Modulation of NK Cell Properties by ESKAPE Group Bacteria
Abstract
1. Introduction
2. Results
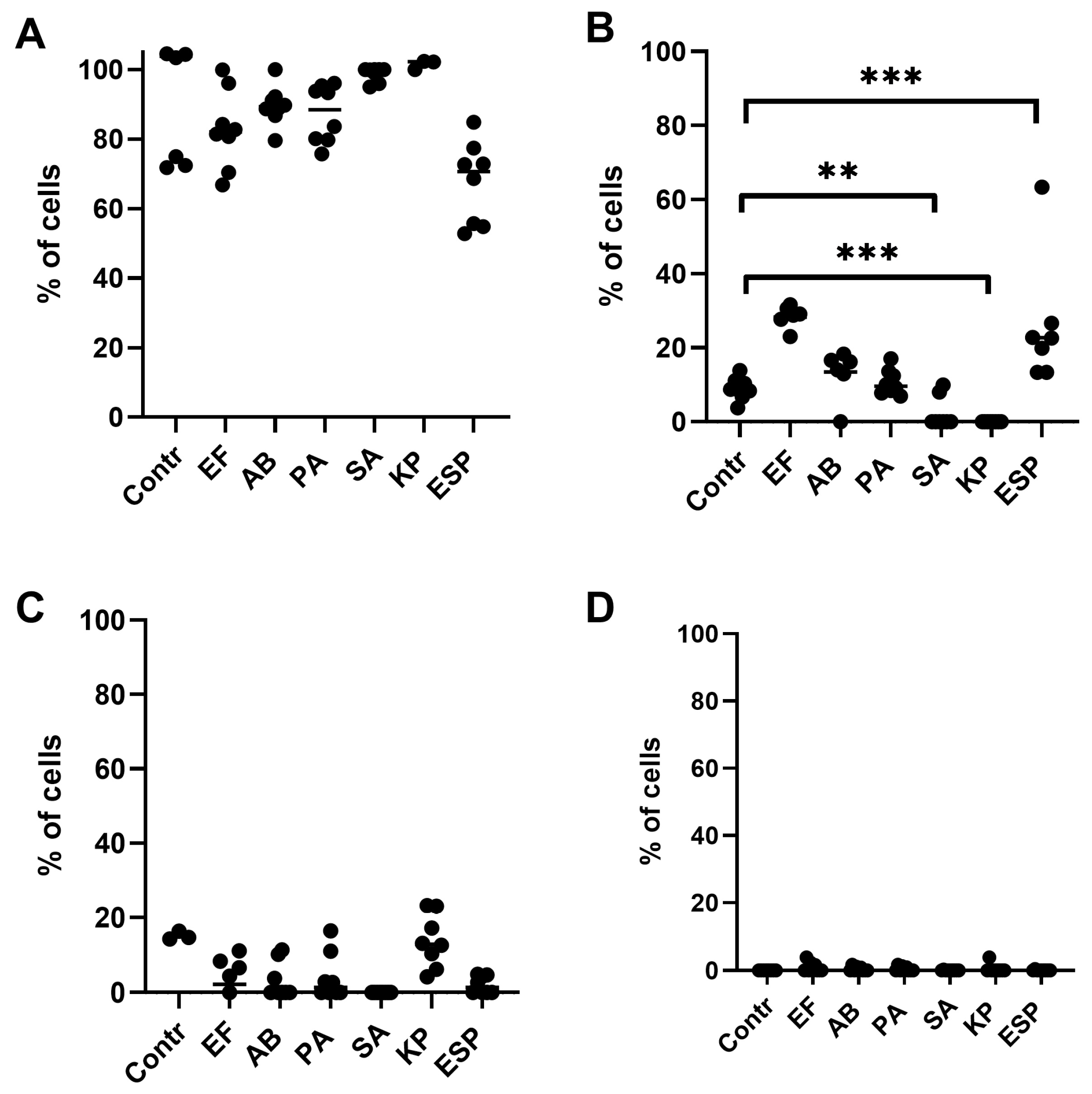
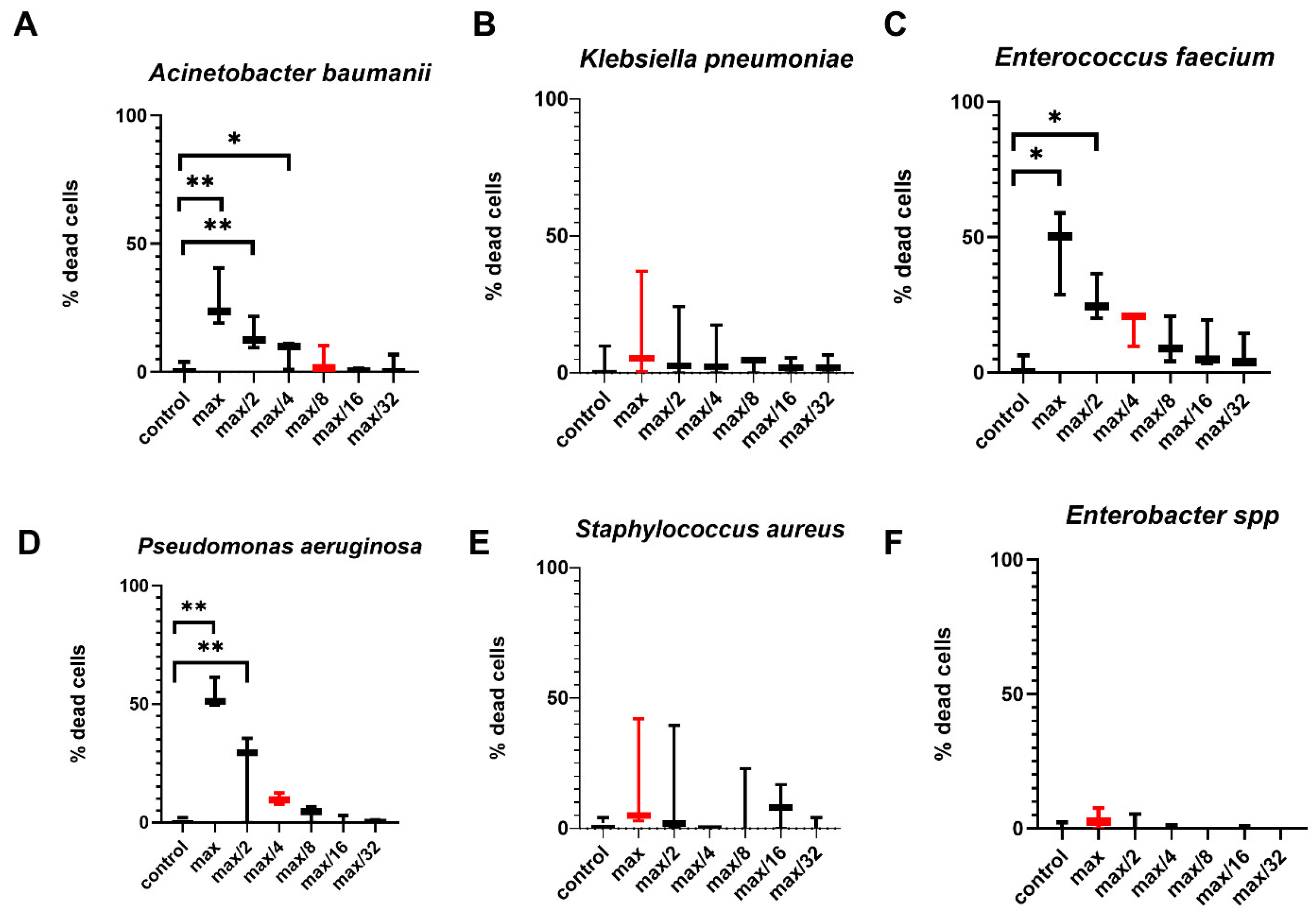
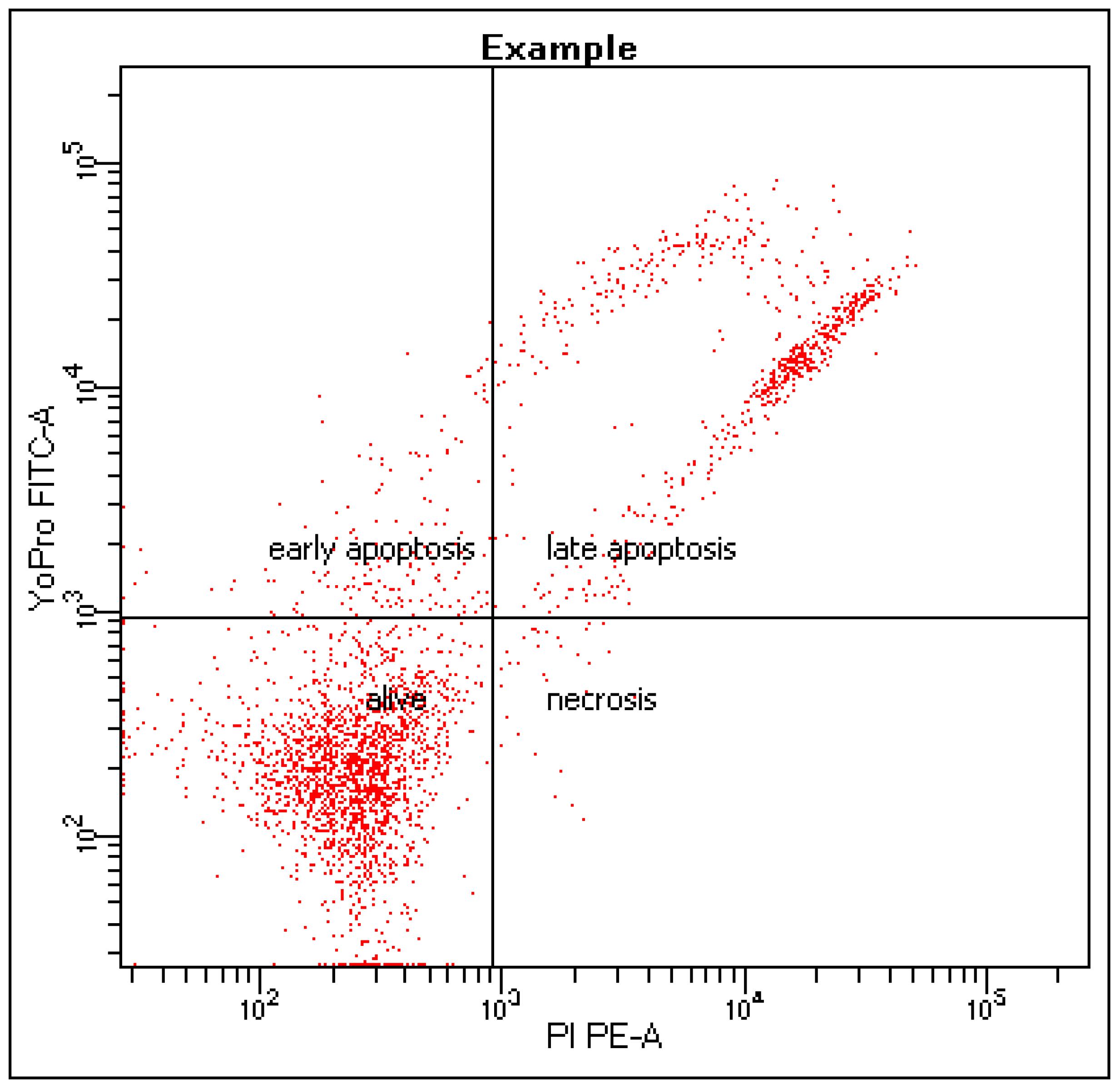
2.1. Effects of Bacterial-Derived Supernatants on the Development of Apoptosis Stages in NK-92 Cells
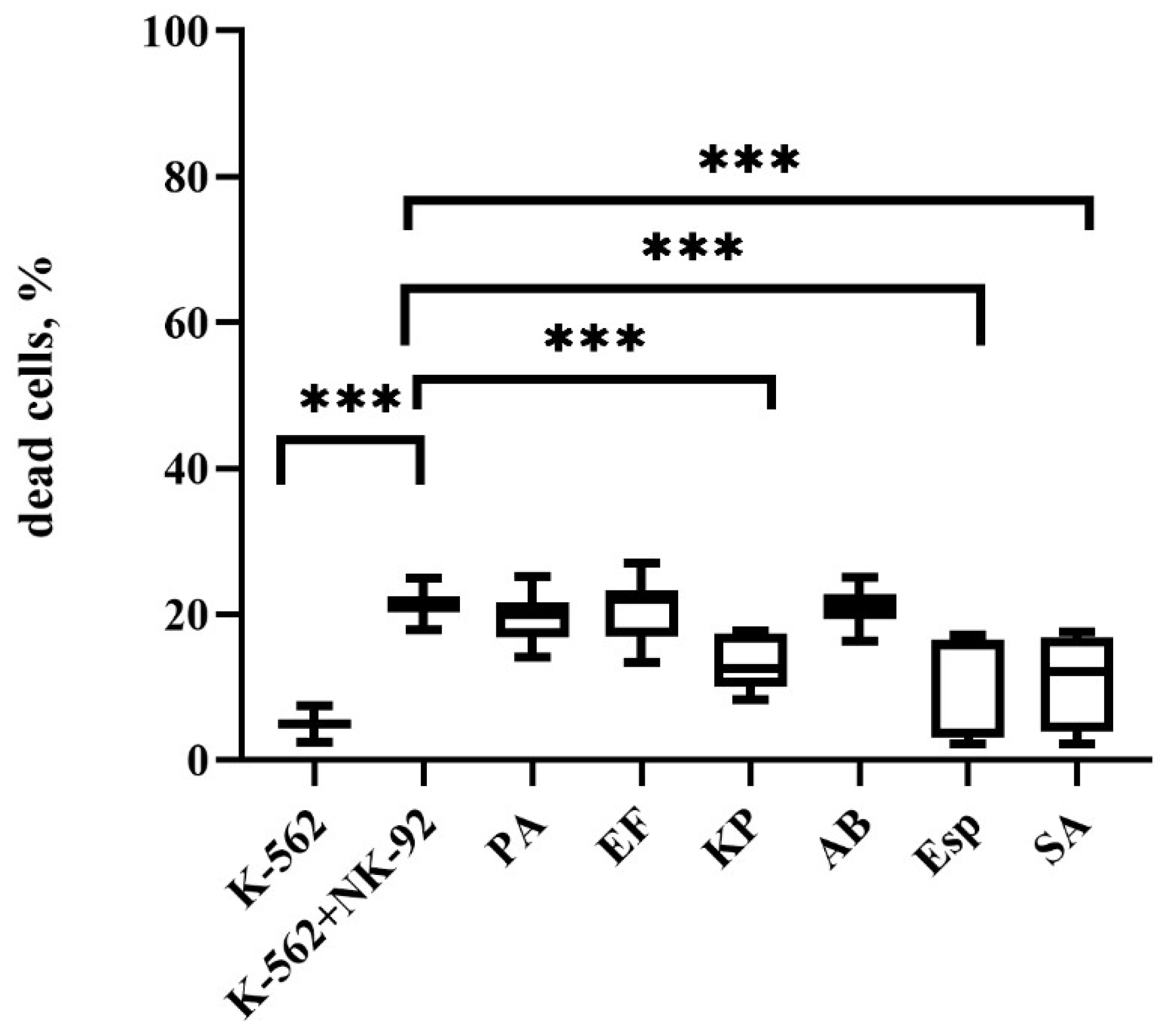
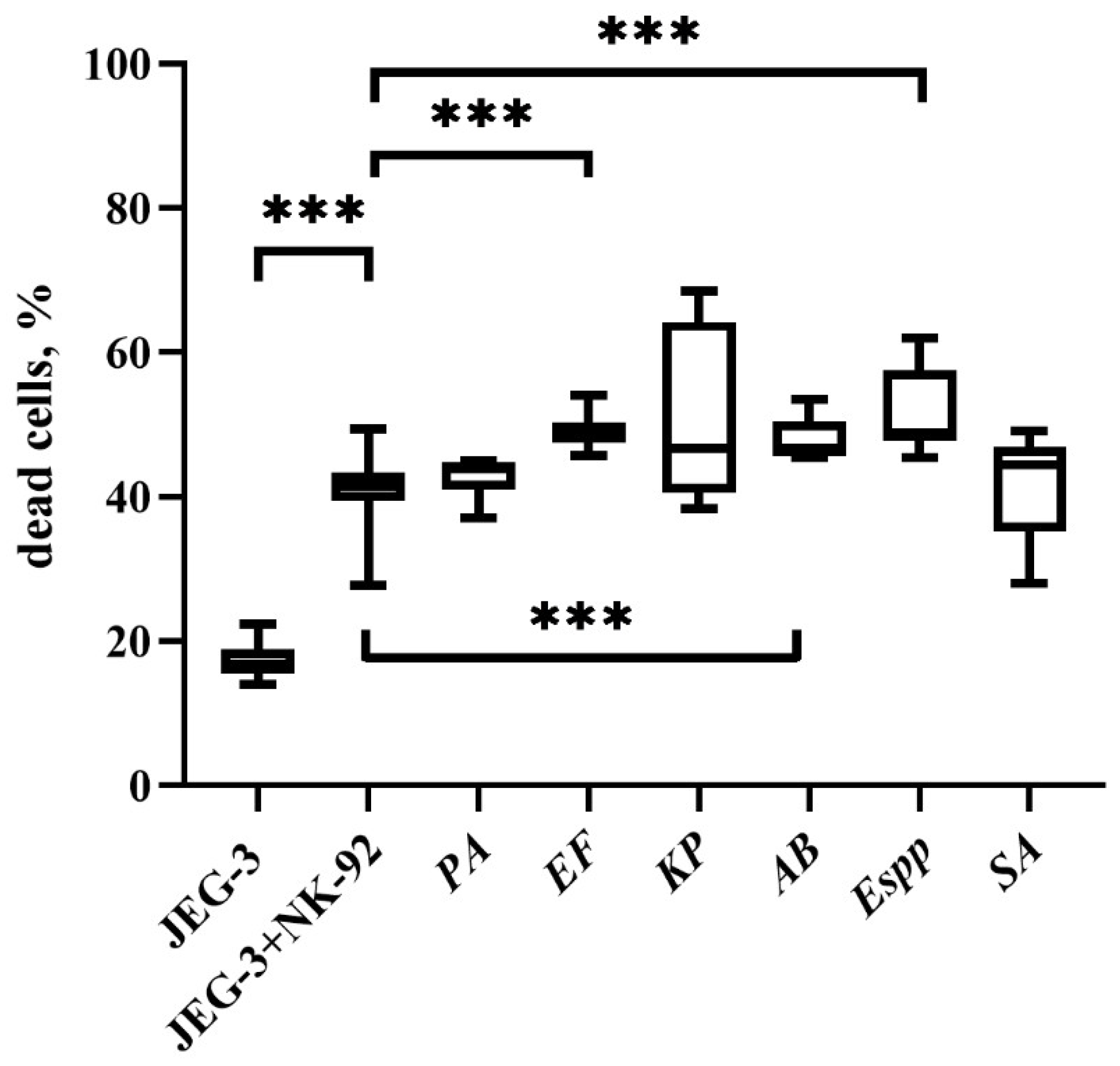
2.2. Changes in the Cytotoxic Activity of NK-92 Cells After Supernatant Exposure
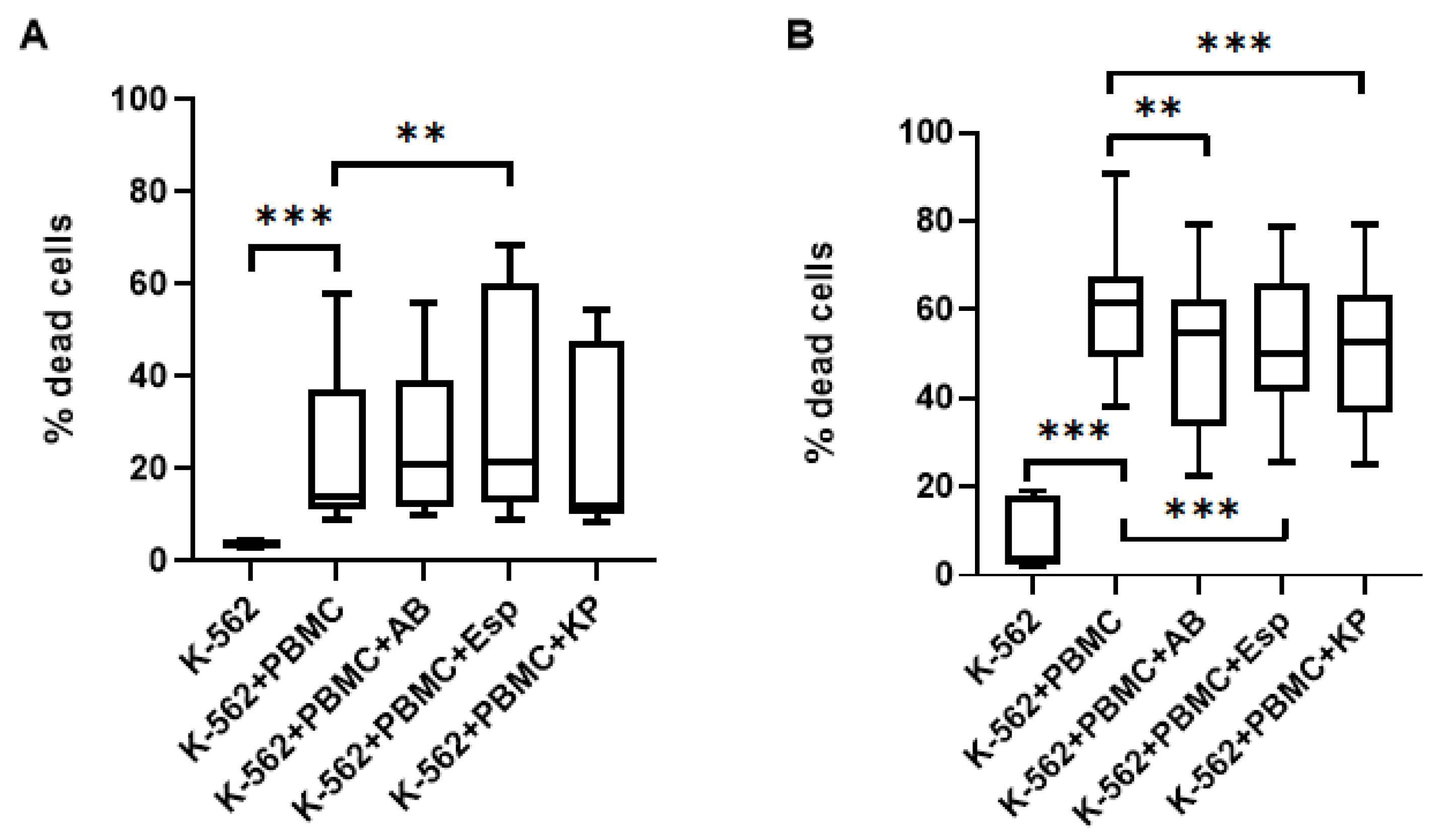
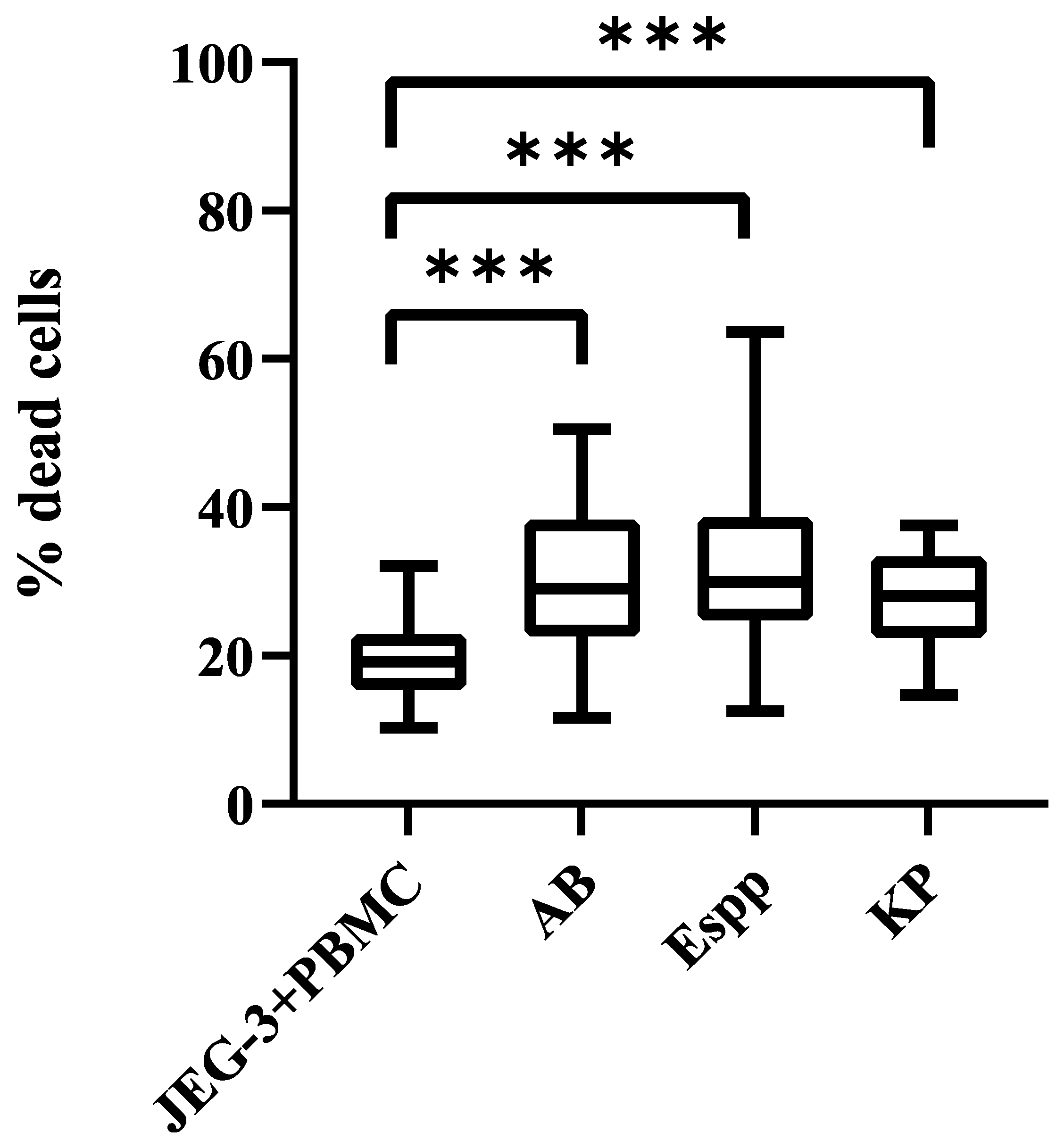
2.3. Changes in the Cytotoxic Activity of NK Cells in PBMC with Supernatants
2.4. Bacterial Supernatant-Induced Changes in Cytokine Gene mRNA Profiles in NK-92 Cells
2.5. Unchanged Cytokine Profile in NK-92-Conditioned Media Following Bacterial Supernatant Treatment
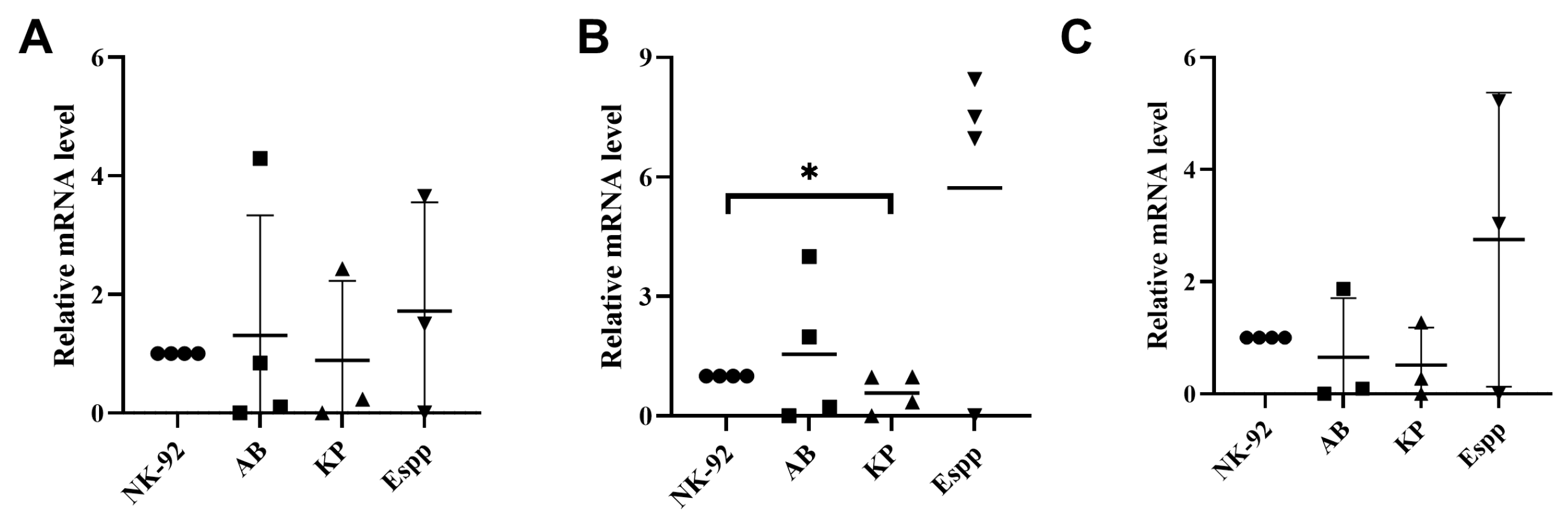
2.6. Bacterial Supernatants Alter the Relative mRNA Content of Receptor-Encoding Genes in NK-92 Cells
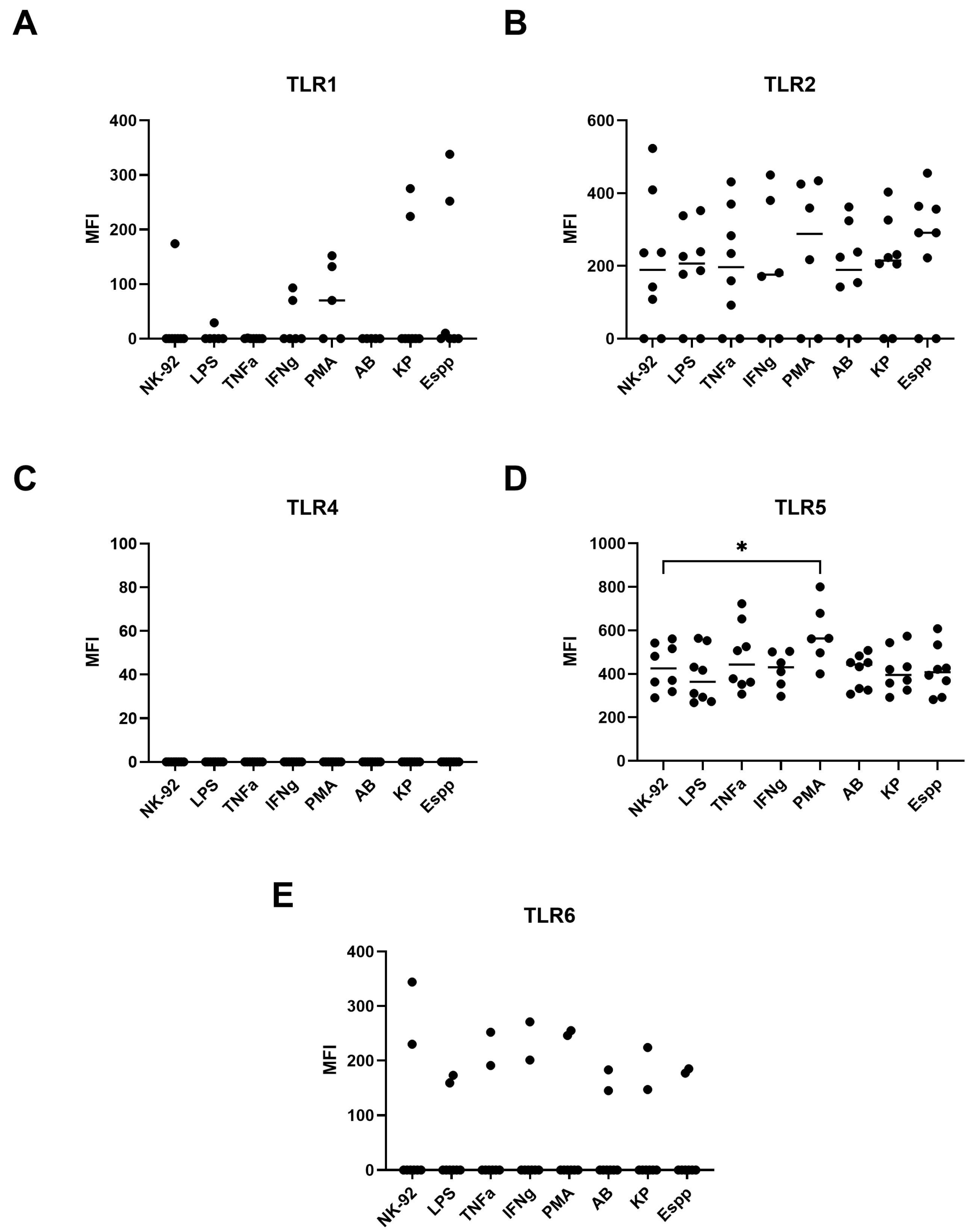
2.7. NK-92 Cells Express TLR Family Molecules
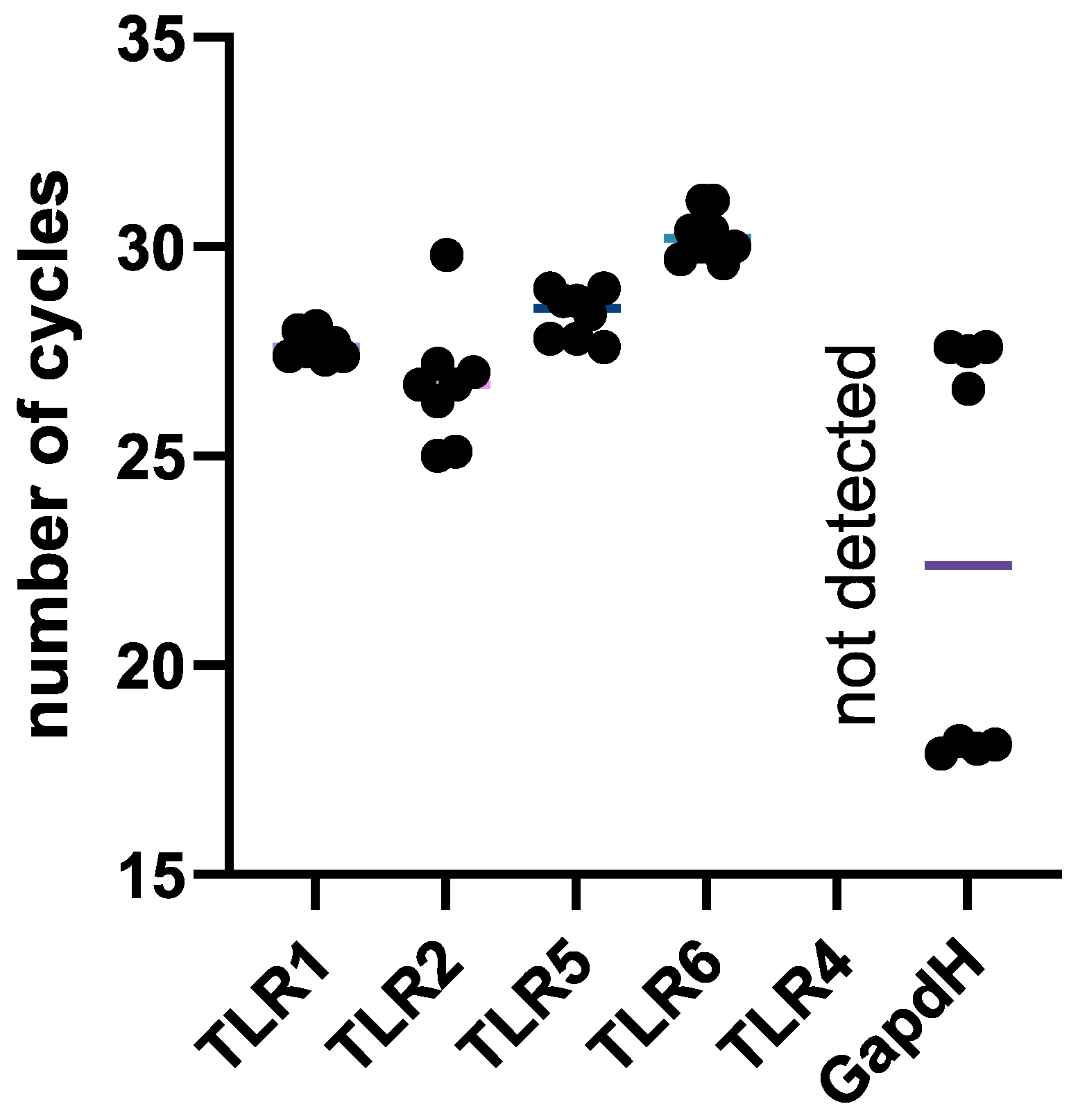
2.8. NK-92 Cells Contain mRNAs of TLR2 and TLR5 Molecules, as Well as TLR1 and TLR6
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Production of Conditioned Media Following the Cultivation of ESKAPE Bacteria
4.3. Optimal Dosage Selection for Supernatants
4.4. Effect of Bacterial Supernatants on NK-92 Cell Death Stages
4.5. Assessment of NK-92 Cytotoxic Activity Against K-562 and JEG-3 Cells with ESKAPE Bacterial Supernatants
4.6. Assessment of NK Cells in PBMC Cytotoxic Activity Against K-562 and JEG-3 Cell Lines with ESKAPE Bacterial Supernatants
4.7. Real-Time PCR Detection of the Relative mRNA Content of Cytokine Genes in NK-92 Cells with ESKAPE Bacterial Supernatants
4.8. Evaluation of Cytokine Production by NK-92 Cells in the Presence of Bacterial Supernatants Using Flow Cytometry
4.9. Expression of NK-92 Activating and Inhibitory Receptors After Bacterial Supernatant Exposure (qPCR Analysis)
4.10. Flow Cytometry Analysis of Innate Immunity Receptor Expression on NK-92 Cells Exposed to Bacterial Supernatants
4.11. Analysis of Innate Immunity Receptor Expression in NK-92 Cells Using Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daruka, L.; Czikkely, M.S.; Szili, P.; Farkas, Z.; Balogh, D.; Grézal, G.; Maharramov, E.; Vu, T.-H.; Sipos, L.; Juhász, S.; et al. ESKAPE pathogens rapidly develop resistance against antibiotics in development in vitro. Nat. Microbiol. 2025, 10, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Stewart, A.G.; Paterson, D.L.; Young, B.; Lye, D.C.; Davis, J.S.; Schneider, K.; Yilmaz, M.; Dinleyici, R.; Runnegar, N.; Henderson, A.; et al. Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC β-Lactamase–Producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2). Open Forum Infect. Dis. 2021, 8, ofab387. [Google Scholar] [CrossRef]
- Wu, W.; Wei, L.; Feng, Y.; Xie, Y.; Zong, Z. Precise Species Identification by Whole-Genome Sequencing of Enterobacter Bloodstream Infection, China. Emerg. Infect. Dis. 2021, 27, 161–169. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Meyers, S.; Lox, M.; Criel, M.; Claes, J.; Peetermans, M.; Trenson, S.; Vande Velde, G.; Vanden Berghe, P.; Baatsen, P.; et al. Staphylococcus aureus endocarditis: Distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur. Hear. J. 2019, 40, 3248–3259. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Davis, C.C. Device-Associated Menstrual Toxic Shock Syndrome. Clin. Microbiol. Rev. 2020, 33, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Bernardy, E.E.; Petit, R.A., 3rd; Raghuram, V.; Alexander, A.M.; Read, T.D.; Goldberg, J.B. Genotypic and Phenotypic Diversity of Staphylococcus aureus Isolates from Cystic Fibrosis Patient Lung Infections and Their Interactions with Pseudomonas aeruginosa. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef]
- Gan, L.; Yan, C.; Cui, J.; Xue, G.; Fu, H.; Du, B.; Zhao, H.; Feng, J.; Feng, Y.; Fan, Z.; et al. Genetic Diversity and Pathogenic Features in Klebsiella pneumoniae Isolates from Patients with Pyogenic Liver Abscess and Pneumonia. Microbiol. Spectr. 2022, 10, e0264621. [Google Scholar] [CrossRef]
- Matheeussen, V.; Xavier, B.B.; Mermans, I.; De Weerdt, A.; Lammens, C.; Goossens, H.; Jansens, H.; Malhotra-Kumar, S. Emergence of colistin resistance during treatment of recurrent pneumonia caused by carbapenemase producing Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2019, 94, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Zainal Abidin, A.; Liew, S.M.; Roberts, J.A.; Sime, F.B. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J. Infect. 2019, 79, 593–600. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef]
- Khalili, Y.; Yekani, M.; Goli, H.R.; Memar, M.Y. Characterization of carbapenem-resistant but cephalosporin-susceptible Pseudomonas aeruginosa. Acta Microbiol. Immunol. Hung. 2019, 66, 529–540. [Google Scholar] [CrossRef]
- Salmanov, A.G.; Ishchak, O.M.; Shostak, Y.M.; Kozachenko, V.V.; Rud, V.O.; Golyanovskiy, O.V.; Shkorbotun, V.O. Bacterial Infection Causes of Pregnancy Loss and Premature Birth in the Women in Ukraine. Wiadomości Lek. 2021, 74, 1355–1359. [Google Scholar] [CrossRef]
- Alsharedeh, R.H.; Yehya, A.; Beni Yonis, O.; Alameri, O.; Alshraiedeh, N. Characterization of ESKAPE pathogens in urinary tract infections among Jordanian patients. J. Infect. Dev. Ctries. 2023, 17, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gurbatri, C.R.; Arpaia, N.; Danino, T. Engineering bacteria as interactive cancer therapies. Science 2022, 378, 858–864. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Ma, Y.; Abulimiti, Y.; Zhou, Y. Lung commensal bacteria promote lung cancer progression through NK cell-mediated immunosuppressive microenvironment. Int. J. Med. Sci. 2025, 22, 1039–1051. [Google Scholar] [CrossRef]
- Ghiringhelli, F.O.; Ménard, C.D.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Yurevna, S.A.; Smirnov, I.V.; Samoylovich, M.P. Stress-induced MICA and MICB molecules in oncology. Med. Immunol. 2022, 24, 433–454. [Google Scholar] [CrossRef]
- Ma, Q.; Song, Z.; Yang, C.; Zhao, Z.; Tang, G.; You, J.; Zeng, C.; Yang, J.; Liu, Q.; Li, H.; et al. The influence of microbiome-derived amino acids metabolites in shaping the glioma immunosuppressive microenvironment. Interdiscip. Med. 2025, 3, e20240070. [Google Scholar] [CrossRef]
- Lv, B.; Zhao, Y.; Li, G.; Jiang, H.; Zhang, M.; Li, Z.; Cao, J. Tumor-Resident Intracellular Bacteria Scavenger Activated In Situ Vaccines for Potent Cancer Photoimmunotherapy. Adv. Healthc. Mater. 2025, 14, e2404271. [Google Scholar] [CrossRef]
- Eckert, L.O.; Moore, D.E.; Patton, D.L.; Agnew, K.J.; Eschenbach, D.A. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect. Dis. Obstet. Gynecol. 2003, 11, 11–17. [Google Scholar] [CrossRef]
- Nelson, D.B.; Hanlon, A.L.; Wu, G.; Liu, C.; Fredricks, D.N. First Trimester Levels of BV-Associated Bacteria and Risk of Miscarriage Among Women Early in Pregnancy. Matern. Child. Health J. 2015, 19, 2682–2687. [Google Scholar] [CrossRef]
- Balle, C.; Esra, R.; Havyarimana, E.; Jaumdally, S.Z.; Lennard, K.; Konstantinus, I.N.; Barnabas, S.L.; Happel, A.U.; Gill, K.; Pidwell, T.; et al. Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms 2020, 8, 1004. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Han, L.; Wang, C.; Yan, Y.; Qi, W.; Fan, A.; Wang, Y.; Xue, F. Aerobic vaginitis in late pregnancy and outcomes of pregnancy. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.A.; Ranjani, A.; Dhanasekaran, D.; Thajuddin, N.; Ramanidevi, T. Surveillance of multidrug resistant bacteria pathogens from female infertility cases. Afr. J. Biotechnol. 2013, 12, 4129–4134. [Google Scholar] [CrossRef]
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Yang, S.; Yang, Z.; Zhou, P.; Peng, T.; Yin, J.; Ye, Z.; Shan, H.; Yu, Y.; Li, R.; et al. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol. Spectr. 2022, 10, e0046222. [Google Scholar] [CrossRef]
- Hasan, Z.; Begum, N.; Ahmed, S.; Yasmin, M. Association of opportunistic bacterial pathogens with female infertility: A case-control study. J. Obstet. Gynaecol. Res. 2025, 51, e16243. [Google Scholar] [CrossRef]
- Abdulla, S.R.; Kareem, S.R.; Hasan, A.H. Vaginal Microbiota Profile in first-trimester miscarriages cases. Cell. Mol. Biol. 2023, 69, 9–17. [Google Scholar] [CrossRef]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC-Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [CrossRef]
- Banfalvi, G. Methods to detect apoptotic cell death. Apoptosis 2017, 22, 306–323. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. 2021, 1, e210. [Google Scholar] [CrossRef]
- Pattarone, G.; Acion, L.; Simian, M.; Mertelsmann, R.; Follo, M.; Iarussi, E. Learning deep features for dead and living breast cancer cell classification without staining. Sci. Rep. 2021, 11, 10304. [Google Scholar] [CrossRef] [PubMed]
- Akolade, J.; Crossley, I.; Farnsworth, N.L. Isolated Pancreatic Islet Treatment and Apoptosis Measurement. J. Vis. Exp. 2025, 219, e67996. [Google Scholar] [CrossRef] [PubMed]
- Beirão, J.; Pérez-Cerezales, S.; Martínez-Páramo, S.; Herráez, M.P. Detection of early damage of sperm cell membrane in Gilthead seabream (Sparus aurata) with the nuclear stain YO-PRO 1. J. Appl. Ichthyol. 2010, 26, 794–796. [Google Scholar] [CrossRef]
- Friedrich, A.; Pechstein, J.; Berens, C.; Lührmann, A. Modulation of host cell apoptotic pathways by intracellular pathogens. Curr. Opin. Microbiol. 2017, 35, 88–99. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Han, M.-L.; Speir, M.; Huang, C.; Schittenhelm, R.B.; Dhital, S.; Emery, J.; Li, J.; Kile, B.T.; et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 2020, 5, 1418–1427. [Google Scholar] [CrossRef]
- Chung, J.W.; Piao, Z.H.; Yoon, S.R.; Kim, M.S.; Jeong, M.; Lee, S.H.; Min, J.K.; Kim, J.W.; Cho, Y.H.; Kim, J.C.; et al. Pseudomonas aeruginosa eliminates natural killer cells via phagocytosis-induced apoptosis. PLoS Pathog. 2009, 5, e1000561. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zheng, R.; Lai, J.; Su, J.; Li, J.; Zhu, B.; Chen, T. Translational selenium nanoparticles boost GPx1 activation to reverse HAdV-14 virus-induced oxidative damage. Bioact. Mater. 2024, 38, 276–291. [Google Scholar] [CrossRef]
- Geske, F.J.; Lieberman, R.; Strange, R.; Gerschenson, L.E. Early stages of p53-induced apoptosis are reversible. Cell Death Differ. 2001, 8, 182–191. [Google Scholar] [CrossRef]
- Masri, C.; Chandrashekhar, Y. Apoptosis: A potentially reversible, meta-stable state of the heart. Hear. Fail. Rev. 2008, 13, 175–179. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, G.; Wu, H.; Zhao, J.; Liu, Y.; Xie, Z.; Yao, Q.; Yu, W.; Ren, W.; Zhao, G. Detection of K562 Leukemia Cells in Different States Using a Graphene-SERS Platform. ACS Appl. Nano Mater. 2021, 4, 8972–8978. [Google Scholar] [CrossRef]
- Thangaraj, J.L.; Phan, M.-T.T.; Kweon, S.; Kim, J.; Lee, J.-M.; Hwang, I.; Park, J.; Doh, J.; Lee, S.-H.; Vo, M.-C.; et al. Expansion of cytotoxic natural killer cells in multiple myeloma patients using K562 cells expressing OX40 ligand and membrane-bound IL-18 and IL-21. Cancer Immunol. Immunother. 2021, 71, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Aye, K.T.; Wattanapongpitak, S.; Supawat, B.; Kothan, S.; Udomtanakunchai, C.; Tima, S.; Tungjai, M. Effect of pre-low-dose irradiation on anticancer activities of gallic acid in leukemic K562 and K562/Dox cells: Cell viability and cellular energetic state studies. Med. Oncol. 2022, 39, 229. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K.L. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J. Oral Microbiol. 2020, 13, 1849493. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Ahrendt, M.; Nattermann, J.; Sauerbruch, T.; Spengler, U. Comparative study of NK cell-mediated cytotoxicity using radioactive and flow cytometric cytotoxicity assays. J. Immunol. Methods 2005, 306, 161–168. [Google Scholar] [CrossRef]
- Vourc’h, M.; David, G.; Gaborit, B.; Broquet, A.; Jacqueline, C.; Chaumette, T.; Caillon, J.; Roquilly, A.; Retiere, C.; Asehnoune, K.; et al. Pseudomonas aeruginosa Infection Impairs NKG2D-Dependent NK Cell Cytotoxicity through Regulatory T-Cell Activation. Infect. Immun. 2020, 88, 10-1128. [Google Scholar] [CrossRef]
- Aku, F.Y.; Akweongo, P.; Nyarko, K.M.; Mensah, L.G.; Amegan-Aho, K.; Kumi, L.; Afari, E.A.; Ameme, D.K.; Kenu, E. Factors associated with culture proven neonatal sepsis in the Ho municipality 2016. Pan Afr. Med. J. 2020, 36, 281. [Google Scholar] [CrossRef]
- Sun, L.; Hong, W.; Wang, S.; Xu, Y.; Li, C. Association of HLA-E single nucleotide polymorphisms with human myeloid leukemia. Hla 2024, 103, e15440. [Google Scholar] [CrossRef]
- Tyshchuk, E.; Grebenkina, P.; Krutetskaya, I.; Smirnov, I.; Stolbovaya, A.; Shashkova, O.; Samoilovich, M.; Bazhenov, D.; Stepanova, O.; Selkov, S.; et al. Endoglin Regulates Intercellular Interactions between Trophoblast and Natural Killer Cells. J. Evol. Biochem. Physiol. 2024, 60, 930–946. [Google Scholar] [CrossRef]
- Hakam, M.S.; Miranda-Sayago, J.M.; Hayrabedyan, S.; Todorova, K.; Spencer, P.S.; Jabeen, A.; Barnea, E.R.; Fernandez, N. Preimplantation Factor (PIF) Promotes HLA-G, -E, -F, -C Expression in JEG-3 Choriocarcinoma Cells and Endogenous Progesterone Activity. Cell. Physiol. Biochem. 2017, 43, 2277–2296. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.-M.; Cao, D.-D.; Liu, X.-F.; Wang, L.; Lin, X.-L.; Duan, Y.-G.; Lee, C.-L.; Chiu, P.C.N.; Yeung, W.S.B.; Yao, Y.-Q. Application of a JEG-3 organoid model to study HLA-G function in the trophoblast. Front. Immunol. 2023, 14, 1130308. [Google Scholar] [CrossRef] [PubMed]
- Grebenkina, P.V.; Mikhailova, V.A.; Bespalova, O.N.; Selkov, S.A.; Sokolov, D.I. Regulation of the cytokine profile of NK cells by the microenvironment factors typical for pregnancy. Med. Immunol. 2024, 27, 445–450. [Google Scholar] [CrossRef]
- Jensen, I.J.; McGonagill, P.W.; Butler, N.S.; Harty, J.T.; Griffith, T.S.; Badovinac, V.P. NK Cell–Derived IL-10 Supports Host Survival during Sepsis. J. Immunol. 2021, 206, 1171–1180. [Google Scholar] [CrossRef]
- Yang, H.-L.; Zhou, W.-J.; Chang, K.-K.; Mei, J.; Huang, L.-Q.; Wang, M.-Y.; Meng, Y.; Ha, S.-Y.; Li, D.-J.; Li, M.-Q. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef]
- Marcon, F.; Zuo, J.; Pearce, H.; Nicol, S.; Margielewska-Davies, S.; Farhat, M.; Mahon, B.; Middleton, G.; Brown, R.; Roberts, K.J.; et al. NK cells in pancreatic cancer demonstrate impaired cytotoxicity and a regulatory IL-10 phenotype. OncoImmunology 2020, 9, 1845424. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Noguera, L.P.; Ahn, D.; Vallejos, O.P.; Castellanos, R.M.; Vazquez, Y.; Salazar-Echegarai, F.J.; González, L.; Suazo, I.; Pardo-Roa, C.; et al. Interleukin-10 Produced by Myeloid-Derived Suppressor Cells Provides Protection to Carbapenem-ResistantKlebsiella pneumoniaeSequence Type 258 by Enhancing Its Clearance in the Airways. Infect. Immun. 2019, 87, 10-1128. [Google Scholar] [CrossRef]
- Guan, Z.; Liu, Y.; Liu, C.; Wang, H.; Feng, J.; Yang, G. Staphylococcus aureus β-Hemolysin Up-Regulates the Expression of IFN-γ by Human CD56bright NK Cells. Front. Cell. Infect. Microbiol. 2021, 11, 658141. [Google Scholar] [CrossRef]
- Alshareef, M.H.; Hartland, E.L.; McCaffrey, K. Effectors Targeting the Unfolded Protein Response during Intracellular Bacterial Infection. Microorganisms 2021, 9, 705. [Google Scholar] [CrossRef]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012, 91, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.M.; Lange, J.R.; Langstein, H.N.; Alexander, H.R.; Buresh, C.M.; Norton, J.A. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J. Immunol. 1992, 149, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Subramanian, A.; Agrawal, D.; Bhoi, S.K.; Pallavi, P.; Mukhopadhayay, A.K. RANTES levels in peripheral blood, CSF and contused brain tissue as a marker for outcome in traumatic brain injury (TBI) patients. BMC Res. Notes 2017, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Fortelny, N.; Overall, C.M.; Pavlidis, P.; Freue, G.V.C. Can we predict protein from mRNA levels? Nature 2017, 547, E19–E20. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polanski, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Davies, K.; Rizek, A.-M.; Kollnberger, S.; Wang, E.C.Y.; Eberl, M.; Underwood, J.; McLaren, J.E. NKG2A-mediated immune modulation of natural killer cells by Staphylococcus aureus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Eriksson, M.; Meadows, S.K.; Basu, S.; Mselle, T.F.; Wira, C.R.; Sentman, C.L. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J. Immunol. 2006, 176, 6219–6224. [Google Scholar] [CrossRef]
- Chalifour, A.; Jeannin, P.; Gauchat, J.F.; Blaecke, A.; Malissard, M.; N’Guyen, T.; Thieblemont, N.; Delneste, Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 2004, 104, 1778–1783. [Google Scholar] [CrossRef]
- Comin, F.; Speziali, E.; Martins-Filho, O.A.; Caldas, I.R.; Moura, V.; Gazzinelli, A.; Correa-Oliveira, R.; Faria, A.M.C. Ageing and Toll-like receptor expression by innate immune cells in chronic human schistosomiasis. Clin. Exp. Immunol. 2007, 149, 274–284. [Google Scholar] [CrossRef]
- Gallardo-Zapata, J.; Pérez-Figueroa, E.; Olivar-López, V.; Medina-Sansón, A.; Jiménez-Hernández, E.; Ortega, E.; Maldonado-Bernal, C. TLR Agonists Modify NK Cell Activation and Increase Its Cytotoxicity in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 7500. [Google Scholar] [CrossRef]
- Duriez, M.; Quillay, H.L.S.; Madec, Y.; El Costa, H.; Cannou, C.; Marlin, R.; de Truchis, C.; Rahmati, M.; Barré-Sinoussi, F.O.; Nugeyre, M.-T.R.S.; et al. Human decidual macrophages and NK cells differentially express Toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front. Microbiol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Barczyk, A.; Bauderlique-Le Roy, H.; Jouy, N.; Renault, N.; Hottin, A.; Millet, R.; Vouret-Craviari, V.; Adriouch, S.; Idziorek, T.; Dezitter, X. Flow cytometry: An accurate tool for screening P2RX7 modulators. Cytom. Part A 2020, 99, 793–806. [Google Scholar] [CrossRef]



| Receptor | Ligand | Potential Object of Interaction |
| TLR2 | Peptidoglycan, triacillipopeptide | Gram-positive, Gram-negative bacteria |
| TLR5 | Flagellin | Gram-positive, Gram-negative bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebenkina, P.; Juchina, V.; Tyshchuk, E.; Gulina, A.; Denisova, E.; Korobova, Z.; Orlov, S.; Totolian, A.; Kraeva, L.; Sokolov, D. Modulation of NK Cell Properties by ESKAPE Group Bacteria. Int. J. Mol. Sci. 2025, 26, 8449. https://doi.org/10.3390/ijms26178449
Grebenkina P, Juchina V, Tyshchuk E, Gulina A, Denisova E, Korobova Z, Orlov S, Totolian A, Kraeva L, Sokolov D. Modulation of NK Cell Properties by ESKAPE Group Bacteria. International Journal of Molecular Sciences. 2025; 26(17):8449. https://doi.org/10.3390/ijms26178449
Chicago/Turabian StyleGrebenkina, Polina, Varvara Juchina, Elizaveta Tyshchuk, Ananstasia Gulina, Elizaveta Denisova, Zoia Korobova, Sergey Orlov, Areg Totolian, Lyudmila Kraeva, and Dmitry Sokolov. 2025. "Modulation of NK Cell Properties by ESKAPE Group Bacteria" International Journal of Molecular Sciences 26, no. 17: 8449. https://doi.org/10.3390/ijms26178449
APA StyleGrebenkina, P., Juchina, V., Tyshchuk, E., Gulina, A., Denisova, E., Korobova, Z., Orlov, S., Totolian, A., Kraeva, L., & Sokolov, D. (2025). Modulation of NK Cell Properties by ESKAPE Group Bacteria. International Journal of Molecular Sciences, 26(17), 8449. https://doi.org/10.3390/ijms26178449








