The Role of ADAM9 and MMP9 in Diabetic Retinopathy: Insights from Ocular Parameters
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Collection and Analysis of Blood Samples
4.3. Ethical Aspects
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAM | A Disintegrin and Metalloprotease |
| DR | Diabetic Retinopathy |
| MMP | Matrix Metalloproteinase |
| IOP | Intraocular pressure |
References
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert Opin. Investig. Drugs 2012, 21, 797–805. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Meng, Y.-A.; Yan, B.; Luo, J. Effect of anti-VEGF treatment on nonperfusion areas in ischemic retinopathy. Int. J. Ophthalmol. 2021, 14, 1647–1652. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Abbas, S.N. Diabetes-induced mitochondrial dysfunction in the retina. Investig. Opthalmol. Vis. Sci. 2003, 44, 5327–5334. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Zhong, Q.; Mohammad, G.; Ho, Y.-S.; Kowluru, R.A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic. Res. 2010, 44, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Mohammad, G.; Zhong, Q.; Kowluru, R.A. Diabetic retinopathy, superoxide damage and antioxidants. Curr. Pharm. Biotechnol. 2011, 12, 352–361. [Google Scholar] [CrossRef]
- Santos, J.M.; Kowluru, R.A. Role of Mitochondria Biogenesis in the Metabolic Memory Associated with the Continued Progression of Diabetic Retinopathy and Its Regulation by Lipoic Acid. Investig. Opthalmol. Vis. Sci. 2011, 52, 8791–8798. [Google Scholar] [CrossRef]
- Xia, H.-Q.; Yang, J.-R.; Zhang, K.-X.; Dong, R.-L.; Yuan, H.; Wang, Y.-C.; Zhou, H.; Li, X.-M. Molecules related to diabetic retinopathy in the vitreous and involved pathways. Int. J. Ophthalmol. 2022, 15, 1180–1189. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Yang, A. The involvement of ACO3 protein in diabetic retinopathy through the PI3k/Akt signaling pathway. Adv. Clin. Exp. Med. 2022, 31, 407–416. [Google Scholar] [CrossRef]
- Pieńczykowska, K.; Bryl, A.; Mrugacz, M. Link Between Metabolic Syndrome, Inflammation, and Eye Diseases. Int. J. Mol. Sci. 2025, 26, 2174. [Google Scholar] [CrossRef]
- Wang, J.; Tsirka, S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 2005, 128 Pt 7, 1622–1633. [Google Scholar] [CrossRef]
- Yang, Y.; Hill, J.W.; Rosenberg, G.A. Multiple roles of metalloproteinases in neurological disorders. Prog. Mol. Biol. Transl. Sci. 2011, 99, 241–263. [Google Scholar]
- Fridman, R.; Toth, M.; Chvyrkova, I.; Meroueh, S.O.; Mobashery, S. Cell surface association of matrix metalloproteinase-9 (gelatinase B). Cancer Metastasis Rev. 2003, 22, 153–166. [Google Scholar] [CrossRef]
- Mishra, M.; Kowluru, R.A. Role of PARP-1 as a novel transcriptional regulator of MMP-9 in diabetic retinopathy. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017, 1863, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2010, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberg, T.G.; Primakoff, P.; Myles, D.G.; White, J.M. ADAM, a novel family of membrane proteins containing A Disintegrin And Metalloprotease domain: Multipotential functions in cell-cell and cell-matrix interactions. J. Cell Biol. 1995, 131, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R. Role of lymphotoxin alpha as a new molecular biomarker in revolutionizing tear diagnostic testing for dry eye disease. Int. J. Ophthalmol. 2023, 16, 1883–1889. [Google Scholar] [CrossRef]
- Masli, S.; Akpek, E.K. Reduced tear thrombospondin-1/matrix metalloproteinase-9 ratio can aid in detecting Sjogren’s syndrome etiology in patients with dry eye. Clin. Transl. Sci. 2022, 15, 1999–2009. [Google Scholar] [CrossRef]
- Haoyuan, M.; Yanshu, L. Regulatory factors and cancer-related physiological effects of ADAM9. Cell Adhes. Migr. 2020, 14, 165–181. [Google Scholar] [CrossRef]
- Cauwe, B.; Van den Steen, P.E.; Opdenakker, G. The Biochemical, Biological, and Pathological Kaleidoscope of Cell Surface Substrates Processed by Matrix Metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Tumour microenvironment—Opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef]
- Drankowska, J.; Kos, M.; Kościuk, A.; Marzęda, P.; Boguszewska-Czubara, A.; Tylus, M.; Święch-Zubilewicz, A. MMP targeting in the battle for vision: Recent developments and future prospects in the treatment of diabetic retinopathy. Life Sci. 2019, 229, 149–156. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian. J. Ophthalmol. 2012, 60, 428–431. [Google Scholar]
- Kour, V.; Swain, J.; Singh, J.; Singh, H.; Kour, H. A Review on Diabetic Retinopathy. Curr. Diabetes Rev. 2024, 20, e201023222418. [Google Scholar] [CrossRef] [PubMed]
- Mounirou, B.A.M.; Adam, N.D.; Yakoura, A.K.; Aminou, M.S.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian J. Endocrinol. Metab. 2022, 26, 111–118. [Google Scholar] [CrossRef]
- Chou, C.W.; Huang, Y.K.; Kuo, T.T.; Liu, J.P.; Sher, Y.P. An Overview of ADAM9: Structure, Activation, and Regulation in Human Diseases. Int. J. Mol. Sci. 2020, 21, 7790. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lu, C.; Feng, T.; Gao, X.; Tu, Y.; Yang, W.; Wang, Y. Circ-ADAM9 Promotes High Glucose-Induced Retinal Pigment Epithelial Cell Injury in DR via Regulating miR-338-3p/CARM1 Axis. J. Ophthalmol. 2022, 2022, 2522249. [Google Scholar] [CrossRef]
- Guaiquil, V.H.; Hewing, N.J.; Chiang, M.F.; Rosenblatt, M.I.; Chan, R.V.P.; Blobel, C.P. A murine model for retinopathy of prematurity identifies endothelial cell proliferation as a potential mechanism for plus disease. Investig. Opthalmol. Vis. Sci. 2013, 54, 5294–5302. [Google Scholar] [CrossRef][Green Version]
- Fabrikantov, O.L.; Lev, I.V.; Agarkov, N.M.; Osmanov, R.E. Prognostic value of matrix metalloproteinases in the development of diabetic retinopathy in the elderly. Adv. Gerontol. 2022, 35, 408–412. [Google Scholar] [PubMed][Green Version]
- Gu, C.; Lhamo, T.; Zou, C.; Zhou, C.; Su, T.; Draga, D.; Luo, D.; Zheng, Z.; Yin, L.; Qiu, Q. Comprehensive analysis of angiogenesis-related genes and pathways in early diabetic retinopathy. BMC Med. Genom. 2020, 13, 142. [Google Scholar] [CrossRef]
- Esparza, J.; Kruse, M.; Lee, J.; Michaud, M.; Madri, J.A. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J. 2004, 18, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J. Cell. Physiol. 2011, 227, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Xu, X.; Xia, X.; Wu, H.; Liu, K.; Zheng, Z.; Zhu, D. MMP9 Is Involved in Glycation End-Products Induced Increase of Retinal Vascular Permeability in Rats and the Therapeutic Effect of Minocycline. Curr. Eye Res. 2008, 33, 977–983. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 30) | NPDR (n = 27) | PDR (n = 32) | p Value | |
|---|---|---|---|---|
| Age (year) | 59.3 ± 1.97 | 59.96 ± 6.02 | 59.84 ± 1.65 | 0.759 a |
| Biomicroscope—right eye | <0.001 b | |||
| Nature | 28 (93.33) | 23 (85.19) | 19 (59.38) | |
| Cataract | 1 (3.33) | 2 (7.41) | 9 (28.13) | |
| Pseudophakia | 1 (3.33) | 2 (7.41) | 4 (12.5) | |
| Biomicroscope—left eye | <0.001 b | |||
| Nature | 29 (96.66) | 25 (92.59) | 19 (59.38) | |
| Cataract | 0 (0) | 0 (0) | 10 (31.25) | |
| Pseudophakia | 1 (3.33) | 2 (7.41) | 3 (9.38) | |
| IOP—right eye (mmHg) | 16.07 ± 2.12 | 16.7 ± 2.45 | 17.28 ± 3.58 | 0.242 a |
| IOP—left eye (mmHg) | 15.67 ± 1.75 | 16.63 ± 2.92 | 18.41 ± 3.97 *† | 0.002 a |
| Visual acuity—right eye | 1 (0.0) | 0.8 (0.6) * | 0.4 (0.3) *† | < 0.001 c |
| Visual acuity—left eye | 1 (0.0) | 0.8 (0.4) * | 0.35 (0.3) *† | < 0.001 c |
| ADAM9 (ng/L) | 1314.28 ± 481.41 | 1096.69 ± 329.54 * | 951.98 ± 280.11 * | 0.001 a |
| MMP9 (ng/L) | 935.04 ± 216.57 | 965.26 ± 161.74 | 811.87 ± 287.78 *† | 0.028 a |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| IOP—right eye | 1.135 | 0.961–1.341 | 0.137 | |||
| IOP—left eye | 1.255 | 1.053–1.495 | 0.011 | 1.237 | 1.021–1.500 | 0.03 |
| ADAM9 | 0.998 | 0.996–0.999 | 0.003 | 0.998 | 0.997–0.999 | 0.007 |
| MMP9 | 0.999 | 0.997–1.001 | 0.322 | |||
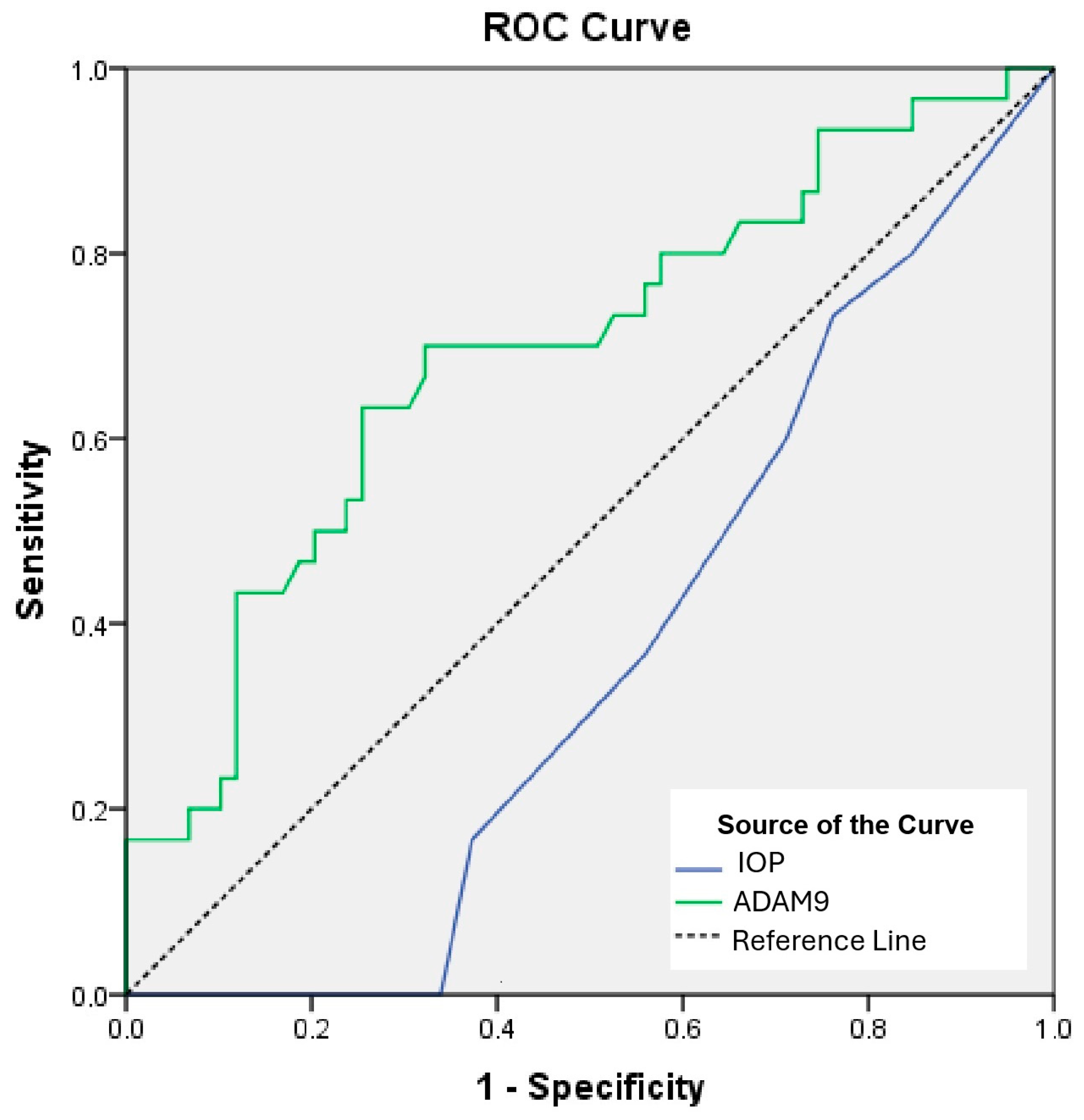
| Risk Factor | AUC | 95% CI | p | Cut-Off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| IOP—left eye | 0.362 | 0.249–0.476 | 0.035 | >18.5 | 33.9 | 100 |
| ADAM9 | 0.693 | 0.573–0.812 | 0.003 | <1194.6 | 63.3 | 74.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, M.A.; Tozcu Yilmaz, D.; Aydin, N.; Tufek, M.; Capraz, M. The Role of ADAM9 and MMP9 in Diabetic Retinopathy: Insights from Ocular Parameters. Int. J. Mol. Sci. 2025, 26, 8436. https://doi.org/10.3390/ijms26178436
Gul MA, Tozcu Yilmaz D, Aydin N, Tufek M, Capraz M. The Role of ADAM9 and MMP9 in Diabetic Retinopathy: Insights from Ocular Parameters. International Journal of Molecular Sciences. 2025; 26(17):8436. https://doi.org/10.3390/ijms26178436
Chicago/Turabian StyleGul, Mehmet Ali, Duygu Tozcu Yilmaz, Nihat Aydin, Melek Tufek, and Mustafa Capraz. 2025. "The Role of ADAM9 and MMP9 in Diabetic Retinopathy: Insights from Ocular Parameters" International Journal of Molecular Sciences 26, no. 17: 8436. https://doi.org/10.3390/ijms26178436
APA StyleGul, M. A., Tozcu Yilmaz, D., Aydin, N., Tufek, M., & Capraz, M. (2025). The Role of ADAM9 and MMP9 in Diabetic Retinopathy: Insights from Ocular Parameters. International Journal of Molecular Sciences, 26(17), 8436. https://doi.org/10.3390/ijms26178436






