Cartilage Regeneration Potential in Early Osteoarthritis of the Knee: A Prospective, Randomized, Open, and Blinded Endpoint Study Comparing Adipose-Derived Mesenchymal Stem Cell (ADSC) Therapy Versus Hyaluronic Acid
Abstract
1. Introduction
2. Results
2.1. Cartilage Regeneration and Structural Changes
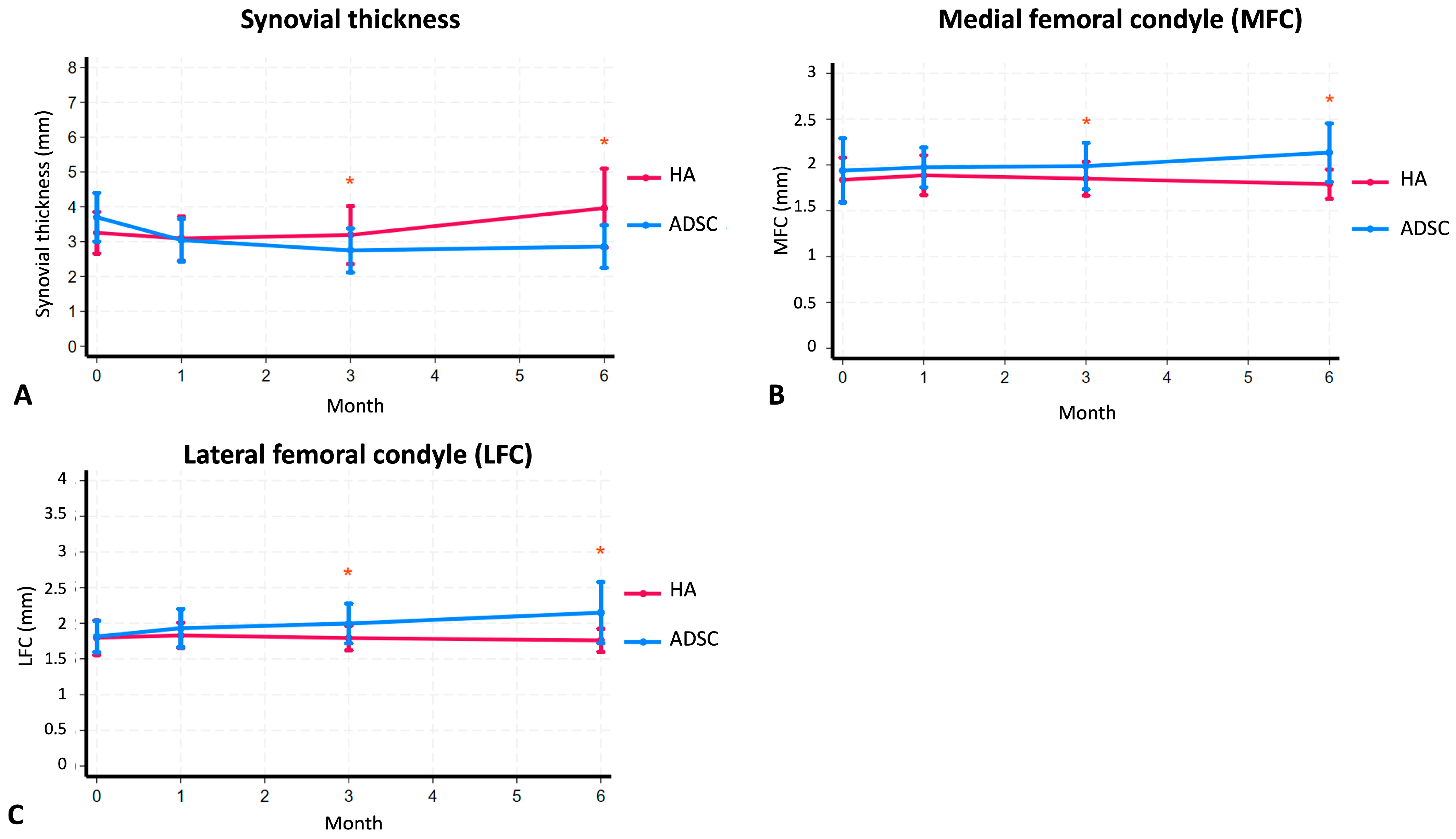
2.2. Reduction in Synovial Thickness
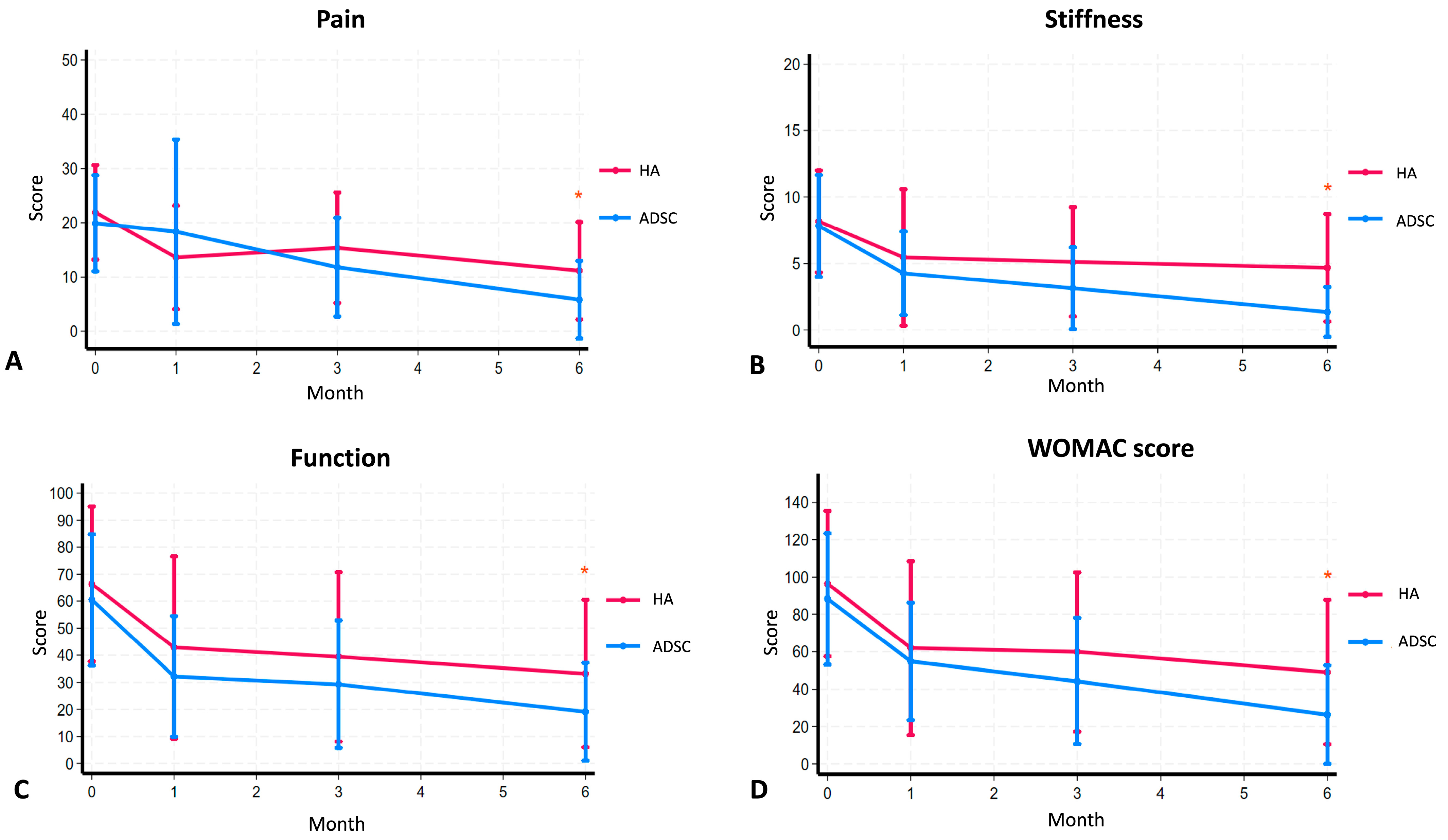
2.3. Clinical Outcomes and WOMAC
2.4. Biomarker Analysis
2.5. Safety and Adverse Events
3. Discussion
4. Materials and Methods
4.1. Patients and Procedures
4.2. ADSC Preparation
4.3. ADSCs and HA Therapy
4.4. MRI Protocol
4.5. Questionnaires
4.6. Urine CTXII Assay
4.7. Statistical Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Knee OA | Knee osteoarthritis |
| ADSCs | Adipose-derived stem cells |
| HA | Hyaluronic acid |
| MSC | Mesenchymal stem cell |
| CTX-II | C-terminal cross-linked telopeptide of type II collagen |
| RCTs | Randomized controlled trials |
| MRT | Magnetic resonance imaging |
| US | Ultrasonography |
| WOMAC | the Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Magnusson, K.; Turkiewicz, A.; Englund, M. Nature vs. nurture in knee osteoarthritis—The importance of age, sex and body mass index. Osteoarthr. Cartil. 2019, 27, 586–592. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.-W.; Park, Y.-B.; Choi, C.-H.; Kyung, H.-S.; Lee, J.-H.; Yoo, J.D.; Yoo, J.-H.; Choi, C.-H.; Kim, C.-W.; Kim, H.-C.; et al. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: A double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet. Disord. 2017, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Natov, N.S.; Dasi, U.R.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis--meta-analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Wang, S.; Xu, H.; Sun, H.; Fan, X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Medicine 2020, 99, e23343. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Khuangsirikul, S.; Yuwapan, A.; Heebthamai, D.; Chotanaphuti, T. Anti-Inflammatory Effect of Cross-Linked Hyaluronic Acids in Osteoarthritic Knee, Detected by Ultrasonography. J. Orthop. Res. Ther. 2020, 7, 1238. [Google Scholar]
- Singh, A.P.; Saran, S.; Thukral, B.B.; Kaushik, R. Ultrasonographic Evaluation of Osteoarthritis-affected Knee Joints: Comparison with Kellgren-Lawrence Grading and Pain Scores. J. Med. Ultrasound 2021, 29, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Arunrukthavon, P.; Heebthamai, D.; Benchasiriluck, P.; Chaluay, S.; Chotanaphuti, T.; Khuangsirikul, S. Can urinary CTX-II be a biomarker for knee osteoarthritis? Arthroplasty 2020, 2, 6. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266, Erratum in Stem Cells 2017, 35, 1651–1652. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Zhao, X.; Ruan, J.; Tang, H.; Li, J.; Shi, Y.; Li, M.; Li, S.; Xu, C.; Lu, Q.; Dai, C. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res. Ther. 2019, 10, 308. [Google Scholar] [CrossRef]
- Ren, B.; Chang, Y.; Liu, R.; Xiao, F.; Xu, J.; Li, L.; Li, T.; Ruan, Z.; Bao, Y.; Lin, J.; et al. Clinical phase I/II trial of SVF therapy for cartilage regeneration: A cellular therapy with novel 3D MRI imaging for evaluating chondral defect of knee osteoarthritis. Front. Cell Dev. Biol. 2023, 11, 1106279. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Yuwapan, A.; Phruetthiphat, O.A.; Khuangsirikul, S.; Heebthamai, D.; Chotanaphuti, T. Effect of highly cross-linked hyaluronic acid in synovitis of knee osteoarthritis. Osteoarthr. Cartil. 2020, 28, S459. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J.; Kwon, S.K.; Kim, Y.S.; Yeo, J.E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef]
- Jiang, P.; Mao, L.; Qiao, L.; Lei, X.; Zheng, Q.; Li, D. Efficacy and safety of mesenchymal stem cell injections for patients with osteoarthritis: A meta-analysis and review of RCTs. Arch. Orthop. Trauma. Surg. 2021, 141, 1241–1251. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample size calculations in clinical research; CRC Biostatistics Series; Chapman & Hall: New York, NY, USA, 2008; Volume 3. [Google Scholar]
- Chagas-Neto, F.A.; Taneja, A.K.; Gregio-Junior, E.; Nogueira-Barbosa, M.H. In-Plane Ultrasound-Guided Knee Injection Through a Lateral Suprapatellar Approach: A Safe Technique. Ultrasound Q. 2017, 33, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chawanvuth, T.; Thanainit, C.; Saradech, K.; Danai, H. Synovial hypertrophy detected using ultrasonogram in primary osteoarthritic knees: Prevalence and correlation with radiographic staging. J. Southeast Asian Med. Res. 2020, 4, 33–40. [Google Scholar]
- Molero-Calafell, J.; Burón, A.; Castells, X.; Porta, M. Intention to treat and per protocol analyses: Differences and similarities. J. Clin. Epidemiol. 2024, 173, 111457. [Google Scholar] [CrossRef] [PubMed]
- StataCorp: Stata Statistical Software, Release 18; StataCorp LLC.: College Station, TX, USA, 2023.
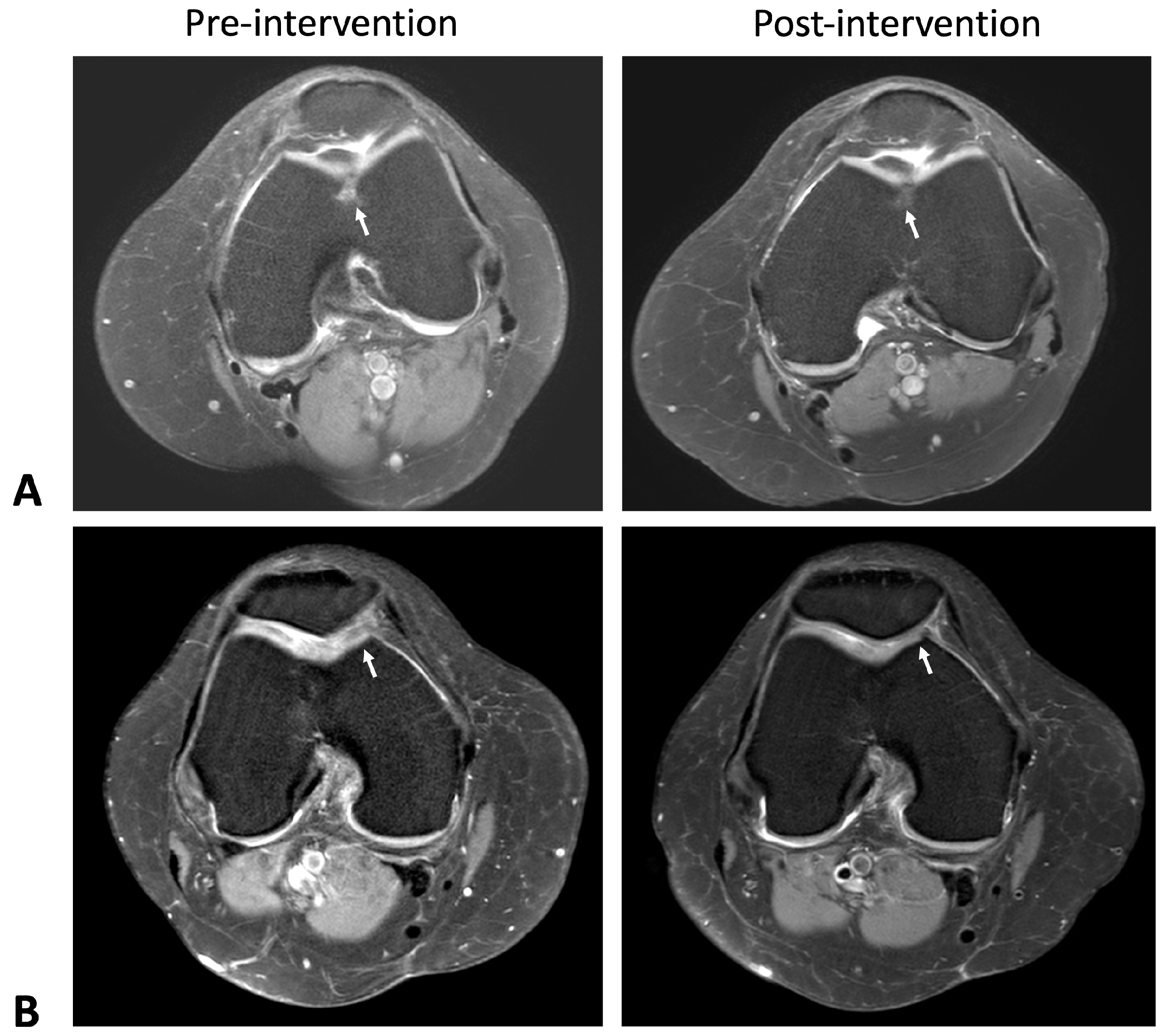
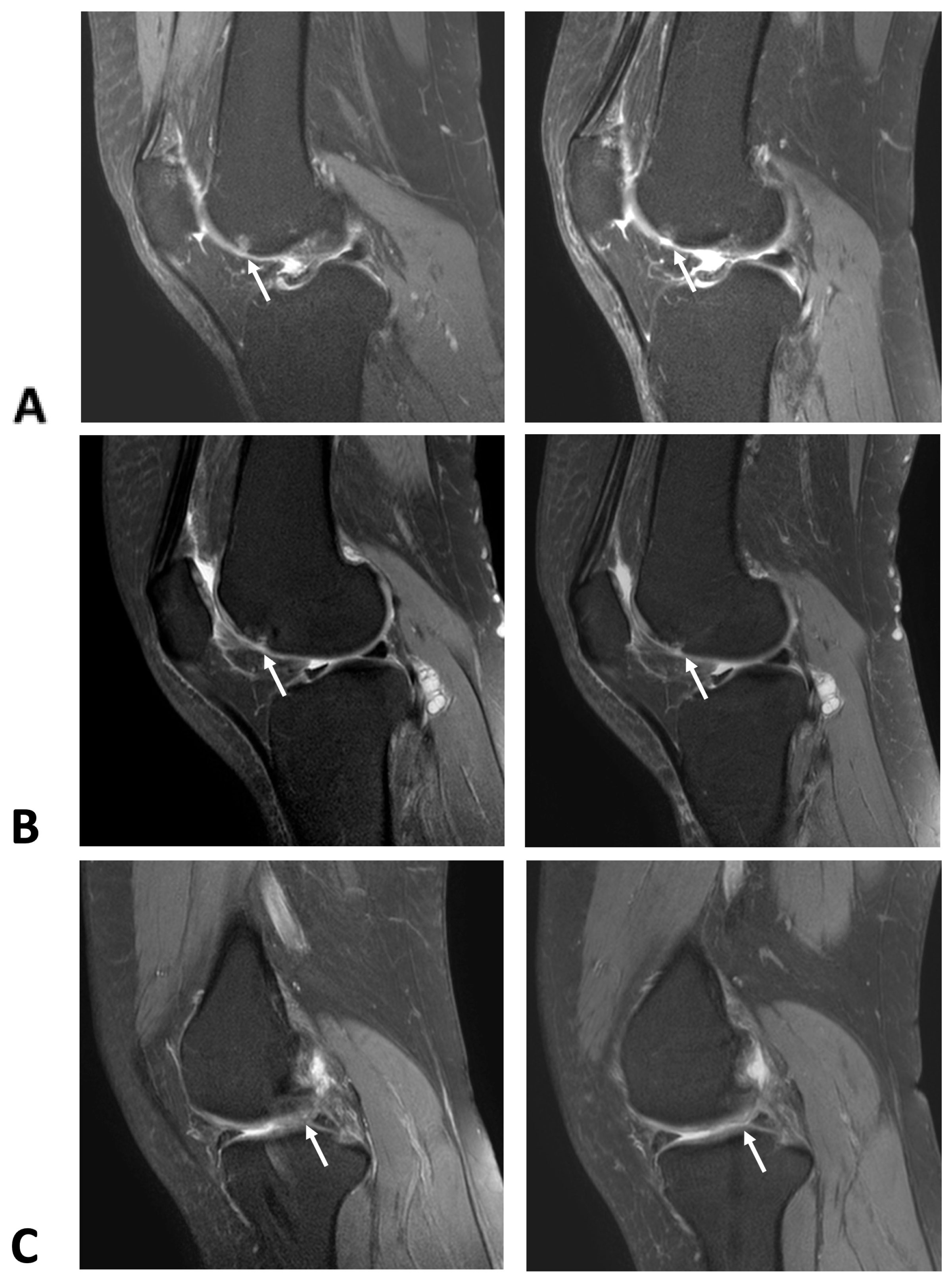


| Characteristics | HA (Control) (n = 24, 51.06%) | ADSC (Intervention) (n = 23, 48.94%) | p-Value |
|---|---|---|---|
| Gender (n, %) | |||
| Male | 4, 8.51% | 4, 8.51% | |
| Female | 20, 44.55% | 19, 40.43% | |
| Age | 59.00 ± 6.69 | 56.91 ± 6.15 | 0.14 |
| Weight (kg) | 61.23 ± 8.97 | 61.73 ± 8.62 | 0.61 |
| Height (cm) | 158.08 ± 7.85 | 156.65 ± 5.43 | 0.24 |
| BMI (kg/m2) | 24.31 ± 2.33 | 25.12 ± 2.99 | 0.84 |
| WOMAC score | |||
| Pain | 21.92 ± 8.70 | 19.91± 8.84 | 0.22 |
| Stiffness | 8.17 ± 3.84 | 7.83 ± 3.83 | 0.38 |
| Function | 66.38 ± 28.68 | 60.52 ± 24.30 | 0.23 |
| Overall WOMAC score | 96.46 ± 38.98 | 88.26 ± 35.21 | 0.23 |
| US | |||
| Synovial thickness (mm) | 3.25 ± 0.60 | 3.70 ± 0.70 | 0.98 |
| MFC (mm) | 1.84 ± 0.24 | 1.94 ± 0.35 | 0.87 |
| LFC (mm) | 1.80 ± 0.24 | 1.81 ± 0.22 | 0.60 |
| Urine CTX (ng/mmol) | 310.45 (145.37–566.75) | 263 (121.53–486.43) | 0.53 |
| Study Outcomes | Before ADSCs | After Injected ADSCs | Before HA | After Injected HA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 m | 1 m | p-Value a | 3 m | p-Value b | 6 m | p-Value c | 0 m | 1 m | p-Value a | 3 m | p-Value b | 6 m | p-Value c | |
| Synovial thickness | 3.70 (95%CI 3.40–4.00) | 3.04 (95%CI 2.78–3.31) | <0.001 * | 2.75 (95%CI 2.48–3.02) | <0.001 * | 2.86 (95%CI 2.60–3.12) | 0.001 * | 3.25 (95%CI 3.00–3.51) | 3.09 (95%CI 2.82–3.36) | 0.320 | 3.19 (95%CI 2.84–3.54) | 0.756 | 3.96 (95%CI 3.46–4.46) | 0.016 * |
| MFC (mm) | 1.94 (95%CI 1.79–2.09) | 1.97 (95%CI 1.88–2.07) | 0.612 | 1.99 (95%CI 1.88–2.10) | 0.634 | 2.13 (95%CI 2.00–2.27) | 0.099 | 1.84 (95%CI 1.73–1.94) | 1.89 (95%CI 1.80–1.98) | 0.261 | 1.85 (95%CI 1.77–1.93) | 0.765 | 1.79 (95%CI 1.72–1.86) | 0.298 |
| LFC (mm) | 1.81 (95%CI 1.72–1.91) | 1.93 (95%CI 1.81–2.05) | 0.053 | 2.00 (95%CI 1.88–2.12) | 0.020 * | 2.15 (95%CI 1.96–2.33) | 0.002 * | 1.80 (95%CI 1.69–1.90) | 1.83 (95%CI 1.75–1.90) | 0.539 | 1.79 (95%CI 1.72–1.86) | 0.934 | 1.76 (95%CI 1.69–1.83) | 0.608 |
| Study Outcomes | Before ADSCs | After Injected ADSCs | Before HA | After Injected HA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 m | 1 m | p-Value a | 3 m | p-Value b | 6 m | p-Value c | 0 m | 1 m | p-Value a | 3 m | p-Value b | 6 m | p-Value c | |
| Pain | 19.91 (95%CI 16.09–23.73) | 18.35 (95%CI 11.02–25.68) | 0.711 | 11.83 (95%CI 7.89–15.76) | 0.007 * | 5.83 (95%CI 2.73–8.93) | <0.001 * | 21.92 (95%CI 18.24–25.59) | 13.63 (95%CI 9.60–17.65) | 0.004 * | 15.38 (95%CI 11.08–19.67) | 0.045 * | 11.18 (95%CI 7.21–15.16) | <0.001 * |
| Stiffness | 7.83 (95%CI 6.17–9.48) | 4.26 (95%CI 2.90–5.62) | 0.003 * | 3.13 (95%CI 1.80–4.46) | 0.001 * | 1.35 (95%CI 0.54–2.16) | <0.001 * | 8.17 (95%CI 6.54–9.79) | 5.46 (95%CI 3.29–7.62) | 0.025 * | 5.13 (95%CI 3.39–6.86) | 0.028 * | 4.68 (95%CI 2.89–6.47) | 0.002 * |
| Function | 60.52 (95%CI 50.02–71.03) | 32.21 (95%CI 22.59–41.84) | 0.002 * | 29.30 (95%CI 19.11–39.50) | <0.001 * | 19.13 (95%CI 11.29–26.97) | <0.001 * | 69.14 (95%CI 56.56–81.72) | 42.88 (95%CI 28.65–57.10) | 0.005 * | 39.42 (95%CI 26.19–52.64) | 0.006 * | 33.23 (95%CI 21.15–45.31) | <0.001 * |
| WOMAC | 88.26 (95%CI 73.03–103.49) | 54.83 (95%CI 41.26–68.39) | 0.009 * | 44.26 (95%CI 29.66–58.86) | <0.001 * | 26.30 (95%CI 14.91–37.70) | <0.001 * | 96.46 (95%CI 80.00–112.92) | 61.96 (95%CI 42.32–81.60) | 0.004 * | 59.92 (95%CI 41.91–77.92) | 0.009 * | 49.09 (95%CI 31.95–66.23) | <0.001 * |
| Study Outcomes | ADSCs | HA | p-Value a |
|---|---|---|---|
| Mean change urine CTX-II (ng/mmol) within 6 months | −82 (95%CI −219 to +119.01) | −69.63 (95%CI −214.44 to +132.36) | 0.8808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangkanjanavelukul, P.; Khuangsirikul, S.; Heebthamai, D.; Yamabhai, M.; Sumphanapai, T.; Khumtong, N.; Chotanaphuti, T. Cartilage Regeneration Potential in Early Osteoarthritis of the Knee: A Prospective, Randomized, Open, and Blinded Endpoint Study Comparing Adipose-Derived Mesenchymal Stem Cell (ADSC) Therapy Versus Hyaluronic Acid. Int. J. Mol. Sci. 2025, 26, 8476. https://doi.org/10.3390/ijms26178476
Tangkanjanavelukul P, Khuangsirikul S, Heebthamai D, Yamabhai M, Sumphanapai T, Khumtong N, Chotanaphuti T. Cartilage Regeneration Potential in Early Osteoarthritis of the Knee: A Prospective, Randomized, Open, and Blinded Endpoint Study Comparing Adipose-Derived Mesenchymal Stem Cell (ADSC) Therapy Versus Hyaluronic Acid. International Journal of Molecular Sciences. 2025; 26(17):8476. https://doi.org/10.3390/ijms26178476
Chicago/Turabian StyleTangkanjanavelukul, Ponthep, Saradej Khuangsirikul, Danai Heebthamai, Montarop Yamabhai, Thitima Sumphanapai, Nattapat Khumtong, and Thanainit Chotanaphuti. 2025. "Cartilage Regeneration Potential in Early Osteoarthritis of the Knee: A Prospective, Randomized, Open, and Blinded Endpoint Study Comparing Adipose-Derived Mesenchymal Stem Cell (ADSC) Therapy Versus Hyaluronic Acid" International Journal of Molecular Sciences 26, no. 17: 8476. https://doi.org/10.3390/ijms26178476
APA StyleTangkanjanavelukul, P., Khuangsirikul, S., Heebthamai, D., Yamabhai, M., Sumphanapai, T., Khumtong, N., & Chotanaphuti, T. (2025). Cartilage Regeneration Potential in Early Osteoarthritis of the Knee: A Prospective, Randomized, Open, and Blinded Endpoint Study Comparing Adipose-Derived Mesenchymal Stem Cell (ADSC) Therapy Versus Hyaluronic Acid. International Journal of Molecular Sciences, 26(17), 8476. https://doi.org/10.3390/ijms26178476







