Integrated Approach for Biochemical and Functional Characterization of Six Clinical Variants of Glucose-6-Phosphate Dehydrogenase
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Analysis
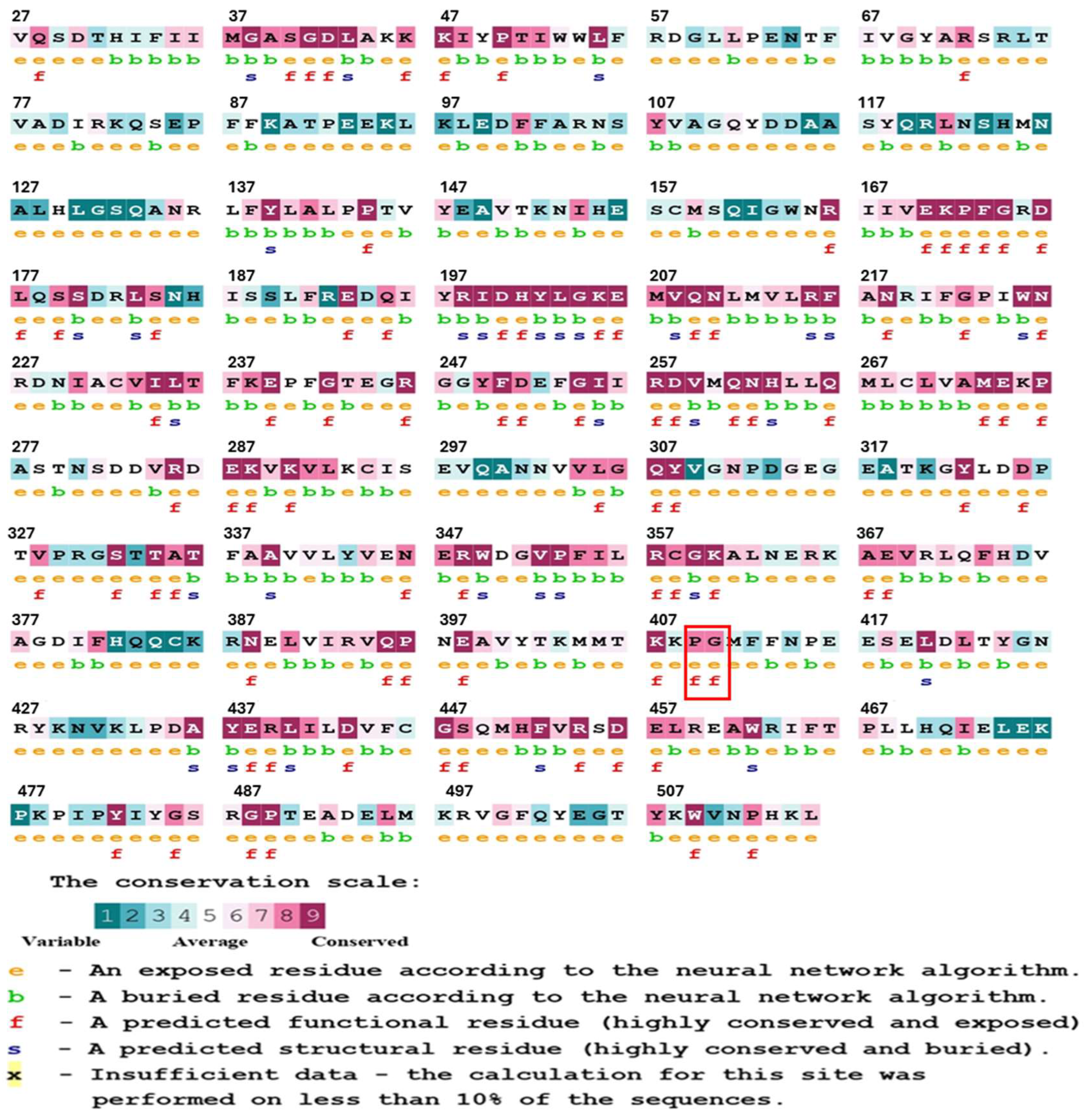
2.1.1. Phylogenetic Examination of the Conservation of G6PD Protein Residues
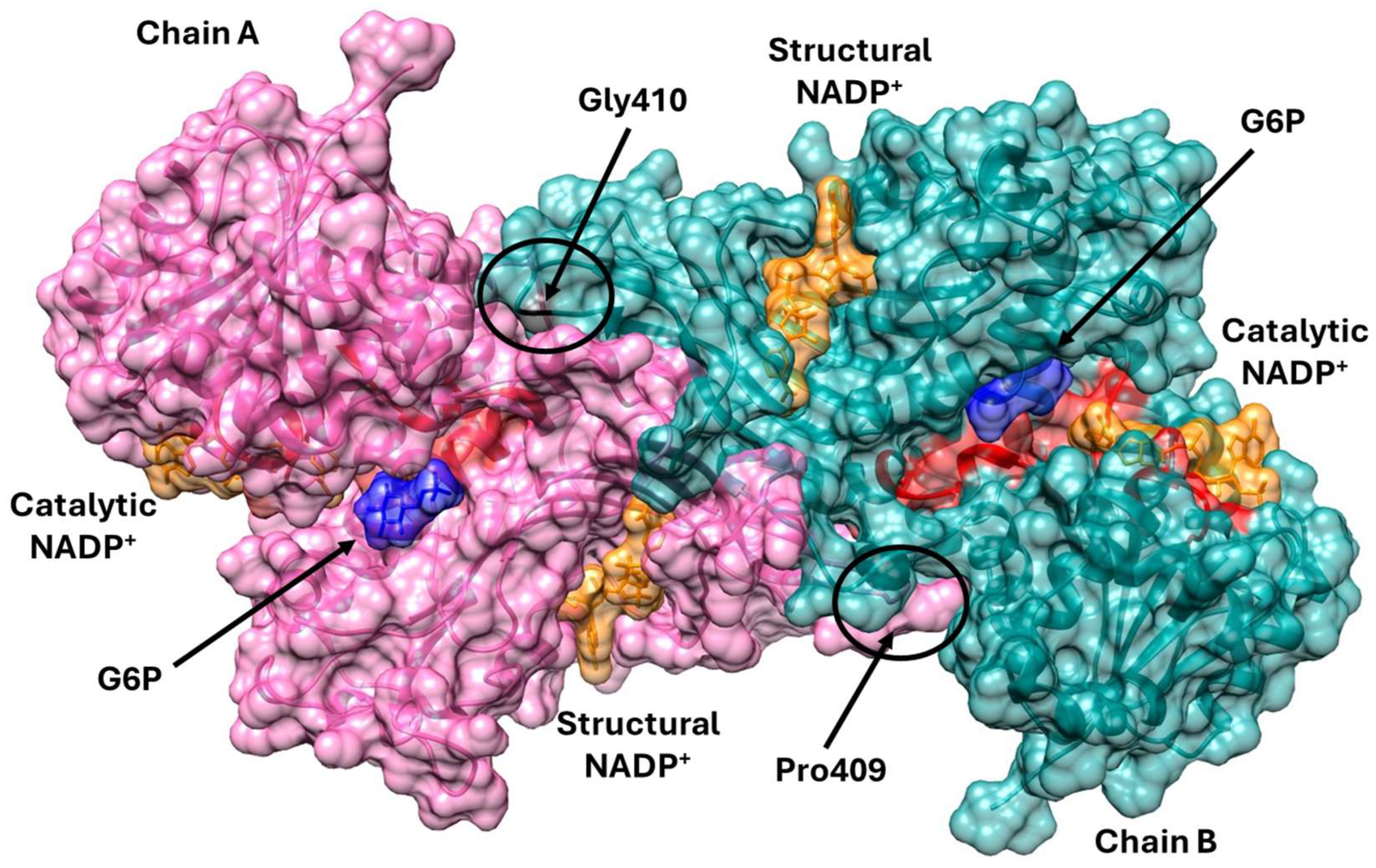
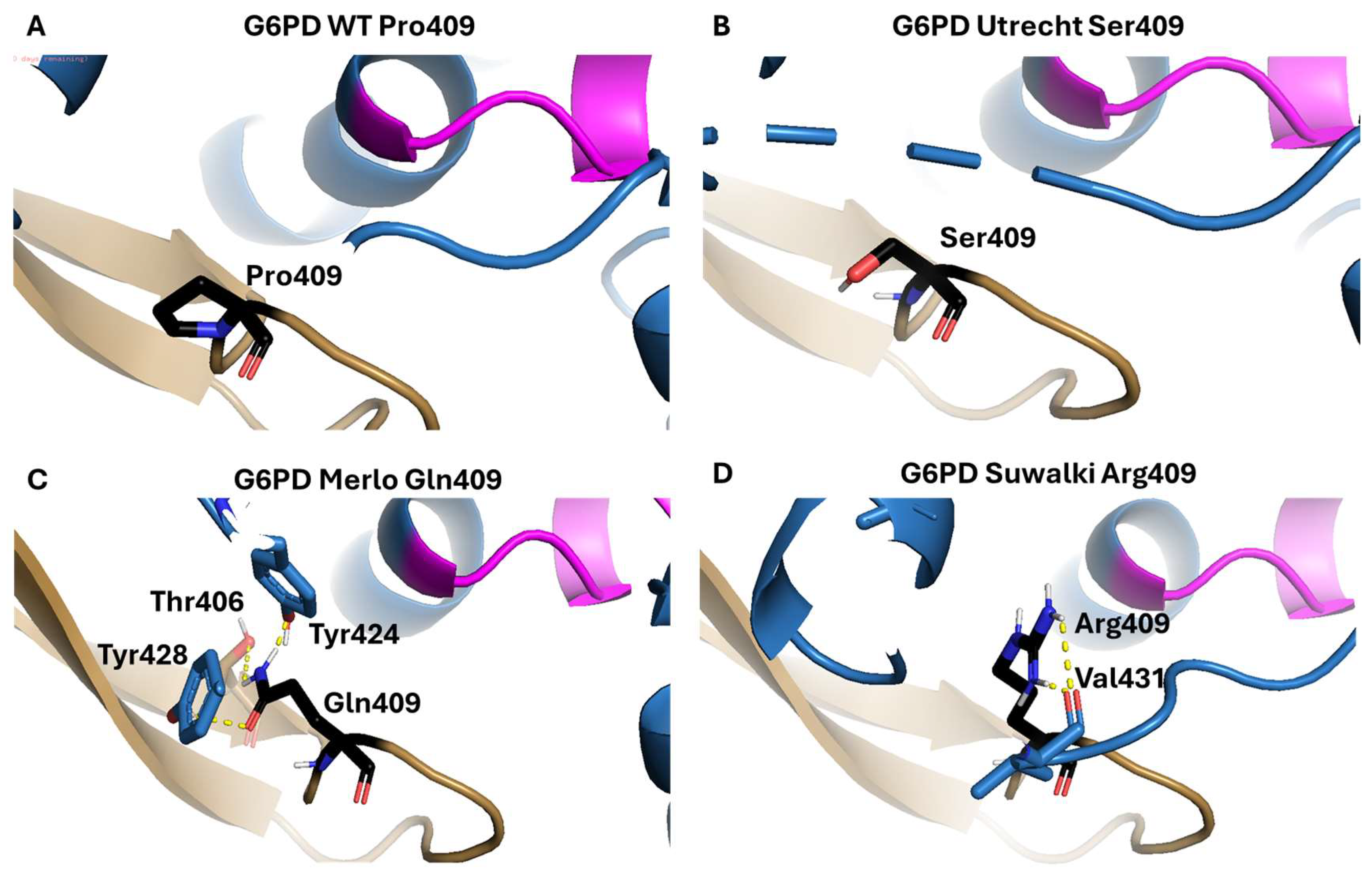
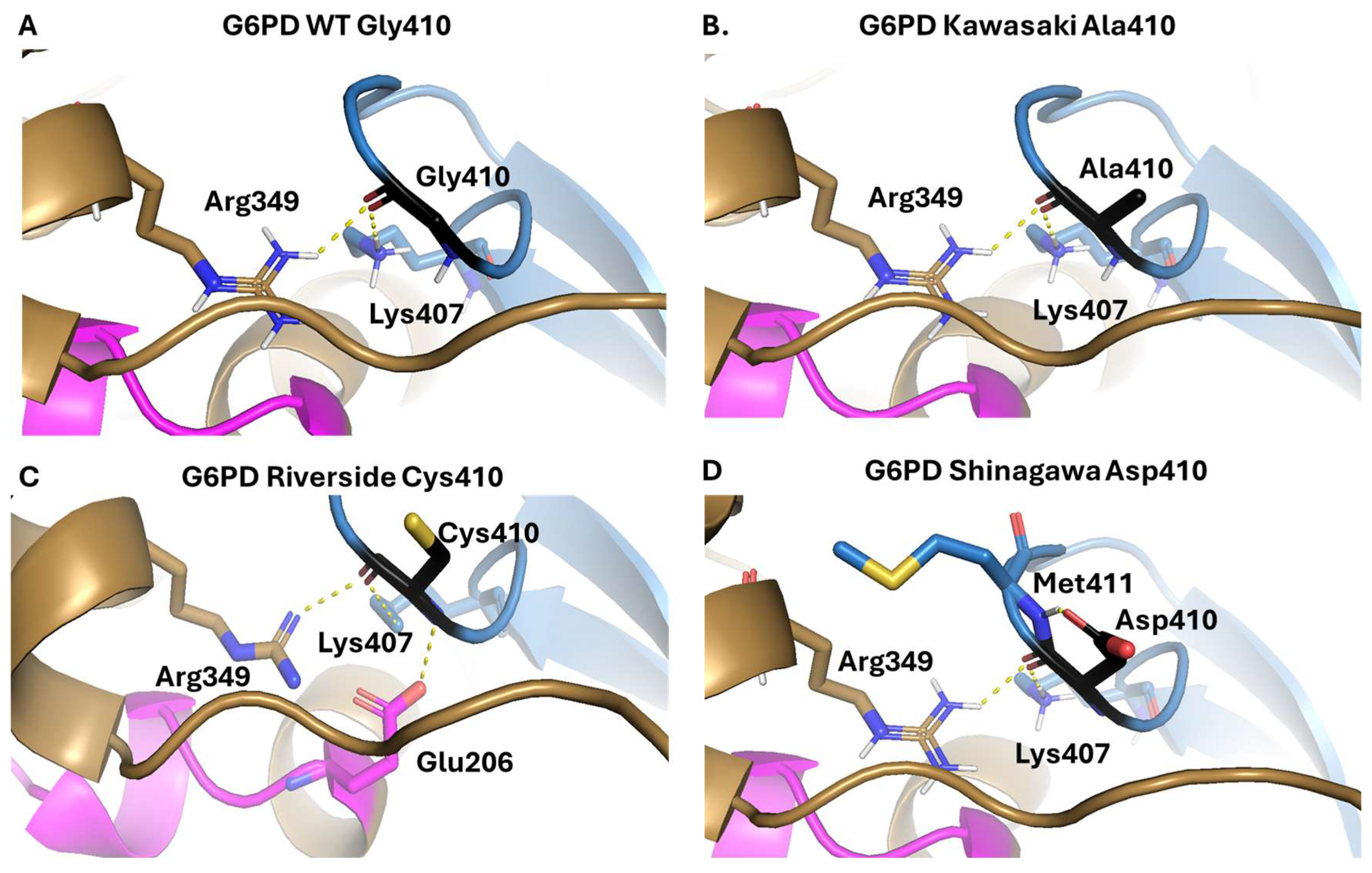
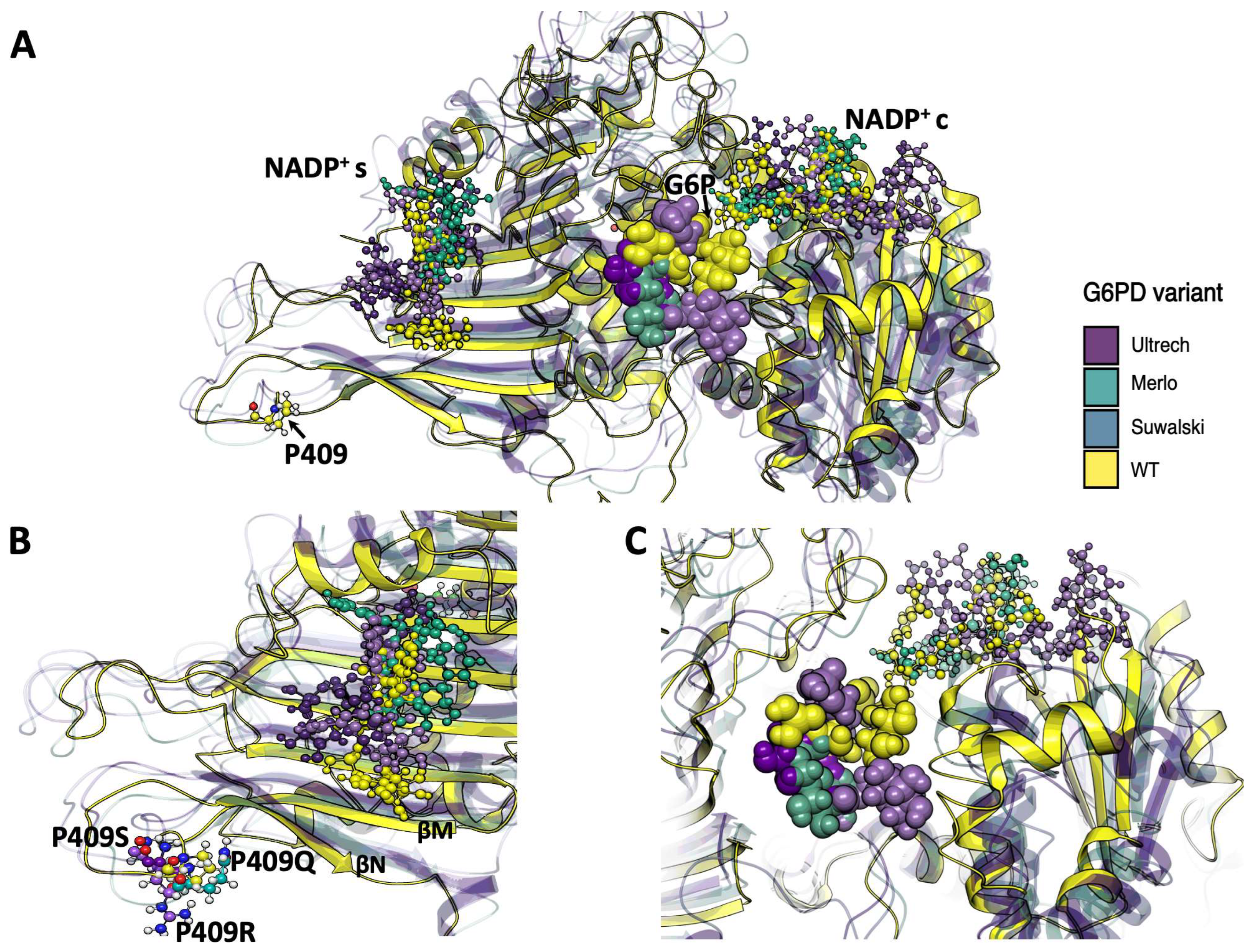
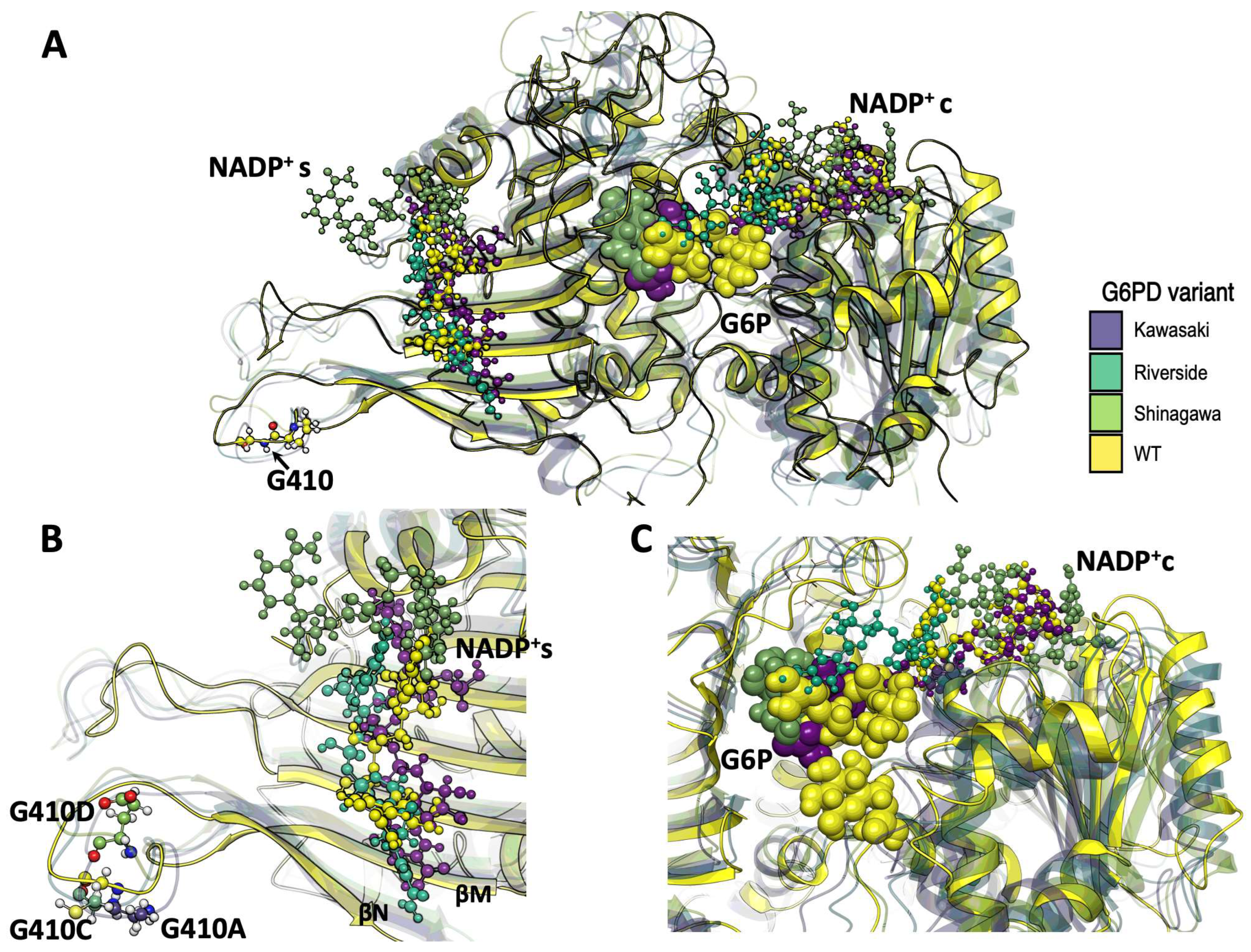
2.1.2. Analysis of the Impact of the Determined Variants on G6PD Protein Structure
2.2. Plasmid Construction and Expression and Purification of Recombinant Human G6PD
2.3. Functional Characterization
2.3.1. Measurement of Steady-State Kinetic Parameters
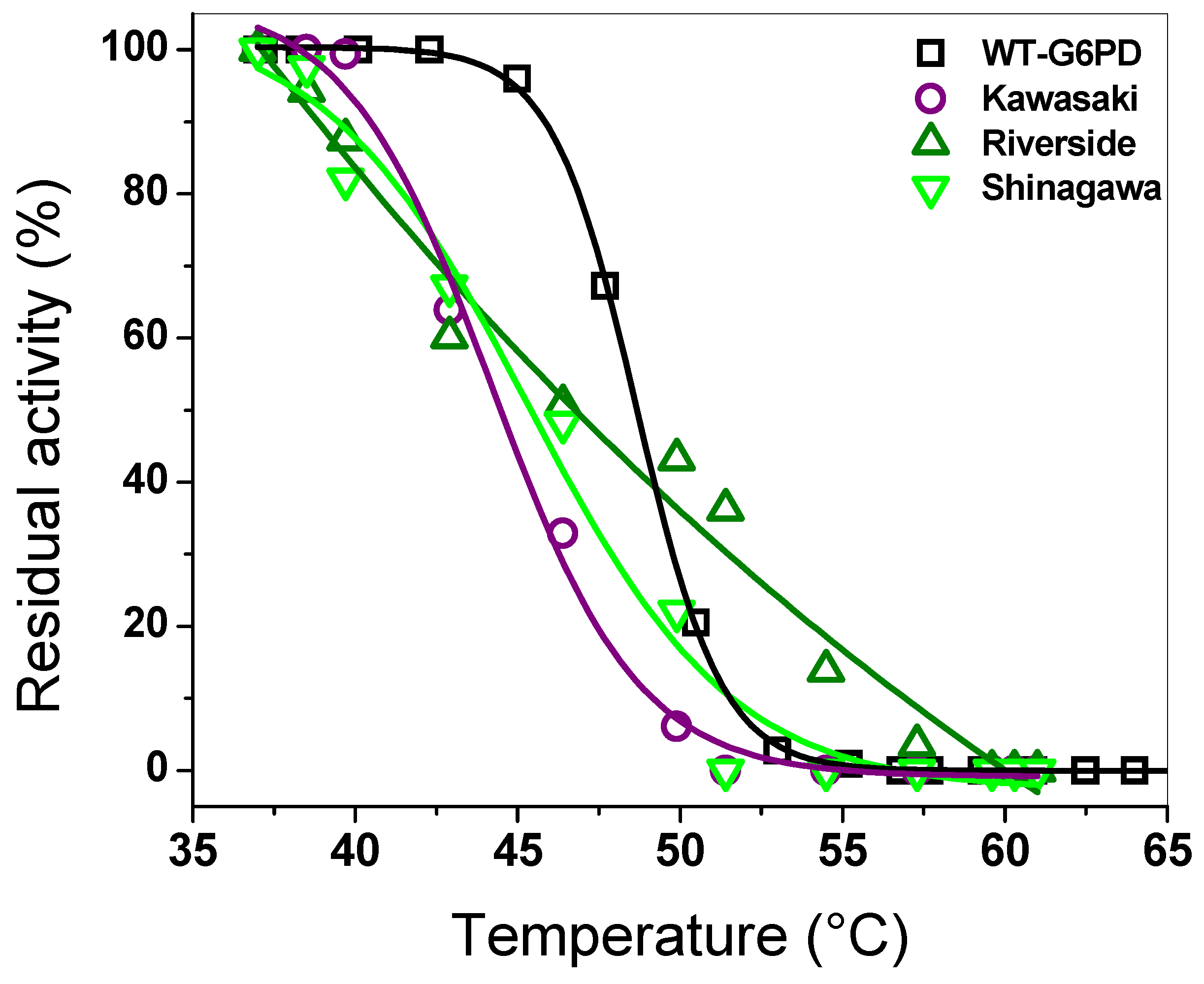
2.3.2. Thermal Inactivation Assays
2.4. Spectroscopic Characterization
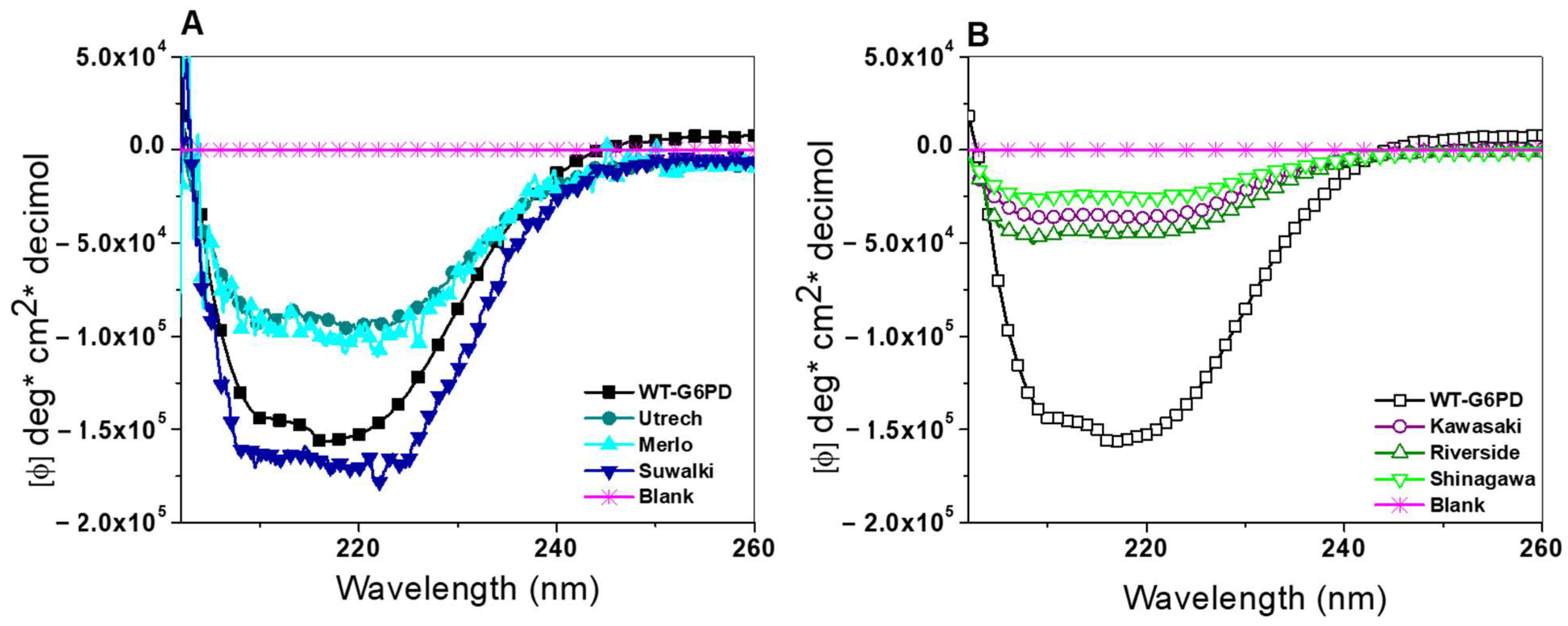
2.4.1. Circular Dichroism (CD) Analysis
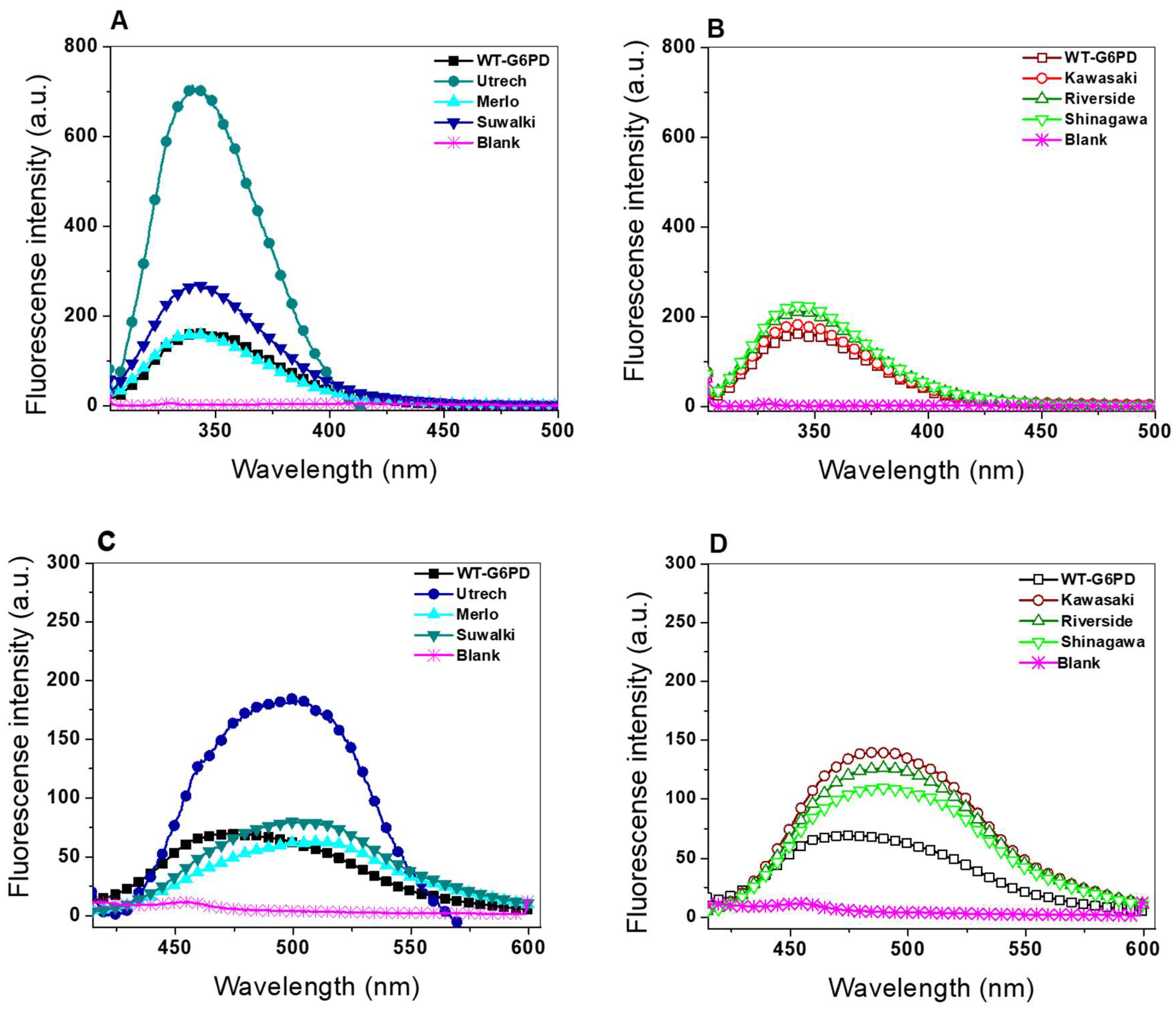
2.4.2. Intrinsic and Extrinsic Fluorescence Assays
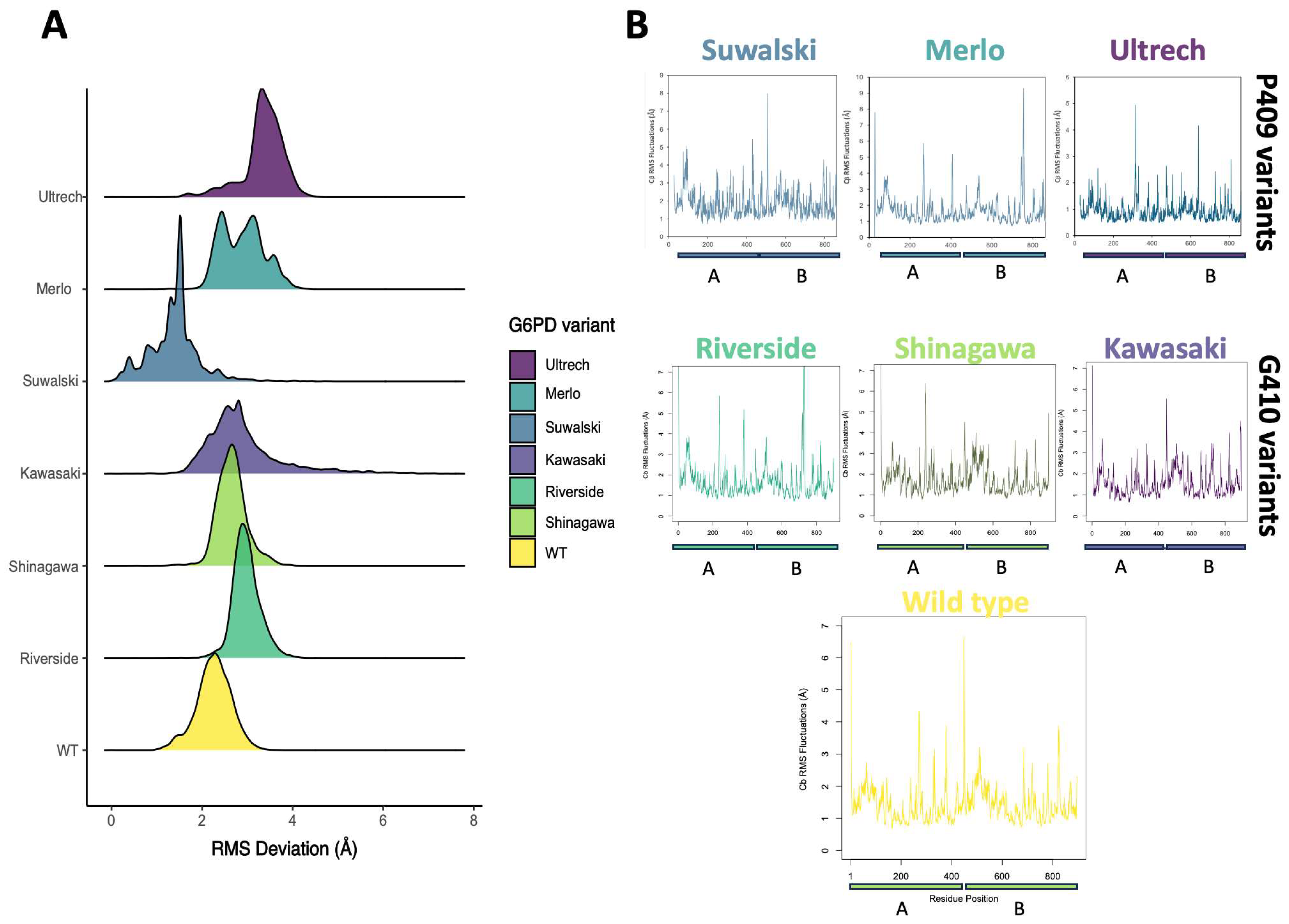
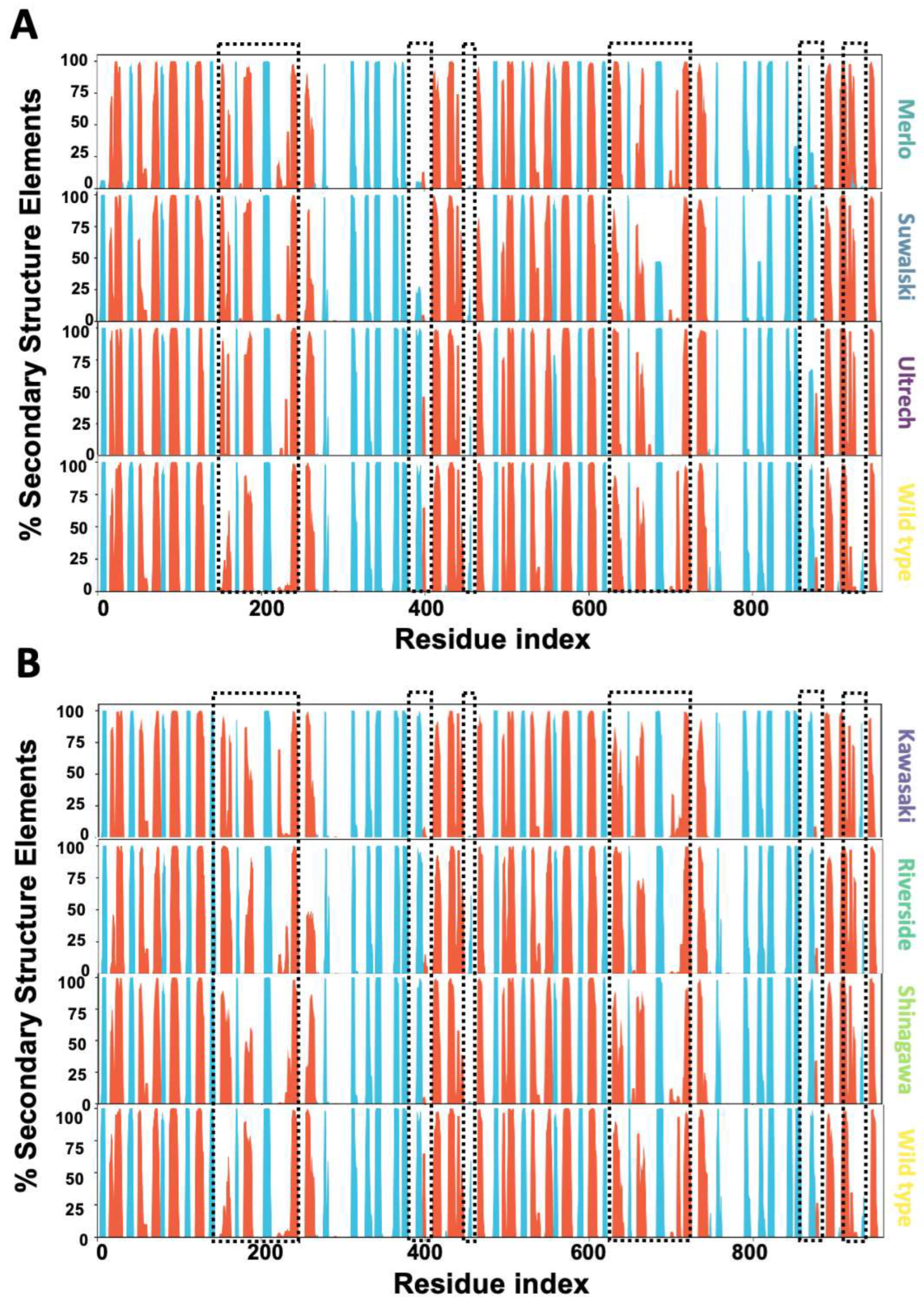
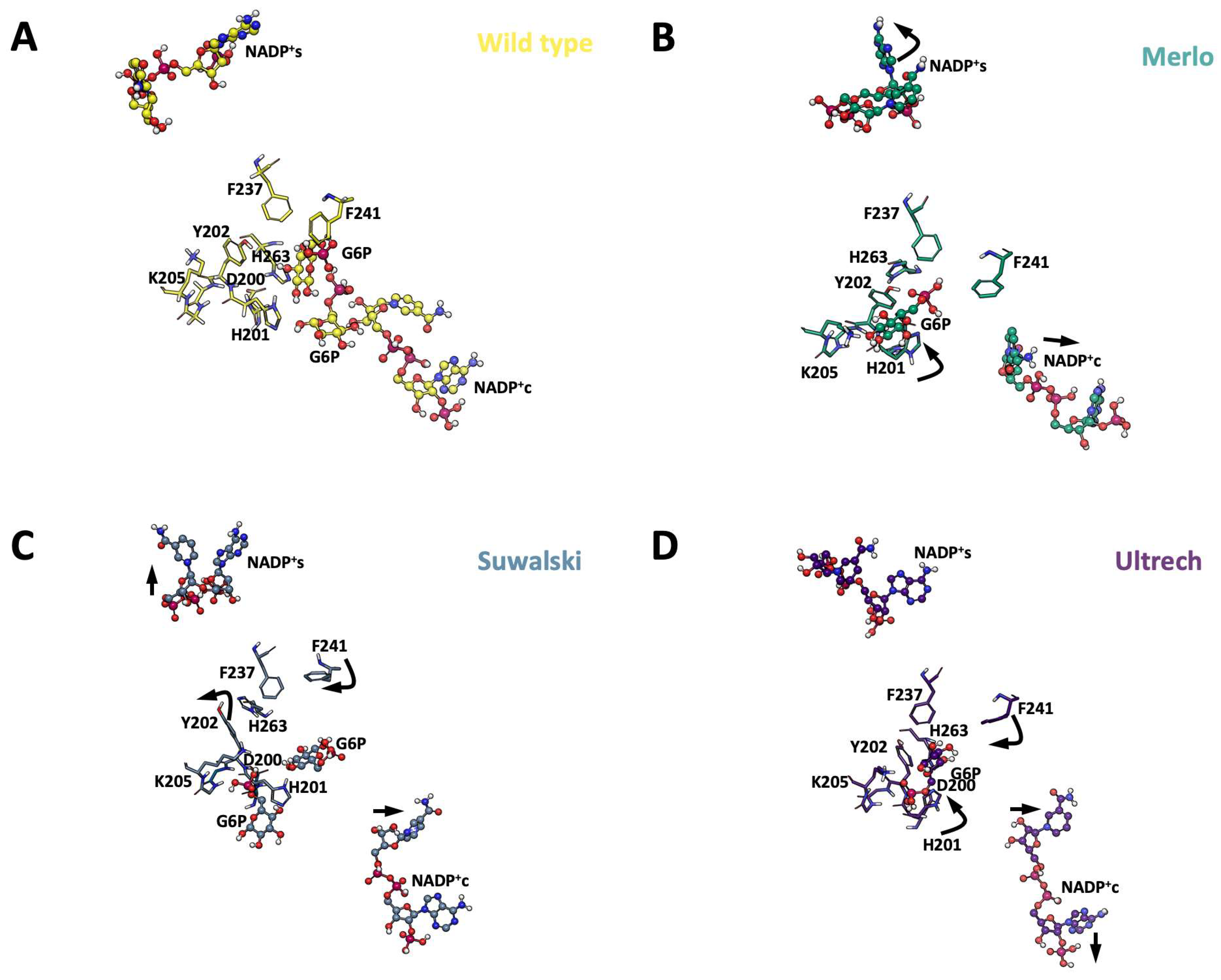
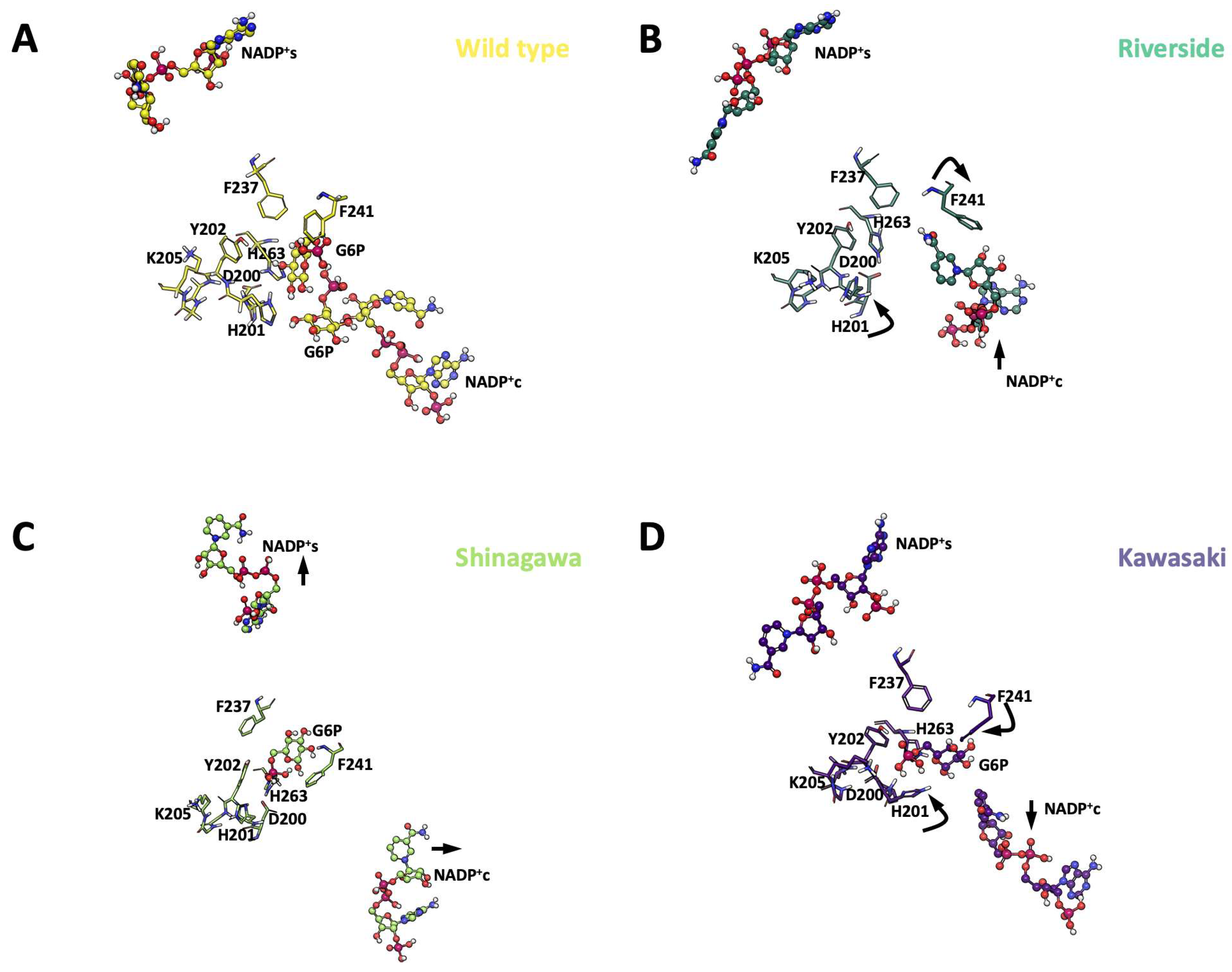
2.5. Conformational Dynamics of G6PD β-Sheet Variants
3. Materials and Methods
3.1. Phylogenetic Analysis of the Conservation of G6PD Protein Residues and Impact on G6PD Protein Structure
In Silico Site-Directed Mutagenesis
3.2. Site-Directed Mutagenesis
3.3. Purification of G6PD Variants
3.4. Functional Characterization Assays
3.4.1. Steady-State Kinetics
3.4.2. Thermal Inactivation Assay
3.5. Spectroscopic Characterization Assays
3.5.1. Circular Dichroism
3.5.2. Intrinsic and Extrinsic Fluorescence
3.6. Molecular Dynamics Simulations (MDSs)
General Simulation Setup and Parameterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Manzo, S.; Terrón-Hernández, J.; de la Mora-de la Mora, I.; González-Valdez, A.; Marcial-Quino, J.; García-Torres, I.; Vanoye-Carlo, A.; López-Velázquez, G.; Hernández-Alcántara, G.; Oria-Hernández, J.; et al. The Stability of G6PD Is Affected by Mutations with Different Clinical Phenotypes. Int. J. Mol. Sci. 2014, 15, 21179–21201. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L. Glucose 6-phosphate dehydrogenase deficiency: From genotype to phenotype. Haematologica 2006, 91, 1303–1306. [Google Scholar] [PubMed]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 9606, 64–74. [Google Scholar] [CrossRef]
- van Wijk, R.; van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Val, F.F.; Siqueira, A.M.; Franca, G.P.; Sampaio, V.S.; Melo, G.C.; Almeida, A.C.; Brito, M.A.; Peixoto, H.M.; Fuller, D.; et al. G6PD deficiency in Latin America: Systematic review on prevalence and variants. Mem. Inst. Oswaldo Cruz 2014, 5, 553–568. [Google Scholar] [CrossRef]
- Luzzatto, L.; Bancone, G.; Dugué, P.A.; Jiang, W.; Minucci, A.; Nannelli, C.; Pfeffer, D.; Prchal, J.; Sirdah, M.; Sodeinde, O.; et al. New WHO classification of genetic variants causing G6PD deficiency. Bull. World Health Organ. 2024, 102, 615–617. [Google Scholar] [CrossRef]
- Luzzatto, L.; Mehta, A.; Meloni, T. Haemoglobinuria and haptoglobin in G6PD deficiency. Br. J. Haematol. 1995, 91, 511–512. [Google Scholar] [CrossRef]
- Minucci, A.; Moradkhani, K.; Hwang, M.; Zuppi, C.; Giardina, B.; Capoluongo, P. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: Review of the “old” and update of the new mutations. Blood Cells Mol. Dis. 2012, 48, 154–165. [Google Scholar] [CrossRef]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Serrano-Posada, H.; González-Valdez, A.; Martínez-Rosas, V.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Castillo-Rodríguez, R.A.; Cuevas-Cruz, M.; et al. Functional and Biochemical Characterization of Three Recombinant Human Glucose-6-Phosphate Dehydrogenase Mutants: Zacatecas, Vanua-Lava and Viangchan. Int. J. Mol. Sci. 2016, 17, 787. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 36, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Notaro, R.; Afolayan, A.; Luzzatto, L. Human mutations in glucose 6-phosphate dehydrogenase reflect evolutionary history. FASEB J. 2000, 14, 485–494. [Google Scholar] [CrossRef]
- Naylor, C.E.; Rowland, P.; Basak, A.K.; Gover, S.; Mason, P.J.; Bautista, J.M.; Vulliamy, T.J.; Luzzatto, L.; Adams, M.J. Glucose 6-phosphate dehydrogenase mutations causing enzyme deficiency in a model of the tertiary structure of the human enzyme. Blood 1996, 87, 2974–2982. [Google Scholar] [CrossRef]
- van Wijk, R.; Huizinga, E.G.; Prins, I.; Kors, A.; Rijksen, G.; Bierings, M.; van Solinge, W.W. Distinct phenotypic expression of two de novo missense mutations affecting the dimer interface of glucose-6-phosphate dehydrogenase. Blood Cells. Mol. Dis. 2004, 32, 112–117. [Google Scholar] [CrossRef]
- Chaves, A.; Eberle, S.E.; Defelipe, L.; Pepe, C.; Milanesio, B.; Aguirre, F.; Fernandez, D.; Turjanski, A.; Feliú-Torres, A. Corrigendum to ‘Two novel DNA variants associated with glucose-6-phosphate dehydrogenase deficiency found in Argentine pediatric patients’. Clin. Biochem. 2018, 58, 131. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, D.; Jablonska-Skwiecinska, E.; Plochocka, D.; Chelstowska, A.; Lewandowska, I.; Witos, I.; Majewska, Z.; Rokicka-Milewska, R.; Burzynska, B. A novel mutation in the glucose-6-phosphate dehydrogenase gene in a subject with chronic nonspherocytic hemolytic anemia--characterization of enzyme using yeast expression system and molecular modeling. Blood Cells. Mol. Dis. 2004, 32, 124–130. [Google Scholar] [CrossRef]
- Vulliamy, T.; Luzzatto, L.; Hirono, A.; Beutler, E. Hematologically important mutations: Glucose-6-phosphate dehydrogenase. Blood Cells. Mol. Dis. 1997, 2, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hirono, A.; Kuhl, W.; Gelbart, T.; Forman, L.; Fairbanks, V.F.; Beutler, E. Identification of the binding domain for NADP+ of human glucose-6-phosphate dehydrogenase by sequence analysis of mutants. Proc. Natl. Acad. Sci. USA 1989, 86, 10015–10017. [Google Scholar] [CrossRef]
- Hirono, A.; Miwa, S.; Fujii, H.; Ishida, F.; Yamada, K.; Kubota, K. Molecular study of eight Japanese cases of glucose-6-phosphate dehydrogenase deficiency by nonradioisotopic single-strand conformation polymorphism analysis. Blood 1994, 83, 3363–3368. [Google Scholar] [CrossRef]
- Beutler, E.; Westwood, B.; Prchal, J.T.; Vaca, G.; Bartsocas, C.S.; Baronciani, L. New glucose-6-phosphate dehydrogenase mutations from various ethnic groups. Blood 1992, 80, 255–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valdar, W.S. Scoring residue conservation. Proteins Struct. Funct. Bioinform. 2002, 48, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Kessel, A.; Ben-Tal, N. Introduction to Proteins: Structure, Function, and Motion, 2nd ed.; Chapman and Hall/CRC (Taylor & Francis Group): Boca Raton, FL, USA, 2018. [Google Scholar]
- Landau, M.; Mayrose, I.; Rosenberg, Y.; Glaser, F.; Martz, E.; Pupko, T.; Ben-Tal, N. ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005, 33, W299–W302. [Google Scholar] [CrossRef] [PubMed]
- Glaser, F.; Pupko, T.; Paz, I.; Bell, R.E.; Bechor-Shental, D.; Martz, E.; Ben-Tal, N. ConSurf: Identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics 2003, 19, 163–164. [Google Scholar] [CrossRef]
- Venselaar, H.; Te Beek, T.A.; Kuipers, R.K.; Hekkelman, M.L.; Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform. 2010, 11, 548. [Google Scholar] [CrossRef]
- Rose, G.D.; Gierasch, L.M.; Smith, J.A. Turns in peptides and proteins. Adv. Protein. Chem. 1985, 37, 1–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Borish, K.; Dey, S.; Nagesh, J.; Das, A. Sequence dependent folding motifs of the secondary structures of Gly-Pro and Pro-Gly containing oligopeptides. Phys. Chem. Chem. Phys. 2022, 30, 18408–18418. [Google Scholar] [CrossRef]
- Lewis, P.N.; Momany, F.A.; Scheraga, H.A. Folding of polypeptide chains in proteins: A proposed mechanism for folding. Proc. Natl. Acad. Sci. USA 1971, 68, 2293–2297. [Google Scholar] [CrossRef]
- Crawford, J.L.; Lipscomb, W.N.; Schellman, C.G. The reverse turn as a polypeptide conformation in globular proteins. Proc. Natl. Acad. Sci. USA 1973, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Deber, C.M.; Brodsky, B.; Rath, A. Proline Residues in Proteins. In eLS; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hwang, S.; Mruk, K.; Rahighi, S.; Raub, A.G.; Chen, C.H.; Dorn, L.E.; Horikoshi, N.; Wakatsuki, S.; Chen, J.K.; Mochly-Rosen, D. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat. Commun. 2018, 9, 4045. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pan, Q.; Pires, D.E.V.; Rodrigues, C.H.M.; Ascher, D.B. DDMut: Predicting effects of mutations on protein stability using deep learning. Nucleic Acids Res. 2023, 51, W122–W128. [Google Scholar] [CrossRef]
- Boonyuen, U.; Chamchoy, K.; Swangsri, T.; Junkree, T.; Day, N.P.; White, N.J.; Imwong, M. A trade off between catalytic activity and protein stability determines the clinical manifestations of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Int. J. Biol. Macromol. 2017, 104, 145–156. [Google Scholar] [CrossRef]
- Praoparotai, A.; Junkree, T.; Imwong, M.; Boonyuen, U. Functional and structural analysis of double and triple mutants reveals the contribution of protein instability to clinical manifestations of G6PD variants. Int. J. Biol. Macromol. 2020, 158, 884–893. [Google Scholar] [CrossRef]
- Nannelli, C.; Bosman, A.; Cunningham, J.; Dugué, P.A.; Luzzatto, L. Genetic variants causing G6PD deficiency: Clinical and biochemical data support new WHO classification. Br. J. Haematol. 2023, 202, 1024–1032. [Google Scholar] [CrossRef]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Enríquez-Flores, S.; De la Mora-De la Mora, I.; González-Valdez, A.; García-Torres, I.; Martínez-Rosas, V.; Sierra-Palacios, E.; Lazcano-Pérez, F.; et al. Mutations of Glucose-6-Phosphate Dehydrogenase Durham, Santa-Maria and A+ Variants Are Associated with Loss Functional and Structural Stability of the Protein. Int. J. Mol. Sci. 2015, 16, 28657–28668. [Google Scholar] [CrossRef]
- Ramírez-Nava, E.J.; Ortega-Cuellar, D.; Serrano-Posada, H.; González-Valdez, A.; Vanoye-Carlo, A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Hernández-Pineda, J.; Rodríguez-Bustamante, E.; Arreguin-Espinosa, R.; et al. Biochemical Analysis of Two Single Mutants that Give Rise to a Polymorphic G6PD A-Double Mutant. Int. J. Mol. Sci. 2017, 18, 2244. [Google Scholar] [CrossRef]
- Cortés-Morales, Y.Y.; Vanoye-Carlo, A.; Castillo-Rodríguez, R.A.; Serrano-Posada, H.; González-Valdez, A.; Ortega-Cuellar, D.; Hernández-Ochoa, B.; Moreno-Vargas, L.M.; Prada-Gracia, D.; Sierra-Palacios, E.; et al. Cloning and biochemical characterization of three glucose 6 phosphate dehydrogenase mutants presents in the Mexican population. Int. J. Biol. Macromol. 2018, 119, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosas, V.; Juárez-Cruz, M.V.; Ramírez-Nava, E.J.; Hernández-Ochoa, B.; Morales-Luna, L.; González-Valdez, A.; Serrano-Posada, H.; Cárdenas-Rodríguez, N.; Ortiz-Ramírez, P.; Centeno-Leija, S.; et al. Effects of Single and Double Mutants in Human Glucose-6-Phosphate Dehydrogenase Variants Present in the Mexican Population: Biochemical and Structural Analysis. Int. J. Mol. Sci. 2020, 21, 2732. [Google Scholar] [CrossRef]
- Alcántara-Ortigoza, M.A.; Hernández-Ochoa, B.; Angel, A.G.-D.; Ibarra-González, I.; Belmont-Martínez, L.; Gómez-Manzo, S.; Vela-Amieva, M. Functional characterization of the p. (Gln195His) or Tainan and novel p. (Ser184Cys) or Toluca glucose-6-phosphate dehydrogenase (G6PD) gene natural variants identified through Mexican newborn screening for glucose-6-phosphatedehydrogenase deficiency. Clin. Biochem. 2022, 109–110, 64–73. [Google Scholar] [CrossRef]
- Luzzatto, L.; Allan, N.C. Different properties of glucose 6-phosphate dehydrogenase from human erythrocytes with normal and abnormal enzyme levels. Biochem. Biophys. Res. Commun. 1965, 21, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Biter, A.B.; Pollet, J.; Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. A method to probe protein structure from UV absorbance spectra. Anal. Biochem. 2019, 587, 113450. [Google Scholar] [CrossRef]
- Costa, S.; Minucci, A.; Kumawat, A.; De Bonis, M.; Prontera, G.; Gelsomino, M.; Tana, M.; Tiberi, E.; Romano, A.; Ruggiero, A.; et al. Pathogenic G6PD Variants: Different Clinical Pictures Arise from Different Missense Mutations in the Same Codon. Br. J. Haematol. 2024, 205, 1985–1994. [Google Scholar] [CrossRef]
- Wang, X.; Chan, T.F.; Lam, V.M.S.; Engel, P.C. What Is the Role of the Second “Structural” NADP+-binding Site in Human Glucose 6-phosphate Dehydrogenase? Protein Sci. 2008, 17, 1403–1411. [Google Scholar] [CrossRef]
- Wei, X.; Kixmoeller, K.; Baltrusaitis, E.; Yang, X.; Marmorstein, R. Allosteric Role of a Structural NADP+ Molecule in Glucose-6-Phosphate Dehydrogenase Activity. Proc. Natl. Acad. Sci. USA 2022, 119, e2119695119. [Google Scholar] [CrossRef]
- Anuar, N.F.S.K.; Wahab, R.A.; Huyop, F.; Amran, S.I.; Hamid, A.A.A.; Halim, K.B.A.; Hood, M.H.M. Molecular docking and molecular dynamics simulations of a mutant acinetobacter haemolyticus alkaline-stable lipase against tributyrin. J. Biomol. Struct. Dyn. 2021, 39, 2079–2091. [Google Scholar] [CrossRef]
- Au, S.W.; Gover, S.; Lam, V.M.; Adams, M.J. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 2000, 8, 293–303. [Google Scholar] [CrossRef]
- Garcia, A.A.; Mathews, I.I.; Horikoshi, N.; Matsui, T.; Kaur, M.; Wakatsuki, S.; Mochly-Rosen, D. Stabilization of Glucose-6-Phosphate Dehydrogenase Oligomers Enhances Catalytic Activity and Stability of Clinical Variants. J. Biol. Chem. 2022, 298, 101610. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Ramírez, A.; González-Valdez, A.; Hernández-Ochoa, B.; Canseco-Ávila, L.M.; López-Roblero, A.; Arreguin-Espinosa, R.; Pérez de la Cruz, V.; Hernández-Urzua, E.; Cárdenas-Rodríguez, N.; Enríquez-Flores, S.; et al. Evaluation of Three Mutations in Codon 385 of Glucose-6-Phosphate Dehydrogenase via Biochemical and In Silico Analysis. Int. J. Mol. Sci. 2024, 25, 12556. [Google Scholar] [CrossRef] [PubMed]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.N.; Lam, V.M.S.; Adams, M.J. Structural Studies of Glucose-6-Phosphate and NADP + Binding to Human Glucose-6-Phosphate Dehydrogenase. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Erez, E.; Martz, E.; Pupko, T.; Ben-Tal, N. ConSurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010, 38, W529–W533. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Shapovalov, M.V.; Dunbrack, R.L., Jr. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 2011, 19, 844–858. [Google Scholar] [CrossRef]
- Feig, M. Local Protein Structure Refinement via Molecular Dynamics Simulations with locPREFMD. J. Chem. Inf. Model. 2016, 56, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Dupradeau, F.-Y.; Cezard, C.; Lelong, R.; Stanislawiak, E.; Pecher, J.; Delepine, J.C.; Cieplak, P. R.E.DD.B.: A Database for RESP and ESP Atomic Charges, and Force Field Libraries. Nucleic Acids Res. 2007, 36, D360–D367. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The Nose–Hoover Thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Martyna, G.J. Remarks on “Constant-Temperature Molecular Dynamics with Momentum Conservation”. Phys. Rev. E 1994, 50, 3234–3236. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Joannopoulos, J.D.; Kleinman, L. Constant-Temperature Molecular Dynamics with Momentum Conservation. Phys. Rev. E 1993, 47, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
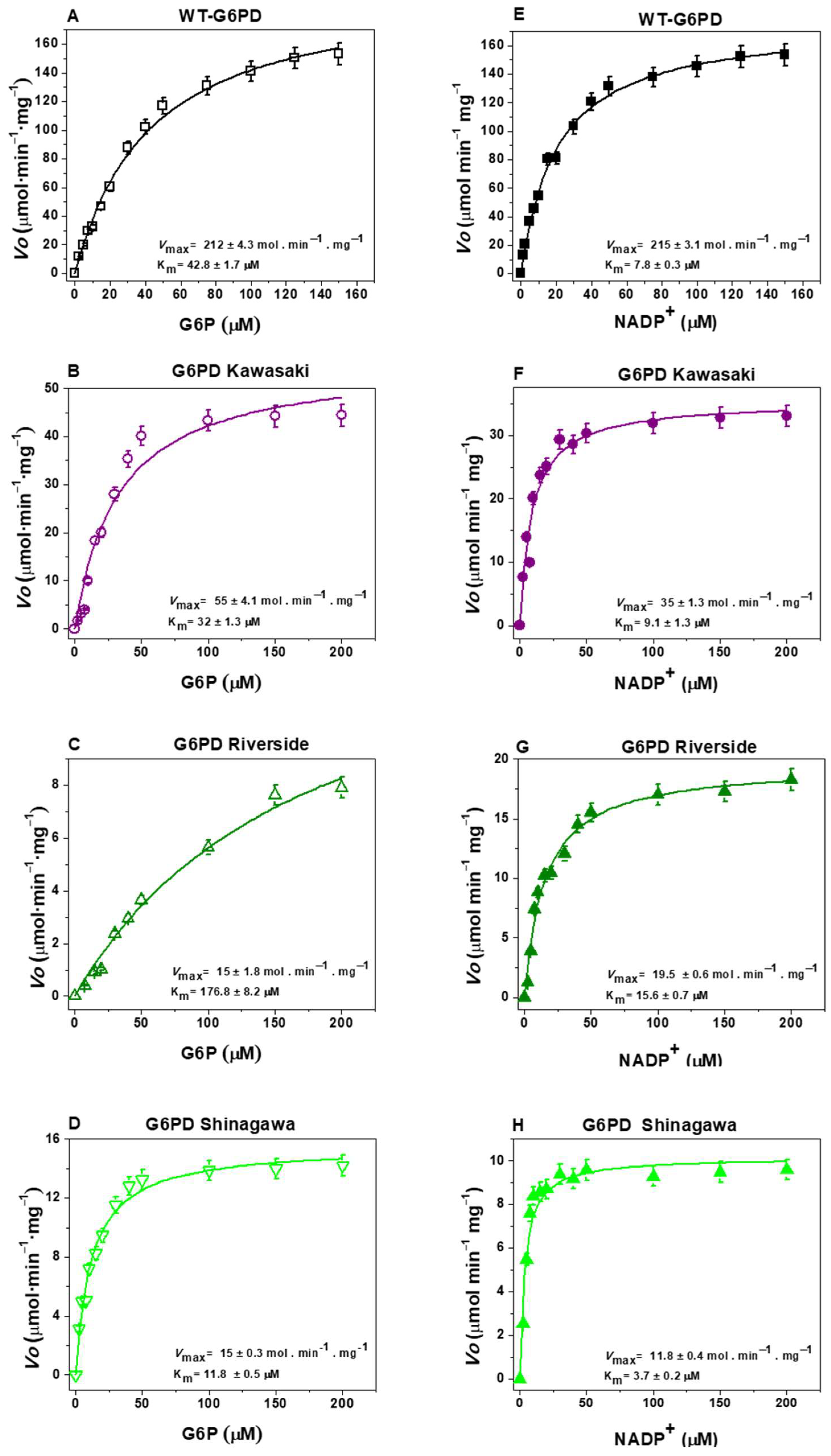
| G6PD | Class | Amino Acid Substitution | kcat (s−1) | Km G6P (µM) | Km NADP+ (µM) | kcat/Km G6P (µM−1 s−1) | kcat/Km NADP+ (µM−1 s−1) |
|---|---|---|---|---|---|---|---|
| WT | - | 211.3 ± 3.1 | 42.8 ± 1.7 | 7.8 ± 0.3 | 4.9 ± 0.3 | 27.1 ± 1.9 | |
| Suwalki | A | Pro409Arg | ND | ND | ND | ND | ND |
| Merlo | A | Pro409Gln | ND | ND | ND | ND | ND |
| Utrecht | A | Pro409Ser | ND | ND | ND | ND | ND |
| Kawasaki | I | Gly410Ala | 34.3 ± 1.2 | 32 ± 1.3 | 9.1 ± 1.3 | 1.1 ± 0.05 | 3.8 ± 0.2 |
| Riverside | I | Gly410Cys | 18.6 ± 1.8 | 176.8 ± 8.2 | 15.6 ± 0.7 | 0.10 ± 0.005 | 1.19 ± 0.05 |
| Shinagawa | I | Gly410Asp | 18.7 ± 0.3 | 11.8 ± 0.5 | 3.7 ± 0.2 | 1.5 ± 0.06 | 5.1 ± 0.18 |
| Mutant | Mutagenic Oligonucleotide | Reference |
|---|---|---|
| Utrecht | Fw: 5′ CCAAGAAGTCGGGCATGTTC-3′ Rv: 5′ GGTTCTTCAGCCCGTACAAG-3′ | This study |
| Suwalki | Fw: 5′ CCAAGAAGCGGGGCATGTTC-3′ Rv: 5′ GGTTCTTCGCCCCGTACAAG-3′ | This study |
| Merlo | Fw: 5′ CCAAGAAGCAGGGCATGTTC-3′ Rv: 5′ GGTTCTTCGTCCCGTACAAG-3′ | This study |
| Riverside | Fw: 5′-CCAAGAAGCCGTGCATGTTC-3′ Rv:5′-GAACATGCACGGCTTCTTGG-3′ | This study |
| Kawasaki | Fw: 5′-CCAAGAAGCCGGCCATGTTC-3′ Rv: 5′-GAACATGGCCGGCTTCTTGG-3′ | This study |
| Shinagawa | Fw: 5′-CCAAGAAGCCGGACATGTTC-3′ Rv: 5′-GAACATGTCCGGCTTCTTGG-3′ | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ochoa, B.; Gualos-González, M.G.; Moreno-Hernández, J.A.; Morales-Luna, L.; Vázquez-Bautista, M.; Canseco-Ávila, L.M.; Pérez de la Cruz, V.; Arreguin-Espinosa, R.; Hernández-Urzua, E.; Enríquez-Flores, S.; et al. Integrated Approach for Biochemical and Functional Characterization of Six Clinical Variants of Glucose-6-Phosphate Dehydrogenase. Int. J. Mol. Sci. 2025, 26, 8464. https://doi.org/10.3390/ijms26178464
Hernández-Ochoa B, Gualos-González MG, Moreno-Hernández JA, Morales-Luna L, Vázquez-Bautista M, Canseco-Ávila LM, Pérez de la Cruz V, Arreguin-Espinosa R, Hernández-Urzua E, Enríquez-Flores S, et al. Integrated Approach for Biochemical and Functional Characterization of Six Clinical Variants of Glucose-6-Phosphate Dehydrogenase. International Journal of Molecular Sciences. 2025; 26(17):8464. https://doi.org/10.3390/ijms26178464
Chicago/Turabian StyleHernández-Ochoa, Beatriz, Mónica Guadalupe Gualos-González, Jhuremy Alexandra Moreno-Hernández, Laura Morales-Luna, Montserrat Vázquez-Bautista, Luis Miguel Canseco-Ávila, Verónica Pérez de la Cruz, Roberto Arreguin-Espinosa, Elizabeth Hernández-Urzua, Sergio Enríquez-Flores, and et al. 2025. "Integrated Approach for Biochemical and Functional Characterization of Six Clinical Variants of Glucose-6-Phosphate Dehydrogenase" International Journal of Molecular Sciences 26, no. 17: 8464. https://doi.org/10.3390/ijms26178464
APA StyleHernández-Ochoa, B., Gualos-González, M. G., Moreno-Hernández, J. A., Morales-Luna, L., Vázquez-Bautista, M., Canseco-Ávila, L. M., Pérez de la Cruz, V., Arreguin-Espinosa, R., Hernández-Urzua, E., Enríquez-Flores, S., De la Mora-De la Mora, I., Cárdenas-Rodríguez, N., Bandala, C., De Franceschi, L., Vidal-Limon, A., & Gómez-Manzo, S. (2025). Integrated Approach for Biochemical and Functional Characterization of Six Clinical Variants of Glucose-6-Phosphate Dehydrogenase. International Journal of Molecular Sciences, 26(17), 8464. https://doi.org/10.3390/ijms26178464










