α-Selective Glycosidation of the Rare Sugar d-Tagatofuranose and the Synthesis of α-d-Tagatofuranosylceramide
Abstract
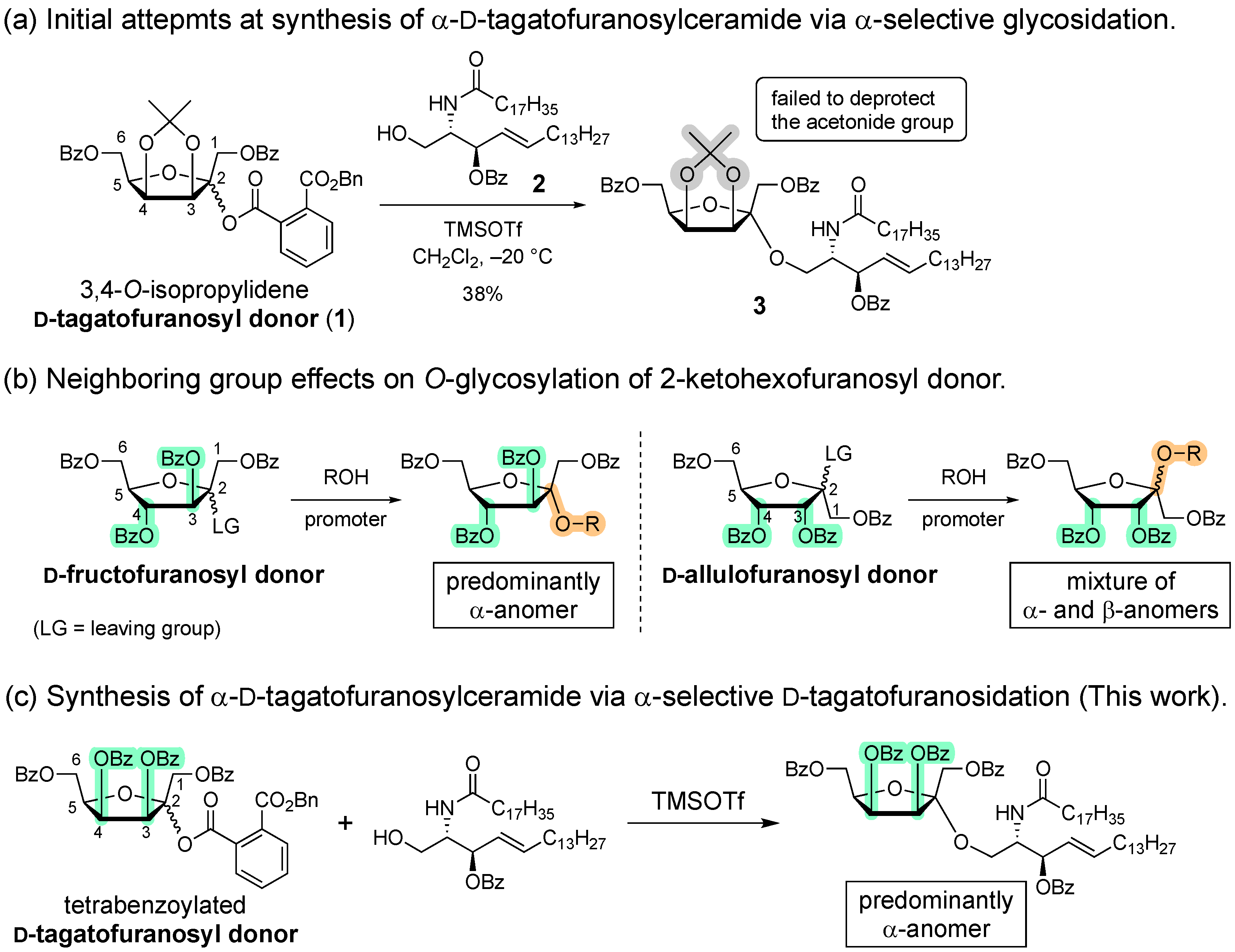
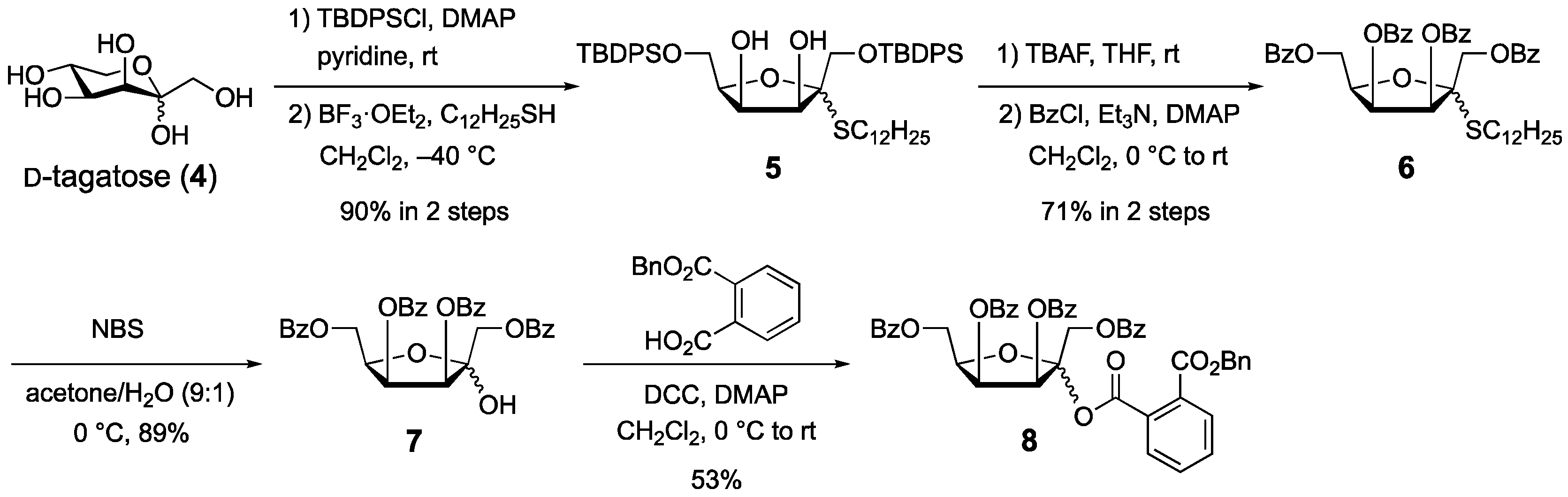
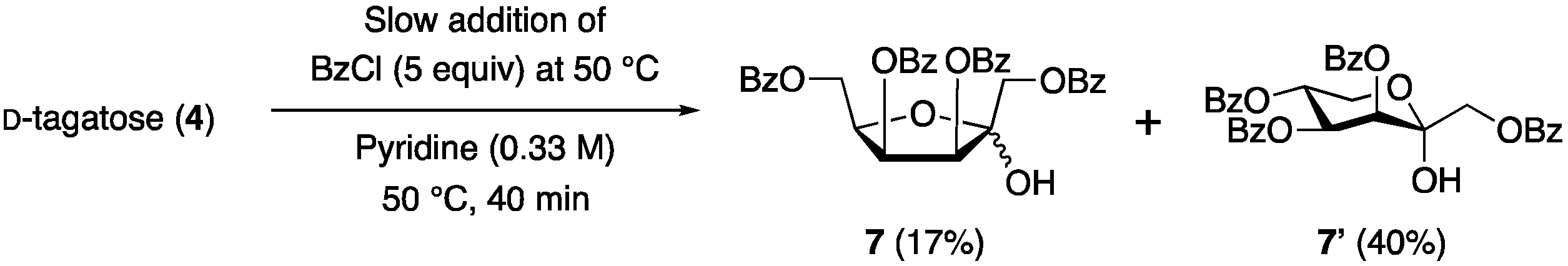
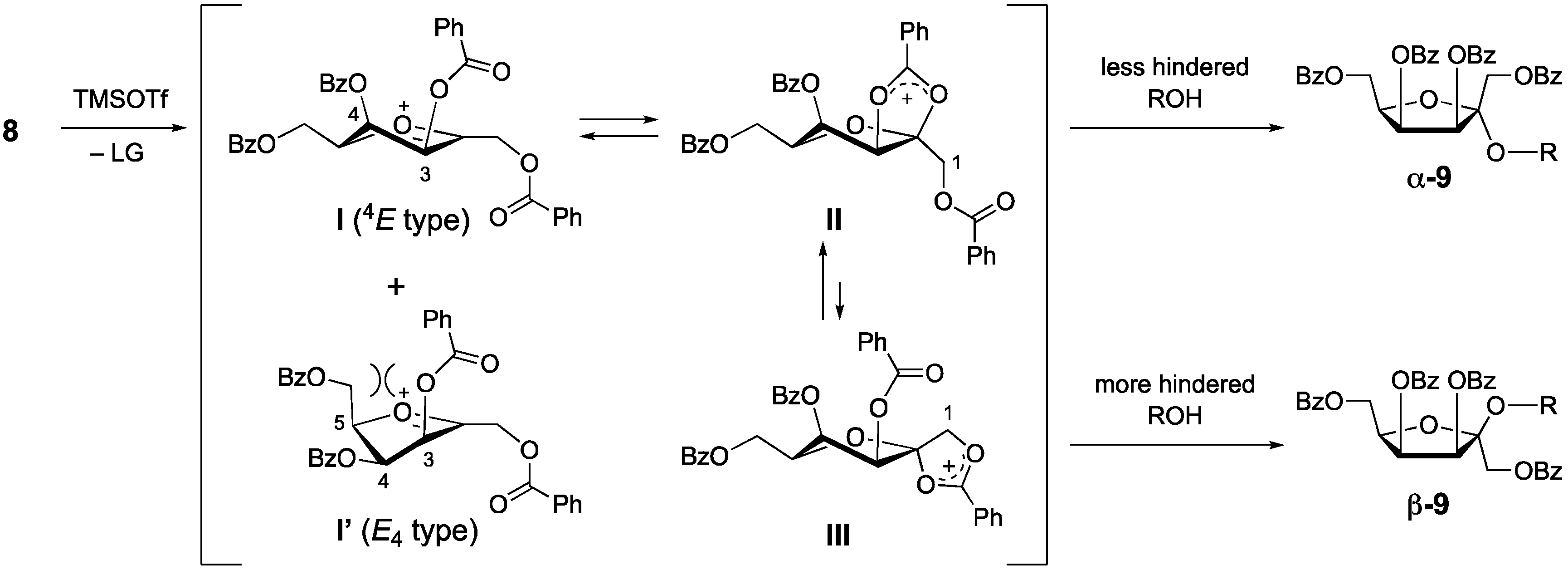
1. Introduction
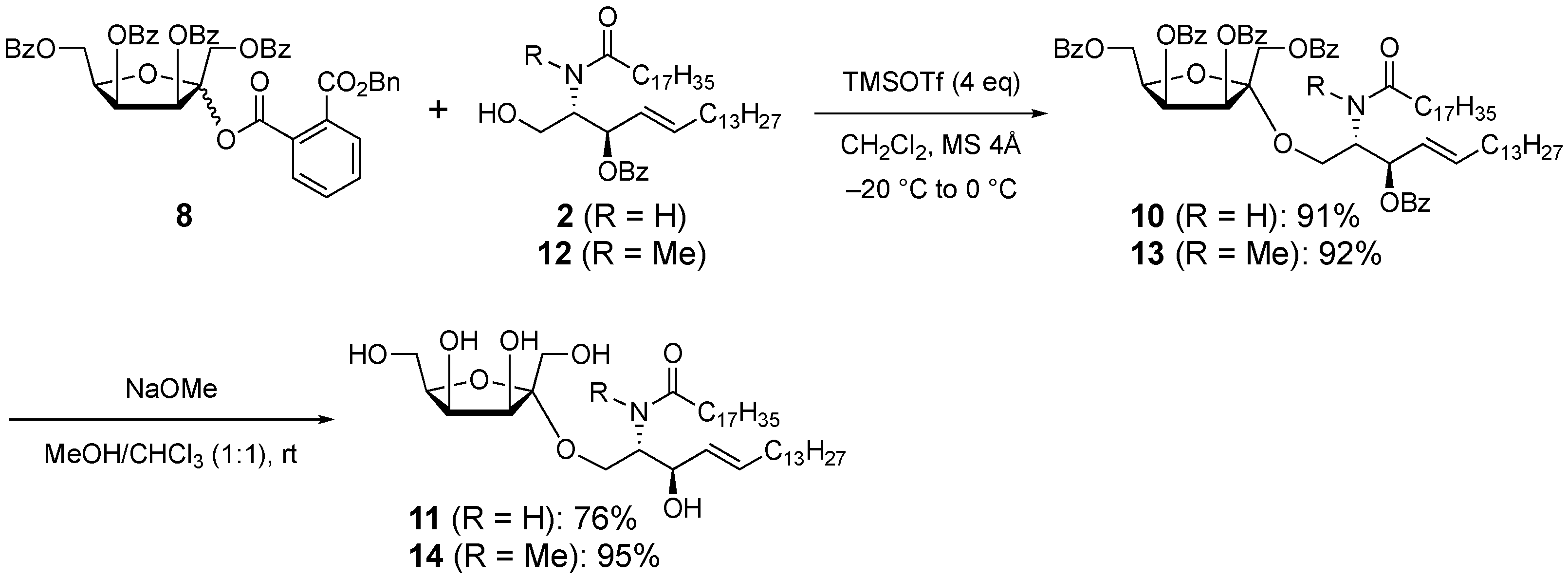
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. 3-O-Benzoyl-1-O-(1,6-Di-O-benzoyl-3,4-O-isopropylidene-α-d-tagatofuranosyl)-N-stearoyl-d-erythro-sphingosine (3)
3.3. (1-Dodecyl) 1,6-Di-O-(tert-butyldiphenylsilyl)-2-thio-d-tagatofuranoside (5)
3.4. (1-Dodecyl) 1,3,4,6-Tetra-O-benzoyl-2-thio-d-tagatofuranoside (6)
3.5. 1,3,4,6-Tetra-O-benzoyl-d-tagatofuranose (7)
3.6. 1,3,4,6-Tetra-O-benzoy-d-tagatofuranosyl phthalate (8)
3.7. General Procedure for the Glycosidation Described in Table 1
3.7.1. (2-Phenethyl) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9a)
3.7.2. (1-Dodecyl) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9b)
3.7.3. (4-Nitrobenzyl) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9c)
3.7.4. Cyclohexyl 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9d)
3.7.5. Isopropyl 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9e)
3.7.6. (4-Methoxyphenyl) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9f)
3.7.7. (2-Isopropylphenyl) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9g)
3.7.8. (Fmoc-L-Ser-OMe) 1,3,4,6-Tetra-O-benzoyl-α-d-tagatofuranoside (9h)
3.7.9. Methyl 5-O-(1,3,4,6-tetra-O-benzoyl-α-d-tagatofuranosyl)-2,3-O-isopropylidene-β-d-ribofuranoside (9i)
3.7.10. Methyl 6-O-(1,3,4,6-tetra-O-benzoyl-α-d-togatofuranosyl)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (9j)
3.8. 3-O-Benzoyl-1-O-(1,3,4,6-tetra-O-benzoyl-α-d-tagatofuranosyl)-N-stearoyl-d-erythro-sphingosine (10)
3.9. 1-O-(α-d-Tagatofuranosyl)-N-stearoyl-d-erythro-sphingosine (11)
3.10. 3-O-Benzoyl-1-O-(1,3,4,6-tetra-O-benzoyl-α-d-tagatofuranosyl)-N-methyl-N-stearoyl-d-erythro-sphingosine (13)
3.11. 1-O-(α-d-Tagatofuranosyl)-N-methyl-N-stearoyl-d-erythro-sphingosine (14)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bz | Benzoyl |
| DMAP | Benzyl |
| TBAF | Tetrabutylammonium fluoride |
| TBDPS | tert-Butyldiphenylsilyl |
| NBS | N-Bromosuccinimide |
| Bn | Benzyl |
| DCC | N,N’-Dicyclohexylcarbodiimide |
| Fmoc | 9-Fluorenylmethyloxycarbonyl |
| TMSOTf | Trimethylsilyl trifluoromethanesulfonate |
References
- Izumori, K. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 2002, 89, 120–124. [Google Scholar] [CrossRef]
- Granstrom, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.K.; Yamaguchi, F.; Nakanose, K.; Watanabe, Y.; Hatano, N.; Tsukamoto, I.; Nagata, M.; Izumori, K.; Tokuda, M. Neuroprotective effect of d-psicose on 6-hydroxydopamine-induced apoptosis in rat pheochromocytoma (PC12) cells. J. Biosci. Bioeng. 2005, 100, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K. d-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Antioxidant properties of custard pudding dessert containing rare hexose, d-psicose. Food Control 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Baráth, M.; Lin, C.-H.; Tvaroška, I.; Hirsch, J. Development of transition state analogue inhibitors for N-acetylglycosyltransferases bearing d-psico- or d-tagatofuranose scaffolds. Chem. Pap. 2015, 69, 348–357. [Google Scholar] [CrossRef]
- Bella, M.; Yan, S.; Šesták, S.; Kozmon, S.; Lin, C.-H.; Mucha, J.; Koóš, M. Synthesis of a β-d-psicofuranosyl sulfone and inhibitory-activity evaluation against N-acetylglucosaminyltransferase I. Eur. J. Org. Chem. 2017, 2017, 6179–6191. [Google Scholar] [CrossRef]
- Matsuo, T.; Izumori, K. d-Psicose inhibits intestinal α-glucosidase and suppresses glycemic response after carbohydrate ingestion in rats. Tech. Bull. Fac. Agric. Kagawa Univ. 2006, 58, 27–32. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Schroeder, W.; Hoeksema, H. A new antibiotic, 6-amino-9-d-psicofuranosylpurine. J. Am. Chem. Soc. 1959, 81, 1767–1768. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of d-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the New GRAS Sweetener and Health Product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Brown, J.C. d-Tagatose Is a Bulk Sweetener with Zero Energy Determined in Rats. J. Nutr. 1996, 126, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chikkerur, J.; Roy, S.C.; Dhali, A.; Kolte, A.P.; Sridhar, M.; Samanta, A.K. Tagatose as a Potential Nutraceutical: Production, Properties, Biological Roles, and Applications. J. Food Sci. 2018, 83, 2699–2709. [Google Scholar] [CrossRef]
- Ortiz, A.D.; Fideles, S.O.; Reis, C.H.; Pagani, B.T.; Bueno, L.M.; Moscatel, M.B.; Buchaim, R.L.; Buchaim, D.V. d-Tagatose: A Rare Sugar with Functional Properties and Antimicrobial Potential against Oral Species. Nutrients 2024, 16, 1943. [Google Scholar] [CrossRef]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. d-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef]
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. d-Tagatose Is a Promising Sweetener to Control Glycaemia: A New Functional Food. BioMed Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Tanaka, K.; Ebihara, K.; Tajima, S.; Gomi, K.; et al. The rare sugar d-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 2020, 3, 423. [Google Scholar] [CrossRef]
- Mijailovic, N.; Richet, N.; Villaume, S.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. d-Tagatose-Based Product Triggers Sweet Immunity and Resistance of Grapevine to Downy Mildew, but Not to Gray Mold Disease. Plants 2022, 11, 296. [Google Scholar] [CrossRef]
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-Activity Relationship of alpha.-Galactosylceramides against B16-Bearing Mice. J. Med. Chem. 1995, 38, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Huang, J.-R.; Tsai, Y.-C.; Hung, J.-T.; Wu, D.; Fujio, M.; Wong, C.-H.; Yu, A.L. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc. Natl. Acad. Sci. USA 2007, 104, 10299–10304. [Google Scholar] [CrossRef] [PubMed]
- Brossay, L.; Naidenko, O.; Burdin, N.; Matsuda, J.; Sakai, T.; Kronenberg, M. Cutting Edge: Structural Requirements for Galactosylceramide Recognition by CD1-Restricted NK T Cells1. J. Immunol. 1998, 161, 5124–5128. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xian, M.-Y.; Wang, X.-F.; Zou, G.-Q.; Luo, R.; Peng, H.; Liu, Z. Conformationally Restricted Analogues of α-Galactosylceramide as Adjuvant in COVID-19 Subunit Vaccine. ACS Med. Chem. Lett. 2023, 14, 1647–1655. [Google Scholar] [CrossRef]
- Bi, J.; Wang, J.; Zhou, K.; Wang, Y.; Fang, M.; Du, Y. Synthesis and Biological Activities of 5-Thio-α-GalCers. ACS Med. Chem. Lett. 2015, 6, 476–480. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Lai, A.C.-Y.; Chi, P.-Y.; Hsu, I.L.; Liu, N.-T.; Wu, K.-C.; García Fernández, J.M.; Chang, Y.-J.; Ortiz Mellet, C. Serine-/Cysteine-Based sp2-Iminoglycolipids as Novel TLR4 Agonists: Evaluation of Their Adjuvancy and Immunotherapeutic Properties in a Murine Model of Asthma. J. Med. Chem. 2023, 66, 4768–4783. [Google Scholar] [CrossRef]
- Angyal, S.J.; Bethell, G.S. Conformational analysis in carbohydrate chemistry. III* The 13C N.M.R. spectra of the hexuloses. Aust. J. Chem. 1976, 29, 1249–1265. [Google Scholar] [CrossRef]
- Angyal, S.; Bodkin, C.; Mills, J.; Pojer, P. Complexes of carbohydrates with metal cations. IX. Synthesis of the methyl d-tagatosides, d-psicosides, d-apiosides and d-erythrosides. Aust. J. Chem. 1977, 30, 1259–1268. [Google Scholar] [CrossRef]
- Hunt-Painter, A.A.; Stocker, B.L.; Timmer, M.S.M. The synthesis of the molecular chaperone 2,5-dideoxy-2,5-imino-d-altritol via diastereoselective reductive amination and carbamate annulation. Tetrahedron 2018, 74, 1307–1312. [Google Scholar] [CrossRef]
- Uenishi, J.; Ueda, A. Synthesis of (+)-sucrose via β-d-psicofuranosylation. Tetrahedron Asymmetry 2008, 19, 2210–2217. [Google Scholar] [CrossRef]
- Uenishi, J.; Ueda, A. Stereoselective β-d-psicofuranosylation and synthesis of β-d-psicofuranosylceramide. Heterocycles 2009, 77, 1297–1305. [Google Scholar] [CrossRef]
- Ueda, A.; Yamashita, T.; Uenishi, J. Chemical synthesis of β-d-psicofuranosyl disaccharides. Carbohydr. Res. 2010, 345, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Yamashita, T.; Uenishi, J. Synthesis of galacto- and mannosucroses. Heterocycles 2010, 81, 1711–1720. [Google Scholar] [CrossRef]
- Makura, Y.; Ueda, A.; Matsuzaki, T.; Minamino, T.; Tanaka, M. α-Selective glycosidation of d-tagatofuranose with a 3,4-O-isopropylidene protection. Tetrahedron 2019, 75, 3758–3766. [Google Scholar] [CrossRef]
- Ueda, A.; Pi, J.; Makura, Y.; Tanaka, M.; Uenishi, J. Stereoselective synthesis of (+)-5-thiosucrose and (+)-5-thioisosucrose. RSC Adv. 2020, 10, 9730–9735. [Google Scholar] [CrossRef]
- Ueda, A.; Nishimura, Y.; Makura, Y.; Tanaka, M.; Uenishi, J. β-Selective d-psicofuranosylation of pyrimidine bases and thiols. Heterocycles 2018, 97, 729–743. [Google Scholar] [CrossRef]
- Duffin, G.R.; Ellames, G.J.; Hartmann, S.; Herbert, J.M.; Smith, D.I. Practical syntheses of [13C]- and [14C]-labelled glucosphingolipids. J. Chem. Soc. Perkin Trans. 1 2000, 2000, 2237–2242. [Google Scholar] [CrossRef]
- Yamanoi, T.; Misawa, N.; Watanabe, M. A highly efficient d-fructofuranosylation catalyzed by scandium(III) triflate. Tetrahedron Lett. 2007, 48, 6458–6462. [Google Scholar] [CrossRef]
- Yamanoi, T.; Ishiyama, T.; Oda, Y.; Matsuda, S.; Watanabe, M. L-Fructo- and d-psicofuranosylation reactions catalyzed by scandium triflate. Heterocycles 2010, 81, 1141–1147. [Google Scholar] [CrossRef]
- Iyoshi, A.; Makura, Y.; Tanaka, M.; Ueda, A. Stereocontrolled synthesis of α-d-allulofuranosides using α-selective d-fructofuranosidation reaction. Carbohydr. Res. 2024, 536, 109044. [Google Scholar] [CrossRef]
- Yamanoi, T.; Oda, Y.; Ishiyama, T.; Watanabe, M. Stereoselectivity of d-psicofuranosylation influenced by protecting groups of psicofuranosyl donors. Heterocycles 2016, 93, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Lavallee, P. The Preparation and Synthetic Utility of tert-Butyldiphenylsilyl Ethers. Can. J. Chem. 1975, 53, 2975–2977. [Google Scholar] [CrossRef]
- Hanessian, S.; Lavallee, P. A stereospecifie, total synthesis of thromboxane B2. Can. J. Chem. 1977, 55, 562–565. [Google Scholar] [CrossRef]
- Clode, D.M.; Laurie, W.A.; McHale, D.; Sheridan, J.B. Synthesis of 6,1′,3′-, 2,6,1′-, 1′,3′,6′-, and 2,1′,6′-tri-O-benzoylsucrose. Carbohydr. Res. 1985, 139, 161–183. [Google Scholar] [CrossRef]
- Ratcliffe, A.J.; Konradsson, P.; Fraser-Reid, B. n-Pentenyl glycosides as efficient synthons for promoter-mediated assembly of n-.alpha.-linked glycoproteins. J. Am. Chem. Soc. 1990, 112, 5665–5667. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.J.; Kim, H.Y.; Kang, S.S.; Kwon, S.Y. Glycosylation with glycosyl benzyl phthalates as a new type of glycosyl donor. Org. Biomol. Chem. 2004, 2, 2408–2410. [Google Scholar] [CrossRef]
- Hu, Y.; Iyoshi, A.; Makura, Y.; Tanaka, M.; Ueda, A. 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose. Molbank 2025, 2025, M2041. [Google Scholar] [CrossRef]
- Hu, Y.; Iyoshi, A.; Tanaka, M.; Ueda, A. Methyl α-d-tagatopyranoside. Molbank 2025, 2025, M2046. [Google Scholar] [CrossRef]
- Fuchs, B. Conformations of Five-Membered Rings. In Topics in Stereochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1979; pp. 1–94. [Google Scholar]
- Larsen, C.H.; Ridgway, B.H.; Shaw, J.T.; Woerpel, K.A. A Stereoelectronic Model To Explain the Highly Stereoselective Reactions of Nucleophiles with Five-Membered-Ring Oxocarbenium Ions. J. Am. Chem. Soc. 1999, 121, 12208–12209. [Google Scholar] [CrossRef]
- Smith, D.M.; Tran, M.B.; Woerpel, K.A. Nucleophilic Additions to Fused Bicyclic Five-Membered Ring Oxocarbenium Ions: Evidence for Preferential Attack on the Inside Face. J. Am. Chem. Soc. 2003, 125, 14149–14152. [Google Scholar] [CrossRef]
- Kaburagi, Y.; Kishi, Y. Operationally Simple and Efficient Workup Procedure for TBAF-Mediated Desilylation: Application to Halichondrin Synthesis. Org. Lett. 2007, 9, 723–726. [Google Scholar] [CrossRef]
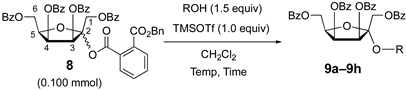 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | 9 | Temp (°C) | Time (min) | Yield (%) | α/β a |
| 1 | 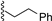 | 9a | −20 | 30 | 80 | 99:1 |
| 2 | 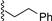 | 9a | rt | 30 | 69 | 85:15 |
| 3 | 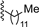 | 9b | 0 | 30 | 72 | 99:1 |
| 4 | 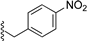 | 9c | −20 to −10 | 60 | 81 | 92:8 |
| 5 | 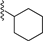 | 9d | −20 to −10 | 60 | 78 | 94:6 |
| 6 |  | 9e | −20 to −10 | 60 | 76 | 93:7 |
| 7 b |  | 9f | −20 to 0 | 60 | 56 | 89:11 |
| 8 b | 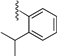 | 9g | −20 to 0 | 60 | 15 (α) + 6 (β) | 71:29 c |
| 9 | 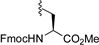 | 9h | −20 | 30 | 81 | 97:3 |
| 10 | 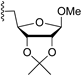 | 9i | −20 | 30 | 82 | 88:12 |
| 11 | 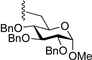 | 9j | −20 | 30 | 78 | 96:4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makura, Y.; Iyoshi, A.; Horiuchi, M.; Hu, Y.; Tanaka, M.; Ueda, A. α-Selective Glycosidation of the Rare Sugar d-Tagatofuranose and the Synthesis of α-d-Tagatofuranosylceramide. Int. J. Mol. Sci. 2025, 26, 8459. https://doi.org/10.3390/ijms26178459
Makura Y, Iyoshi A, Horiuchi M, Hu Y, Tanaka M, Ueda A. α-Selective Glycosidation of the Rare Sugar d-Tagatofuranose and the Synthesis of α-d-Tagatofuranosylceramide. International Journal of Molecular Sciences. 2025; 26(17):8459. https://doi.org/10.3390/ijms26178459
Chicago/Turabian StyleMakura, Yui, Akihiro Iyoshi, Makito Horiuchi, Yiming Hu, Masakazu Tanaka, and Atsushi Ueda. 2025. "α-Selective Glycosidation of the Rare Sugar d-Tagatofuranose and the Synthesis of α-d-Tagatofuranosylceramide" International Journal of Molecular Sciences 26, no. 17: 8459. https://doi.org/10.3390/ijms26178459
APA StyleMakura, Y., Iyoshi, A., Horiuchi, M., Hu, Y., Tanaka, M., & Ueda, A. (2025). α-Selective Glycosidation of the Rare Sugar d-Tagatofuranose and the Synthesis of α-d-Tagatofuranosylceramide. International Journal of Molecular Sciences, 26(17), 8459. https://doi.org/10.3390/ijms26178459






