Redefining Normal: Cytokine Dysregulation in Long COVID and the Post-Pandemic Healthy Donors
Abstract
1. Introduction
2. Results
2.1. Baseline Cohorts’ Characteristics
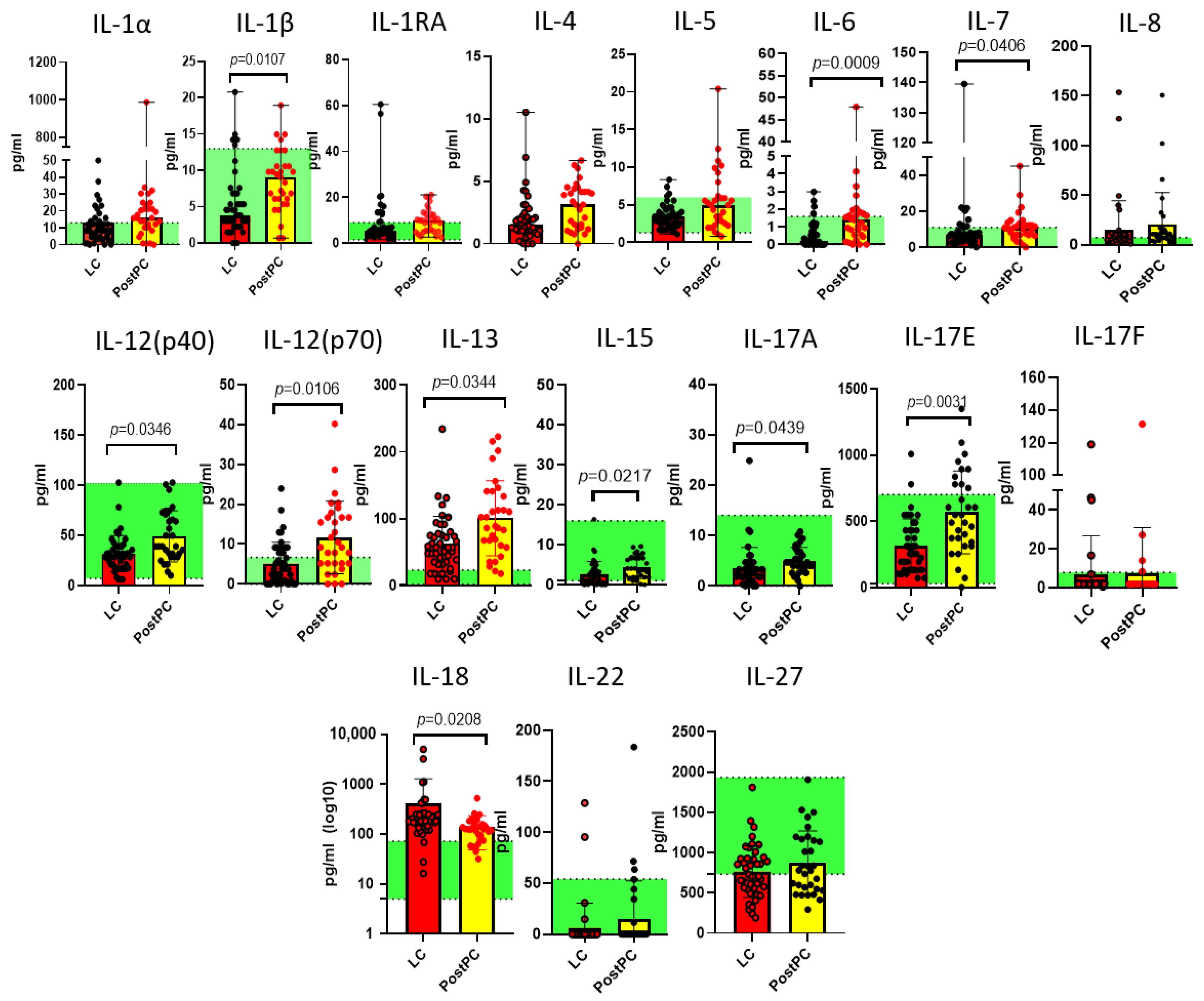
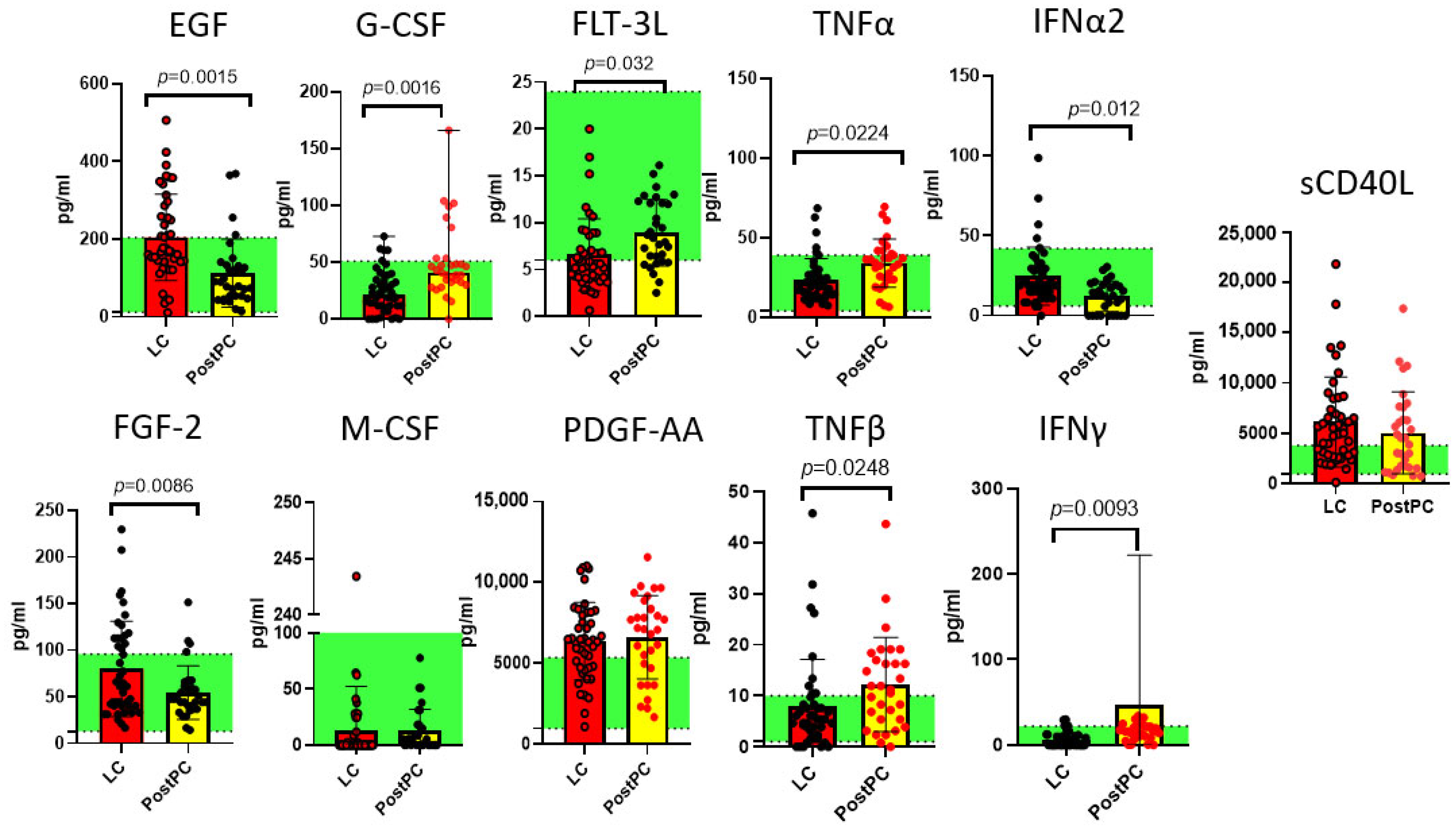
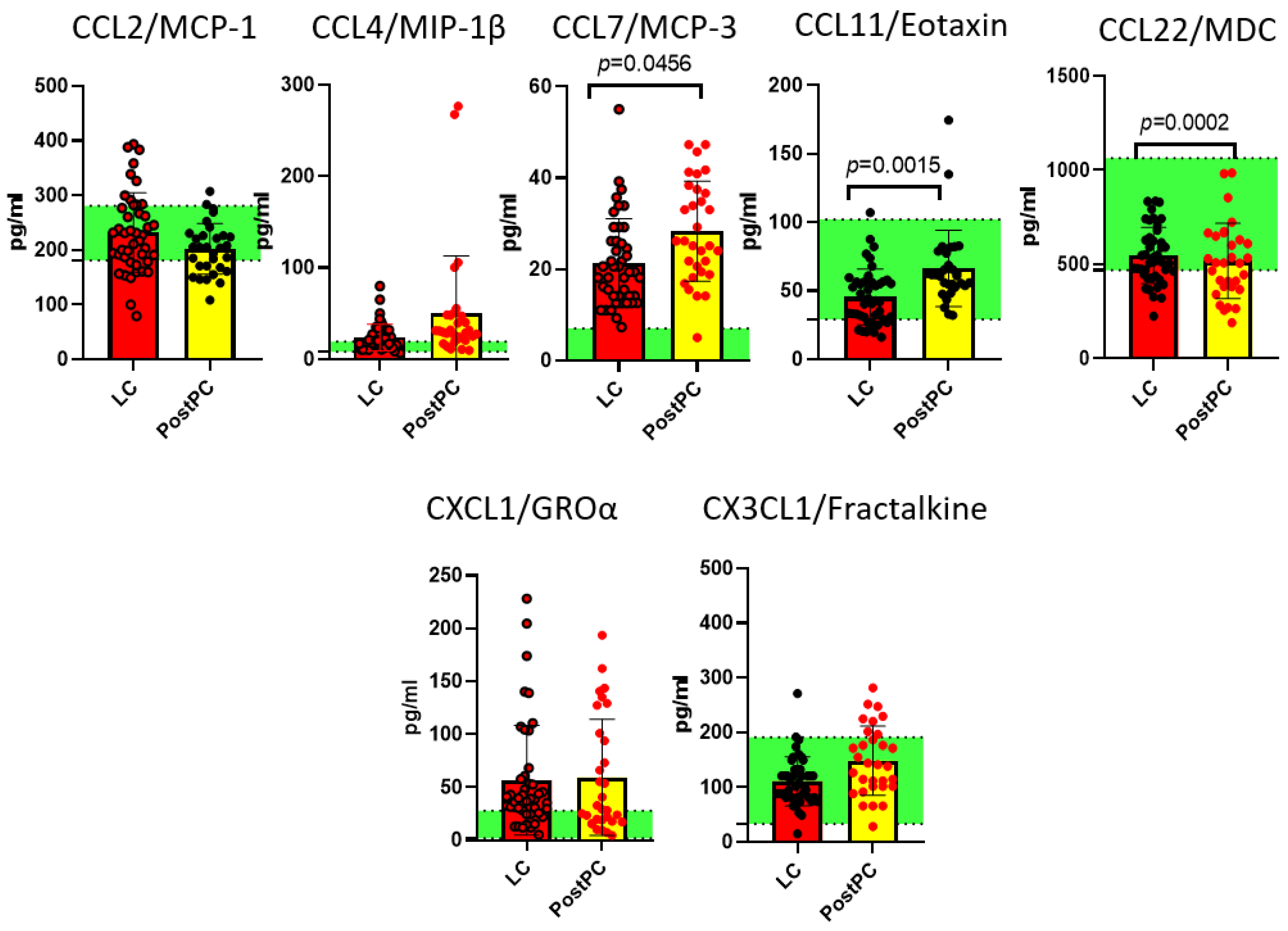
2.2. Measurements of Cytokines in Long COVID and Post- and Pre-Pandemic Donors
2.3. Comparison of Pre-Pandemic Healthy Controls and Post-Pandemic Recovered Individuals
3. Discussion
4. Materials and Methods
4.1. Study Cohorts and Ethical Considerations
- Long COVID (LC) Group (n = 44): Individuals meeting WHO criteria for diagnosis, with persistent psychoneurological symptoms (e.g., cognitive impairment via Montreal Cognitive Assessment; anxiety/depression via Hospital Anxiety and Depression Scale) and somatic complaints (fatigue, dyspnea) lasting >12 weeks post-acute infection. All participants had PCR-confirmed prior SARS-CoV-2 infection.
- Post-Pandemic Recovered Individuals (n = 33): Asymptomatic individuals with confirmed prior mild COVID-19 (PCR-positive) and full recovery >1 year before sampling.
- Pre-Pandemic Naïve Controls (n = 30): Healthy donors sampled in early 2020 (pre-COVID-19 pandemic), confirmed SARS-CoV-2-naïve via nucleocapsid IgG seronegativity.
4.2. Blood Collection and Plasma Isolation
4.3. Cytokine Assessment
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FC | Fold Change |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| IFN | Interferon |
| IL | Interleukin |
| IP-10 | Interferon Gamma-Induced Protein 10 |
| LC | Long COVID |
| MCP | Monocyte Chemoattractant Protein |
| M-CSF | Macrophage Colony-Stimulating Factor |
| MDC | Macrophage-Derived Chemokine |
| MIP | Macrophage Inflammatory Protein |
| PDGF | Platelet-Derived Growth Factor |
| PostPC | Post-Pandemic Recovered Individuals |
| PrePC | Pre-Pandemic Controls |
| TGF | Transforming Growth Factor |
| Th | T Helper |
| TNF | Tumor Necrosis Factor |
| VEGF | Vascular Endothelial Growth Factor |
References
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 31 July 2025).
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID After Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long COVID in People Infected with Severe Acute Respiratory Syndrome Coronavirus 2 After 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac464. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological Dysfunction Persists for 8 Months Following Initial Mild-to-Moderate SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Savchenko, A.A.; Tikhonova, E.; Kudryavtsev, I.; Kudlay, D.; Korsunsky, I.; Beleniuk, V.; Borisov, A. TREC/KREC Levels and T and B Lymphocyte Subpopulations in COVID-19 Patients at Different Stages of the Disease. Viruses 2022, 14, 646. [Google Scholar] [CrossRef]
- Cabaro, S.; D’Esposito, V.; Di Matola, T.; Sale, S.; Cennamo, M.; Terracciano, D.; Parisi, V.; Oriente, F.; Portella, G.; Beguinot, F.; et al. Cytokine Signature and COVID-19 Prediction Models in the Two Waves of Pandemics. Sci. Rep. 2021, 11, 20793. [Google Scholar] [CrossRef]
- Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Korobova, Z.R.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; Vorobyev, E.A.; Vorobieva, S.V.; et al. Predictive Value of Specific Cytokines for Lethal COVID-19 Outcome. Russ. J. Infect. Immun. 2022, 12, 859–868. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Corrigendum: Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front. Neurol. 2019, 10, 316. [Google Scholar] [CrossRef]
- Yildiz Gulhan, P.; Eroz, R.; Ozturk, C.E.; Yekenkurul, D.; Altinsoy, H.B.; Balbay, E.G.; Ercelik, M.; Davran, F.; Yildiz, S. Determination of Both the Expression and Serum Levels of Epidermal Growth Factor and Transforming Growth Factor β1 Genes in COVID-19. Sci. Rep. 2025, 15, 9771. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Camporesi, A.; Di Sante, G.; Sali, M.; Boza, M.d.C.P.; Morello, R.; Valentini, P.; Raffaelli, F.; Rodriguez, L.; Gonzalez, L.; et al. Cytokine Profile in Children Following SARS-CoV-2 Infection: Preliminary Findings. Pediatr. Infect. Dis. J. 2025, 44, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Korobova, Z.R.; Stanevich, O.V.; Lebedeva, A.A.; Vorobyov, E.A.; Vorobyova, S.V.; Kulikov, A.N.; Lioznov, D.A.; et al. Plasma Cytokines in Patients with COVID-19 During Acute Phase of the Disease and Following Complete Recovery. Med. Immunol. 2021, 23, 311–326. [Google Scholar] [CrossRef]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Immunotherapy for Cancer: Harnessing the T Cell Response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Haidar, J.H.; Bazarbachi, A.; Mahfouz, R.; Haidar, H.A.; Jaafar, H.; Daher, R. Serum Flt3 Ligand Variation as a Predictive Indicator of Hematopoietic Stem Cell Mobilization. J. Hematother. Stem Cell Res. 2002, 11, 533–538. [Google Scholar] [CrossRef]
- Donlan, A.N.; Sutherland, T.E.; Marie, C.; Preissner, S.; Bradley, B.T.; Carpenter, R.M.; Sturek, J.M.; Ma, J.Z.; Moreau, G.B.; Donowitz, J.R.; et al. IL-13 Is a Driver of COVID-19 Severity. JCI Insight 2021, 6, e150107. [Google Scholar] [CrossRef]
- Tanabe, T.; Fujimoto, K.; Yasuo, M.; Tsushima, K.; Yoshida, K.; Ise, H.; Yamaya, M. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. Clin. Exp. Allergy 2008, 38, 122–134. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saftli, R.; Ochieng, P.J.; Aborode, A.T.; Suzuki, H.; Oyelami, F.A. Interleukin-18 Cytokine in Immunity, Inflammation, and Autoimmunity: Biological Role in Induction, Regulation, and Treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Andrejčinová, I.; Blažková, G.; Papatheodorou, I.; Bendíčková, K.; Bosáková, V.; Skotáková, M.; Panovský, R.; Opatřil, L.; Vymazal, O.; Kovačovicová, P.; et al. Persisting IL-18 Levels After COVID-19 Correlate with Markers of Cardiovascular Inflammation Reflecting Potential Risk of CVDs Development. Heliyon 2024, 10, e25938. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Schlickeiser, S.; Feldmann, S.; Ober, V.; Grüner, E.; Pleimelding, C.; Gilberg, L.; Brand, I.; Weigl, N.; Ahmed, M.I.M.; et al. Persistent Immune Abnormalities Discriminate Post-COVID Syndrome from Convalescence. Infection 2024, 52, 1087–1097. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Adish, Z.; Chernykh, E.I.; Kaschenko, V.A.; et al. Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int. J. Mol. Sci. 2022, 23, 14146. [Google Scholar] [CrossRef]
- Hsu, R.-J.; Yu, W.-C.; Peng, G.-R.; Ye, C.-H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.-C.; Yu, S.-H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Low, R.J.; Akrami, A. A Review of Cytokine-Based Pathophysiology of Long COVID Symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef] [PubMed]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Chernykh, E.I.; Kashchenko, V.A.; Ratnikov, V.A.; Gorelov, V.P.; et al. A Comparative Study of the Plasma Chemokine Profile in COVID-19 Patients Infected with Different SARS-CoV-2 Variants. Int. J. Mol. Sci. 2022, 23, 9058. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, S.; Dong, L.; Qiao, S.; Wang, X.; Yu, D.; Gao, P.; Hou, Y.; Quan, S.; Li, Y.; et al. Long-Term Effects of Omicron BA.2 Breakthrough Infection on Immunity-Metabolism Balance: A 6-Month Prospective Study. Nat. Commun. 2024, 15, 2444. [Google Scholar] [CrossRef]
- Espíndola, O.; Resende, P.C.; Guaraldo, L.; Calvet, G.A.; Fuller, T.L.; Penetra, S.L.S.; Santos, H.F.P.; Pina-Costa, A.; da Silva, M.F.B.; Moraes, I.C.V.; et al. Long COVID-19 Syndrome Associated with Omicron XBB.1.5 Infection: A Case Report. Mem. Inst. Oswaldo Cruz 2023, 118, e230069. [Google Scholar] [CrossRef]
- Ganesh, R.; Ghosh, A.K.; Nyman, M.A.; Croghan, I.T.; Grach, S.L.; Anstine, C.V.; Salonen, B.R.; Hurt, R.T. PROMIS Scales for Assessment of Persistent Post-COVID Symptoms: A Cross Sectional Study. J. Prim. Care Community Health 2021, 12, 21501327211030413. [Google Scholar] [CrossRef]
- Murray, H.C.; Muleme, M.; Cooper, D.; McNamara, B.J.; Hussain, M.A.; Bartolo, C.; O’BRien, D.P.; Athan, E. Prevalence, Risk Factors, and Outcomes of Secondary Infections Among Hospitalized Patients with COVID-19 or Post-COVID-19 Conditions in Victoria, 2020–2023. Int. J. Infect. Dis. 2024, 145, 107078. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID Manifests with T Cell Dysregulation, Inflammation and an Uncoordinated Adaptive Immune Response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Kratzer, B.; Gattinger, P.; Trapin, D.; Ettel, P.; Körmöczi, U.; Rottal, A.; Stieger, R.B.; Sehgal, A.N.A.; Feichter, M.; Borochova, K.; et al. Differential Decline of SARS-CoV-2-Specific Antibody Levels, Innate and Adaptive Immune Cells, and Shift of Th1/Inflammatory to Th2 Serum Cytokine Levels Long After First COVID-19. Allergy 2024, 79, 2482–2501. [Google Scholar] [CrossRef]
- Sievers, B.L.; Cheng, M.T.K.; Csiba, K.; Meng, B.; Gupta, R.K. SARS-CoV-2 and Innate Immunity: The Good, the Bad, and the “Goldilocks”. Cell. Mol. Immunol. 2024, 21, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Lu, P.; Monteiro, V.S.; Tabachnikova, A.; Wang, K.; Hooper, W.B.; Bastos, V.; Greene, K.; Sawano, M.; Guirgis, C.; et al. Immunological and Antigenic Signatures Associated with Chronic Illnesses After COVID-19 Vaccination. medRxiv 2025. medRxiv:2025.02.18.25322379. [Google Scholar] [CrossRef]
- Maciel, A.B.S.; Pinto, A.S.; Maia Silva, B.; Goulart, C.L.; Silva, L.F.A.; Chaves, A.S.; Mouta, G.S.; Sato, C.M.S.; Valente, J.; Mwangi, V.I.; et al. Inflammatory Discoveries Two Years After Acute Severe COVID-19: A Longitudinal Biomarker Profile Assessment in Long COVID Individuals in the Brazilian Amazon. Front. Immunol. 2024, 15, 1520193. [Google Scholar] [CrossRef] [PubMed]
- Skevaki, C.; Moschopoulos, C.D.; Fragkou, P.C.; Grote, K.; Schieffer, E.; Schieffer, B. Long COVID: Pathophysiology, Current Concepts, and Future Directions. J. Allergy Clin. Immunol. 2025, 155, 1059–1070. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Arsentieva, N.A.; Totolian, A.A. Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19. Int. J. Mol. Sci. 2023, 24, 13083. [Google Scholar] [CrossRef]

| Group | Long COVID | Post-Pandemic Recovered Individuals | Pre-Pandemic Naïve Controls |
|---|---|---|---|
| n | 44 | 33 | 30 |
| Age (Me, Q25–Q75) | 38, 29–48 | 40, 35–48 | 44, 38–52 |
| Gender distribution (% female/% male) | 72.7/27.3 | 60.1/0.39 | 80/20 |
| Anti-SARS-CoV-2 IgG | + (Positive) | + (Positive) | − (Negative) |
| COVID-19 severity | 84.1% mild, 15.9% moderate/severe | 100% Mild | n/a |
| Vaccination status | 40.9% vaccinated with Sputnik V | 100% vaccinated with Sputnik V | n/a |
| Health status and existing comorbidities | 100% hypertension, 56.8% hair loss, 52.3% poor appetite, 45.5% nonspecific abdominal pain. 75% had comorbidities: gastrointestinal (31.8%), renal/urinary (18.2%), cardiovascular disorders (6.8%); 22.7% BMI > 25 kg/m2. | No residual symptoms or active comorbidities reported. | No residual symptoms or active comorbidities reported. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobova, Z.R.; Arsentieva, N.A.; Lyubimova, N.E.; Totolian, A.A. Redefining Normal: Cytokine Dysregulation in Long COVID and the Post-Pandemic Healthy Donors. Int. J. Mol. Sci. 2025, 26, 8432. https://doi.org/10.3390/ijms26178432
Korobova ZR, Arsentieva NA, Lyubimova NE, Totolian AA. Redefining Normal: Cytokine Dysregulation in Long COVID and the Post-Pandemic Healthy Donors. International Journal of Molecular Sciences. 2025; 26(17):8432. https://doi.org/10.3390/ijms26178432
Chicago/Turabian StyleKorobova, Zoia R., Natalia A. Arsentieva, Natalia E. Lyubimova, and Areg A. Totolian. 2025. "Redefining Normal: Cytokine Dysregulation in Long COVID and the Post-Pandemic Healthy Donors" International Journal of Molecular Sciences 26, no. 17: 8432. https://doi.org/10.3390/ijms26178432
APA StyleKorobova, Z. R., Arsentieva, N. A., Lyubimova, N. E., & Totolian, A. A. (2025). Redefining Normal: Cytokine Dysregulation in Long COVID and the Post-Pandemic Healthy Donors. International Journal of Molecular Sciences, 26(17), 8432. https://doi.org/10.3390/ijms26178432







