The Neurotropic Activity of Novel Dermorphin Analogs Active at Systemic and Noninvasive Administration
Abstract
1. Introduction
2. Results
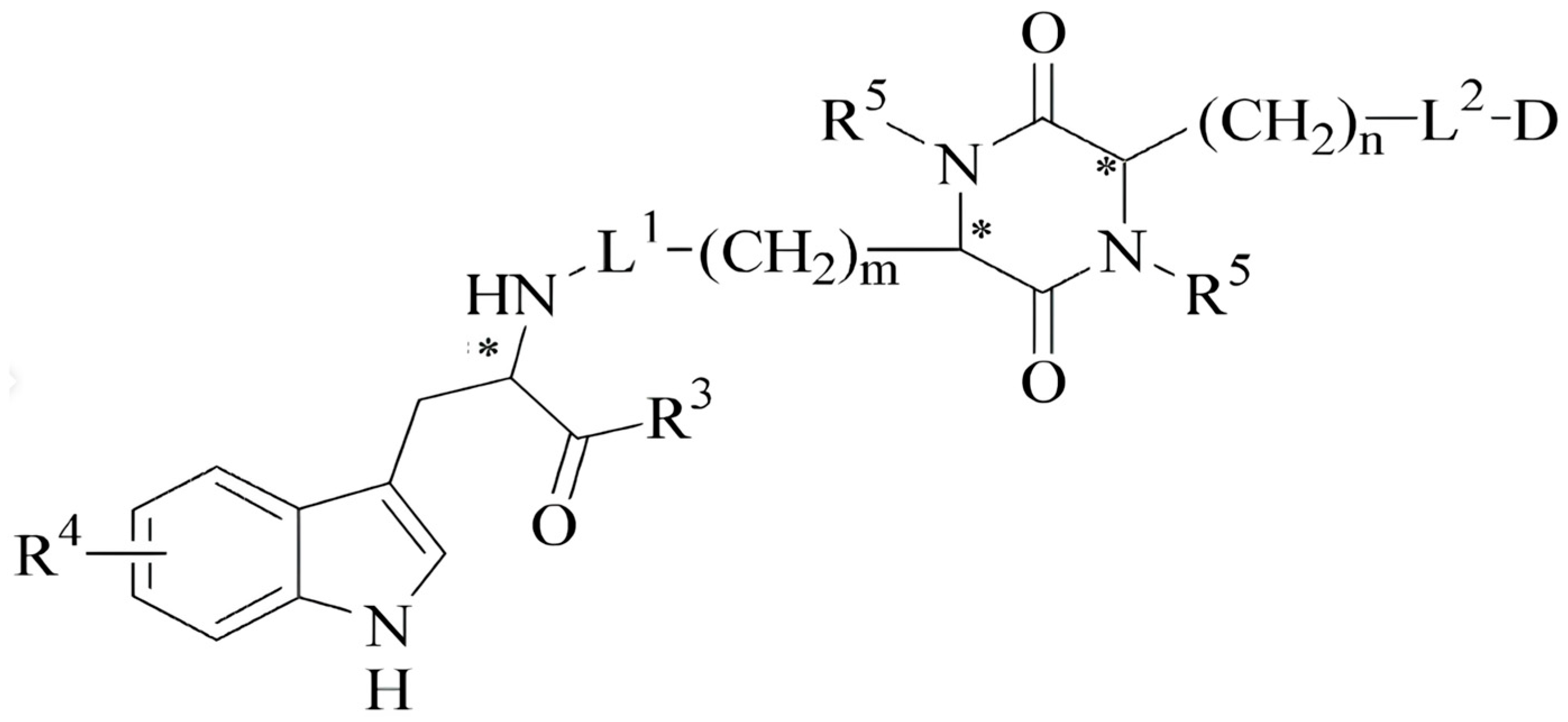
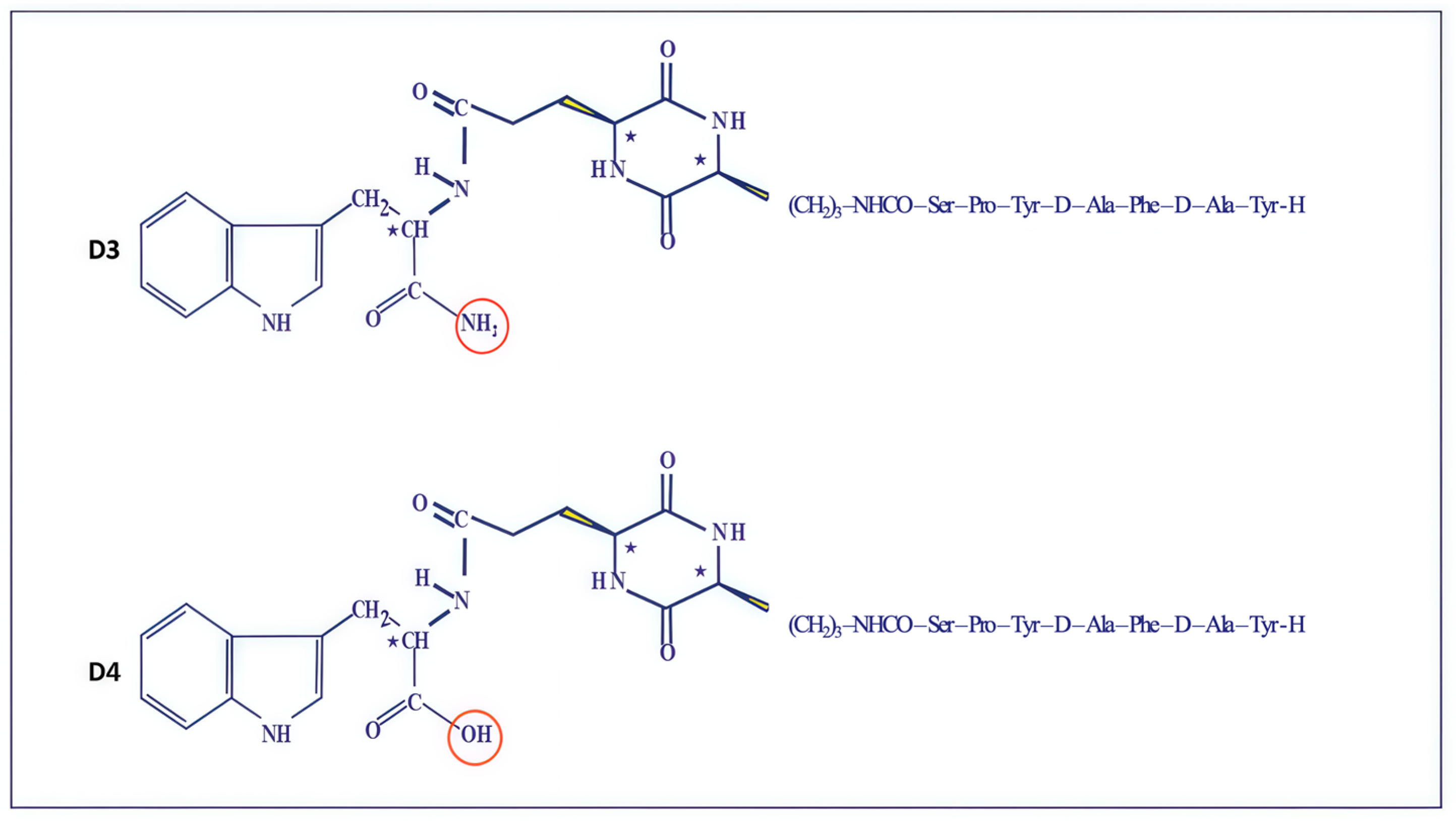
2.1. Synthetic Analogs of Dermorphin and Their Biological Activity In Vitro
2.2. Opioid Activity Studies of New Dermorphin Analogs on Isolated Guinea Pig Ileum
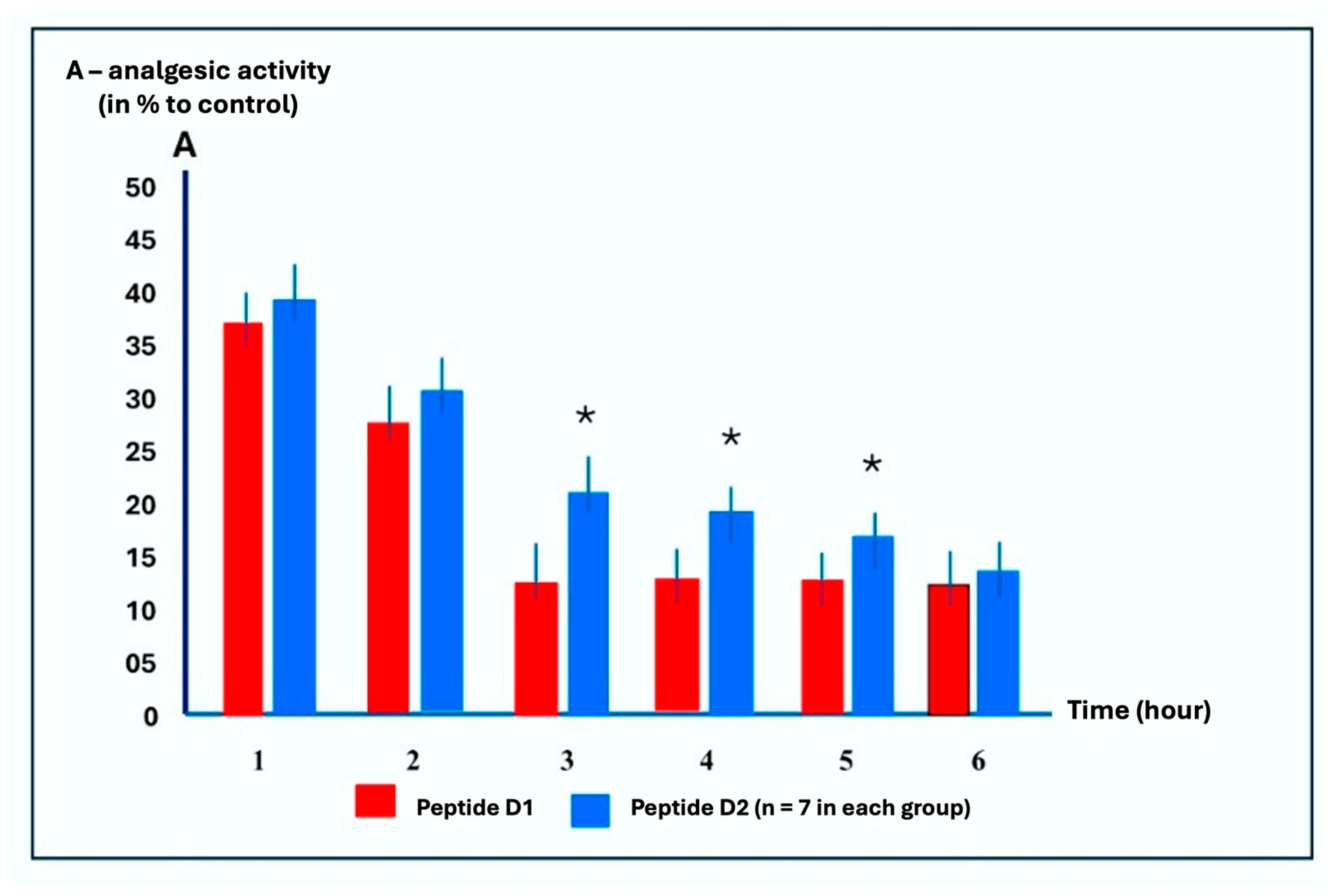
2.3. Time-Dependent Analgesic Activity Study of Peptides D1 and D2
2.4. Study Animals’ Behavioral Responses to Peptide D2 in Rats Using the “Open Field” Test with Intranasal Administration
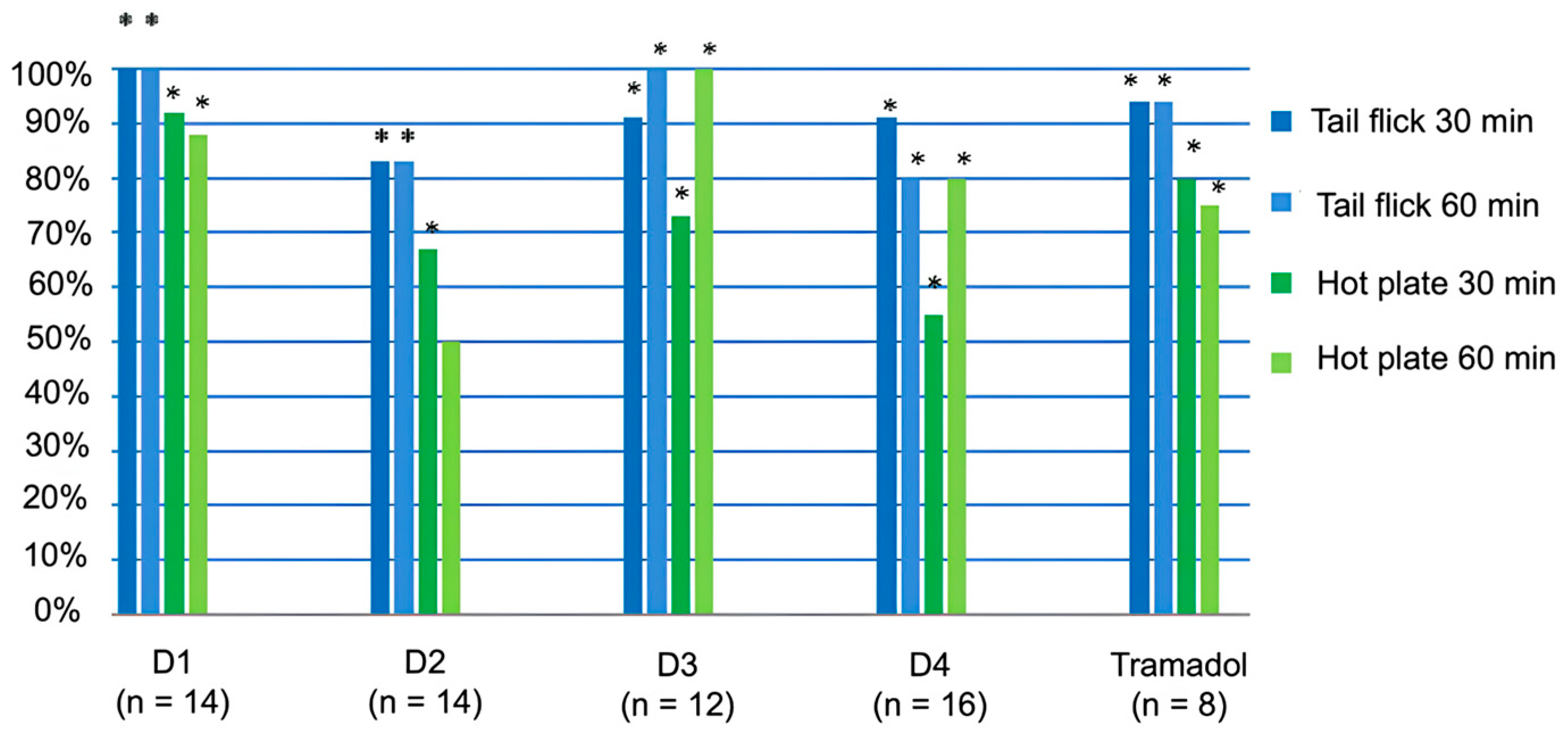
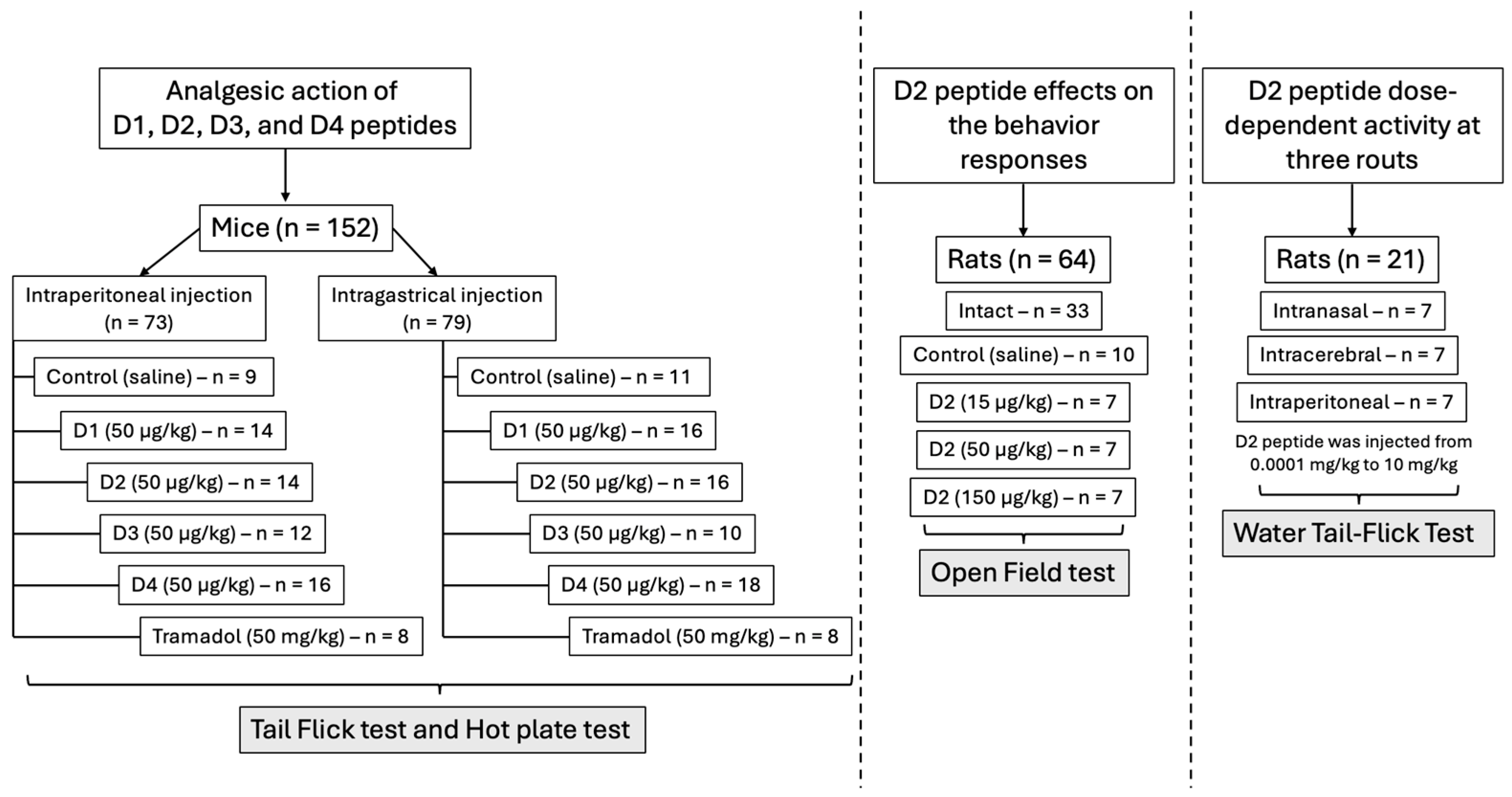
2.5. Analgesic Action of Peptides D1, D2, D3, and D4 in Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Synthesis of Linear Peptides
4.3. Synthesis of Cyclopeptides and Development of Noninvasive Forms of Dermorphin Analogs
4.4. Peripheral Opioid Activity of Peptides on Isolated Guinea Pig Ileum
4.5. Analgesic Action of Peptide D2 Using Different Routes of Administration on Rats
4.5.1. Comparative Study of Time-Dependent Analgesic Activity of Peptides D1 and D2 at Intraperitoneal Injection
- t1—time of relief from pain stimulus after the introduction of substances,
- t0—time of relief from pain stimulus as the baseline,
- tmax—maximal duration of pain stimulus (30 sec).
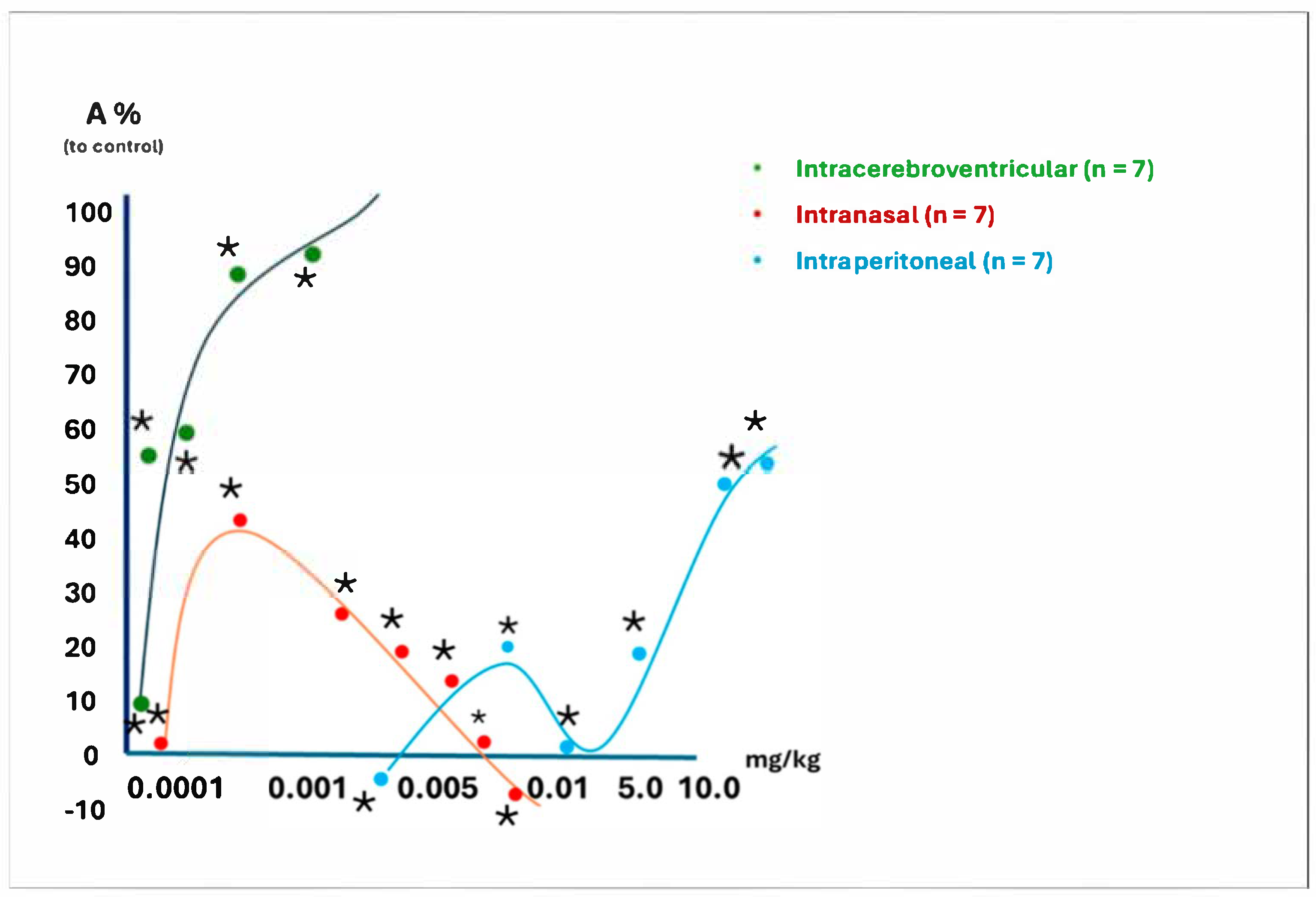
4.5.2. The Dose-Dependent Activity of D2 at Three Administration Routes in Rats
4.6. The Effect of D2 on the Central Nervous System
4.6.1. The Effect of Intranasal Administration of D2 on the Behavioral Responses of Rats in the “Open Field” Model
- In the intact group, animals without training were injected with 0.05 mL of physiological solution one minute before being placed in the “open field.” (n = 33).
- The control group consisted of animals adapted after a 5-day administration of 0.5 mL of saline (n = 10).
- Single drug action at a 15 μg/kg dose was administered to adapted animals (n = 7).
- Adapted animals (n = 7) received a 50 μg/kg dose of a single drug action.
- Single drug action at a 150 μg/kg dose was administered to adapted animals (n = 7).
4.6.2. The Analgesic Action of Peptides D1, D2, D3, and D4 in Mice After Intraperitoneal and Intragastric Administration
- Control (saline)—n = 11, analgesic action was not observed;
- D1 (50 μg/kg)—n = 16;
- D2 (50 μg/kg)—n = 16;
- D3 (50 μg/kg)—n = 10;
- D4 (50 μg/kg)—n = 18;
- Tramadol (50 μg/kg)—n = 18.
- Control (saline)—n = 9, analgesic action was not observed;
- D1 (50 μg/kg)—n = 14;
- D2 (50 μg/kg)—n = 14;
- D3 (50 μg/kg)—n = 12;
- D4 (50 μg/kg)—n = 16;
- Tramadol (50 μg/kg)—n = 18.
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Appendix A
Appendix A.1
| Code | Structure | MW | HPLCtr | MS |
|---|---|---|---|---|
| D1 | H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 (dermorphin) | 802 | 3.14 | 803 |
| D2 | H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser-NH-CH3 | 830 | 3.28 | 831 |
| D3 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu- (L-Trp-NH2)] | 1242 | 19.0 | 1243 |
| D4 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu-(L-Trp-OH)] | 1241 | 16.0 | 1242 |
| D5 | H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser-OH | 817 | 3.19 | 818 |
| D6 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)-L-Glu(OH)] | 801 | 17.4 | 802 |
| D7 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)-L-Glu- (L-TrpOMe)] | 986 | 19.8 | 987 |
| D8 | Arg-Tyr-D-Ala-Phe-Gly-OH | 613 | 1.76 | 614 |
| D9 | Arg-Tyr-D-Ala-Phe-D-Ala-OH | 627 | 1.79 | 628 |
| D10 | H-Tyr-D-Ala-Phe-D-Ala-OH | 471 | 2.68 | 472 |
Appendix A.2
- The synthesis of Boc-Phe-D-Ala-OBzl.
- 2.
- The synthesis of TFA. H-Phe-D-Ala-OBzl
- 3.
- The synthesis of Boc-D-Ala-Phe-D-Ala-OBzl
- 4.
- The synthesis of TFA. H-Phe-D-Ala-OBzl
- 5.
- The synthesis of Boc-D-Ala-Phe-D-Ala-OBzl
- 6.
- The synthesis of TFA. H-D-Ala-Phe-D-Ala-OBzl
- 7.
- The synthesis of Boc-Tyr-D-Ala-Phe-D-Ala-OBzl.
- 8.
- The synthesis of Boc2-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser(Bzl)-NH2.
- 9.
- The synthesis of TFA. H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser(Bzl)-NHMe
References
- Mizusawa, K. Subchapter 9E—Dermorphin. In Handbook of Hormones, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 1095–1113. [Google Scholar] [CrossRef]
- Melchiorri, P.; Negri, L. The Dermorphin Peptide Family. Gen. Pharmacol. 1996, 27, 1099–1107. [Google Scholar] [CrossRef]
- Hochrainer, N.; Serafin, P.; D’Ingiullo, S.; Mollica, A.; Granica, S.; Brytan, M.; Kleczkowska, P.; Spetea, M. In vitro and In Vivo Pharmacological Profiles of LENART01, a Dermorphin-Ranatensin Hybrid Peptide. Int. J. Mol. Sci. 2024, 25, 4007. [Google Scholar] [CrossRef] [PubMed]
- Vardy, E.; Sassano, M.F.; Rennekamp, A.J.; Kroeze, W.K.; Mosier, P.D.; Westkaemper, R.B.; Stevens, C.W.; Katritch, V.; Stevens, R.C.; Peterson, R.T.; et al. Single Amino Acid Variation Underlies Species-Specific Sensitivity to Amphibian Skin-Derived Opioid-like Peptides. Chem. Biol. 2015, 22, 764–775. [Google Scholar] [CrossRef]
- Thompson, C.; Williams, M.L. Review of the Physiological Effects of Phyllomedusa Bicolor Skin Secretion Peptides on Humans Receiving Kambô. Toxicol. Res. Appl. 2022, 6, 23978473221085746. [Google Scholar] [CrossRef]
- Hesselink, J.K.; Schatman, M. Rediscovery of old drugs: The forgotten case of dermorphin for postoperative pain and palliation. J. Pain Res. 2018, 11, 2991–2995. [Google Scholar] [CrossRef]
- Tiwari, V.; Yang, F.; He, S.-Q.; Shechter, R.; Zhang, C.; Shu, B.; Zhang, T.; Tiwari, V.; Wang, Y.; Dong, X.; et al. Activation of Peripheral μ-Opioid Receptors by Dermorphin [D-Arg2, Lys4] (1–4) Amide Leads to Modality-Preferred Inhibition of Neuropathic Pain. Anesthesiology 2016, 124, 706–720. [Google Scholar] [CrossRef]
- Bedini, A.; Cuna, E.; Baiula, M.; Spampinato, S. Quantitative Systems Pharmacology and Biased Agonism at Opioid Receptors: A Potential Avenue for Improved Analgesics. Int. J. Mol. Sci. 2022, 23, 5114. [Google Scholar] [CrossRef]
- Asfour, M.H. Advanced Trends in Protein and Peptide Drug Delivery: A Special Emphasis on Aquasomes and Microneedle Techniques. Drug Deliv. Transl. Res. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V. Multifunctional Bioactive Compounds. U.S. Patent US8637521B2, 4 August 2006. [Google Scholar]
- Deigin, V.; Ksenofontova, O.; Khrushchev, A.; Yatskin, O.; Goryacheva, A.; Ivanov, V. Chemical Platform for the Preparation of Synthetic Orally Active Peptidomimetics with Hemoregulating Activity. ChemMedChem 2016, 11, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.; Ksenofontova, O.; Yatskin, O.; Goryacheva, A.; Ignatova, A.; Feofanov, A.; Ivanov, V. Novel Platform for the Preparation of Synthetic Orally Active Peptidomimetics with Hemoregulating Activity. II. Hemosuppressor Activity of 2,5-Diketopiperazine-Based Cyclopeptides. Int. Immunopharmacol. 2020, 81, 106185. [Google Scholar] [CrossRef]
- Stewart, M.J.; Watson, I.D. Standard units for expressing drug concentrations in biological fluids. Br. J. Clin. Pharmacol. 1983, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Gyang, E.A.; Kosterlitz, H.W. Agonist and antagonist actions of morphine-like drugs on the guinea-pig isolated ileum. Brit. J. Pharmac. Chemother. 1966, 27, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Attila, M.; Salvadori, S.; Balboni, G.; Bryant, S.D.; Lazarus, L.H. Synthesis and receptor binding analysis of dermorphin hepta-, hexa- and pentapeptide analogues. Evidence for one- and two-sided binding models for the mu-opioid receptor. Int. J. Pept. Protein Res. 1993, 42, 550–559. [Google Scholar] [CrossRef]
- Aburas, K.; Misbah, A.; Fehelbum, H.; Abukhdir, A. Analgesic Effect by Using Hot Plate and Tail Flick Test in Rats Models for Aqueous Moringa oleifera Extract. Libyan J. Med. Res. 2023, 17, 107–119. [Google Scholar] [CrossRef]
- Krumins, S.A. Characterization of dermorphin binding to membranes of rat brain and heart. Neuropeptides 1987, 9, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Symeon, I.; Polissidis, A.; Balafas, E.; Stasinopoulou, M.; Alexakos, P.; Voyiatzaki, C.; Kostomitsopoulos, N. Evaluation of the effects of tramadol on analgesic response and locomotor activity in two different strains of laboratory mice. J. Hell. Vet. Med. Soc. 2017, 68, 89–96. [Google Scholar] [CrossRef][Green Version]
- Morse, J.S.; Stockbridge, H.; Egan, K.B.; Mai, J.; Wickizer, T.; Franklin, G.M. Primary care survey of the value and effectiveness of the Washington State Opioid Dosing Guideline. J. Opioid Manag. 2011, 7, 427–433. [Google Scholar] [CrossRef]
- Zupanets, I.A.; Shebeko, S.K.; Popov, S.B.; Zupanets, K.O.; Zimin, S.M. Analgesic properties of the dry combined extract BNO 1016 in leukotriene-dependent hyperalgesia. Pharmazie 2022, 77, 27–31. [Google Scholar]
- Machelska, H.; Celik, M.Ö. Opioid Receptors in Immune and Glial Cells- Implications for Pain Control. Front. Immunol. 2020, 11, 300. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug. Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yao, J.; Kong, J.; Yu, A.; Wei, J.; Dong, Y.; Song, R.; Shan, D.; Zhong, X.; Lu, F.; et al. 2,5-Diketopiperazines: A Review of Source, Synthesis, Bioactivity, Structure, and MS Fragmentation. Curr. Med. Chem. 2023, 30, 1060–1085. [Google Scholar] [CrossRef]
- Lee, Y.S. Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery. Biomolecules 2022, 12, 1241. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Cunliffe, G.; Ho, R.C.; Lee, S.S.; Jung, S. Potentials of Neuropeptides as Therapeutic Agents for Neurological Diseases. Biomedicines 2022, 10, 343. [Google Scholar] [CrossRef]
- Doti, N.; Ruvo, M. Synthetic Peptides and Peptidomimetics: From Basic Science to Biomedical Applications- Second Edition. Int. J. Mol. Sci. 2024, 25, 1083. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Nazimov, I.V.; Gretskaya, N.M.; Deigin, V.I.; Bezuglov, V.V. Investigation of Peptide Stability upon Hydrolysis by Fragments of the Organs of the Gastrointestinal Tract of Rats. Russ. J. Bioorg. Chem. 2010, 36, 690–695. [Google Scholar] [CrossRef]
- Skotland, T.; Iversen, T.G.; Torgersen, M.L.; Sandvig, K. Cell-Penetrating Peptides: Possibilities and Challenges for Drug Delivery in vitro and in Vivo. Molecules 2015, 20, 13313–13323. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Cecchi, M.; Capriles, N.; Watson, S.J.; Akil, H. Differential responses to morphine-induced analgesia in the tail-flick test. Behav. Brain Res. 2008, 194, 146–151. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Thorn-Gray, B.E.; Levitt, R.A.; Hill, J.; Ward, K. A neuroanatomical study of analgesia and catatonia induced by etorphine in the rat. Neuropharmacology 1981, 20, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Remesic, M.; Lee, Y.S.; Hruby, V.J. Cyclic Opioid Peptides. Curr. Med. Chem. 2016, 23, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Guardiani, G.; Davis, P.; Ma, S.W.; Porreca, F.; Lai, J.; Mannina, L.; Sobolev, A.P.; Hruby, V.J. Synthesis of stable and potent delta/mu opioid peptides: Analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH by ring-closing metathesis. J. Med. Chem. 2007, 50, 3138–3142. [Google Scholar] [CrossRef] [PubMed]
- Berezowska, I.; Chung, N.N.; Lemieux, C.; Wilkes, B.C.; Schiller, P.W. Dicarba analogues of the cyclic enkephalin peptides H-Tyr-c[D-Cys-Gly-Phe-D(or L)-Cys]NH(2) retain high opioid activity. J. Med. Chem. 2007, 50, 1414–1417. [Google Scholar] [CrossRef]
- Herrera, C.; Bolton, F.; Arias, A.S.; Harrison, R.A.; Gutiérrez, J.M. Analgesic effect of morphine and tramadol in standard toxicity assays in mice injected with venom of the snake Bothrops asper. Toxicon 2018, 154, 35–41. [Google Scholar] [CrossRef]

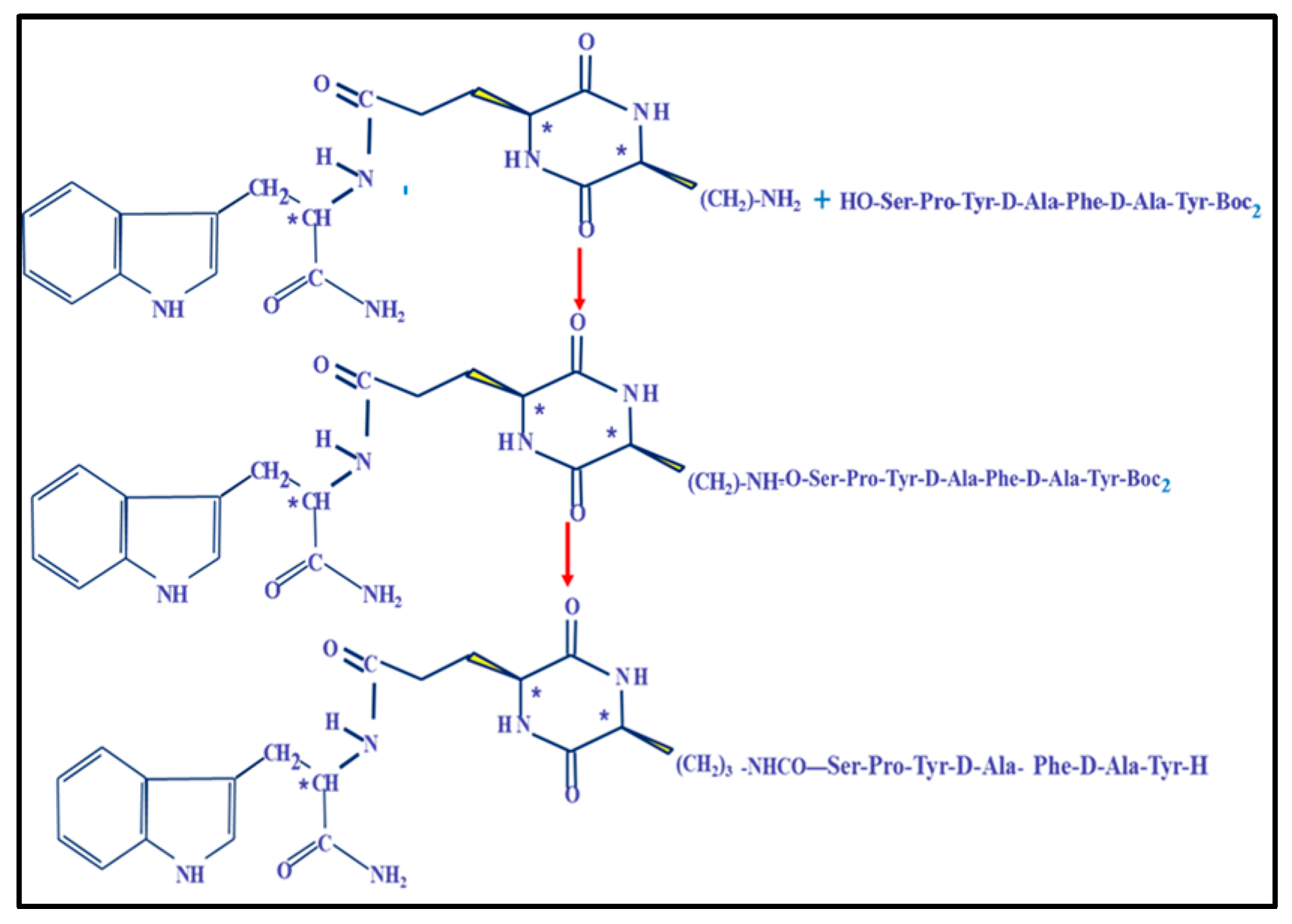
| Code | Structure | Index IC50 mol/L * |
|---|---|---|
| D1 | H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 (dermorphin) | 2.5 × 10−9 |
| D2 | H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser-NH-CH3 | 9.5 × 10−9 |
| D3 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu(L-Trp-NH2)] | 3.3 × 10−8 |
| D4 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu(L-Trp-OH)] | 1.1 × 10−8 |
| D5 | H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser-OH | 7.6 × 10−7 |
| D6 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)-L-Glu(OH)] | 5.7 × 10−7 |
| D7 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)-L-Glu-(L-TrpOMe)] | 2.0 × 10−6 |
| D8 | Arg-Tyr-D-Ala-Phe-Gly-OH | 3.0 × 10−6 |
| D9 | H-Arg-Tyr-D-Ala-Phe-D-AlaOH | 1.0 × 10−6 |
| D10 | H-Tyr-D-Ala-Phe-D-Ala-OH | 1.0 × 10−6 |
| Action | Intact (n = 33) | Control (Trained n = 10) | Peptide D2 (dose, µg/kg) | ||
|---|---|---|---|---|---|
| 15 (n = 7) | 50 (n = 7) | 150 (n = 7) | |||
| Path | 100.75 ± 6.31 | 65.80 ± 13.00 * | 23.83 ± 7.16 ** | 21.80 ± 6.16 ** | 14.80 ± 3.80 ** |
| Dive | 25.63 ± 2.04 | 11.70 ± 2.12 * | 6.17 ± 1.63 ** | 3.40 ± 0.76 ** | 2.90 ± 0.99 ** |
| Stand | 33.51 ± 2.42 | 16.30 ± 4.37 * | 8.83 ± 3.03 ** | 4.50 ± 1.35 ** | 4.70 ± 1.45 ** |
| Grooming | 7.16 ± 0.68 | 6.80 ± 1.82 * | 1.58 ± 0.42 ** | 0.90 ± 0.46 ** | 1.00 ± 0.39 ** |
| Defecation | 2.73 ± 0.23 | 2.10 ± 0.43 * | 0.92 ± 0.26 ** | 1.10 ± 0.18 ** | 0.60 ± 0.22 ** |
| Urination | 0.820 ± 0.12 | 0.30 ± 0.15 | 0.25 ± 0.13 | 0.20 ± 0.13 | 0.10 ± 0.10 |
| Path | 100.75 ± 6.31 | 65.80 ± 13.00 * | 23.83 ± 7.16 ** | 21.80 ± 6.16 ** | 14.80 ± 3.80 ** |
| Dive | 25.63 ± 2.04 | 11.70 ± 2.12 * | 6.17 ± 1.63 ** | 3.40 ± 0.76 ** | 2.90 ± 0.99 ** |
| Stand | 33.51 ± 2.42 | 16.30 ± 4.37 * | 8.83 ± 3.03 ** | 4.50 ± 1.35 ** | 4.70 ± 1.45 ** |
| Code | Structure |
|---|---|
| D1 | H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 (dermorphin) |
| D2 | H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser-NH-CH3 |
| D3 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu(L-Trp-NH2)] |
| D4 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala-Tyr-Pro-Ser)-L-Glu(L-Trp-OH)] |
| D6 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)-L-Glu(OH)] |
| D7 | Cyclo-[L-Lys(H-Tyr-D-Ala-Phe-D-Ala)- L-Glu-(L-TrpOMe)] |
| Tyr-c2,5 (-S-) [DVal-Gly-he-DAla]-OH | |
| Tyr-c2,5 (-S-) [DAla-Gly-Phe-DAla]-OH | |
| Tyr-c2,5 (-CH2CH2-) [DAla-Gly-Phe-Ala]-NH2 | |
| Tyr c2,5 (-cisCH=CH-) [DAla-Gly-Phe-DAla]-OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deigin, V.; Korobov, N.; Volpina, O.; Linkova, N.; Diatlova, A.; Medvedev, D.; Krasichkov, A.; Polyakova, V. The Neurotropic Activity of Novel Dermorphin Analogs Active at Systemic and Noninvasive Administration. Int. J. Mol. Sci. 2025, 26, 8437. https://doi.org/10.3390/ijms26178437
Deigin V, Korobov N, Volpina O, Linkova N, Diatlova A, Medvedev D, Krasichkov A, Polyakova V. The Neurotropic Activity of Novel Dermorphin Analogs Active at Systemic and Noninvasive Administration. International Journal of Molecular Sciences. 2025; 26(17):8437. https://doi.org/10.3390/ijms26178437
Chicago/Turabian StyleDeigin, Vladislav, Nikolay Korobov, Olga Volpina, Natalia Linkova, Anastasiia Diatlova, Dmitrii Medvedev, Alexander Krasichkov, and Victoria Polyakova. 2025. "The Neurotropic Activity of Novel Dermorphin Analogs Active at Systemic and Noninvasive Administration" International Journal of Molecular Sciences 26, no. 17: 8437. https://doi.org/10.3390/ijms26178437
APA StyleDeigin, V., Korobov, N., Volpina, O., Linkova, N., Diatlova, A., Medvedev, D., Krasichkov, A., & Polyakova, V. (2025). The Neurotropic Activity of Novel Dermorphin Analogs Active at Systemic and Noninvasive Administration. International Journal of Molecular Sciences, 26(17), 8437. https://doi.org/10.3390/ijms26178437








