Non-Alcoholic Fatty Liver Disease in Poultry: Risk Factors, Mechanism of Development, and Emerging Strategies
Abstract
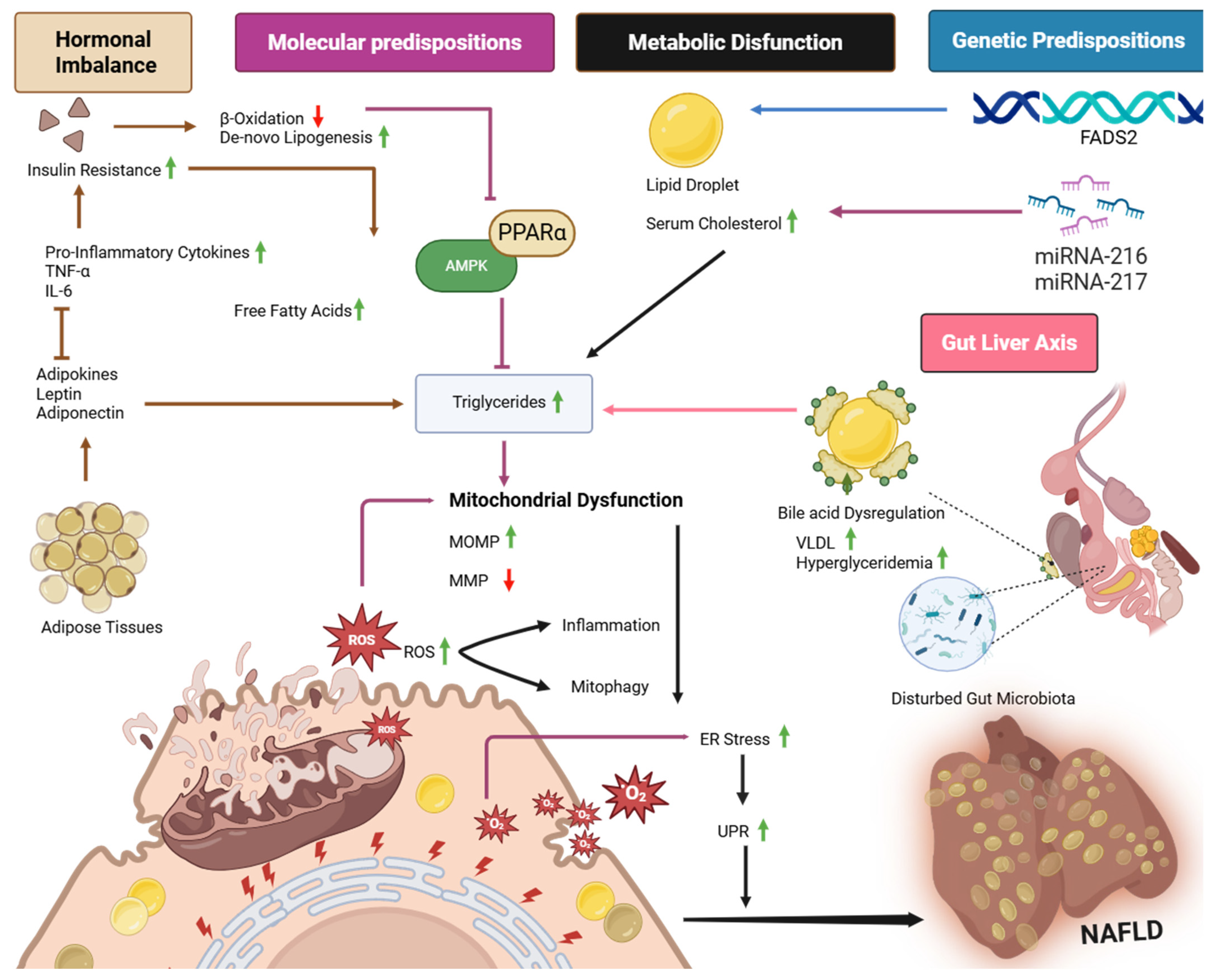
1. Introduction
| Risk Factor | Impact | Reference |
|---|---|---|
| Dietary Factors | Energy-dense and high-carbohydrate diets, while intended to support high egg yield, contribute to excessive hepatic fat accumulation when not nutritionally balanced. Moreover, factors like overfeeding, vitamin deficiency, and mycotoxins are also associated with liver fattening conditions in poultry. | [32] |
| prolonged metabolic disease | Birds with prolonged metabolic disease patterns show severe hepatic pathologies such as fibrosis, cirrhosis, or hepatic failure, which can ultimately lead to mortality in layers. | [33] |
| Environmental Stress | Adverse conditions such as heat stress, poor ventilation, and overcrowding exacerbate metabolic disturbances and promote lipid deposition in the liver. | [34] |
| Gut Microbiota | In chickens, dysbiosis of the gut microbiota increases intestinal permeability, allowing the translocation of endotoxins such as lipopolysaccharide (LPS) into the blood stream. This promotes hepatic inflammation, disrupts lipid metabolism, and contributes to the progression of NAFLD. | [31,35] |
| Metabolic and Inflammatory Factors | Metabolic factors such as amino acid imbalance and insulin resistance in poultry are linked to NAFLD, whereas oxidative stress and ROS have a strong association with inflammatory pathways leading to NAFLD. | [36,37,38] |
| Genetic Predispositions | Different poultry breeds and genetic selection of high-producing layers and fast-growing broilers, genetically selected for traits like rapid growth or high egg production, leads to increased metabolic demand, hormonal imbalances, and a greater tendency for hepatic lipogenesis, making them more prone to fat accumulation in the liver. | [39] |
2. Lipid Metabolism in Poultry
3. Bile Acid Metabolism in Poultry
4. Mechanisms of Fatty Liver Development in Poultry
4.1. Role of Nutritional Imbalance in Development of NAFLD
4.2. Role of Hepatic Lipid Accumulation and Bile Acid Dysregulation in NAFLD
4.3. Molecular Basis of NAFLD Development in Poultry
4.3.1. Molecular Markers in NAFLD in Poultry
4.3.2. Signaling Pathways
4.4. The Role of ER-Mitochondrial Interplay in NAFLD
4.5. Genetic Predispositions of NAFLD in Poultry
4.6. Role of Hormonal Dysregulation in Progression of NAFLD
| Adipokine | Effect | Reference |
|---|---|---|
| Leptin | In poultry, elevated leptin levels are associated with leptin resistance, a state in which hepatic and peripheral tissues fail to respond to leptin signaling. This impairment disrupts lipid homeostasis by reducing fatty acid oxidation while sustaining lipogenesis. Consequently, excess lipid deposition occurs in the liver, predisposing birds to NAFLD progression. | [107] |
| Adiponectin | Adiponectin significantly inhibits adipocyte development in chickens by downregulating key adipogenic transcription factors such as C/EBPα and FAS, while simultaneously upregulating lipolytic genes like ATGL and its receptor AdipoR1. This suppression of adipogenesis is further mediated through activation of the p38 MAPK/ATF-2 and TOR/p70S6K signaling pathways, collectively leading to reduced lipid accumulation and fat deposition in chickens’ hepatic tissues. | [109] |
| Visfatin | Visfatin protein expression is primarily localized around the central vein in hepatic lobules exhibiting mild steatosis. Elevated levels of Visfatin in serum and liver tissue appear early in the disease progression, suggesting its potential involvement in the pathogenesis of NAFLD. | [110] |
| Resistin | Resistin alters mitochondrial morphology, reduces mitochondrial content, and promotes lipid accumulation under high-fat diet conditions. | [111] |
| Chemerin | In chickens, chemerin is implicated in the control of lipid metabolism, exhibiting a negative correlation between its plasma levels and fattening, as well as hepatic expression. It has a modulatory role in hepatic lipid accumulation and an impact on the onset of fatty liver disease. | [105] |
| Tumor Necrosis Factor-α (TNF α) | TNF-α induces insulin resistance by disrupting post-receptor insulin signaling pathways, originates from adipose tissues, and acts as important cytokine in pro-inflammatory pathways. | [112] |
| Interleukin-6 (IL-6) | Elevated IL-6 levels activate hepatic inflammatory signaling pathways, disrupt lipid metabolism, and promote hepatic steatosis, thereby facilitating the NAFLD. | [112,113] |
4.7. Role of Environmental Stress in NAFLD
4.8. Influence of Production Phases on NAFLD
4.9. Role of Gut Microbiota in NAFLD Development and Prevention
5. Disease Diagnosis and Emerging Strategies to Prevent NAFLD in Poultry
6. Knowledge Gaps and Future Directions in NAFLD Research in Poultry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NASH | Non-Alcoholic Steatohepatitis |
| T2DM | Type-2 Diabetes Mellitus |
| FLHS | Fatty Liver Hemorrhagic Syndrome |
| ACC | Acetyl Co-A Carboxylase |
| FAS | Fatty Acid Synthase |
| PUFA | Polyunsaturated Fatty Acid |
| VLDL | Very Low-Density Lipoprotein |
| SREBP-1c | Sterol Regulatory Element-Binding Protein 1c |
| FXR | Farnesoid X Receptor |
| DEG | Differentially Expressed Gene |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin 6 |
| AACS | Acetoacetyl-CoA Synthetase |
| GST | Glutathione S-Transferase |
| AMPK | AMP-Activated Protein Kinase |
| PPAR α | Peroxisome Proliferator-Activated Receptor alpha |
| ER | Endoplasmic Reticulum |
| ROS | Reactive Oxygen Species |
| MOMP | Mitochondrial Outer Membrane Permeability |
| MMP | Mitochondrial Membrane Potential |
| C/EBP | CCAAT/Enhancer-Binding Protein |
| CHOP | C/EBP Homologous Protein |
| H2AX | Histone 2A.X |
| FADS2 | Fatty Acid Desaturase 2 |
| BMAL1 | Brain and Muscle Arnt-Like Protein 1 |
| CLOCK | Circadian Locomotor Output Cycles Protein Kinase |
| PER | Period (family of genes involved in circadian rhythms) |
| CRY | Cryptochrome |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| LXR | Liver X Receptors |
References
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of Non-Alcoholic and Alcoholic Fatty Liver Diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Motta, B.M.; Masarone, M.; Torre, P.; Persico, M. From Non-Alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): Epidemiology, Incidence, Predictions, Risk Factors, and Prevention. Cancers 2023, 15, 5458. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Laddha, A.P.; Dzielak, L.; Lewis, C.; Xue, R.; Manautou, J.E. Impact of Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) on the Expression and Function of Hepatobiliary Transporters: A Comprehensive Mechanistic Review. Biochim. Biophys. Acta-Mol. Basis Dis. 2024, 1870, 167037. [Google Scholar] [CrossRef]
- Wu, W.; Ma, X.; Chen, R.; Fan, J.; Ye, W.; Chen, Z.; Huang, Q.; Qian, L. Effects of Phytosterol Ester Supplementation on Egg Characteristics, Eggshell Ultrastructure, Antioxidant Capacity, Liver Function and Hepatic Metabolites of Laying Hens during Peak Laying Period. Antioxidants 2024, 13, 458. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.; Chuppava, B.; Dimitri, R.; Visscher, C. Hepatic Lipidosis in Fattening Turkeys: A Review. Ger. J. Vet. Res. 2021, 1, 48–66. [Google Scholar] [CrossRef]
- Crespo, R. Fatty Liver Hemorrhagic Syndrome in Poultry. Vet. Man 2020, 25, 1–3. [Google Scholar]
- Wu, Q.; Tang, H.; Wang, H. The Anti-Oxidation and Mechanism of Essential Oil of Paederia Scandens in the NAFLD Model of Chicken. Animals 2019, 9, 850. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Zhang, Y.; Huang, Z.; Huang, Z.; Chen, X.; Sun, C.; Qi, Y.; Hu, Y.; Chen, T.; et al. Tartary Buckwheat Flavonoids and 25-Hydroxyvitamin D3 Mitigate Fatty Liver Syndrome in Laying Hens: Association with Cecal Microbiota Remodeling and Lipid Metabolic Homeostasis. Animals 2025, 15, 2210. [Google Scholar] [CrossRef]
- Trott, K.A.; Giannitti, F.; Rimoldi, G.; Hill, A.; Woods, L.; Barr, B.; Anderson, M.; Mete, A. Fatty Liver Hemorrhagic Syndrome in the Backyard Chicken: A Retrospective Histopathologic Case Series. Vet. Pathol. 2014, 51, 787–795. [Google Scholar] [CrossRef]
- Anene, D.O.; Akter, Y.; Groves, P.J.; Horadagoda, N.; Liu, S.Y.; Moss, A.; Hutchison, C.; O’Shea, C.J. Association of Feed Efficiency with Organ Characteristics and Fatty Liver Haemorrhagic Syndrome in Laying Hens. Sci. Rep. 2023, 13, 5872. [Google Scholar] [CrossRef]
- Usturoi, M.G.; Rațu, R.N.; Crivei, I.C.; Veleșcu, I.D.; Usturoi, A.; Stoica, F.; Radu Rusu, R.M. Unlocking the Power of Eggs: Nutritional Insights, Bioactive Compounds, and the Advantages of Omega-3 and Omega-6 Enriched Varieties. Agriculture 2025, 15, 242. [Google Scholar] [CrossRef]
- Rozenboim, I.; Mahato, J.; Cohen, N.A.; Tirosh, O. Low Protein and High-Energy Diet: A Possible Natural Cause of Fatty Liver Hemorrhagic Syndrome in Caged White Leghorn Laying Hens. Poult. Sci. 2016, 95, 612–621. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty Liver Haemorrhagic Syndrome Occurrence in Laying Hens: Impact of Production System. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Chaudhury, T.; Brodosi, L.; Marchesini, G.; Mitra, S.K.; Petroni, M.L. Chapter 22—NAFLD, the Hepatic Manifestation of the Metabolic Syndrome. In Metabolic Syndrome; Mukhopadhyay, S., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 279–291. ISBN 978-0-323-85732-1. [Google Scholar]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites 2024, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Iqbal Yatoo, M.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 Fatty Acids in Poultry Nutrition: Effect on Production Performance and Health. Animals 2019, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Nalbandov, A.V. Endocrine Control of Physiological Functions. Poult. Sci. 1953, 32, 88–103. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; He, Y.; Dou, T.; Jia, J.; Ge, C. Endocrine and Genetic Factors Affecting Egg Laying Performance in Chickens: A Review. Br. Poult. Sci. 2020, 61, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, W.; Ding, H.; Zhang, G.; Xie, K.; Zhang, T. Integrated Metabolomic and Transcriptomic Analysis Reveals Potential Gut-Liver Crosstalks in the Lipogenesis of Chicken. Animals 2023, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei, C.P.; Selamat, J.; Nordin, N.; Idris, L.H.; Razis, A.F.A. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical Response: Effects of Heat Stress-induced Oxidative Stress on Lipid Metabolism in the Avian Liver. Antioxidants 2021, 10, 35. [Google Scholar] [CrossRef]
- Nega1, T.E.; Assefa1, T.; Bekele, D.; Tesema, A. Influence of Daylength, Light Color, Light Intensity, and Sources on the Performance of Growers, and Layers of Different Strains of Chicken: A Review. EC Nutr. 2024, 19, 1–17. [Google Scholar]
- Huang, Y.; Cai, H.; Han, Y.; Yang, P. Mechanisms of Heat Stress on Neuroendocrine and Organ Damage and Nutritional Measures of Prevention and Treatment in Poultry. Biology 2024, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Arciello, M.; Gori, M.; Maggio, R.; Barbaro, B.; Tarocchi, M.; Galli, A.; Balsano, C. Environmental Pollution: A Tangible Risk for NAFLD Pathogenesis. Int. J. Mol. Sci. 2013, 14, 22052–22066. [Google Scholar] [CrossRef]
- Adetunji, A.G.; Obeng-Gyasi, E. Investigating the Interplay of Toxic Metals and Essential Elements in Liver Disease. Int. J. Environ. Res. Public Health 2024, 21, 762. [Google Scholar] [CrossRef]
- Tauson, R. Management and Housing Systems for Layers—Effects on Welfare and Production. World’s Poult. Sci. J. 2005, 61, 477–490. [Google Scholar] [CrossRef]
- Berenjian, A.; Sharifi, S.D.; Mohammadi-Sangcheshmeh, A.; Bakhtiarizadeh, M.R. Omega-3 Fatty Acids Reduce the Negative Effects of Dexamethasone-Induced Physiological Stress in Laying Hens by Acting through the Nutrient Digestibility and Gut Morphometry. Poult. Sci. 2021, 100, 100889. [Google Scholar] [CrossRef]
- Beldowska, A.; Barszcz, M.; Dunislawska, A. State of the Art in Research on the Gut-Liver and Gut-Brain Axis in Poultry. J. Anim. Sci. Biotechnol. 2023, 14, 37. [Google Scholar] [CrossRef]
- van Eck, L.M.; Enting, H.; Carvalhido, I.J.; Chen, H.; Kwakkel, R.P. Lipid Metabolism and Body Composition in Long-Term Producing Hens. Worlds. Poult. Sci. J. 2023, 79, 243–264. [Google Scholar] [CrossRef]
- Borchert, P.; Zellmer-Bruhn, D.M. Reproduced with Permission of the Copyright Owner. Further Reproduction Prohibited Without. J. Allergy Clin. Immunol. 2010, 130, 556. [Google Scholar]
- Kumari, K.N.R.; Nath, D.N.; Venkateswra, S. Ameliorative Measures to Counter Heat Stress in Poultry. Worlds. Poult. Sci. J. 2019, 74, 117–130. [Google Scholar] [CrossRef]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut–Liver Axis, Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Kuo, T.-C.; Ho, C.-T.; Kuo, C.-H.; Tseng, Y.J.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Sheen, L.-Y. Mass-Spectrometry-Based Serum Metabolomics of a C57BL/6J Mouse Model of High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease Development. J. Agric. Food Chem. 2015, 63, 7873–7884. [Google Scholar] [CrossRef]
- Jian, H.; Xu, Q.; Wang, X.; Liu, Y.; Miao, S.; Li, Y.; Mou, T.; Dong, X.; Zou, X. Amino Acid and Fatty Acid Metabolism Disorders Trigger Oxidative Stress and Inflammatory Response in Excessive Dietary Valine-Induced NAFLD of Laying Hens. Front. Nutr. 2022, 9, 849767. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Gontijo, R.; Han, C.; Zhang, L.; Zhang, V.; Hosseini, M.; Mekeel, K.; Schnabl, B.; Loomba, R.; Karin, M.; Brenner, D.A.; et al. Metabolic Injury of Hepatocytes Promotes Progression of NAFLD and AALD. Semin. Liver Dis. 2022, 42, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wang, H.; Shi, X.; Zhang, X.; Zhu, Y.; Chen, W.; Zhang, H.; Huang, Y. A Comparative Study to Determine the Effects of Breed and Feed Restriction on Glucose Metabolism of Chickens. Anim. Nutr. 2023, 13, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary Flavonoids as Modulators of Lipid Metabolism in Poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef]
- Krogdahl, Å. Digestion and Absorption of Lipids in Poultry. J. Nutr. 1985, 115, 675–685. [Google Scholar] [CrossRef]
- Ni, Y.D.; Wei, X.J.; Zhang, C.X.; Zhong, Y.; Lu, L.Z.; Grossmann, R.; Zhao, R.Q. The Effect of Equol Injection in Ovo on Lipid Metabolism and Hepatic Lipogenic Gene Expression in Broilers. Animal 2012, 6, 1444–1450. [Google Scholar] [CrossRef][Green Version]
- Guillou, H.; Martin, P.G.P.; Pineau, T. Transcriptional Regulation of Hepatic Fatty Acid Metabolism. Subcell. Biochem. 2008, 49, 3–47. [Google Scholar] [CrossRef]
- Saeed, M.; Alagawany, M.; Arain, M.A.; El-Hack, M.E.A.; Dhama, K. Beneficial Impacts of Choline in Animal and Human With Special Reference To Its Role Against Fatty Liver Syndrome. J. Exp. Biol. Agric. Sci. 2017, 5, 589–598. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef]
- Machado, M.L.V.M.; Diehl, A.M. Animal Models of Nonalcoholic Fatty Liver Disease. In Alcoholic and Non-Alcoholic Fatty Liver Disease: Bench to Bedside; Chalasani, N., Szabo, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 121–145. ISBN 978-3-319-20538-0. [Google Scholar]
- Cartoni Mancinelli, A.; Di Veroli, A.; Mattioli, S.; Cruciani, G.; Dal Bosco, A.; Castellini, C. Lipid Metabolism Analysis in Liver of Different Chicken Genotypes and Impact on Nutritionally Relevant Polyunsaturated Fatty Acids of Meat. Sci. Rep. 2022, 12, 1888. [Google Scholar] [CrossRef]
- Randolph, G.J.; Miller, N.E. Lymphatic Transport of High-Density Lipoproteins and Chylomicrons. J. Clin. Investig. 2014, 124, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Cheng ZhangRui, C.Z.; Marei, W.; Fouladi-Nashta, A. Polyunsaturated Fatty Acids and Fertility in Female Mammals: An Update. CABI Rev. 2013, 8, 1–14. [Google Scholar] [CrossRef]
- Davis, R.A. Evolution of Processes and Regulators of Lipoprotein Synthesis: From Birds to Mammals. J. Nutr. 1997, 127, 795S–800S. [Google Scholar] [CrossRef]
- Ma, Z.; Li, H.; Zheng, H.; Jiang, K.; Yan, F.; Tian, Y.; Kang, X.; Wang, Y.; Liu, X. Hepatic ELOVL6 MRNA Is Regulated by the Gga-MiR-22-3p in Egg-Laying Hen. Gene 2017, 623, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of Differentially Expressed Genes and Pathways for Intramuscular Fat Metabolism between Breast and Thigh Tissues of Chickens. BMC Genom. 2018, 19, 55. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.H.; Abo Ghanima, M.M.; Kamal, M.; Abd El-Hack, M.E.; Alagawany, M. Chapter 8—The Use of Bile Acids Supplement in Poultry Feed. In Organic Feed Additives for Livestock; Alagawany, M., Sallam, S.M., Abd El-Hack, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 127–138. ISBN 978-0-443-13510-1. [Google Scholar]
- Zhao, L.; Jiang, Q.; Lei, J.; Cui, J.; Pan, X.; Yue, Y.; Zhang, B. Bile Acid Disorders and Intestinal Barrier Dysfunction Are Involved in the Development of Fatty Liver in Laying Hens. Poult. Sci. 2024, 103, 104422. [Google Scholar] [CrossRef]
- Schwarz, M.; Lund, E.G.; Setchell, K.D.R.; Kayden, H.J.; Zerwekh, J.E.; Björkhem, I.; Herz, J.; Russell, D.W. Disruption of Cholesterol 7α-Hydroxylase Gene in Mice. II. Bile Acid Deficiency Is Overcome by Induction of Oxysterol 7α-Hydroxylase. J. Biol. Chem. 1996, 271, 18024–18031. [Google Scholar] [CrossRef]
- Jia, H.; Dong, N. Effects of Bile Acid Metabolism on Intestinal Health of Livestock and Poultry. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1258–1269. [Google Scholar] [CrossRef]
- Sun, X.-H.; Lv, M.-W.; Zhao, Y.-X.; Zhang, H.; Ullah Saleem, M.A.; Zhao, Y.; Li, J.-L. Nano-Selenium Antagonized Cadmium-Induced Liver Fibrosis in Chicken. J. Agric. Food Chem. 2023, 71, 846–856. [Google Scholar] [CrossRef]
- Kalbande, V.H.; Ravikanth, K.; Maini, S.; Rekhe, D.S. Methionine Supplementation Options in Poultry. Int. J. Poult. Sci. 2009, 8, 588–591. [Google Scholar] [CrossRef]
- Peng, J.L.; Bai, S.P.; Wang, J.P.; Ding, X.M.; Zeng, Q.F.; Zhang, K.Y. Methionine Deficiency Decreases Hepatic Lipid Exportation and Induces Liver Lipid Accumulation in Broilers. Poult. Sci. 2018, 97, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lan, F.; Zhou, Q.; Guo, X.; Jin, J.; Wen, C.; Guo, Y.; Hou, Z.; Zheng, J.; Wu, G.; et al. Mechanisms of Hepatic Steatosis in Chickens: Integrated Analysis of the Host Genome, Molecular Phenomics and Gut Microbiome. Gigascience 2024, 13, giae023. [Google Scholar] [CrossRef]
- Klabunde, M.; Collado, D.; Bohon, C. S-Adenosylmethionine (SAMe) for Liver Health: A Systematic Review. J. Psychiatr. Res. 2017, 94, 36–46. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Liu, R.; Zhao, G.; Zhang, Y.; Zheng, M.; Cui, H.; Li, P.; Cui, X.; Liu, J.; et al. Expression and Methylation of Microsomal Triglyceride Transfer Protein and Acetyl-CoA Carboxylase Are Associated with Fatty Liver Syndrome in Chicken. Poult. Sci. 2016, 95, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Vargas, C.R. Evidence That Oxidative Disbalance and Mitochondrial Dysfunction Are Involved in the Pathophysiology of Fatty Acid Oxidation Disorders. Cell. Mol. Neurobiol. 2022, 42, 521–532. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhou, X.; Chen, W.; Zhou, H. Dysregulated Bile Acid Homeostasis: Unveiling Its Role in Metabolic Diseases. Med. Rev. 2024, 4, 262–283. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Xu, C.; Wang, D.; Ren, J.; Li, Y.; Tian, Y.; Wang, Y.; Jiao, Y.; Kang, X.; et al. Transcriptome Profile of Liver at Different Physiological Stages Reveals Potential Mode for Lipid Metabolism in Laying Hens. BMC Genom. 2015, 16, 763. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological Alterations of Poultry to the High Environmental Temperature. J. Therm. Biol. 2018, 76, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, Y.; Amevor, F.K.; Ning, Z.; Deng, X.; Wu, Y.; Wei, S.; Cao, X.; Xu, D.; Tian, Y.; et al. Effect of High Energy Low Protein Diet on Lipid Metabolism and Inflammation in the Liver and Abdominal Adipose Tissue of Laying Hens. Animals 2024, 14, 1199. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Das, B. Nutritional and Management Practices of Poultry Under Extreme Hot and Humid Condition. In Impact of Climate Change on Livestock Health and Production; CRC Press: Boca Raton, FL, USA, 2022; pp. 221–229. [Google Scholar]
- Bist, R.B.; Bist, K.; Poudel, S.; Subedi, D.; Yang, X.; Paneru, B.; Mani, S.; Wang, D.; Chai, L. Sustainable Poultry Farming Practices: A Critical Review of Current Strategies and Future Prospects. Poult. Sci. 2024, 103, 104295. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein Metabolism in Poultry. Worlds. Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Shneider, B.L. Intestinal Bile Acid Transport: Biology, Physiology, and Pathophysiology. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 407–417. [Google Scholar] [CrossRef]
- Tsuei, J.; Chau, T.; Mills, D.; Wan, Y.-J.Y. Bile Acid Dysregulation, Gut Dysbiosis, and Gastrointestinal Cancer. Exp. Biol. Med. 2014, 239, 1489–1504. [Google Scholar] [CrossRef]
- Bing, H.; Li, Y.L. The Role of Bile Acid Metabolism in the Occurrence and Development of NAFLD. Front. Mol. Biosci. 2022, 9, 1089359. [Google Scholar] [CrossRef]
- Yang, W.Y.; Chang, P.E.; Li, S.J.; Ding, S.T.; Lin, Y.Y. Exploring Bile-Acid Changes and Microflora Profiles in Chicken Fatty Liver Disease Model. Animals 2024, 14, 992. [Google Scholar] [CrossRef]
- Wallace, M.; Metallo, C.M. Tracing Insights into De novo Lipogenesis in Liver and Adipose Tissues. Semin. Cell Dev. Biol. 2020, 108, 65–71. [Google Scholar] [CrossRef]
- Ashraf, N.U.; Sheikh, T.A. Endoplasmic Reticulum Stress and Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Free Radic. Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef]
- Moon, Y.S. Lipid Metabolism and Fatty Liver in Poultry. Korean J. Poult. Sci. 2018, 45, 109–118. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Ni, H.; Yin, Y.; Gao, A.; Cui, B.; Zhang, W.; Li, Y.; Yang, Y. Core Competing Endogenous RNA Network Based on MRNA and Non-Coding RNA Expression Profiles in Chicken Fatty Liver. Anim. Genet. 2024, 55, 772–778. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, Z.; Liu, R.R.; Wang, J.; Zheng, M.Q.; Li, Q.H.; Cui, H.X.; Zhao, G.P.; Wen, J. Alteration of Hepatic Gene Expression along with the Inherited Phenotype of Acquired Fatty Liver in Chicken. Genes 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cerullo, G. Investigating the Link between Ketogenic Diet, NAFLD, Mitochondria, and Oxidative Stress: A Narrative Review. Antioxidants 2023, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Chen, Y.J.; Chen, C.Y.; Tsai, M.H.; Han, C.L.; Chen, Y.J.; Mersmann, H.J.; Ding, S.T. Identification of Potential Plasma Biomarkers for Nonalcoholic Fatty Liver Disease by Integrating Transcriptomics and Proteomics in Laying HENS. J. Nutr. 2017, 147, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK Activation Reduces Hepatic Lipid Content by Increasing Fat Oxidation in Vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of Nonalcoholic Fatty Liver Disease: Role of AMPK. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Navidshad, B.; Royan, M. Peroxisome Proliferator-Activated Receptor Alpha (PPARa), a Key Regulator of Lipid Metabolism in Avians. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 303–308. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by Pparα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPARα Action and Its Impact on Lipid Metabolism, Inflammation and Fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Tian, W.; Gonzales, G.B.; Wang, H.; Yang, Y.; Tang, C.; Zhao, Q.; Zhang, J.; Zhang, H.; Qin, Y. Caffeic Acid and Chlorogenic Acid Mediate the ADPN-AMPK-PPARα Pathway to Improve Fatty Liver and Production Performance in Laying Hens. J. Anim. Sci. Biotechnol. 2025, 16, 49. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Group, C.S.; Hospital, H.; Diabetes, M. AMPK, Insulin Resistance, and the Metabolic Syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Li, N.; Yi, B.J.; Saleem, M.A.U.; Li, X.N.; Li, J.L. Autophagy Protects against Cd-Induced Cell Damage in Primary Chicken Hepatocytes via Mitigation of Oxidative Stress and Endoplasmic Reticulum Stress. Ecotoxicol. Environ. Saf. 2023, 259, 115056. [Google Scholar] [CrossRef]
- Shirihai, O.S.; Song, M.; Dorn, G.W. How Mitochondrial Dynamism Orchestrates Mitophagy. Circ. Res. 2015, 116, 1835–1849. [Google Scholar] [CrossRef]
- Dong, H.; Czaja, M.J. Regulation of Lipid Droplets by Autophagy. Trends Endocrinol. Metab. 2011, 22, 234–240. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between Heat Stress and Oxidative Stress in Poultry; Mitochondrial Dysfunction and Dietary Interventions with Phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Shreya, S.; Grosset, C.F.; Jain, B.P. Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef] [PubMed]
- Baral, A. Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses 2024, 4, 481–504. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-Associated Cardiac Pathogenesis in Broiler Breeder Hens: Development of Metabolic Cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, C.R.; Wang, Y.X.; Xu, Z.M.; Li, S.Q.; Li, J.C.; Sun, X.Q.; Wang, H.W.; Wang, Q.; Zhang, Y.; et al. Fads2b Plays a Dominant Role in ∆6/∆5 Desaturation Activities Compared with Fads2a in Common Carp (Cyprinus carpio). Int. J. Mol. Sci. 2023, 24, 10638. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Insight into Potential Interactions of Thyroid Hormones, Sex Hormones and Their Stimulating Hormones in the Development of Non-Alcoholic Fatty Liver Disease. Metabolites 2022, 12, 718. [Google Scholar] [CrossRef]
- Tramunt, B.; Montagner, A.; Tan, N.S.; Gourdy, P.; Rémignon, H.; Wahli, W. Roles of Estrogens in the Healthy and Diseased Oviparous Vertebrate Liver. Metabolites 2021, 11, 502. [Google Scholar] [CrossRef]
- Shini, S.; Shini, A.; Bryden, W.L. Unravelling Fatty Liver Haemorrhagic Syndrome: 1. Oestrogen and Inflammation. Avian Pathol. 2020, 49, 87–98. [Google Scholar] [CrossRef]
- Kosmalski, M.; Śliwińska, A.; Drzewoski, J. Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus—The Chicken or the Egg Dilemma. Biomedicines 2023, 11, 1097. [Google Scholar] [CrossRef]
- Wu, X.L.; Zou, X.Y.; Zhang, M.; Hu, H.Q.; Wei, X.L.; Jin, M.L.; Cheng, H.W.; Jiang, S. Osteocalcin Prevents Insulin Resistance, Hepatic Inflammation, and Activates Autophagy Associated with High-Fat Diet–Induced Fatty Liver Hemorrhagic Syndrome in Aged Laying Hens. Poult. Sci. 2021, 100, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Savastano, S.; Colao, A. Hepatic Steatosis, Low-Grade Chronic Inflammation and Hormone/Growth Factor/Adipokine Imbalance. World J. Gastroenterol. 2010, 16, 4773–4783. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Bertolani, C. Adipokines in Liver Diseases. Hepatology 2009, 50, 957–969. [Google Scholar] [CrossRef]
- Mellouk, N.; Ramé, C.; Barbe, A.; Grandhaye, J.; Froment, P.; Dupont, J. Chicken Is a Useful Model to Investigate the Role of Adipokines in Metabolic and Reproductive Diseases. Int. J. Endocrinol. 2018, 2018, 4579734. [Google Scholar] [CrossRef]
- Stojsavljević, S.; Palčić, M.G.; Jukić, L.V.; Duvnjak, L.S.; Duvnjak, M. Adipokines and Proinflammatory Cytokines, the Key Mediators in the Pathogenesis of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 18070–18091. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xing, C.; Cao, H.; Zhang, C.; Luo, J.; Guo, X. Insulin Resistance and Metabonomics Analysis of Fatty Liver Haemorrhagic Syndrome in Laying Hens Induced by a High- Energy Low-Protein Diet. Sci. Rep. 2019, 9, 10141. [Google Scholar] [CrossRef]
- Yan, J.; Gan, L.; Chen, D.; Sun, C. Adiponectin Impairs Chicken Preadipocytes Differentiation through P38 MAPK/ATF-2 and TOR/P70 S6 Kinase Pathways. PLoS ONE 2013, 8, e77716. [Google Scholar] [CrossRef]
- Qu, H.-L.; Bai, X.-Y.; Liu, T.-S.; Li, Y.-N.; Li, X.-Y. Expression and Significance of Visfatin in the Course of the Development of NAFLD. Life Sci. Res. 2012, 16, 511–515. [Google Scholar]
- Wen, F.; Shi, Z.; Liu, X.; Tan, Y.; Wei, L.; Zhu, X.; Zhang, H.; Zhu, X.; Meng, X.; Ji, W.; et al. Acute Elevated Resistin Exacerbates Mitochondrial Damage and Aggravates Liver Steatosis Through AMPK/PGC-1α Signaling Pathway in Male NAFLD Mice. Horm. Metab. Res. 2020, 53, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Sanz, M.J.; Real, J.T.; Marques, P.; Capuozzo, M.; Eldjoudi, D.A.; Gualillo, O. Adipokines in Non-Alcoholic Fatty Liver Disease: Are We on the Road toward New Biomarkers and Therapeutic Targets? Biology 2022, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Shini, A.; Bryden, W.L. Unravelling Fatty Liver Haemorrhagic Syndrome: 2. Inflammation and Pathophysiology. Avian Pathol. 2020, 49, 131–143. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Review Article Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Olgun, O. The Importance of Nutrition in Alleviating High Stocking Density Stress in Poultry—A Review. Ann. Anim. Sci. 2022, 22, 855–863. [Google Scholar] [CrossRef]
- Park, B.; Um, K.; Park, S.; Zammit, V.A. Effect of Stocking Density on Behavioral Traits, Blood Biochemical Parameters and Immune Responses in Meat Ducks Exposed to Heat Stress. Arch. Anim. Breed. 2018, 61, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shu, G.; Liu, Y.; Qin, P.; Zheng, Y.; Tian, Y.; Zhao, X. Farm Environmental Enrichments Improve the Welfare of Layer Chicks and Pullets: A Comprehensive Review. Anamals 2022, 12, 2610. [Google Scholar] [CrossRef] [PubMed]
- Mench, J.A.; Sumner, D.A. Sustainability of Egg Production in the United States—The Policy and Market Context 1. Poult. Sci. 2007, 90, 229–240. [Google Scholar] [CrossRef]
- Majewski, E.; Potori, N.; Sulewski, P.; Adam, W.; Martyna, M.; Monika, G.; Malak-rawlikowska, A.; Grontkowska, A.; Szili, V. End of the Cage Age? A Study on the Impacts of the Transition from Cages on the EU Laying Hen Sector. Agriculture 2024, 14, 111. [Google Scholar] [CrossRef]
- Geng, A.L.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Yan, Z.X.; Liu, H.G. Effects of Light Regime on Circadian Rhythmic Behavior and Reproductive Parameters in Native Laying Hens. Poult. Sci. 2022, 101, 101808. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Huang, P.Y.; Chai, C.Y.; Yu, S.; Hsieh, Y.L.; Chang, H.C.; Kuo, C.W.; Lee, Y.C.; Yu, H.S. Early Detection of the Initial Stages of LED Light-Triggered Non-Alcoholic Fatty Liver Disease by Wax Physisorption Kinetics-Fourier Transform Infrared Imaging. Analyst 2022, 148, 643–653. [Google Scholar] [CrossRef]
- Langmesser, S.; Tallone, T.; Bordon, A.; Rusconi, S.; Albrecht, U. Interaction of Circadian Clock Proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol. 2008, 9, 41. [Google Scholar] [CrossRef]
- Gooley, J.J. Circadian Regulation of Lipid Metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, F.; Sun, Y.; Li, Y.; Xie, X.; Du, X.; Liu, L.; Wu, Y.; Song, D.; Xiong, H.; et al. Novel Insights into the Circadian Modulation of Lipid Metabolism in Chicken Livers Revealed by RNA Sequencing and Weighted Gene Co-Expression Network Analysis. Poult. Sci. 2017, 103, 104321. [Google Scholar] [CrossRef]
- Scheele, C.W. Pathological Changes in Metabolism of Poultry Related to Increasing Production Levels Pathological Changes in Metabolism of Poultry. Vet. Q. 1977, 19, 127–130. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, L.; Liu, L.; Chen, Q.; Lin, Z.; Zhang, D.; Wang, Y.; Liu, Y. Comparative Analysis of Different Proteins and Metabolites in the Liver and Ovary of Local Breeds of Chicken and Commercial Chickens in the Later Laying Period. Int. J. Mol. Sci. 2023, 24, 14394. [Google Scholar] [CrossRef]
- You, M.; Zhang, S.; Shen, Y.; Zhao, X.; Chen, L.; Liu, J.; Ma, N. Quantitative Lipidomics Reveals Lipid Perturbation in the Liver of Fatty Liver Hemorrhagic Syndrome in Laying Hens. Poult. Sci. 2023, 102, 102352. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zheng, J.; Zhang, S.; Wang, B.; Wu, C.; Guo, X. Advances in the Involvement of Gut Microbiota in Pathophysiology of NAFLD. Front. Med. 2020, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut Microbiome Analysis as a Tool towards Targeted Non-Invasive Biomarkers for Early Hepatocellular Carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Xiao, C.; Zhu, G.; Wu, N.; Tu, Y.; Yang, C. Potential Biomarker for Fatty Liver Hemorrhagic Syndrome in Laying Hens. J. World’s Poult. Res. 2020, 10, 545–555. [Google Scholar] [CrossRef]
- Diaz, G.J.; Squires, E.J.; Julian, R.J. The Use of Selected Plasma Enzyme Activities for the Diagnosis of Fatty Liver-Hemorrhagic Syndrome in Laying Hens. Avian Dis. 1999, 43, 768–773. [Google Scholar] [CrossRef]
- Dong, X.; Tong, J. Different Susceptibility to Fatty Liver-Haemorrhagic Syndrome in Young and Older Layers and the Interaction on Blood LDL-C Levels between Oestradiols and High Energy-Low Protein Diets. Br. Poult. Sci. 2019, 60, 265–271. [Google Scholar] [CrossRef]
- Haghighi-Rad, F.; Polin, D. The Relationship of Plasma Estradiol and Progesterone Levels to the Fatty Liver Hemorrhagic Syndrome in Laying Hens. Poult. Sci. 1981, 60, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Hansen, R.J.; Williams, D.L.; Hamilton, R.L. Estrogen Induction of VLDLy Assembly in Egg-Laying Hens. J. Nutr. 1999, 129, 467S–472S. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.; You, M.; Shen, Y.; Zhang, S.; Li, J.; He, X.; Zhao, X.; Ma, N. Quantitative Lipidomics Reveals the Changes of Lipids and Antioxidant Capacity in Egg Yolk from Laying Hens with Fatty Liver Hemorrhagic Syndrome. Poult. Sci. 2024, 103, 103785. [Google Scholar] [CrossRef] [PubMed]
- Mangus, L.M.; Franca, M.S.; Shivaprasad, H.L.; Wolf, J.C. Research-Relevant Background Lesions and Conditions in Common Avian and Aquatic Species. ILAR J. 2021, 62, 169–202. [Google Scholar] [CrossRef]
- Bourganou, M.V.; Chondrogianni, M.E.; Kyrou, I.; Flessa, C.M.; Chatzigeorgiou, A.; Oikonomou, E.; Lambadiari, V.; Randeva, H.S.; Kassi, E. Unraveling Metabolic Dysfunction-Associated Steatotic Liver Disease Through the Use of Omics Technologies. Int. J. Mol. Sci. 2025, 26, 1589. [Google Scholar] [CrossRef]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The role of lipid sensing nuclear receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
| Feature | Poultry | Mammals |
|---|---|---|
| Lipoprotein transport | Portomicrons facilitate the direct transfer of lipids from the intestines to the liver through the portal vein, circumventing the lymphatic system [47]. | Chylomicrons transport undigested fat- and fat-soluble vitamins via the lymphatic system to the blood stream [48]. |
| Fatty acid composition | Polyunsaturated fatty acids (PUFA) can be synthesized by poultry birds, a characteristic unique to avian species [49]. | Mammals are unable to synthesize PUFAs, and it is induced via diet [50]. |
| Site of lipogenesis and storage | Liver is the primary site of lipogenesis and adipose tissues are the sites of storage in poultry [51]. | De novo lipogenesis occurs in adipose tissues, and fat is stored in the liver [51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, A.; Bin Tahir, M.T.; Zhao, M.; Gong, D.; Ge, J.; Geng, T. Non-Alcoholic Fatty Liver Disease in Poultry: Risk Factors, Mechanism of Development, and Emerging Strategies. Int. J. Mol. Sci. 2025, 26, 8460. https://doi.org/10.3390/ijms26178460
Imtiaz A, Bin Tahir MT, Zhao M, Gong D, Ge J, Geng T. Non-Alcoholic Fatty Liver Disease in Poultry: Risk Factors, Mechanism of Development, and Emerging Strategies. International Journal of Molecular Sciences. 2025; 26(17):8460. https://doi.org/10.3390/ijms26178460
Chicago/Turabian StyleImtiaz, Aneeqa, Muhammad Talha Bin Tahir, Minmeng Zhao, Daoqing Gong, Jing Ge, and Tuoyu Geng. 2025. "Non-Alcoholic Fatty Liver Disease in Poultry: Risk Factors, Mechanism of Development, and Emerging Strategies" International Journal of Molecular Sciences 26, no. 17: 8460. https://doi.org/10.3390/ijms26178460
APA StyleImtiaz, A., Bin Tahir, M. T., Zhao, M., Gong, D., Ge, J., & Geng, T. (2025). Non-Alcoholic Fatty Liver Disease in Poultry: Risk Factors, Mechanism of Development, and Emerging Strategies. International Journal of Molecular Sciences, 26(17), 8460. https://doi.org/10.3390/ijms26178460









