ZnO-Based Nanoparticles for Targeted Cancer Chemotherapy and the Role of Tumor Microenvironment: A Systematic Review
Abstract
1. Introduction
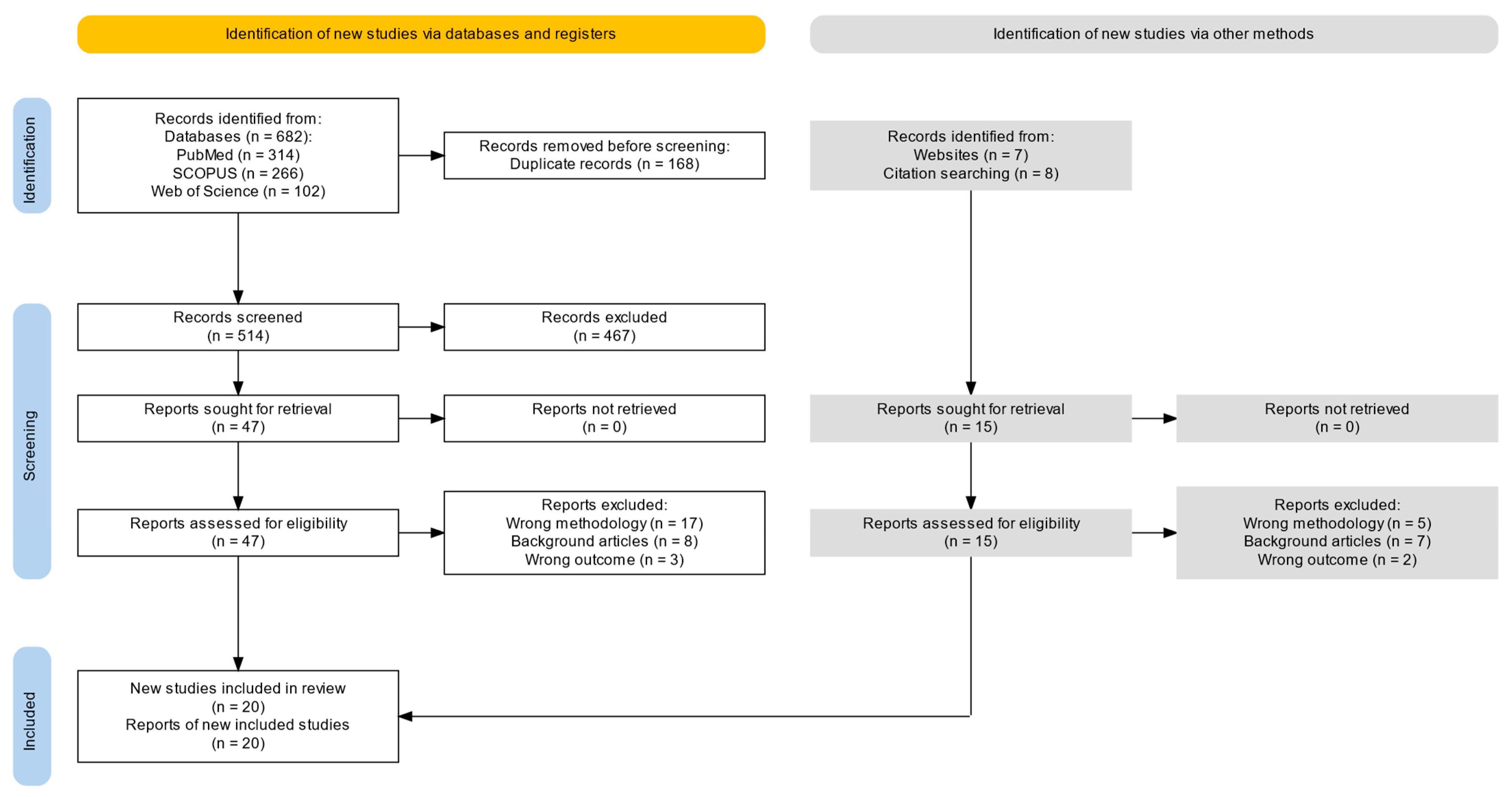
2. Methods
2.1. Search Strategy and Information Sources
2.2. Eligibility Criteria
2.3. Data Management
2.4. Study Selection
2.5. Data Extraction and Quality Assessment
2.6. Qualitative Synthesis
3. Results
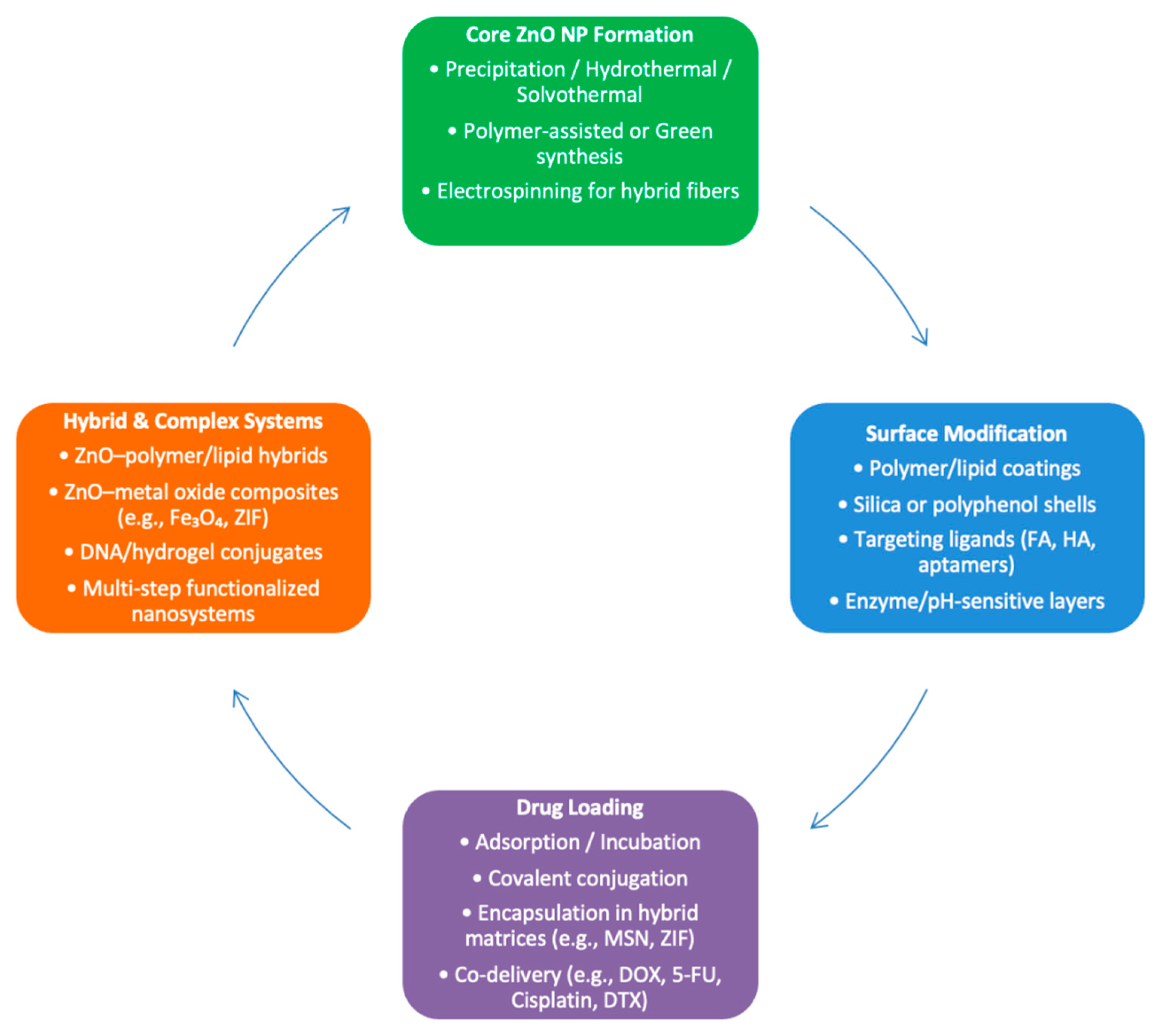
3.1. NP Composition and Synthesis Methods
3.2. Physicochemical Characterization of ZnO-Based NPs
3.3. Cancer Models and Experimental Systems
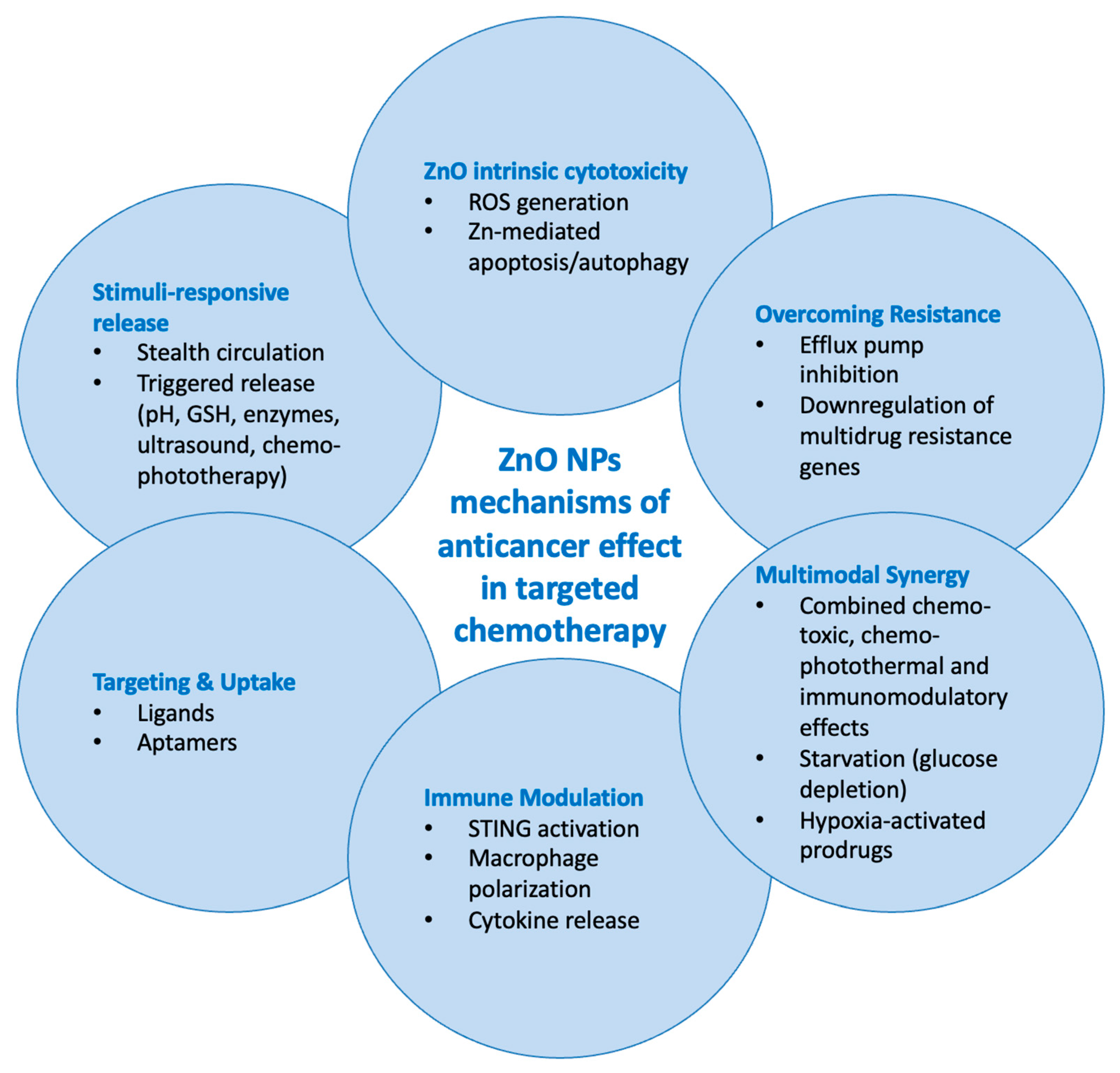
3.4. Mechanisms of Anticancer Action and pH-Responsiveness
3.5. Anticancer Performance
3.6. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Xia, F.; Lin, R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980–2021: A systematic analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef]
- Bi, J.H.; Tuo, J.Y.; Xiao, Y.X.; Tang, D.D.; Zhou, X.H.; Jiang, Y.F.; Ji, X.W.; Tan, Y.T.; Yuan, H.Y.; Xiang, Y.B. Observed and relative survival trends of lung cancer: A systematic review of population-based cancer registration data. Thorac. Cancer 2024, 15, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhang, H.; Swarbrick, A. TiME for a change: The tumor microenvironment as the missing piece in cancer therapeutics. PLoS Biol. 2025, 23, e3003276. [Google Scholar] [CrossRef]
- El-Tanani, M.; Rabbani, S.A.; Babiker, R.; Rangraze, I.; Kapre, S.; Palakurthi, S.S.; Alnuqaydan, A.M.; Rizzo, M.; El-Tanani, Y.; Tambuwala, M.M. Unraveling the tumor microenvironment: Insights into cancer metastasis and therapeutic strategies. Cancer Lett. 2024, 591, 216894. [Google Scholar] [CrossRef]
- Guo, Y.; Morshedi, M. Cutting-edge nanotechnology: Unveiling the role of zinc oxide nanoparticles in combating deadly gastrointestinal tumors. Front. Bioeng. Biotechnol. 2025, 13, 1547757. [Google Scholar] [CrossRef]
- DeLong, R.K.; Cheng, Y.H.; Pearson, P.; Lin, Z.; Coffee, C.; Mathew, E.N.; Hoffman, A.; Raelene, M.W.; Higginbotham, M.L. Translating Nanomedicine to Comparative Oncology-the Case for Combining Zinc Oxide Nanomaterials with Nucleic Acid Therapeutic and Protein Delivery for Treating Metastatic Cancer. J. Pharmacol. Exp. Ther. 2019, 370, 671–681. [Google Scholar] [CrossRef]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Mohammadi, M.; Hashemzadeh, M.S. Zinc Oxide Nanoparticles and Cancer Chemotherapy: Helpful Tools for Enhancing Chemo-sensitivity and Reducing Side Effects? Biol. Trace Elem. Res. 2024, 202, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Ravi, P.; Reddy, M.C.; Thummala, C.; Mandal, T.K. Zinc Oxide Nanoparticles in Modern Science and Technology: Multifunctional Roles in Healthcare, Environmental Remediation, and Industry. Nanomaterials 2025, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Percivalle, N.M.; Conte, M.; Dumontel, B.; Racca, L.; Carofiglio, M.; Cauda, V. Development of doped ZnO-based biomimicking and tumor-targeted nanotheranostics to improve pancreatic cancer treatment. Cancer Nanotechnol. 2022, 13, 37. [Google Scholar] [CrossRef]
- Chen, W.H.; Luo, G.F.; Qiu, W.X.; Lei, Q.; Hong, S.; Wang, S.B.; Zheng, D.W.; Zhu, C.H.; Zeng, X.; Feng, J.; et al. Programmed Nanococktail for Intracellular Cascade Reaction Regulating Self-Synergistic Tumor Targeting Therapy. Small 2016, 12, 733–744. [Google Scholar] [CrossRef]
- Cui, T.; Yan, Z.; Qin, H.; Sun, Y.; Ren, J.; Qu, X. A Sequential Target-Responsive Nanocarrier with Enhanced Tumor Penetration and Neighboring Effect In Vivo. Small 2019, 15, 1903323. [Google Scholar] [CrossRef] [PubMed]
- Delong, R.K.; Comer, J.; Mathew, E.N.; Jaberi-Douraki, M. Comparative Molecular Immunological Activity of Physiological Metal Oxide Nanoparticle and its Anticancer Peptide and RNA Complexes. Nanomaterials 2019, 9, 1670. [Google Scholar] [CrossRef]
- Ekambaram, R.; Saravanan, S.; Babu, V.P.S.; Dharmalingam, S. Fabrication and evaluation of Docetaxel doped ZnO nanoparticles incorporated PCL nanofibers for its hemocompatibility, cytotoxicity and apoptotic effects against A549. Materialia 2022, 21, 101278. [Google Scholar] [CrossRef]
- Gomaa, S.; Nassef, M.; Tabl, G.; Zaki, S.; Abdel-Ghany, A. Doxorubicin and folic acid-loaded zinc oxide nanoparticles-based combined anti-tumor and anti-inflammatory approach for enhanced anti-cancer therapy. BMC Cancer 2024, 24, 34. [Google Scholar] [CrossRef]
- He, L.; Sun, X.; Nan, X.; Wang, T.; Bai, P. Thermal and pH responsive ZnO-based nanoparticles for efficient drug delivery. AIP Adv. 2019, 9, 125026. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Liu, M.; Zhong, T.; Li, H.; Wang, J.; Zhao, H.; Tian, Y.; Wang, H.; Wang, J.; et al. Antitumor Activity of the Zinc Oxide Nanoparticles Coated with Low-Molecular-Weight Heparin and Doxorubicin Complex In Vitro and In Vivo. Mol. Pharm. 2022, 19, 4179–4190. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Rui, Q.; Liu, P.; Cai, W.; Kong, Y.; Zuo, X. Biodegradable Controlled Drug Delivery Platform Based on Carboxylated Mesoporous Silica Nanoparticles—Zinc Oxide Quantum Dots. Ind. Eng. Chem. Res. 2024, 63, 13459–13468. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Wang, X.; Tian, C.; Shen, Y.; Zhu, M. A dual-targeting Fe3O4@C/ZnO-DOX-FA nanoplatform with pH-responsive drug release and synergetic chemo-photothermal antitumor in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111455. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Martinez, N.L.; Cadena-Galeana, A.D.; Villanueva-Sanchez, F.G.; Perez-Cornejo, N.; Avelar-Juarez, K.M.; Ramos-Baena, J.D.; Cruz-Monroy, E.A.; Vazquez-Zuniga, U.; Garcia-Contreras, R. Efficacy of Antineoplastic Nanocarriers on 3D Oral Cancer Spheroids. Vivo 2023, 37, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.R.; Prabhu, A. Acid Degradable Zinc Oxide Nanoparticle Loaded Etoposide Nanoformulation for Targeting Lung Adenocarcinoma via Drug Metabolism and Crosstalk Between Angiogenesis and Akt Pathways. Bionanoscience 2024, 14, 4406–4417. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Freire, B.M.; Armentano, G.M.; Melo Santana, B.D.; Batista, B.L.; Carneiro-Ramos, M.S.; Seabra, A.B. Chronic exposure to nitric oxide sensitizes prostate cancer cells and improved ZnO/CisPt NPs cytotoxicity and selectivity. Int. J. Pharm. 2023, 640, 122998. [Google Scholar] [CrossRef]
- Saravanan, K.; Manickam, R.; Govindasamy, C.; El-Newehy, A.S.; Hussein-Al-Ali, S.H.; Senthilkumar, S. Fabrication of verrucarin-a-loaded zinc oxide nanocomposite for inducing apoptosis in triple-negative breast cancer cells. Process Biochem. 2024, 145, 13–21. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, H.; Li, B.; Feng, C.; Dai, Y. An Ultrasound-Triggered STING Pathway Nanoagonist for Enhanced Chemotherapy-Induced Immunogenic Cell Death. Small 2024, 20, 2309850. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef]
- Xiao, X.; Liang, S.; Zhao, Y.; Huang, D.; Xing, B.; Cheng, Z.; Lin, J. Core–shell structured 5-FU@ZIF-90@ZnO as a biodegradable nanoplatform for synergistic cancer therapy. Nanoscale 2020, 12, 3846–3854. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, X.; Zhang, J.; Wang, X.Y.; Bi, S. Functional Nucleic Acids-Engineered Bio-Barcode Nanoplatforms for Targeted Synergistic Therapy of Multidrug-Resistant Cancer. ACS Nano 2023, 17, 13533–13544. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, X.; Lu, Y.; Zhang, R.; Lv, K.; Gong, J.; Feng, J.; Zhang, H. A pH-Responsive Charge-Convertible Drug Delivery Nanocarrier for Precise Starvation and Chemo Synergistic Oncotherapy. Chempluschem 2023, 88, e202200394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Li, Y.; Wang, J.; He, X.; Zhang, J.; Qiao, Y.; Wu, H.; Zhu, L. Targeting and sensitizing MDR cancer by an MMP2 and pH dual-responsive ZnO-based nanomedicine. Cancer Nanotechnol. 2023, 14, 56. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidian, H.; Omidi, Y. Advanced nanoscale drug delivery systems for bone cancer therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166739. [Google Scholar] [CrossRef]
- Kapoor, D.; Maheshwari, N.; Soni, N.; Singhai, N.J.; Sharma, M.C.; Prajapati, B.; Yele, S.; Maheshwari, R. Metallic nanoparticles in cancer: Types, green synthesis, applications, tumor microenvironment and toxicity considerations. J. Drug Deliv. Sci. Technol. 2024, 92, 105307. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Emerging nanomedicines for effective breast cancer immunotherapy. J. Nanobiotechnol. 2020, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, M.K.; Sharma, A.K.; Jayaprakash, G.K.; Tonk, R.K.; Chellappan, D.K.; Singh, S.K.; Dua, K.; Ahmed, F.; Bhattacharyya, S.; et al. Metal-based nanomaterials and nanocomposites as promising frontier in cancer chemotherapy. MedComm 2023, 4, e253. [Google Scholar] [CrossRef]
- Al-Shehaby, N.; Elshoky, H.A.; Zidan, M.; Salaheldin, T.A.; Gaber, M.H.; Ali, M.A.; El-Sayed, N.M. In vitro localization of modified zinc oxide nanoparticles showing selective anticancer effects against colorectal carcinoma using biophysical techniques. Sci. Rep. 2025, 15, 16811. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Yap, P.K.; Lim, G.L.X.; Mehta, M.; Chan, Y.; Ng, S.W.; Kapoor, D.N.; Negi, P.; Anand, K.; Singh, S.K.; et al. Perspectives and advancements in the design of nanomaterials for targeted cancer theranostics. Chem. Biol. Interact. 2020, 329, 109221. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Fang, G.; Yan, S.; Alkafaas, S.S.; El Nasharty, M.A.; Khedr, S.A.; Hussien, A.M.; Ghosh, S.; Dladla, M.; Elkafas, S.S.; et al. Green Synthesis of Zinc Oxide Nanoparticles: Preparation, Characterization, and Biomedical Applications—A Review. Int. J. Nanomed. 2024, 19, 12889–12937. [Google Scholar] [CrossRef]
- Xiao, M.; Tang, Q.; Zeng, S.; Yang, Q.; Yang, X.; Tong, X.; Zhu, G.; Lei, L.; Li, S. Emerging biomaterials for tumor immunotherapy. Biomater. Res. 2023, 27, 47. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.Q.; Rodrigues, C.F.; Fernandes, N.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Metal-Polymer Nanoconjugates Application in Cancer Imaging and Therapy. Nanomaterials 2022, 12, 3166. [Google Scholar] [CrossRef]
- Shammeri, A.; Abu-Huwaij, R.; Hamed, R. Development and characterization of magnetic hydrogels loaded with greenly synthesized iron-oxide nanoparticles conjugated with cisplatin. Pharm. Dev. Technol. 2024, 29, 383–392. [Google Scholar] [CrossRef]

| Study | Nanoparticle | Synthesis Method | Physicochemical Characterization Methods | Cancer Type Studied | Model (In Vitro/In Vivo) and Route of Administration | Ph-Responsive Value * | Mechanism | Important Findings/Anticancer Performance |
|---|---|---|---|---|---|---|---|---|
| Chen et al., 2016 [17] | ZnO-DOX/R8@HA | Sequential functionalization protocol (forming ZnO-DOX/R8@HA):
| HRTEM, XPS, EDX, FT-IR spectroscopy, DLS | Squamous cell carcinoma | In vitro: SCC-7 cell line with CD44 receptor overexpressed on the cell membrane and COS7 cell line with negative CD44 receptor expression In vivo: SCC-7 tumor-bearing mice (IV tail injection) | 5 | Stealthy circulation Targeted delivery Controlled release Synergistic therapeutic effects |
|
| Wang et al., 2017 [31] | ZnO-DOX | DOX loading by ZnO-NPs in aqueous DOX solution (5 mg/mL) under stirring for 24 h in the dark, followed by centrifugation, washing, and freeze-drying. | TEM, DLS, Zeta potential measurement, UV–Vis spectroscopy, fluorescence microscopy, flow cytometry | Breast cancer, cervical cancer, ovarian cancer, and uterine sarcoma | In vitro: MDA-MB-231 (Dox-sensitive); HeLa (Dox-sensitive); NCI/ADR-RES; MES-SA/Dx5 In vivo: NA | 5 | pH-responsive drug release; Enhanced cellular uptake via endocytosis; ROS generation and Zn2+-mediated apoptosis; CD44 downregulation and CSC sensitization; Immunomodulation via M1-like macrophage polarization; Synergistic therapeutic effects |
|
| Cui et al., 2019 [18] | ZnO-DOX | DOX loading onto commercially obtained ZnO-NPs by mixing ZnO-NPs with DOX solution and incubating at 37 °C for 24 h in the dark; the mixture was then centrifuged, washed, and dried under vacuum. | TEM, DLS, Zeta potential measurement, UV–Vis spectroscopy, FT-IR spectroscopy, XRD, fluorescence microscopy | Colon cancer | In vitro: Human colon cancer cell line HCT116 In vivo: HCT116 tumor-bearing BALB/c nude mice (IV tail injection) | 5.5 | pH-responsive drug release; ROS generation and oxidative damage; Zn2+-mediated apoptosis and autophagy induction; Secretion of proinflammatory cytokines (TNF-α, IL-6, IFN-γ); Synergistic effects |
|
| DeLong et al., 2019 [19] | ZnO-LL37/carboplatin | Suspension of ZnO-NPs in serum-free culture medium, followed by incubation with LL-37 peptide (10 μg/mL) to allow surface adsorption. For combination treatment, carboplatin was added separately to the ZnO–LL37 complex prior to administration. | TEM, DLS, Zeta potential measurement, UV–Vis spectroscopy, XRD | Lung cancer | In vitro: Human non-small cell lung cancer cell line A549 (Intratumoral injection) In vivo: A549 xenograft tumors in immunocompromised mice | NA | ROS generation and Zn2+-mediated cytotoxicity; Immune stimulation via LL-37–induced proinflammatory cytokines; Immune infiltration in the TME; Synergistic chemosensitization |
|
| He et al., 2019 [22] | ZnO@PMNE | Synthesis of ZnO@PMNE NPs via
| HRTEM, FT-IR spectroscopy, UV–Vis spectroscopy, photoluminescence spectroscopy, XRD, DLS, confocal fluorescence microscopy | Hepatocellular carcinoma | In vitro: HepG2 cells In vivo: HepG2 tumor-bearing nude mice (xenograft model—IV tail injection) | 5.4 | pH-responsive drug release; ROS generation and Zn2+-mediated apoptosis; Thermo- and pH-sensitive polymer shell enabling stimuli-responsive delivery; Enhanced cellular uptake; Synergistic anticancer effects |
|
| Xiao et al., 2019 [32] | 5-FU@ZIF-90@ZnO (FZZ) | Solution preparation for porous ZIF-90 NPs under stirring, encapsulation of 5-FU, coating with ZnO through the addition of TEA and zinc acetate into the ZIF-90 solution. | TEM, EDS, XRD, FT-IR, DLS, Zeta potential measurement | Human cervical cancer; Hepatocellular carcinoma | In vitro: HeLa cells In vivo: H22 xenograft tumor model (Balb/c mice—IV tail injection) | 5.5 | ZnO core–shell structure; Controlled drug release; Synergistic effect (zinc ions and 5-FU) |
|
| Liu et al., 2021 [25] | Fe3O4@C/ZnO-DOX-FA | Solvothermal synthesis of Fe3O4 NPs, MAA functionalization, and sequential ZIF-8 shell growth. Fe3O4@C/ZnO obtained via calcination, followed by PEI modification and FA conjugation through crosslinking. DOX loading achieved via ultrasonication. | SEM, TEM, FT-IR, UV–Vis/XRD/Raman spectroscopy, BET analysis, EDS, IR thermal imaging, SQUID magnetometry, fluorescence microscopy, MTT assay | Human cervical cancer; Hepatoma | In vitro: HeLa cell line for photothermal, chemo-photothermal assays, and cellular uptake studies In vivo: H22 hepatoma tumor-bearing female mice (IV tail injection) | 5 | Magnetic targeting; Cancer cell-specific targeting via FA conjugation; pH-responsive drug release; Synergistic chemo-photothermal therapy |
|
| Li et al., 2022 [23] | ZnO-LD NPs (ZnO-NPs coated with LMHP-DOX complex) | Dispersing ZnO-NPs in DMSO, LMHP-DOX complex formation, ZnO-NPs incubation with LMHP-DOX, dropwise addition, dialysis for the formation of ZnO-LD NPs. | TEM, DLS, XRD, FT-IR spectroscopy, UV–Vis spectroscopy, fluorescence spectroscopy | Prostate cancer, breast cancer | In vitro: PC-3M, PC-3 (high and low metastatic human prostate cancer), and 4T1 (mammary breast cancer) cell lines (IV tail injection) In vivo: PC-3M tumor-bearing BALB/c nude male mice mice, 4T1 tumor-bearing BALB/c female mice | 4.5 | pH-sensitive drug release, ROS generation to induce apoptosis; Enhanced penetration into tumor tissues for treatment and imaging with second-order nonlinearity techniques |
|
| Barui et al., 2022 [16] | EV-Lipo-pep-NCs-Gem (NCs constists of Gd-doped ZnO NCs) |
| FESEM, X-ray detector for energy-dispersive spectroscopy (EDS) analysis, DC magnetometer, DLS, Zeta potential measurements, NP tracking analysis (NTA), TEM, fluorescence microscopy analyses | Pancreatic cancer (ATCC) | In vitro: BxPC3 (non metastatic cell line) and AsPC-1(metastatic cell line) In vivo: NA | - | ZnO NCs: cytotoxicity mechanisms preferably affecting cancer cells (mainly based on reactive oxygen species, ROS, generation and Zn2+ ions release), reach the tumor area in a selective way, sparing healthy cells, and thus minimizing toxicity toward them, enhancing the response to chemotherapy (Gemcitabine) |
|
| Ekambaram et al., 2022 [20] | PCL + ZnO + DTX nanofibers |
ZnO-NPs + docetaxel (DTX) + PCL were weighed and stirred in magnetic stirrer. Then Electrospinning was carried out. | SEM, EDAX, FT-IR, XRD, TGA, DSC, UV–Vis spectroscopy, HPLC | Human lung cancer | In vitro: Human lung cancer A549 cell line In vivo: NA | 5.5 | Electrospun nanofiber matrix for sustained release; ZnO-mediated cytotoxicity; DTX-induced apoptosis; pH-responsive release in acidic tumor microenvironment; Synergistic chemo-nanotoxic effect |
Improved mechanical and release properties, supporting use in post-operative cancer therapy. |
| Zhou et al., 2022 [34] | MTGZ@PPD (MSN-TPZ-GOx@ZnO@PAH-PEG-DMMA) |
| XRD, TEM, UV–Vis spectroscopy, FT-IR spectroscopy, TGA, ICP-AES | 4T1 cell line mimics human breast cancer | In vitro: 4T1 (mouse breast cancer) cells, L929 (normal fibroblast) cells In vivo: 4T1 cells tumor-bearing BALB/c female mice (IV tail injection) | 6.5 | Stealth circulation via charge-convertible PPD shell; pH-responsive charge reversal promotes tumor cell uptake; ZnO QDs decompose under acidic TME, triggering controlled release; Glucose depletion via GOx and hypoxia-activated TPZ enable starvation–chemo synergistic therapy |
|
| Pieretti et al., 2023 [28] | ZnO/Cisplatin NPs |
| TEM, XRD, FT-IR, SEM/EDS, DLS, Zeta potential measurement, ICP-MS, MTT assay, fluorescence microscopy | Prostate cancer | In vitro: PC3 cell line In vivo: NA | 5.5 | pH-triggered release; NP-mediated cytotoxicity; NO-enhanced chemosensitivity; Sustained platinum release; ZnO intrinsic toxicity (oxidative stress induction); improved selectivity |
|
| Mendoza-Martinez et al., 2023 [26] | 5-FU + ZnO-NPs; Cis + ZnO-NPs |
| TEM; DLS; XRD; FT-IR; Zeta potential measurement | Lymphoid cancer | In vitro: 2D Monolayer Cell Culture; 3D Spheroid Culture In vivo: NA | NA | Nanocarrier Drug Delivery (ZnO as a carrier for 5-FU and Cis); ROS generation and apoptosis induction; endocytosis-mediated uptake; Synergistic therapeutic effects between ZnO and chemotherapeutic agents |
|
| Li et al., 2024 [24] | ZnO−BMSN−MTX |
| FT-IR spectroscopy, XRD, TEM, DLS, nitrogen adsorption/desorption isotherms | Hepatocellular carcinoma | In vitro: SMMC-7721 tumor cells (human hepatocellular carcinoma cell line) In vivo: NA | 5 | pH-responsive drug release; GSH-responsive drug release; synergistic therapeutic effects (MTX and Zn2+) |
|
| Pavan et al., 2024 [27] | ZnO-ETP |
| SEM, DLS, XRD, FT-IR, UV–Vis spectroscopy, Zeta potential measurement, MTT assay | Lung adenocarcinoma | In vitro: A549 lung adenocarcinoma cell line In vivo: NA | 5 | pH-responsive drug release; Inhibition of Cytochrome P450 (CYP3A4), reducingmetabolism and excretion of etoposide; Induction of apoptosis in cancer cells by activating the caspase-7 and caspase-9 pathways; Anti-angiogenesis |
|
| Saravanan et al., 2024 [29] | Ver-A@ZnNCs | Co-precipitation method for synthesis of ZnO-NPs (zinc acetate and lithium hydroxide as precursors in 100% ethanol) Chitosan Coating (dropwise addition of chitosan solution in 0.1% acetic acid) Verrucarin-A Loading with dropwise addition of an ethanolic solution of Verrucarin-A and removal of unbound drug. | TEM, XRD, FT-IR, UV–Vis spectroscopy, DLS, Zeta potential measurement, ICP-MS, MTT assay | Breast cancer | In vitro: MDA-MB-231 TNBC cell line In vivo: NA | 5.5 | Targeted delivery; pH-responsive drug release; Induction of apoptosis; Synergistic therapeutic effect through ROS generation and inhibition of cancer cell proliferation |
|
| Tian et al., 2024 [30] | PZnO@DOX |
| TEM, DLS, XRD, FT-IR, UV–Vis spectroscopy, Zeta potential measurement, MTT assay | Breast cancer | In vitro: 4T1 breast cancer cell line In vivo: 4T1 tumor-bearing BALB/c mice (IV tail injection) | 5 | Targeted delivery; Ultrasound-triggered DOX release; STING pathway activation; pH-responsive drug release; Synergistic therapeutic effects |
|
| Zhi et al., 2023 [33] | Cisplatin-loaded ZnO@BBCs |
| TEM, DLS, Zeta potential measurement, FT-IR spectroscopy, 1 H NMR spectroscopy, MALDI-TOF MS, HAADF-STEM with EDX mapping, PXRD, EDS, XPS, native PAGE, MTT assay | MDR lung adenocarcinoma | In vitro: A549/DDP cells of MDR human lung adenocarcinoma In vivo:xenograft- tumor models were established by subcutaneously injecting A549/DDP cells into nude BALB/c mice (IV injection) | 4.5−5.5 | pH-responsive drug release (acidic tumor conditions); ROS generation and Zn2+-mediated cytotoxicity; Circumvention of multidrug resistance by enhancing intracellular drug accumulation and reducing drug efflux |
|
| Zhou et al., 2023 [35] | ZnO/DPPG/PEG-pp-PE/DOX |
| DLS, Zeta potential measurement, TEM, LC–MS/MS, 1 H NMR spectroscopy | Breast cancer | In vitro: MDA-MB-231 triple-negative breast cancer cell line In vivo: MDA-MB-231 xenograft tumor model in BALB/c nude mice (IV tail injection) | 5 | Stealthy circulation; Tumor-targeted delivery via MMP2-cleavable PEG; pH- and enzyme-responsive drug release; Efflux pump evasion; Synergistic cytotoxicity via Zn2+-induced mitochondrial dysfunction and DOX-induced apoptosis |
|
| Gomaa et al., 2024 [21] | ZnO-NPs/DOX/FA |
| UV–Vis spectroscopy, TEM, MTT assay | EAC | In vitro: EAC cells In vivo: EAC tumor-bearing Swiss albino mice (female, 6–8 weeks old) injected intraperitoneally with 2.5 × 105 EAC cells per mouse (IP injection) | NA | FA-mediated targeting for enhanced uptake by tumor cells; Controlled cytotoxicity through DOX release and Zn2+-induced ROS generation; Synergistic antitumor effect via combined chemotoxic and immunomodulatory mechanisms |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseriotis, V.-S.; Ampazis, D.; Karachrysafi, S.; Papamitsou, T.; Petrakis, G.; Kouvelas, D.; Mavropoulos, P.; Lallas, K.; Sič, A.; Fouskas, V.; et al. ZnO-Based Nanoparticles for Targeted Cancer Chemotherapy and the Role of Tumor Microenvironment: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8417. https://doi.org/10.3390/ijms26178417
Tseriotis V-S, Ampazis D, Karachrysafi S, Papamitsou T, Petrakis G, Kouvelas D, Mavropoulos P, Lallas K, Sič A, Fouskas V, et al. ZnO-Based Nanoparticles for Targeted Cancer Chemotherapy and the Role of Tumor Microenvironment: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(17):8417. https://doi.org/10.3390/ijms26178417
Chicago/Turabian StyleTseriotis, Vasilis-Spyridon, Dimitrios Ampazis, Sofia Karachrysafi, Theodora Papamitsou, Georgios Petrakis, Dimitrios Kouvelas, Paraskevas Mavropoulos, Konstantinos Lallas, Aleksandar Sič, Vasileios Fouskas, and et al. 2025. "ZnO-Based Nanoparticles for Targeted Cancer Chemotherapy and the Role of Tumor Microenvironment: A Systematic Review" International Journal of Molecular Sciences 26, no. 17: 8417. https://doi.org/10.3390/ijms26178417
APA StyleTseriotis, V.-S., Ampazis, D., Karachrysafi, S., Papamitsou, T., Petrakis, G., Kouvelas, D., Mavropoulos, P., Lallas, K., Sič, A., Fouskas, V., Stergiou, K., Pavlidis, P., & Arnaoutoglou, M. (2025). ZnO-Based Nanoparticles for Targeted Cancer Chemotherapy and the Role of Tumor Microenvironment: A Systematic Review. International Journal of Molecular Sciences, 26(17), 8417. https://doi.org/10.3390/ijms26178417










