracts Are Non-Cytotoxic to VeroE6/TMPRSS2 and MDCK Cells up to 200 µg/mL
2.7. EC50, EC90, CC50, and Selectivity Index (SI) of Active P. mume Extracts
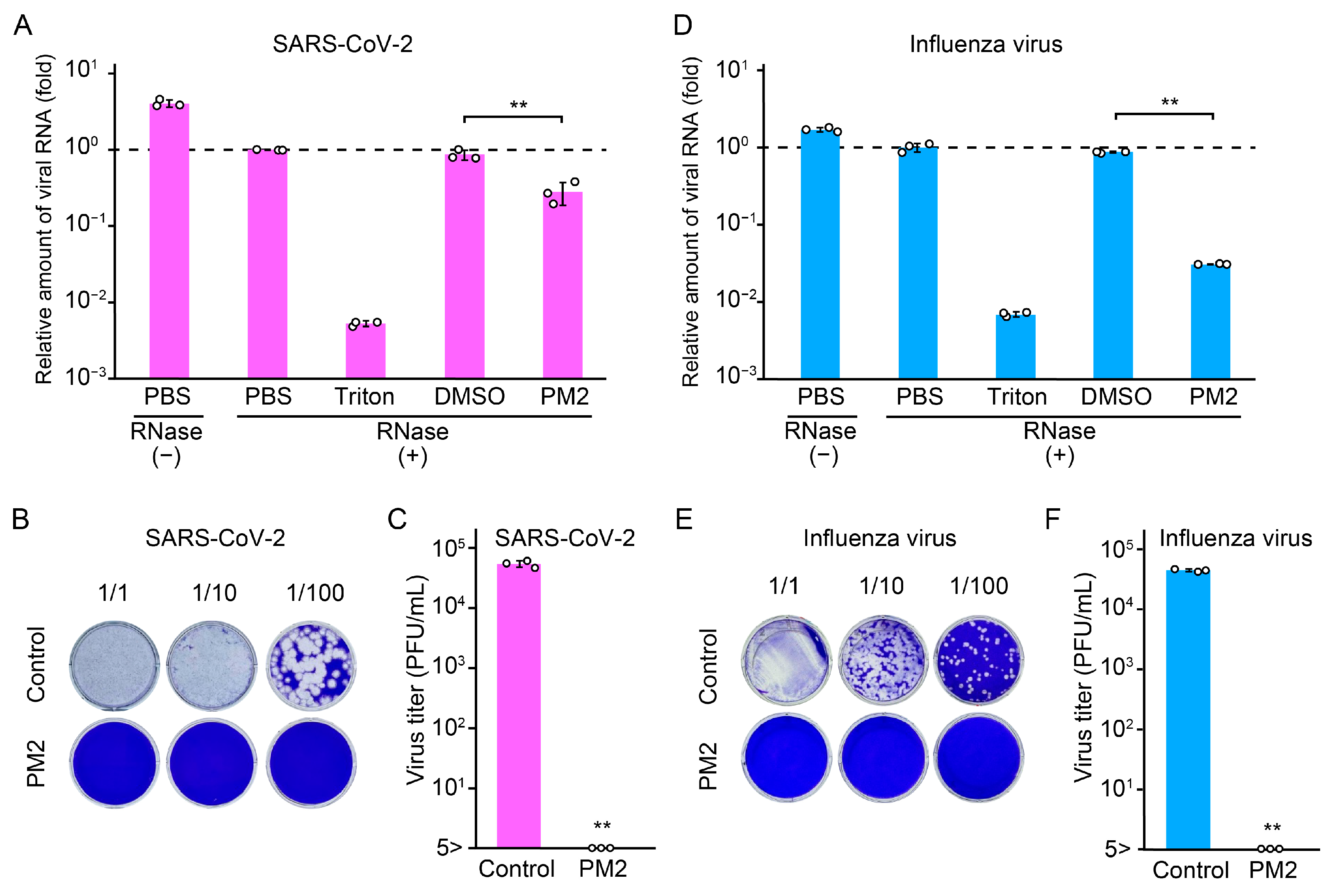
2.8. PM2 Directly Damages SARS-CoV-2 and Influenza Virus Particles
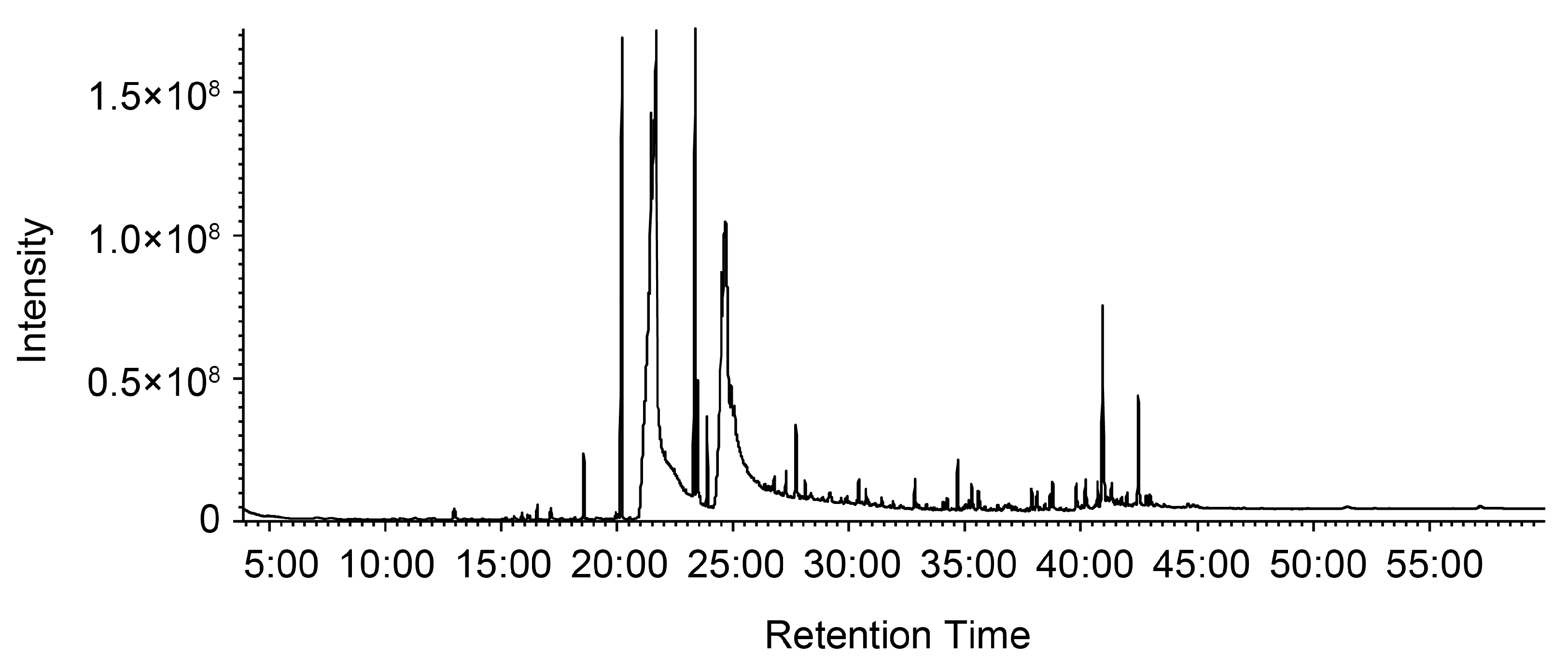
2.9. Gas Chromatograpy–Mass Spectrometry (GC-MS) Analysis of PM2 to Identify Antiviral Compounds
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Extraction of Fractions from P. mume Fruit (PM1–PM5)
4.3. Extraction of Fractions from P. mume Pickling Brine (PM6–PM8)
4.4. Cytopathic Effect (CPE) Assay with Full-Time Treatment of Viruses and Host Cells with P. mume Extracts
4.5. RNA Extraction
4.6. Reverse Transcription Quantitative PCR (RT-qPCR)
- Forward primer: NIID_2019-nCOV_N_F2, 5′-AAATTTTGGGGACCAGGAAC-3′
- Reverse primer: NIID_2019-nCOV_N_R2v3, 5′-TGGCACCTGTGTAGGTCAAC-3′
- Probe: NIID_2019-nCOV_N_P2, 5′-FAM-ATGTCGCGCATTGGCATGGA-BHQ-3′
- Thermal cycling: 55 °C for 10 min, 95 °C for 3 min, followed by 40 cycles at 95 °C for 15 s and 58 °C for 30 s.
- Influenza forward primer: FluV-F, 5′-CACCTGATATTGTGGATTACTGATCG-3′
- Influenza reverse primer: FluV-R, 5′-CACTCTGCTGTTCCTGTTGATATTC-3′
- Influenza probe: FluV-P, 5′-FAM-CCTCATGGACTCAGGCACTCCTTCCG-TAMRA-3′
- 18S forward primer: 18S-F, 5′-GTAACCCGTTGAACCCCATT-3′
- 18S reverse primer: 18S-R, 5′-CCATCCAATCGGTAGTAGCG-3′
- 18S probe: 18S-P, 5′-FAM-TGCGTTGATTAAGTCCCTGCCCTTTGTA-TAMRA-3′
- Thermal cycling: 50 °C for 5 min, 95 °C for 20 s, followed by 40 cycles at 95 °C for 1 s and 60 °C for 20 s.
4.7. Time-of-Addition Assay
4.8. Plaque Assay
4.9. MTS Assay
4.10. Transmission Electron Microscopy (TEM) of Virions Treated with PM2
4.11. Immunoelectron Microscopy (IEM) of Virions Treated with PM2
4.12. Virion Integrity Assay
4.13. Statistical Analysis and Calculation of 50% Effective Concentrations (EC50)
4.14. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 580, E7, Erratum in Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Nicholson, K.G. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol. Infect. 1996, 116, 51–63. [Google Scholar] [CrossRef]
- Hayden, F.G. Update on influenza and rhinovirus infections. Adv. Exp. Med. Biol. 1999, 458, 55–67. [Google Scholar] [CrossRef]
- Glezen, W.P.; Greenberg, S.B.; Atmar, R.L.; Piedra, P.A.; Couch, R.B. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000, 283, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Andrews, N.J.; Trotter, C.L.; Edmunds, W.J. Estimating deaths due to influenza and respiratory syncytial virus. JAMA 2003, 289, 2499, author reply 2500–2492. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1262, Correction in Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 13 June 2025).
- McGeoch, D.; Fellner, P.; Newton, C. Influenza virus genome consists of eight distinct RNA species. Proc. Natl. Acad. Sci. USA 1976, 73, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Yavuz, S. COVID-19: An Update on Epidemiology, Prevention and Treatment, September-2023. Infect. Dis. Clin. Microbiol. 2023, 5, 165–187. [Google Scholar] [CrossRef]
- Swierczynska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Sabu, J.M.; Zahid, I.; Jacob, N.; Alele, F.O.; Malau-Aduli, B.S. Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1880. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Tsvetnitsky, V.; Donnelly, J.J. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016, 34, 2926–2933. [Google Scholar] [CrossRef]
- Keshavarz, M.; Mirzaei, H.; Salemi, M.; Momeni, F.; Mousavi, M.J.; Sadeghalvad, M.; Arjeini, Y.; Solaymani-Mohammadi, F.; Sadri Nahand, J.; Namdari, H.; et al. Influenza vaccine: Where are we and where do we go? Rev. Med. Virol. 2019, 29, e2014. [Google Scholar] [CrossRef]
- Cao, Y.; Lai, K.M.; Fu, K.C.; Kuo, C.L.; Tan, Y.J.; Yu, L.L.; Huang, D. Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism. Foods 2024, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.R.; Fan, C.L.; Chen, Y.; Quan, J.Y.; Shi, L.Z.; Tian, C.Y.; Shang, X.; Xu, N.S.; Ye, W.C.; Yu, L.Z.; et al. Anti-inflammatory, anti-angiogenetic and antiviral activities of dammarane-type triterpenoid saponins from the roots of Panax notoginseng. Food Funct. 2022, 13, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.H.; Lee, H.N.; Park, T. Prunus mume extract ameliorates exercise-induced fatigue in trained rats. J. Med. Food 2008, 11, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, H.D.; Won, Y.S.; Hong, S.M.; Moon, K.D.; Seo, K.I. Anti-Fatigue Effect of Prunus mume Vinegar in High-Intensity Exercised Rats. Nutrients 2020, 12, 1205. [Google Scholar] [CrossRef]
- Sheo, H.J.; Lee, M.Y.; Chung, D.L. Effect of Prunus mume extract on gastric secretion in rats and carbon tetrachloride induced liver damage of rabbits. J. Korean Soc. Food Nutr. J. 1993, 19, 21–26. [Google Scholar]
- Wang, L.; Zhang, H.Y.; Wang, L. Comparison of pharmacological effects of Fructus Mume and its processed products. Zhong Yao Cai 2010, 33, 353–356. [Google Scholar]
- Jang, I.; Kang, S.; Ko, Y. Influence of Plum (Prunus mume Siebold and Zucc.) Products on Growth Performance, Intestinal Function and Immunity in Broiler Chicks. J. Poult. Sci. 2013, 50, 28–36. [Google Scholar] [CrossRef][Green Version]
- Na, J.R.; Oh, K.N.; Park, S.U.; Bae, D.; Choi, E.J.; Jung, M.A.; Choi, C.Y.; Lee, D.W.; Jun, W.; Lee, K.Y.; et al. The laxative effects of Maesil (Prunus mume Siebold & Zucc.) on constipation induced by a low-fibre diet in a rat model. Int. J. Food Sci. Nutr. 2013, 64, 333–345. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.W.; Yang, L.J. Relation between Helicobacter pylori and pathogenesis of chronic atrophic gastritis and the research of its prevention and treatment. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992, 12, 515–526. [Google Scholar]
- Otsuka, T.; Tsukamoto, T.; Tanaka, H.; Inada, K.; Utsunomiya, H.; Mizoshita, T.; Kumagai, T.; Katsuyama, T.; Miki, K.; Tatematsu, M. Suppressive effects of fruit-juice concentrate of Prunus mume Sieb. et Zucc. (Japanese apricot, Ume) on Helicobacter pylori-induced glandular stomach lesions in Mongolian gerbils. Asian Pac. J. Cancer Prev. 2005, 6, 337–341. [Google Scholar] [PubMed]
- Nakajima, S.; Fujita, K.; Inoue, Y.; Nishio, M.; Seto, Y. Effect of the folk remedy, Bainiku-ekisu, a concentrate of Prunus mume juice, on Helicobacter pylori infection in humans. Helicobacter 2006, 11, 589–591. [Google Scholar] [CrossRef]
- Enomoto, S.; Yanaoka, K.; Utsunomiya, H.; Niwa, T.; Inada, K.; Deguchi, H.; Ueda, K.; Mukoubayashi, C.; Inoue, I.; Maekita, T.; et al. Inhibitory effects of Japanese apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis. Eur. J. Clin. Nutr. 2010, 64, 714–719. [Google Scholar] [CrossRef]
- Lee, J.H.; Stein, B.D. Antimicrobial activity of a combination of Mume fructus, Schizandrae fructus, and Coptidis rhizoma on enterohemorrhagic Escherichia coli O26, O111, and O157 and its effect on Shiga toxin releases. Foodborne Pathog. Dis. 2011, 8, 643–646. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Wong, R.W.; Hagg, U.; Chen, Y.; Herath, T.D.; Samaranayake, P.L.; Kao, R. Prunus mume extract exhibits antimicrobial activity against pathogenic oral bacteria. Int. J. Paediatr. Dent. 2011, 21, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus mume Against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Nishide, M.; Ikeda, K.; Mimura, H.; Yoshida, M.; Mitani, T.; Hajime Koyama, A. Antiviral and virucidal activities against herpes simplex viruses of umesu phenolics extracted from Japanese apricot. Microbiol. Immunol. 2019, 63, 359–366. [Google Scholar] [CrossRef]
- Ikeda, K.; Nishide, M.; Tsujimoto, K.; Nagashima, S.; Kuwahara, T.; Mitani, T.; Koyama, A.H. Antiviral and Virucidal Activities of Umesu Phenolics on Influenza Viruses. Jpn. J. Infect. Dis. 2020, 73, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, R.A.; Bi, G.; Alderton, J.M. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 1994, 263, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Miyake, K.; McNeil, P.L. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J. Cell Biol. 1997, 139, 63–74. [Google Scholar] [CrossRef]
- McNeil, P.L.; Steinhardt, R.A. Plasma membrane disruption: Repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 2003, 19, 697–731. [Google Scholar] [CrossRef]
- Meldolesi, J. Surface wound healing: A new, general function of eukaryotic cells. J. Cell. Mol. Med. 2003, 7, 197–203. [Google Scholar] [CrossRef]
- McNeil, P.L.; Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005, 6, 499–505. [Google Scholar] [CrossRef]
- Mitani, T.; Horinishi, A.; Kishida, K.; Kawabata, T.; Yano, F.; Mimura, H.; Inaba, N.; Yamanishi, H.; Oe, T.; Negoro, K.; et al. Phenolics profile of mume, Japanese apricot (Prunus mume Sieb. et Zucc.) fruit. Biosci. Biotechnol. Biochem. 2013, 77, 1623–1627. [Google Scholar] [CrossRef]
- Yan, X.T.; Lee, S.H.; Li, W.; Sun, Y.N.; Yang, S.Y.; Jang, H.D.; Kim, Y.H. Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunus mume. Food Chem. 2014, 156, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shao, J.; Sun, H.; Zhong, W.; Zhuang, W.; Zhang, Z. Evaluation of different kinds of organic acids and their antibacterial activity in Japanese Apricot fruits. Afr. J. Agric. Res. 2012, 7, 4911–4918. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathe, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Wu, X.; Shi, J.; Yang, Q.; Zhang, Y. Phenolic compounds from the edible seeds extract of Chinese Mei (Prunus mume Sieb. et Zucc) and their antimicrobial activity. LWT-Food Sci. Technol. 2011, 44, 347–349. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.; Gitelman, J.; Inbar, M. Unsaturated free fatty acids inactivate animal enveloped viruses. Arch. Virol. 1980, 66, 301–307. [Google Scholar] [CrossRef]
- Kohn, A.; Gitelman, J.; Inbar, M. Interaction of polyunsaturated fatty acids with animal cells and enveloped viruses. Antimicrob. Agents Chemother. 1980, 18, 962–968. [Google Scholar] [CrossRef]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Hodgson, L.; Williamson, M.K.; Buzas, D.; Borucu, U.; Powers, K.; Stenner, R.; Vasileiou, K.; et al. The free fatty acid-binding pocket is a conserved hallmark in pathogenic beta-coronavirus spike proteins from SARS-CoV to Omicron. Sci. Adv. 2022, 8, eadc9179. [Google Scholar] [CrossRef]
- Shokry, S.; Hegazy, A.; Abbas, A.M.; Mostafa, I.; Eissa, I.H.; Metwaly, A.M.; Yahya, G.; El-Shazly, A.M.; Aboshanab, K.M.; Mostafa, A. Phytoestrogen beta-Sitosterol Exhibits Potent In Vitro Antiviral Activity against Influenza A Viruses. Vaccines 2023, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, I.; Takaku, H.; Tashiro, M.; Yamamoto, N. High yield production of influenza virus in Madin Darby canine kidney (MDCK) cells with stable knockdown of IRF7. PLoS ONE 2013, 8, e59892. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Matsuyama, S.; Hoshino, T.; Yamamoto, N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of Genetic Diagnostic Methods for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020, 73, 304–307. [Google Scholar] [CrossRef]
- Mizukoshi, T.; Tateishi, K.; Tokusanai, M.; Yoshinaka, Y.; Yamamoto, A.; Yamamoto, N.; Yamamoto, N. Targeted Elimination of Influenza Virus and Infected Cells with Near-Infrared Antiviral Photoimmunotherapy (NIR-AVPIT). Pharmaceutics 2025, 17, 173. [Google Scholar] [CrossRef]
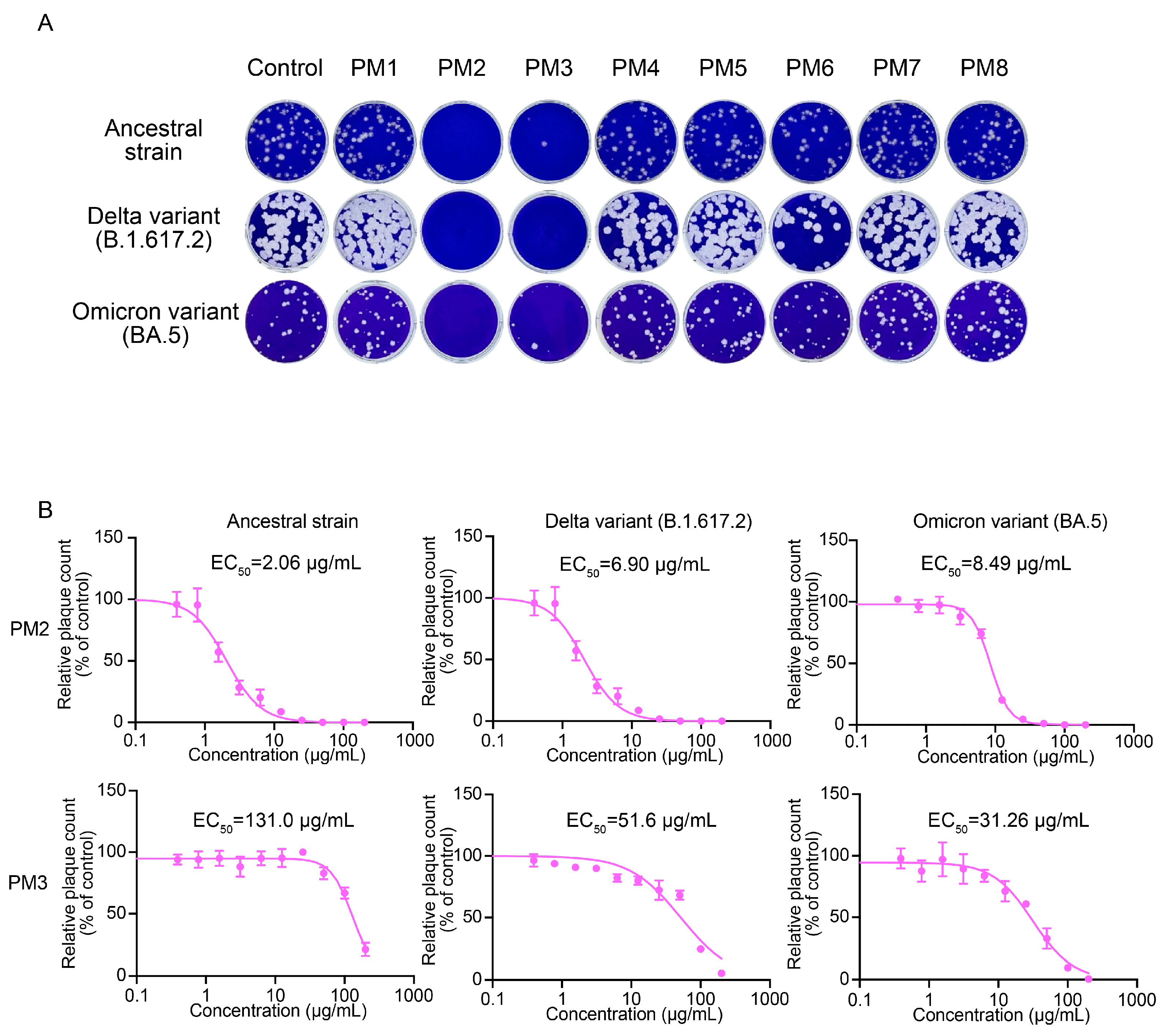
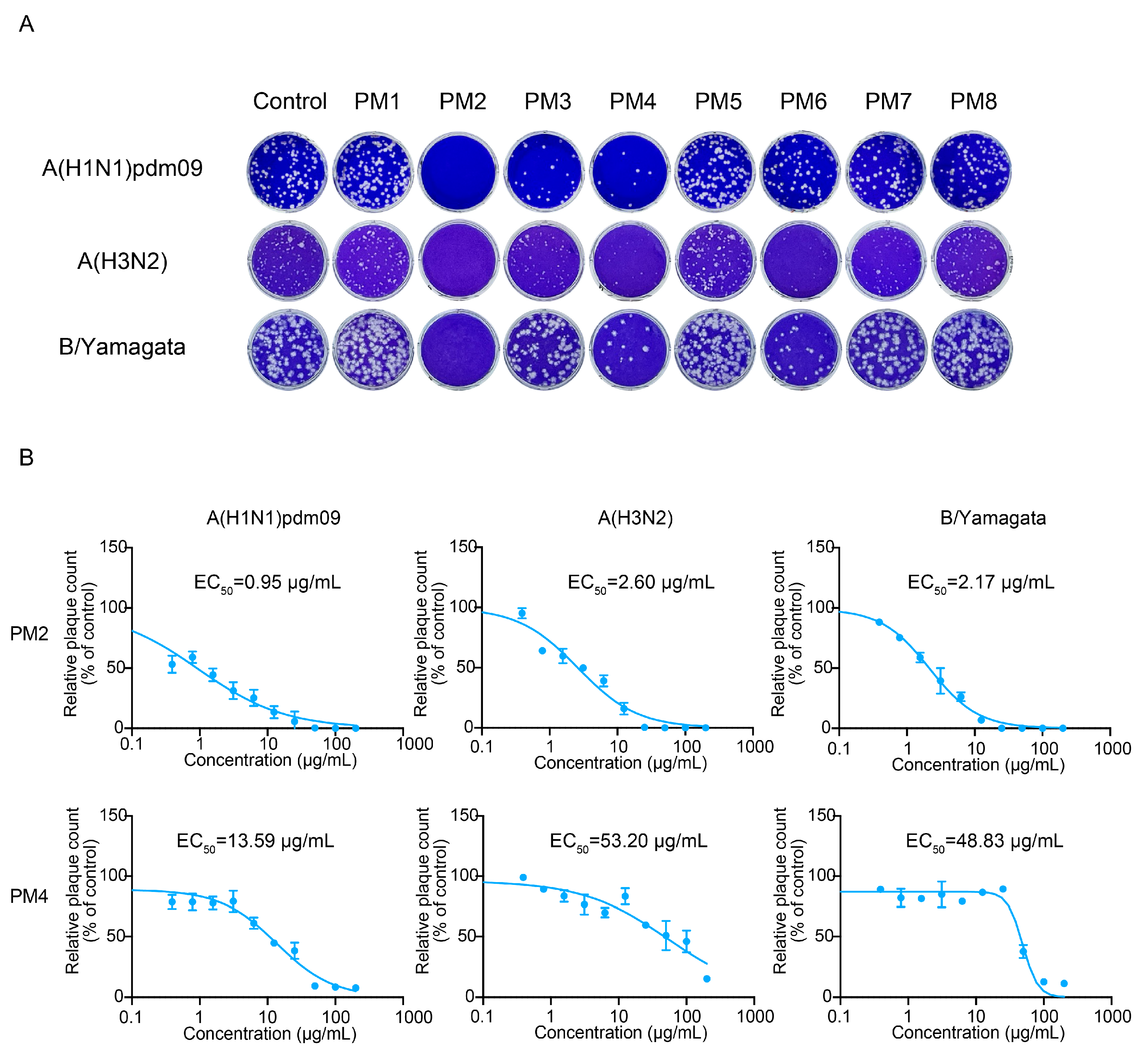

| Extract | Virus | EC50 (µg/mL) | EC90 (µg/mL) | CC50 (µg/mL) | Selectivity Index |
|---|---|---|---|---|---|
| PM2 | SARS-CoV-2 Ancestral strain | 2.06 | 7.23 | >200 | >97.09 |
| PM2 | SARS-CoV-2 Delta variant (B.1.617.2) | 6.90 | 20.87 | >200 | >28.99 |
| PM2 | SARS-CoV-2 Omicron variant (BA.5) | 8.49 | 16.74 | >200 | >23.56 |
| PM3 | SARS-CoV-2 Ancestral strain | 131.0 | >200 | >200 | >1.53 |
| PM3 | SARS-CoV-2 Delta variant (B.1.617.2) | 51.60 | >200 | >200 | >3.88 |
| PM3 | SARS-CoV-2 Omicron variant (BA.5) | 31.26 | 131.3 | >200 | >6.40 |
| PM2 | Influenza virus A(H1N1)pdm09 | 0.95 | 23.77 | >200 | >209.4 |
| PM2 | Influenza virus A(H3N2) | 2.60 | 23.71 | >200 | >76.92 |
| PM2 | Influenza virus B/Yamagata | 2.17 | 13.05 | >200 | >92.17 |
| PM4 | Influenza virus A(H1N1)pdm09 | 13.59 | 110.2 | >200 | >14.72 |
| PM4 | Influenza virus A(H3N2) | 53.20 | >200 | >200 | >3.76 |
| PM4 | Influenza virus B/Yamagata | 48.83 | 85.38 | >200 | >4.10 |
| No. | RT | Compounds | MF | MW | Area |
|---|---|---|---|---|---|
| 1 | 12:57 | Dodecanoic acid | C12H24O2 | 200.32 | 0.25 |
| 2 | 15:32 | Hexadecylene oxide | C16H32O | 240.42 | 0.03 |
| 3 | 15:52 | Hexadecanal | C16H32O | 240.42 | 0.083 |
| 4 | 16:06 | Methyl tetradecanoate | C15H30O2 | 242.40 | 0.06 |
| 5 | 16:31 | Octadecyl vinyl ether | C20H40O | 296.53 | 0.15 |
| 6 | 17:06 | Tetradecanoic acid | C14H28O2 | 228.37 | 0.20 |
| 7 | 18:31 | Hexahydrofarnesyl acetone | C18H36O | 268.48 | 0.62 |
| 8 | 20:00 | Methyl (7E)-7-hexadecenoate | C17H32O2 | 268.43 | 0.07 |
| 9 | 20:10 | Methyl hexadecanoate | C17H34O2 | 270.45 | 6.67 |
| 10 | 21:39 | Hexadecanoic acid | C16H32O2 | 256.42 | 44.34 |
| 11 | 23:19 | Methyl 9,12-octadecadienoate | C19H34O2 | 294.47 | 5.66 |
| 12 | 23:24 | Methyl-9,12,15-octadecatrienoate | C19H32O2 | 292.46 | 1.38 |
| 13 | 23:32 | Methyl octadecanoate | C19H38O2 | 298.50 | 0.06 |
| 14 | 24:39 | 9,12-Octadecadienoic acid | C18H32O2 | 280.45 | 27.14 |
| 15 | 26:30 | 8,11,14-Eicosatrienoic Acid | C20H34O2 | 306.48 | 0.03 |
| 16 | 26:39 | Methyl 11-(3-pentyl-2-oxiranyl)undecanoate | C19H36O3 | 312.49 | 0.06 |
| 17 | 26:44 | Eicosane | C20H42 | 282.55 | 0.15 |
| 18 | 27:15 | Methyl eicosanoate | C21H42O2 | 326.56 | 0.30 |
| 19 | 27:41 | 4,8,12,16-Tetramethylheptadecan-4-olide | C21H40O2 | 324.54 | 0.83 |
| 20 | 28:04 | Butyl hexadecanoate | C20H40O2 | 312.53 | 0.18 |
| 21 | 28:19 | Ethyl docosanoate | C24H48O2 | 368.64 | 0.06 |
| 22 | 30:17 | Farnesyl acetate | C17H28O2 | 264.40 | 0.05 |
| 23 | 30:22 | Methyl 5-(2-undecylcyclopropyl)pentanoate | C20H38O2 | 310.51 | 0.28 |
| 24 | 30:41 | Methyl 9-(2-[(2-butylcyclopropyl)methyl]cyclopropyl)nonanoate | C21H38O2 | 322.53 | 0.18 |
| 25 | 31:52 | 2-Tetradecen-1-ol | C14H28O | 212.37 | 0.07 |
| 26 | 32:48 | 2-Hexyldecanol | C16H34O | 242.44 | 0.36 |
| 27 | 33:18 | Methyl heptacosanoate | C28H56O2 | 424.74 | 0.06 |
| 28 | 34:01 | 9-Octadecenamide | C18H35NO | 281.48 | 0.07 |
| 29 | 34:39 | Supraene | C30H50 | 410.72 | 0.50 |
| 30 | 35:08 | Unknown | 0.10 | ||
| 31 | 35:15 | Unknown | 0.33 | ||
| 32 | 35:32 | Eicosane | C20H42 | 282.55 | 0.19 |
| 33 | 35:35 | Octadecanol | C18H38O | 270.49 | 0.11 |
| 34 | 35:49 | Unknown | 0.04 | ||
| 35 | 36:23 | Unknown (sterols) | 0.05 | ||
| 36 | 36:50 | Heptacosane | C27H56 | 380.73 | 0.04 |
| 37 | 36:56 | Heptatriacotanol | C37H76O | 537.00 | 0.02 |
| 38 | 37:02 | Unknown | 0.03 | ||
| 39 | 37:09 | Geranylgeraniol | C20H34O | 290.48 | 0.03 |
| 40 | 37:50 | Cholesta-4,6-dien-3-ol | C27H44O | 384.64 | 0.25 |
| 41 | 38:05 | Stigmastan-3,5-diene | C29H48 | 396.69 | 0.31 |
| 42 | 38:25 | α-Tocopherol | C29H50O2 | 430.71 | 0.07 |
| 43 | 38:44 | Unknown | 0.33 | ||
| 44 | 39:46 | Campesterol | C28H48O | 400.68 | 0.38 |
| 45 | 40:10 | Stigmasterol | C29H48O | 412.69 | 0.42 |
| 46 | 40:55 | β-Sitosterol | C29H50O | 414.71 | 2.86 |
| 47 | 41:05 | 24-Propylidenecholesterol | C30H50O | 426.72 | 0.04 |
| 48 | 41:17 | Cycloeucalenyl acetate | C32H52O2 | 468.75 | 0.25 |
| 49 | 41:43 | Cycloartenyl acetate | C32H52O2 | 468.75 | 0.07 |
| 50 | 41:57 | Stigmasta-3,5-dien-7-one | C29H46O | 410.67 | 0.14 |
| 51 | 42:26 | Cycloeucalenyl acetate | C32H52O2 | 468.75 | 1.48 |
| 52 | 42:47 | Cholesta-4,6-dien-3-one | C27H42O | 382.62 | 0.12 |
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokusanai, M.; Tateishi, K.; Hirata, K.; Fukunishi, N.; Suzuki, Y.; Kono, R.; Natsumi, S.; Kato, C.; Takekoshi, S.; Okuno, Y.; et al. Prunus mume Extract Inhibits SARS-CoV-2 and Influenza Virus Infection In Vitro by Directly Targeting Viral Particles. Int. J. Mol. Sci. 2025, 26, 8487. https://doi.org/10.3390/ijms26178487
Tokusanai M, Tateishi K, Hirata K, Fukunishi N, Suzuki Y, Kono R, Natsumi S, Kato C, Takekoshi S, Okuno Y, et al. Prunus mume Extract Inhibits SARS-CoV-2 and Influenza Virus Infection In Vitro by Directly Targeting Viral Particles. International Journal of Molecular Sciences. 2025; 26(17):8487. https://doi.org/10.3390/ijms26178487
Chicago/Turabian StyleTokusanai, Mizuki, Koichiro Tateishi, Kanako Hirata, Nahoko Fukunishi, Yusuke Suzuki, Ryohei Kono, Sorama Natsumi, Chikara Kato, Susumu Takekoshi, Yoshiharu Okuno, and et al. 2025. "Prunus mume Extract Inhibits SARS-CoV-2 and Influenza Virus Infection In Vitro by Directly Targeting Viral Particles" International Journal of Molecular Sciences 26, no. 17: 8487. https://doi.org/10.3390/ijms26178487
APA StyleTokusanai, M., Tateishi, K., Hirata, K., Fukunishi, N., Suzuki, Y., Kono, R., Natsumi, S., Kato, C., Takekoshi, S., Okuno, Y., Utsunomiya, H., & Yamamoto, N. (2025). Prunus mume Extract Inhibits SARS-CoV-2 and Influenza Virus Infection In Vitro by Directly Targeting Viral Particles. International Journal of Molecular Sciences, 26(17), 8487. https://doi.org/10.3390/ijms26178487





