Natural Polysaccharide-Based Nanoparticles Enhance Intracellular Delivery and Cytotoxicity of Antrodia camphorata in Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of AC-Loaded NPs (AC-NPs)
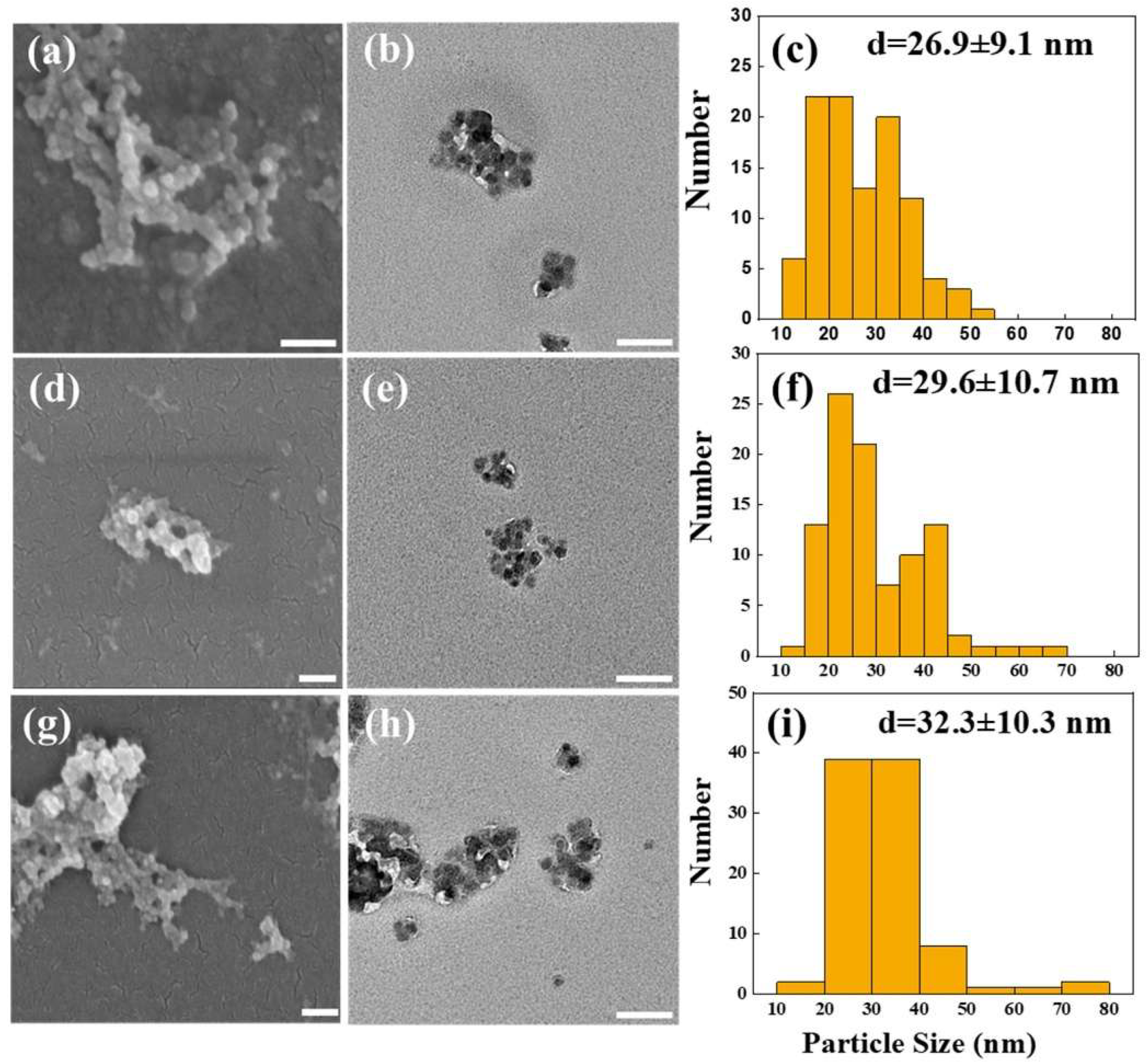
2.1.1. Surface Morphology and Particle Size of Synthesized AC-NPs
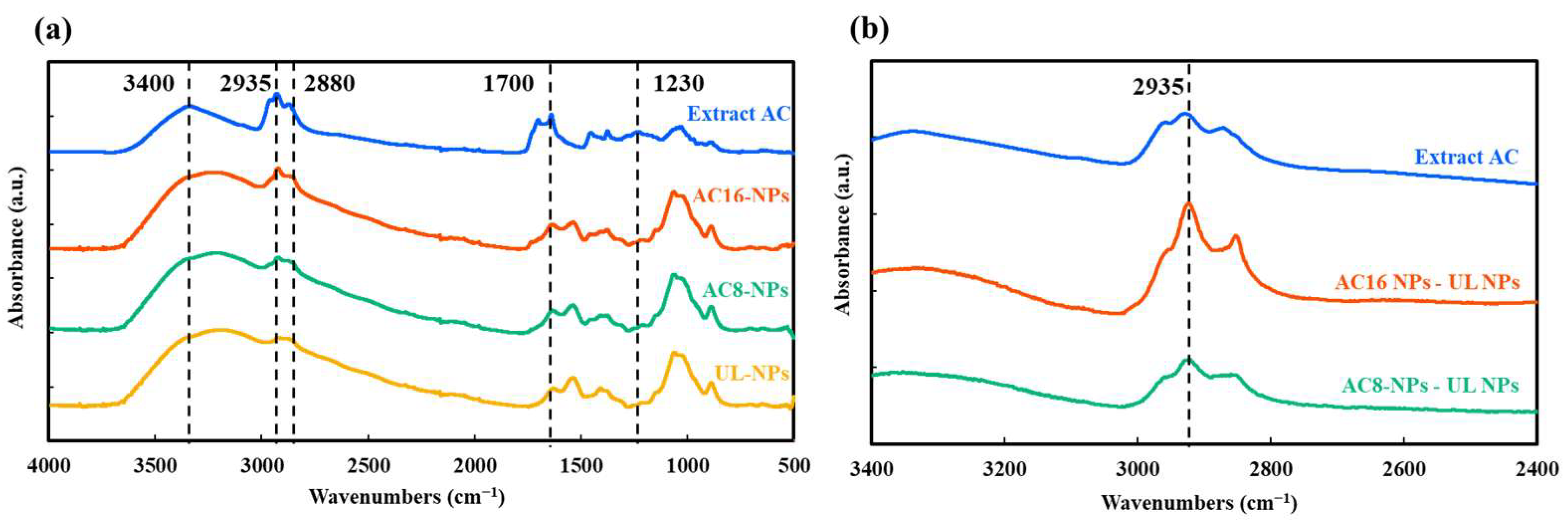
2.1.2. Fourier-Transform Infrared (FTIR) Analysis
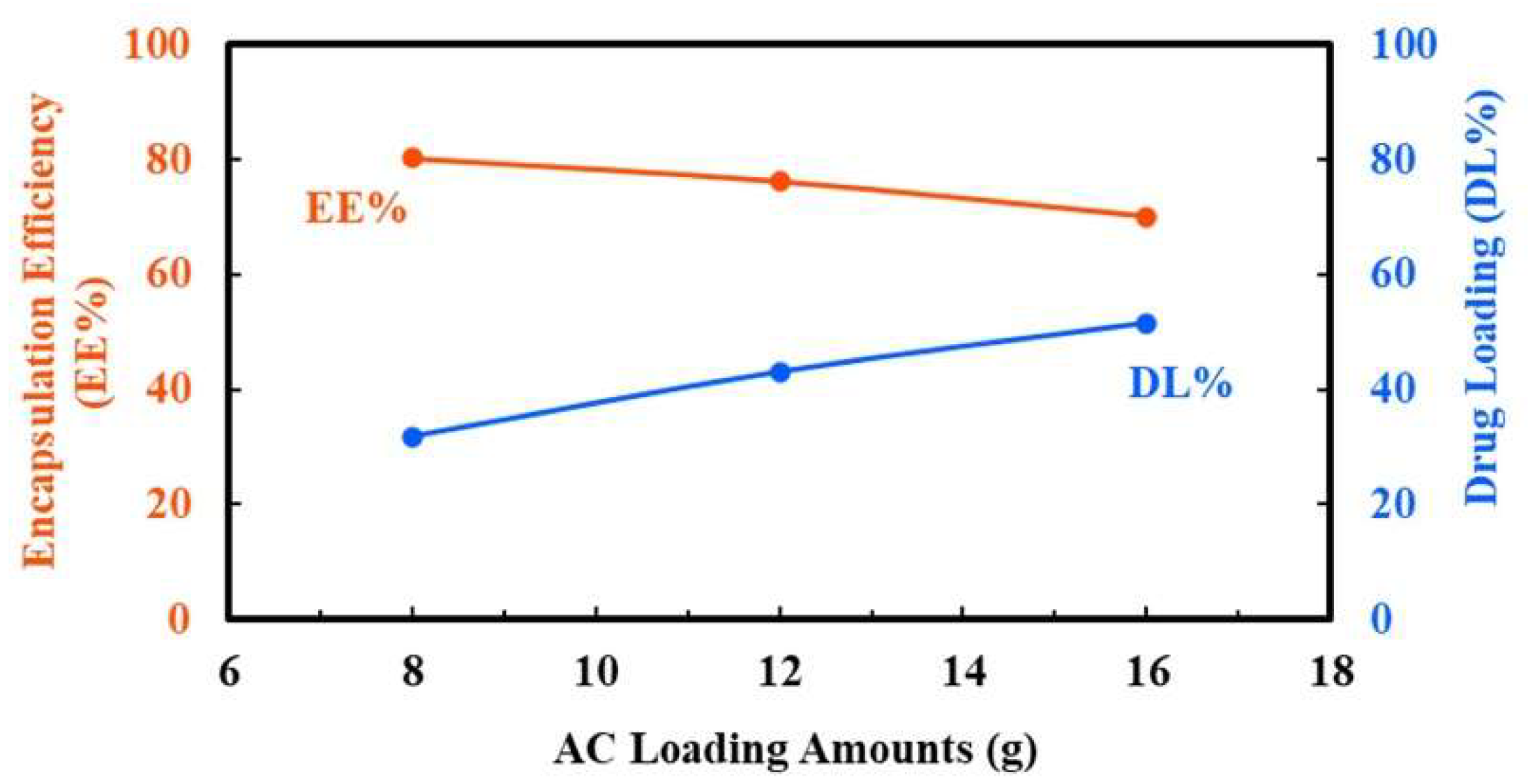
2.1.3. Determination of Drug Loading (DL%) and Encapsulation Efficiency (EE%)
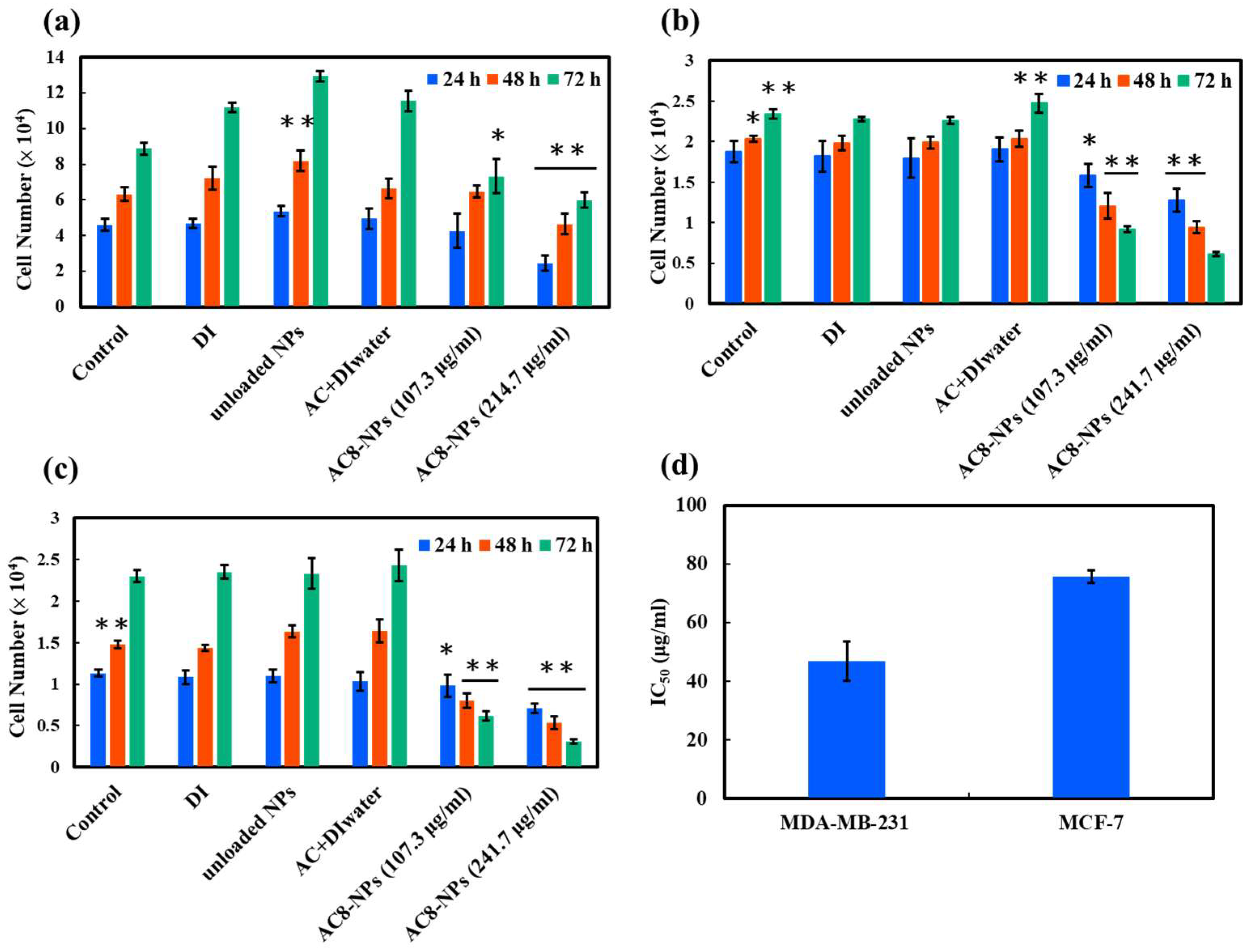
2.2. In Vitro Cytotoxicity Analysis
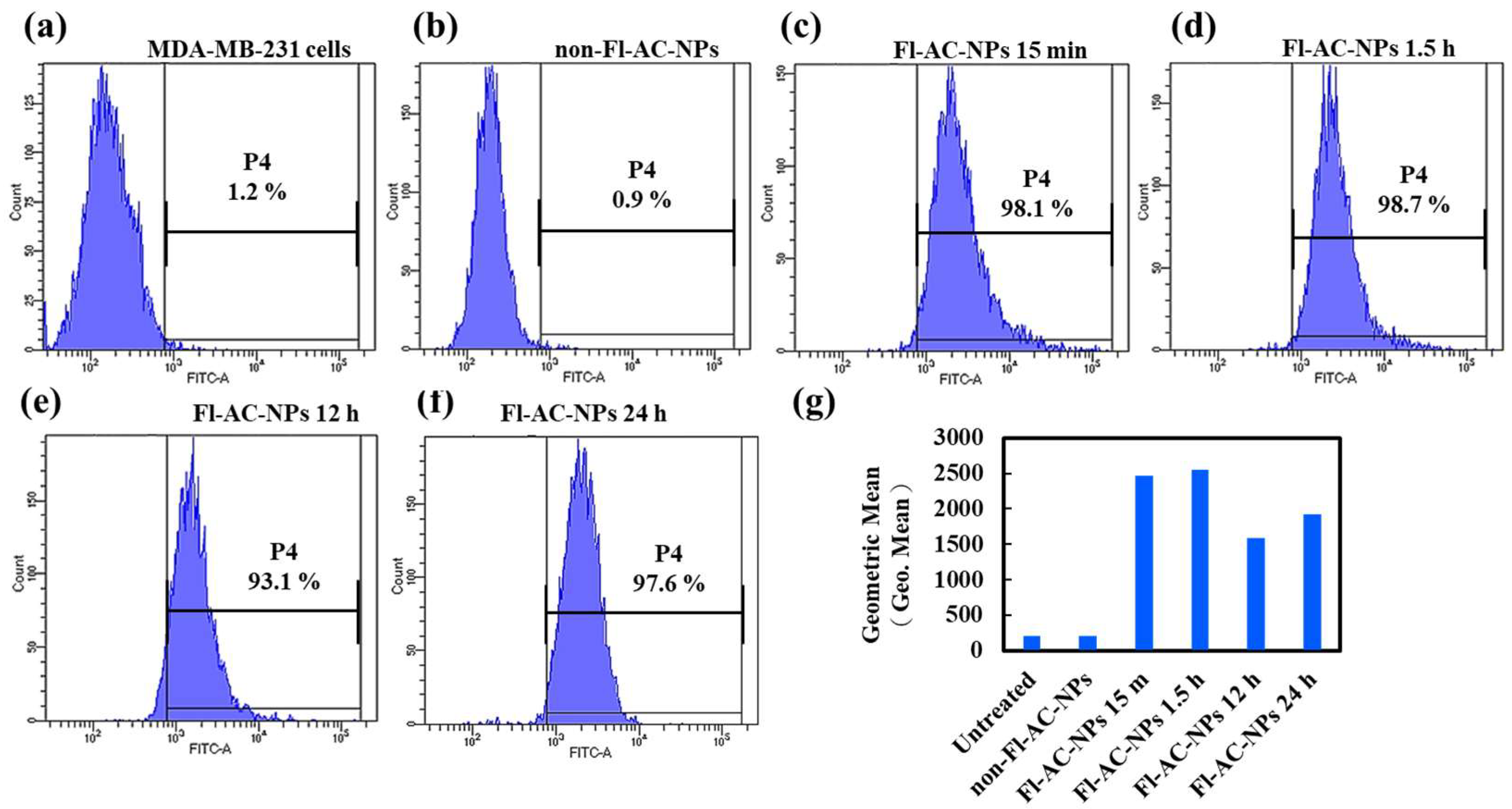
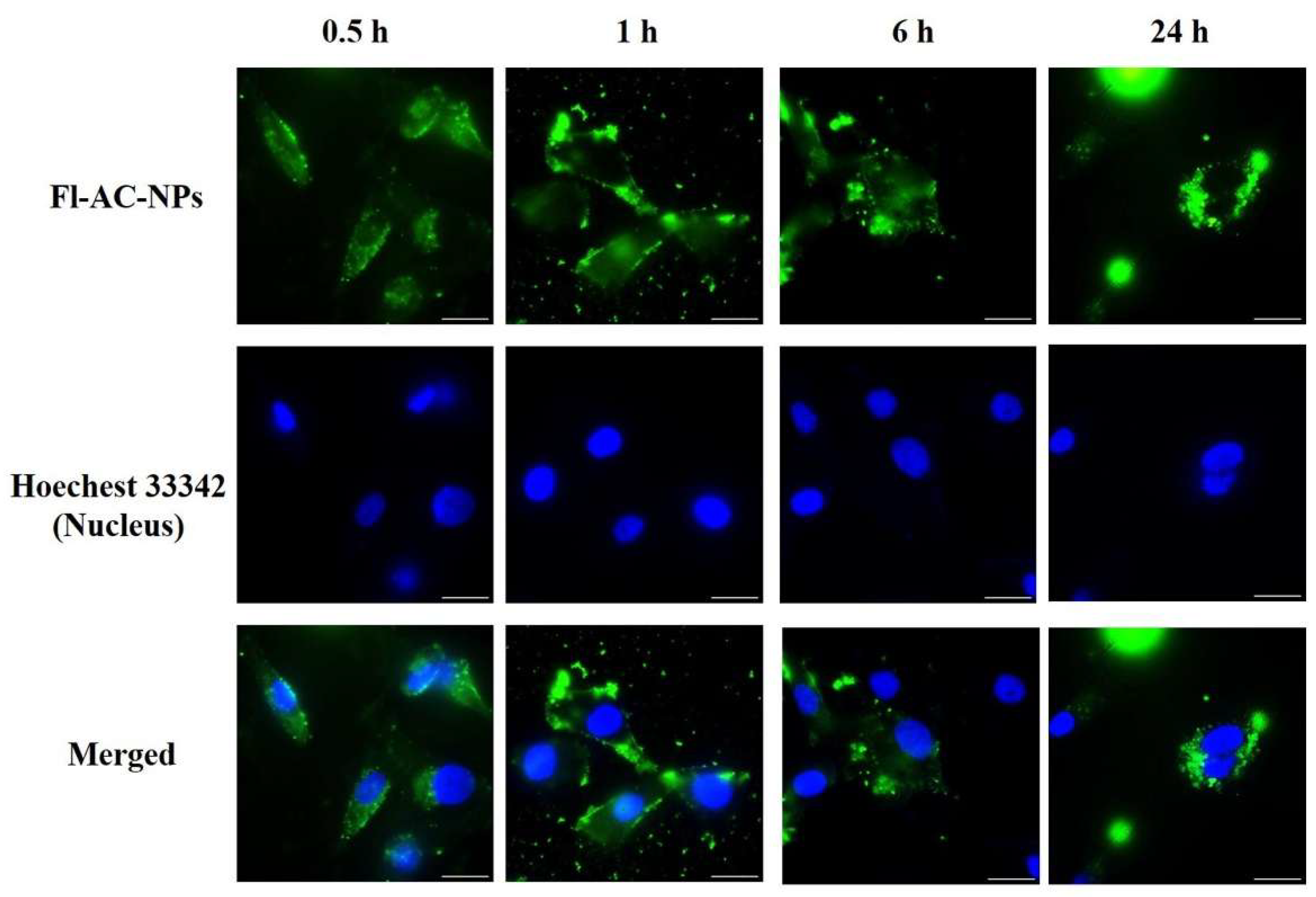
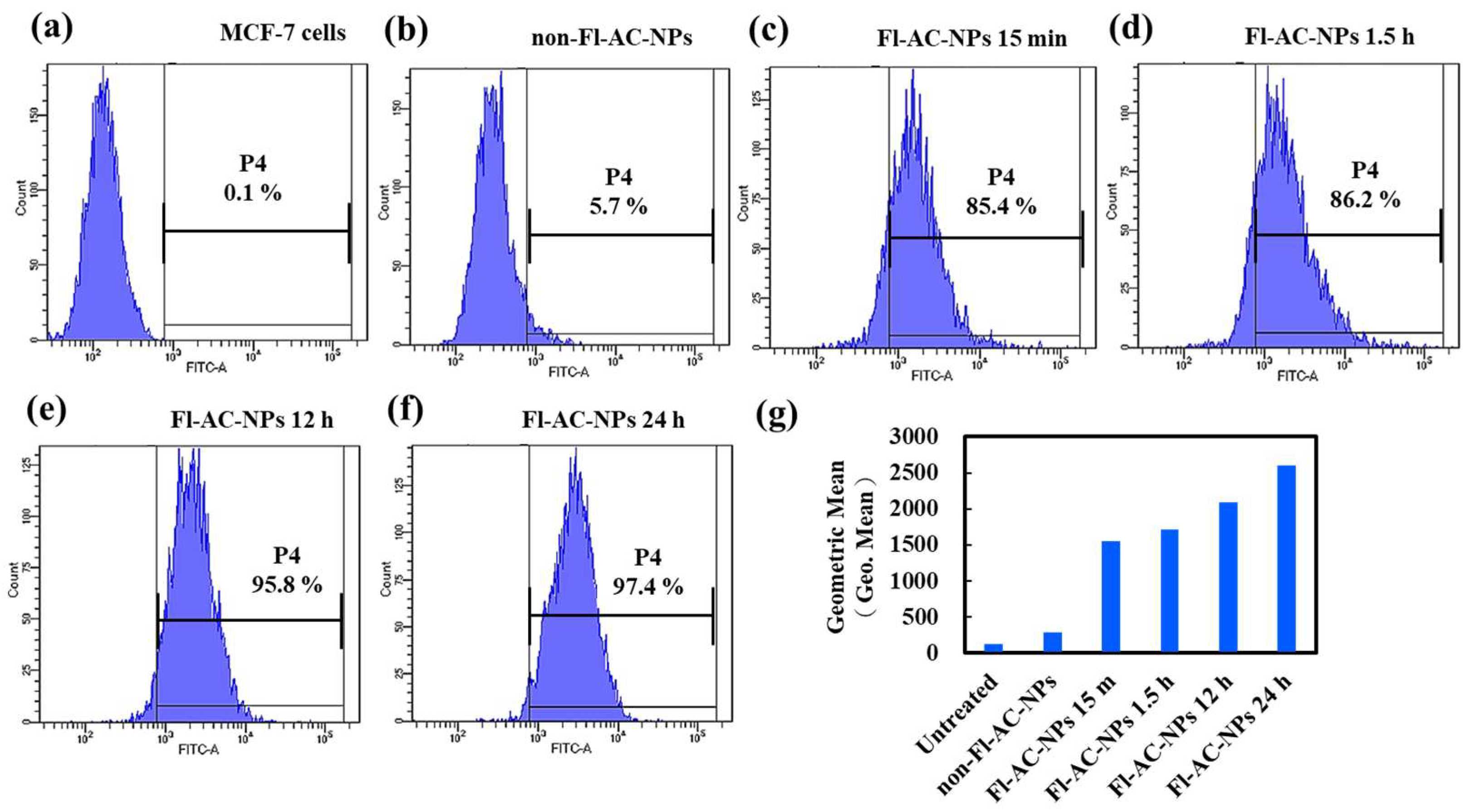
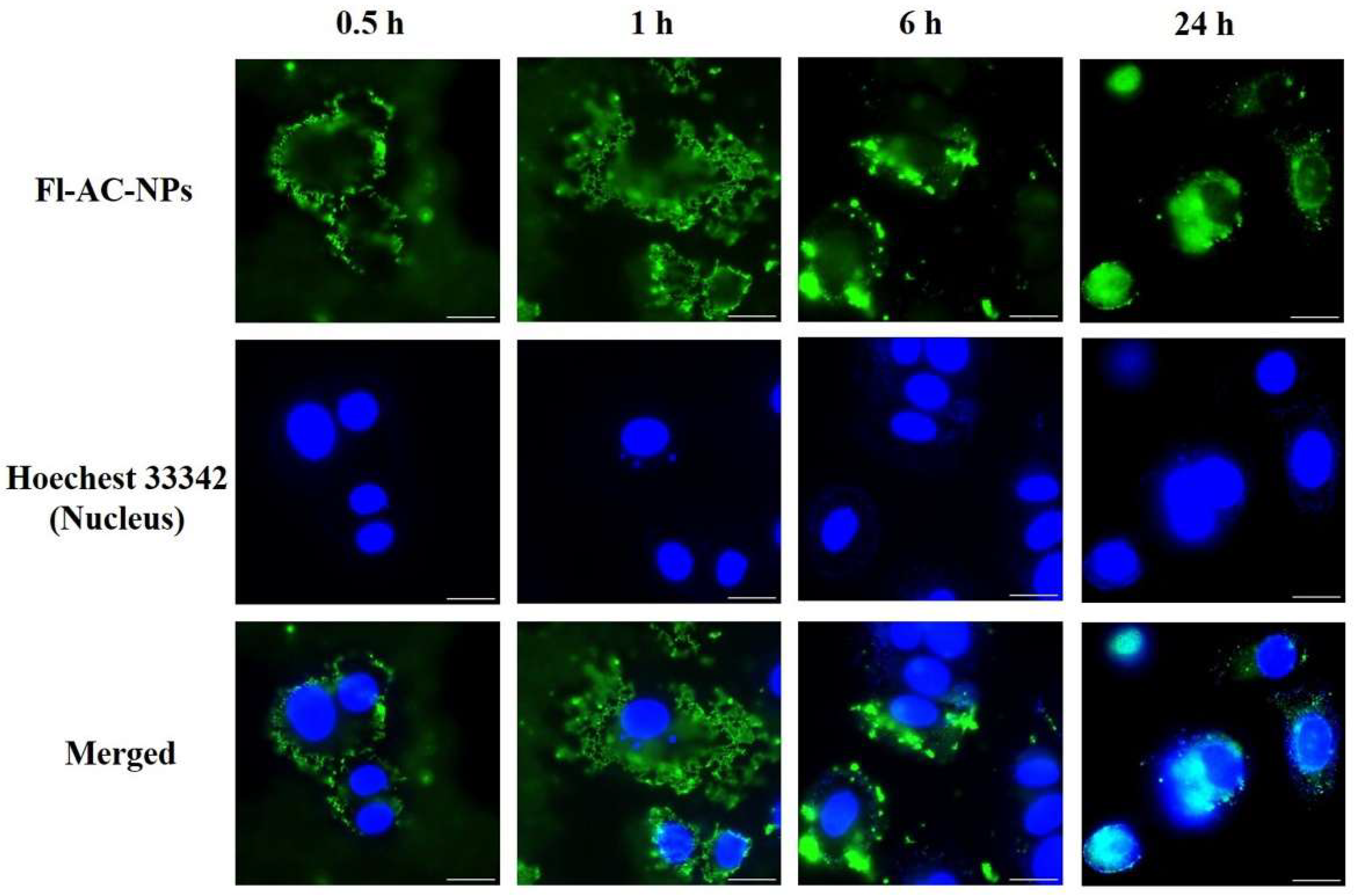
2.3. Cellular Internalization of AC-NPs
3. Discussion
3.1. Design of AC-Loaded NPs
3.2. Characterization of AC-Loaded NPs
3.3. Formulation Optimization
3.4. Cytotoxicity
3.5. In Vitro Cellular Uptake Analysis
4. Materials and Methods
4.1. Materials
4.2. Characterization of Prepared NPs
4.3. EE% and DL% of Prepared NPs
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Preparation of Fluorescent AC-NP Conjugates
4.7. Visualization of AC-NP Cellular Uptake Using Confocal Microscopy
4.8. Quantification of AC-NP Internalization by Flow Cytometry
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Antrodia camphorata |
| DL | Drug loading |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EE | Encapsulation efficiency |
| ER | Estrogen receptor |
| FBS | Fetal bovine serum |
| FTIR | Fourier-transform infrared |
| HA | Hyaluronic acid |
| NPs | Nanoparticles |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TNBC | Triple-negative breast cancer |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, J.; Wang, G.; Wang, L.; Liu, J.; Ouyang, L.; Liu, B. Small-molecule drug discovery in triple negative breast cancer: Current situation and future directions. J. Med. Chem. 2021, 64, 2382–2418. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Moon, H.R.; Ospina-Muñoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS ONE 2020, 15, e0234012. [Google Scholar] [CrossRef] [PubMed]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic acid-coated chitosan nanoparticles as targeted-carrier of tamoxifen against MCF7 and TMX-resistant MCF7 cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lu, K.H.; Ho, C.T.; Sheen, L.Y. Protective effects of Antrodia cinnamomea against liver injury. J. Tradit. Complement. Med. 2012, 2, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chen, S.C.; Chen, H.C.; Liao, J.W.; Yang, H.L. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 2680–2688. [Google Scholar] [CrossRef]
- Peng, C.C.; Chen, K.C.; Peng, R.Y.; Su, C.H.; Hsieh-Li, H.M. Human urinary bladder cancer T24 cells are susceptible to the Antrodia camphorata extracts. Cancer Lett. 2006, 243, 109–119. [Google Scholar] [CrossRef]
- Yang, P.Y.; Hu, D.N.; Liu, F.S. Cytotoxic effect and induction of apoptosis in human cervical cancer cells by Antrodia camphorata. Am. J. Chin. Med. 2013, 41, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, Y.C.; Kuo, P.L.; Ng, L.T.; Kuo, Y.H.; Lin, C.C. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005, 221, 77–89, Erratum in Cancer Lett. 2014, 349, 153. [Google Scholar] [CrossRef]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for delivery of pentacyclic triterpenoids in anticancer therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, L.; Ning, E.; Fan, H.; Liang, J.; Lai, X.; Zhang, L.; Gao, Y.; Li, Y.; et al. Forsythia suspensa leaves triterpenoids induce breast cancer cell apoptosis via the mitochondrial pathway. Food Sci. Nutr. 2025, 13, e70664. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Gupta, P.; Tripathi, N.; Chakrabarty, S.; Verma, A.; Kumari, S.; Gautam, V.; Ravikanth, G.; Jain, S.K. New ring-A modified cycloartane triterpenoids from Dysoxylum malabaricum bark: Isolation, structure elucidation and their cytotoxicity. Steroids 2024, 205, 109390. [Google Scholar] [CrossRef]
- Teng, W.; Zhou, Z.; Cao, J.; Guo, Q. Recent advances of natural pentacyclic triterpenoids as bioactive delivery system for synergetic biological applications. Foods 2024, 13, 2226. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- da Silva, M.C.; da Silva, H.N.; Alves Leal Cruz, R.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-acetyl-D-glucosamine-loaded chitosan filaments biodegradable and biocompatible for use as absorbable surgical suture materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Aminu, N.; Bello, I.; Umar, N.M.; Tanko, N.; Aminu, A.; Audu, M.M. The influence of nanoparticulate drug delivery systems in drug therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101961. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharmacol. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Kutty, R.V.; Feng, S.S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171. [Google Scholar] [CrossRef]
- Tran, P.; Nguyen, T.N.; Lee, Y.; Tran, P.N.; Park, J.S. Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells. Korean J. Physiol. Pharmacol. 2021, 25, 479–488. [Google Scholar] [CrossRef]
- Yin, Y.; Dang, Q.; Liu, C.; Yan, J.; Cha, D.; Yu, Z.; Cao, Y.; Wang, Y.; Fan, B. Itaconic acid grafted carboxymethyl chitosan and its nanoparticles: Preparation, characterization and evaluation. Int. J. Biol. Macromol. 2017, 102, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Wu, H.; Guo, T.; Nan, J.; Yang, L.; Liao, G.; Park, H.J.; Li, J. Hyaluronic-acid-coated chitosan nanoparticles for insulin oral delivery: Fabrication, characterization, and hypoglycemic ability. Macromol. Biosci. 2022, 22, e2100493. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Rades, T.; Chaiyana, W. Chitosan alginate nanoparticles of protein hydrolysate from Acheta domesticus with enhanced stability for skin delivery. Pharmaceutics 2024, 16, 724. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Jiang, K.; Liu, Y.; Yang, L.; Park, H.J. Alginate calcium microbeads containing chitosan nanoparticles for controlled insulin release. Appl. Biochem. Biotechnol. 2021, 193, 463–478. [Google Scholar] [CrossRef]
- Krishnan, V.; Peng, K.; Sarode, A.; Prakash, S.; Zhao, Z.; Filippov, S.K.; Todorova, K.; Sell, B.R.; Lujano, O.; Bakre, S.; et al. Hyaluronic acid conjugates for topical treatment of skin cancer lesions. Sci. Adv. 2021, 7, eabe6627. [Google Scholar] [CrossRef]
- Tang, H.; Nie, W.; Xiao, J.; Zha, Z.; Chen, Q.; Yin, H. Structural characterization and anti-inflammatory effect in hepatocytes of a galactoglucan from Antrodia camphorata mycelium. RSC Adv. 2019, 9, 7664–7672. [Google Scholar] [CrossRef]
- Chiu, C.H.; Peng, C.C.; Ker, Y.B.; Chen, C.C.; Lee, A.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Physicochemical characteristics and anti-inflammatory activities of Antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia. Molecules 2013, 19, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-L.; Chang, J.-S.; Chang, K.L.B. Antiproliferative effect of Antrodia camphorata polysaccharides encapsulated in chitosan–silica nanoparticles strongly depends on the metabolic activity type of the cell line. J. Nanopart. Res. 2013, 15, 1945. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in research of chitosan chemical modification technologies and their applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sotiropoulos, K. Current advances of polysaccharide-based nanogels and microgels in food and biomedical sciences. Polymers 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Ngawhirunpat, T.; Wonglertnirant, N.; Opanasopit, P.; Ruktanonchai, U.; Yoksan, R.; Wasanasuk, K.; Chirachanchai, S. Incorporation methods for cholic acid chitosan-g-mPEG self-assembly micellar system containing camptothecin. Colloids Surf. B Biointerfaces 2009, 74, 253–259. [Google Scholar] [CrossRef]
- Poshina, D.N.; Rakshina, A.D.; Skorik, Y.A. Hydrophobic chitosan derivatives for gene and drug delivery in cancer therapies. Polysaccharides 2025, 6, 11. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Q.; Riviere, J.E.; Lin, Z. Pharmacokinetics and tumor delivery of nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 83, 104404. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Yang, B.; Campbell, A.; Booth, R.; Booth, J.; Belton, P.; Qi, S. Using a systematic and quantitative approach to generate new insights into drug loading of PLGA nanoparticles using nanoprecipitation. Nanoscale Adv. 2024, 6, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Febrina, E.; Nurhasanah, S.; Gozali, D.; Elamin, K.M.; Wathoni, N. Drug loading in chitosan-based nanoparticles. Pharmaceutics 2024, 16, 1043. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of high-drug-loading nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chen, S.C.; Tsai, P.C.; Chen, C.S.; Lu, F.J.; Chang, N.W.; Yang, H.L. Inhibition of cyclooxygenase-2 and induction of apoptosis in estrogen-nonresponsive breast cancer cells by Antrodia camphorata. Food Chem. Toxicol. 2007, 45, 1107–1115. [Google Scholar] [CrossRef]
- Chang, C.T.; Korivi, M.; Huang, H.C.; Thiyagarajan, V.; Lin, K.Y.; Huang, P.J.; Liu, J.Y.; Hseu, Y.C.; Yang, H.L. Inhibition of ROS production, autophagy or apoptosis signaling reversed the anticancer properties of Antrodia salmonea in triple-negative breast cancer (MDA-MB-231) cells. Food Chem. Toxicol. 2017, 103, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Jiang, Y.; Jiang, Y.; Mao, R. Angiogenesis and immune microenvironment in triple-negative breast cancer: Targeted therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167880. [Google Scholar] [CrossRef]
- Lee, C.C.; Yang, H.L.; Way, T.D.; Kumar, K.J.S.; Juan, Y.C.; Cho, H.J.; Lin, K.Y.; Hsu, L.S.; Chen, S.C.; Hseu, Y.C. Inhibition of cell growth and induction of apoptosis by Antrodia camphorata in HER-2/neu-overexpressing breast cancer cells through the induction of ROS, depletion of HER-2/neu, and disruption of the PI3K/Akt signaling pathway. Evid. Based Complement. Alternat. Med. 2012, 2012, 702857. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Rao, Y.K.; Whang-Peng, J.; Huang, C.Y.F.; Shyue, S.K.; Hsu, S.L.; Tzeng, Y.M. Antcin B and its ester derivative from Antrodia camphorata induce apoptosis in hepatocellular carcinoma cells involves enhancing oxidative stress coincident with activation of intrinsic and extrinsic apoptotic pathway. J. Agric. Food Chem. 2011, 59, 10943–10954. [Google Scholar] [CrossRef]
- Badana, A.; Chintala, M.; Varikuti, G.; Pudi, N.; Kumari, S.; Kappala, V.R.; Malla, R.R. Lipid raft integrity is required for survival of triple negative breast cancer cells. J. Breast Cancer 2016, 19, 372–384. [Google Scholar] [CrossRef]
- Voronovic, E.; Skripka, A.; Jarockyte, G.; Ger, M.; Kuciauskas, D.; Kaupinis, A.; Valius, M.; Rotomskis, R.; Vetrone, F.; Karabanovas, V. Uptake of upconverting nanoparticles by breast cancer cells: Surface coating versus the protein corona. ACS Appl. Mater. Interfaces 2021, 13, 39076–39087. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Wang, M.; Zhang, C.; Zhang, T.; Zhou, H.; Zhao, W.; Zhao, W.; Xia, G.; Shao, R. Endocytosis and organelle targeting of nanomedicines in cancer therapy. Int. J. Nanomed. 2020, 15, 9447–9467. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Mugundhan, S.L.; Mohan, M. Hyaluronic acid-coated capecitabine nanostructures for CD44 receptor-mediated targeting in breast cancer therapy. RSC Adv. 2025, 15, 12653–12670. [Google Scholar] [CrossRef]
- Deng, M.; Rao, J.-D.; Guo, R.; Li, M.; He, Q. Size-adjustable nano-drug delivery systems for enhanced tumor retention and penetration. Pharm. Front. 2021, 3, e98–e112. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.-Y.; Kwon, S.-H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef]
- Kim, M.L.; Sorg, I.; Arrieumerlou, C. Endocytosis-independent function of clathrin heavy chain in the control of basal NF-κB activation. PLoS ONE 2011, 6, e17158. [Google Scholar] [CrossRef] [PubMed]
| Name | AC Input Amount (g) | Description |
|---|---|---|
| Unloaded-NPs (UL-NPs) | 0 | Nanoparticles without AC (blank control) |
| AC8-NPs | 8 | Nanoparticles loaded with 8 g of AC extract |
| AC12-NPs | 12 | Nanoparticles loaded with 12 g of AC extract |
| AC16-NPs | 16 | Nanoparticles loaded with 16 g of AC extract |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Miyajima, H.; Chou, M.-Y.; Fujita, S. Natural Polysaccharide-Based Nanoparticles Enhance Intracellular Delivery and Cytotoxicity of Antrodia camphorata in Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8420. https://doi.org/10.3390/ijms26178420
Tsai Y-C, Miyajima H, Chou M-Y, Fujita S. Natural Polysaccharide-Based Nanoparticles Enhance Intracellular Delivery and Cytotoxicity of Antrodia camphorata in Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(17):8420. https://doi.org/10.3390/ijms26178420
Chicago/Turabian StyleTsai, Yu-Chen, Hiroki Miyajima, Ming-Yang Chou, and Satoshi Fujita. 2025. "Natural Polysaccharide-Based Nanoparticles Enhance Intracellular Delivery and Cytotoxicity of Antrodia camphorata in Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 17: 8420. https://doi.org/10.3390/ijms26178420
APA StyleTsai, Y.-C., Miyajima, H., Chou, M.-Y., & Fujita, S. (2025). Natural Polysaccharide-Based Nanoparticles Enhance Intracellular Delivery and Cytotoxicity of Antrodia camphorata in Breast Cancer Cells. International Journal of Molecular Sciences, 26(17), 8420. https://doi.org/10.3390/ijms26178420







