Heat Stress and Anthropogenic Substrates: Molecular and Behavioral Adaptation of Metridium senile in Human-Modified Marine Environments
Abstract
1. Introduction
2. Results
2.1. Substrate Selectivity and Attachment Ability of M. senile
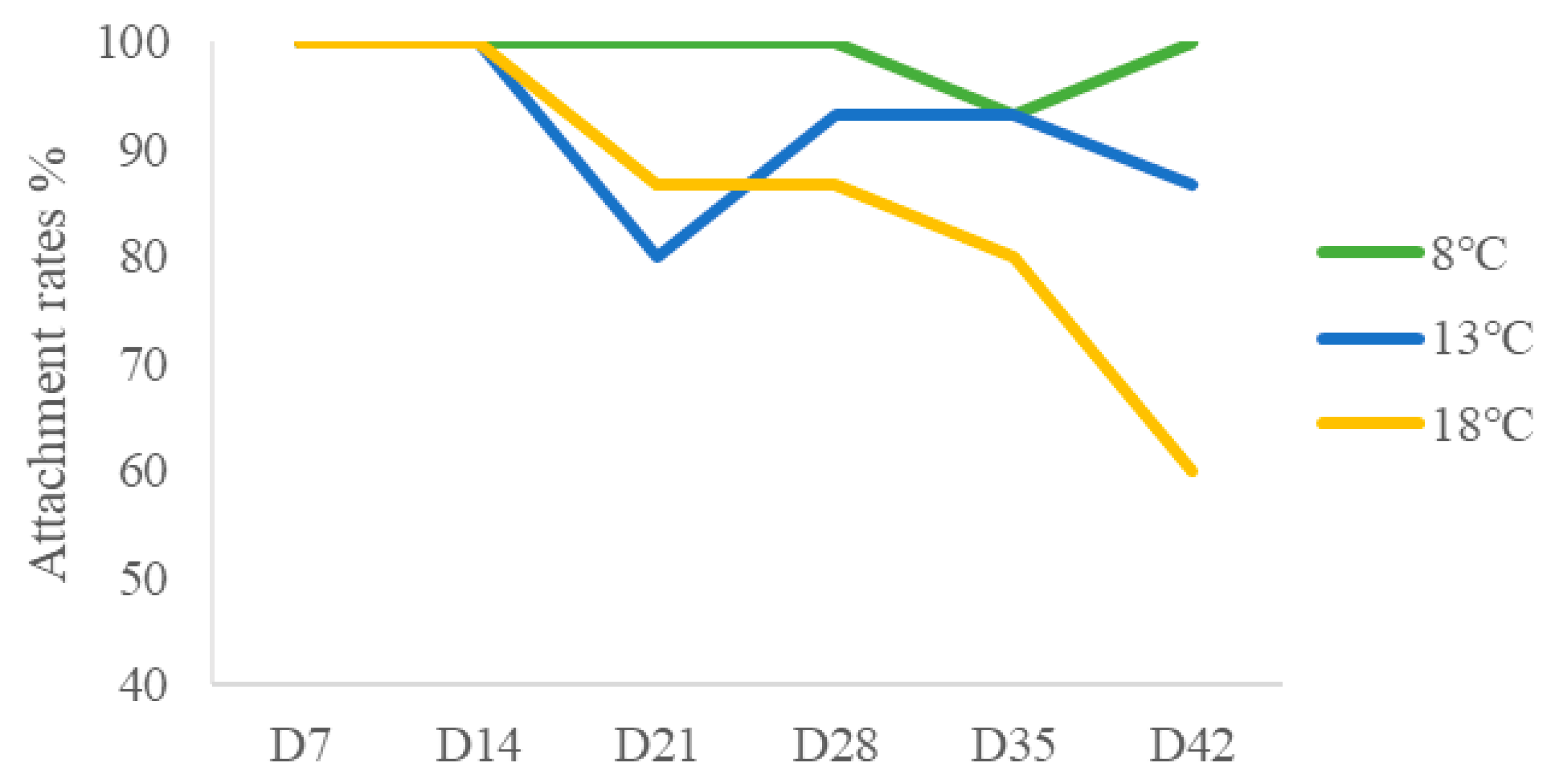
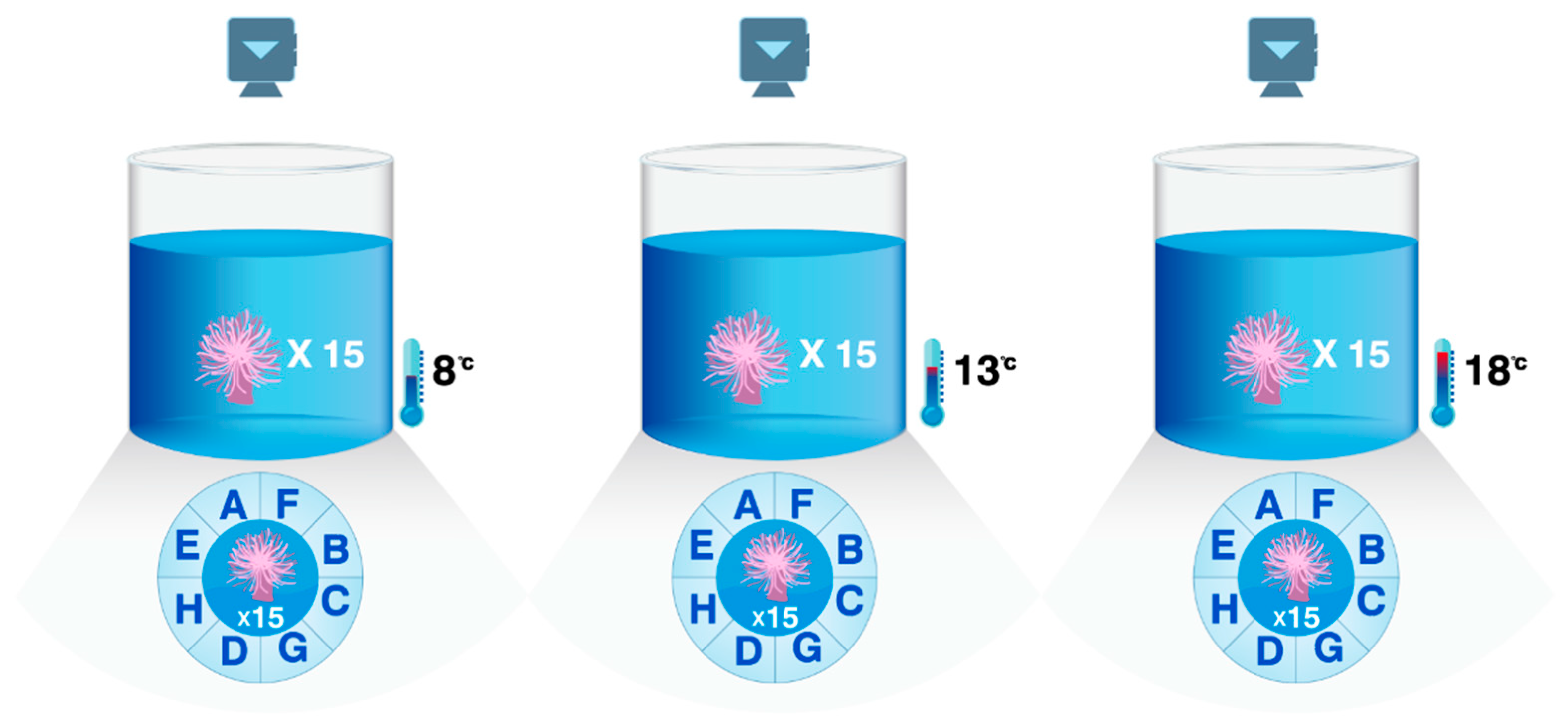
2.1.1. Changes in the Attachment Ability of M. senile
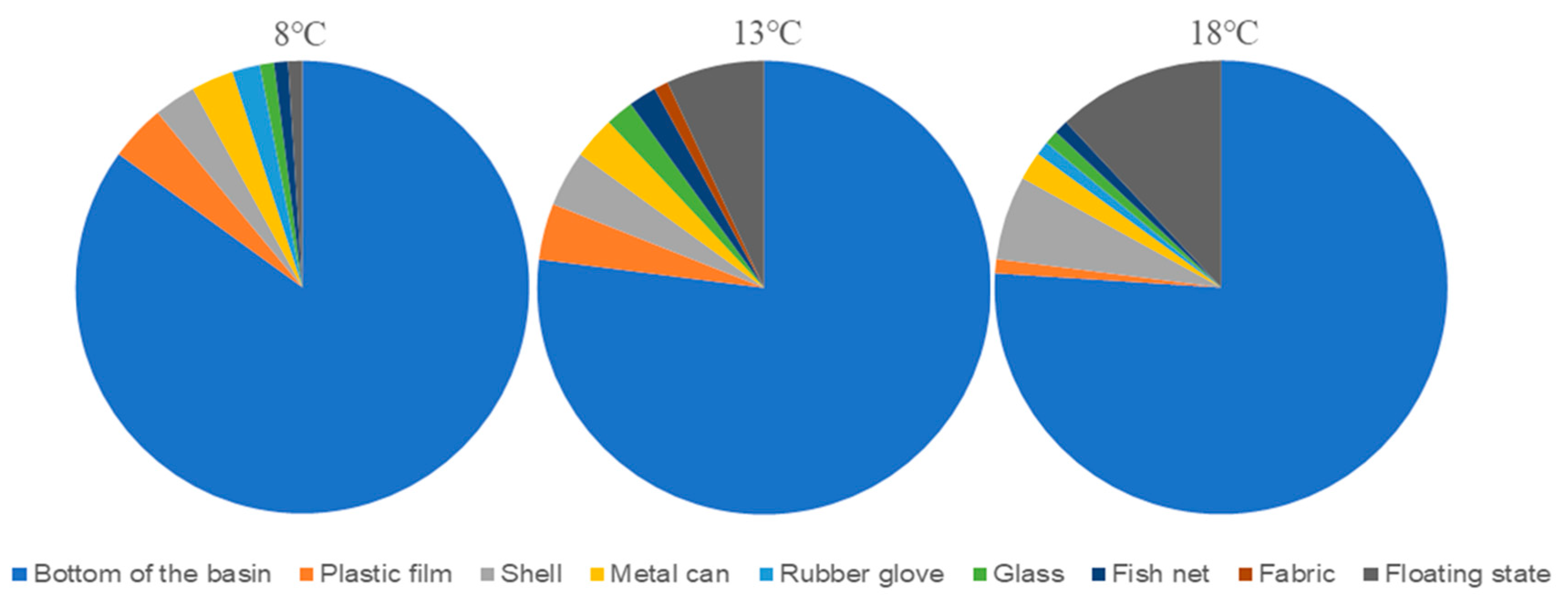
2.1.2. Selectivity of M. senile for Different Substrate Materials
2.2. Transcriptomic Analysis Results
2.2.1. Transcriptome Data Quality and Gene Statistics
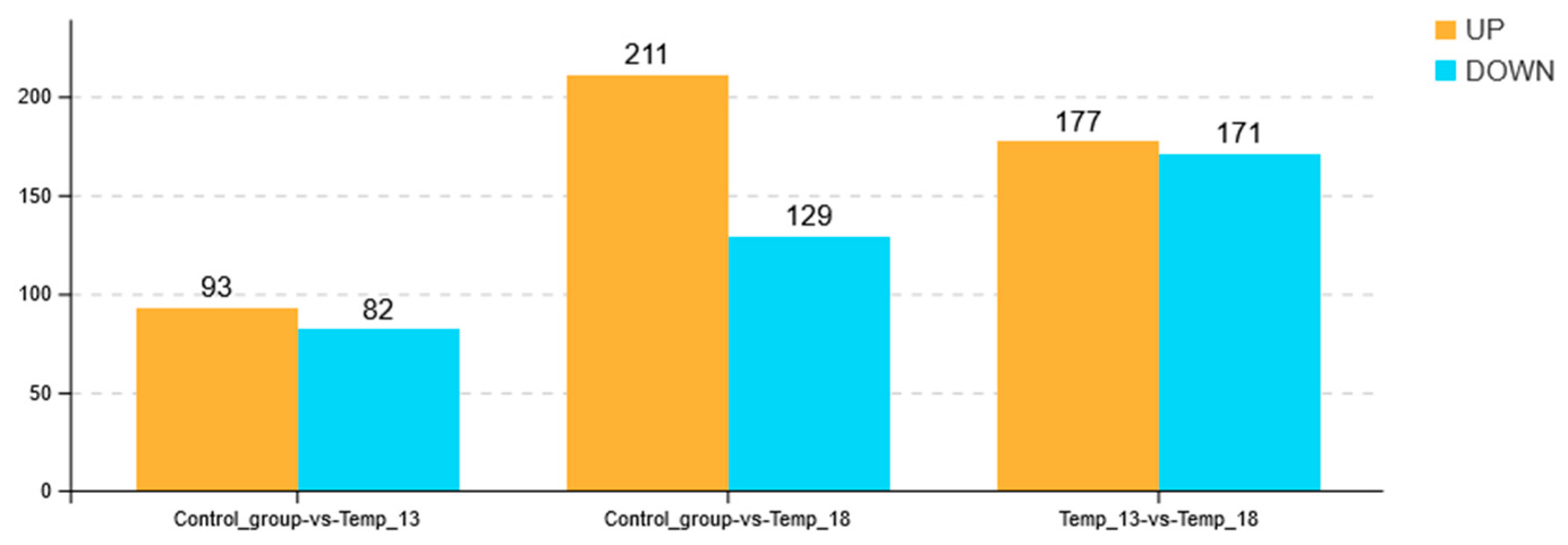
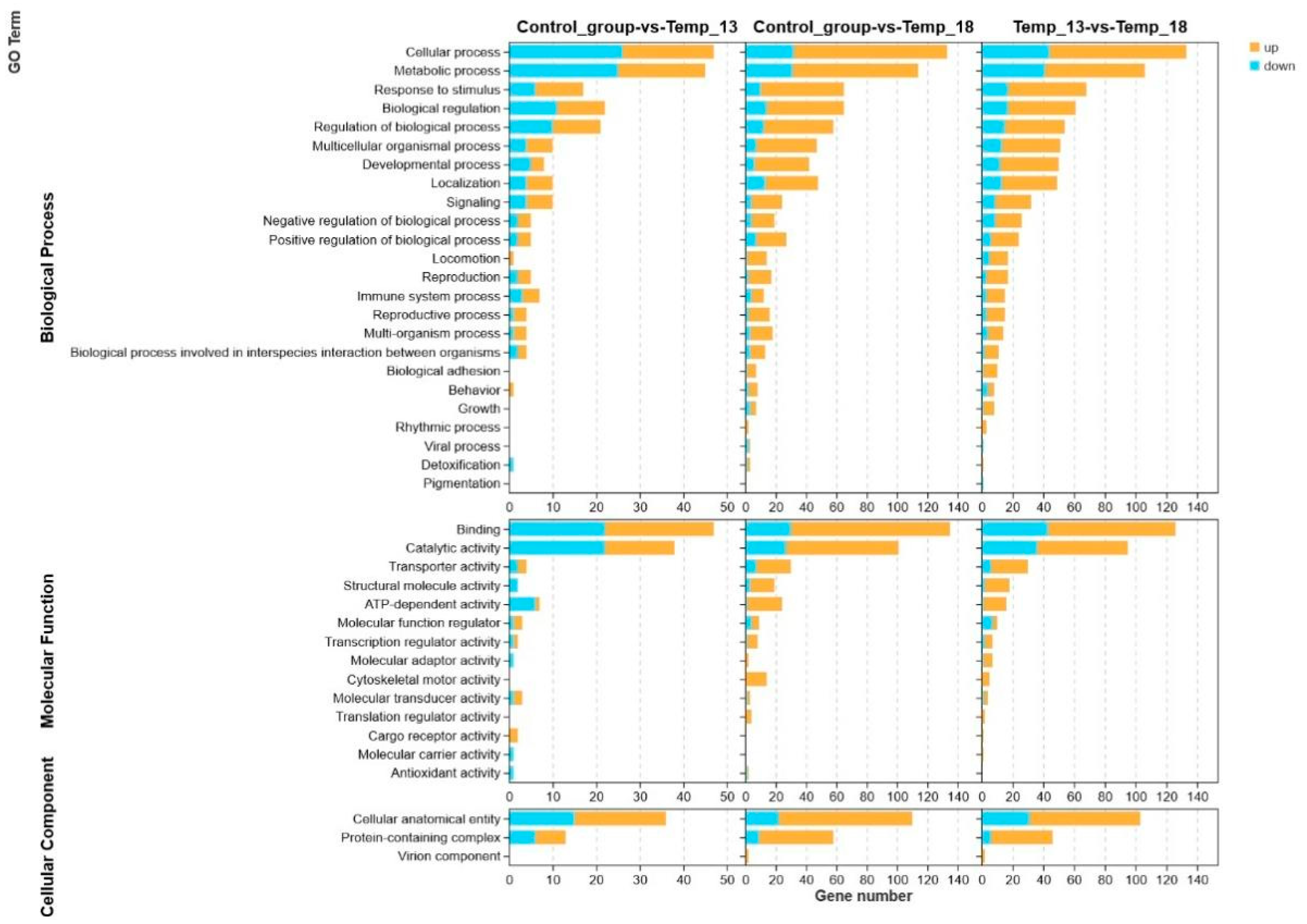
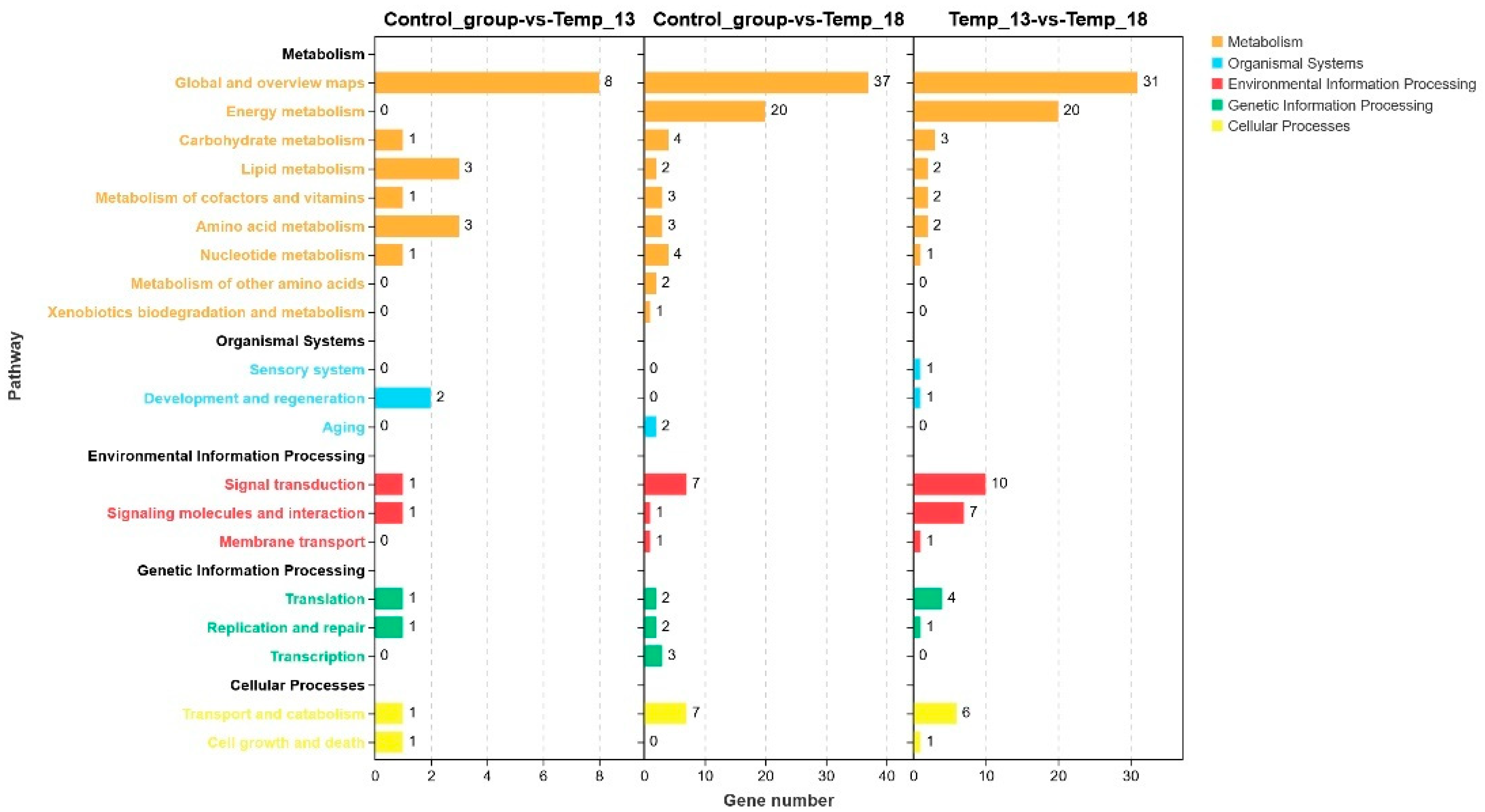
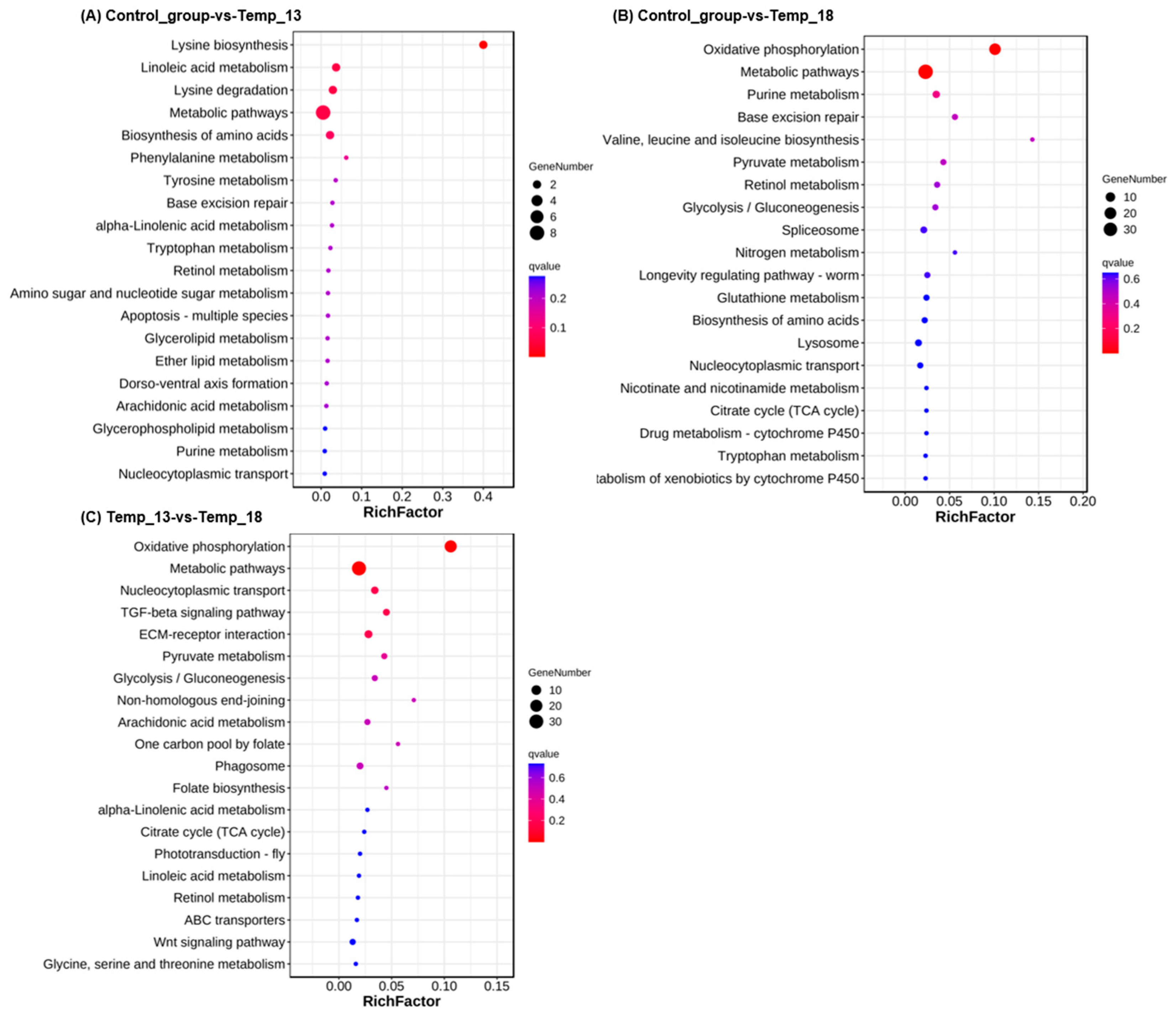
2.2.2. DEG Analysis and Annotation
2.2.3. Screening of DEGs Related to the Adaptive Response
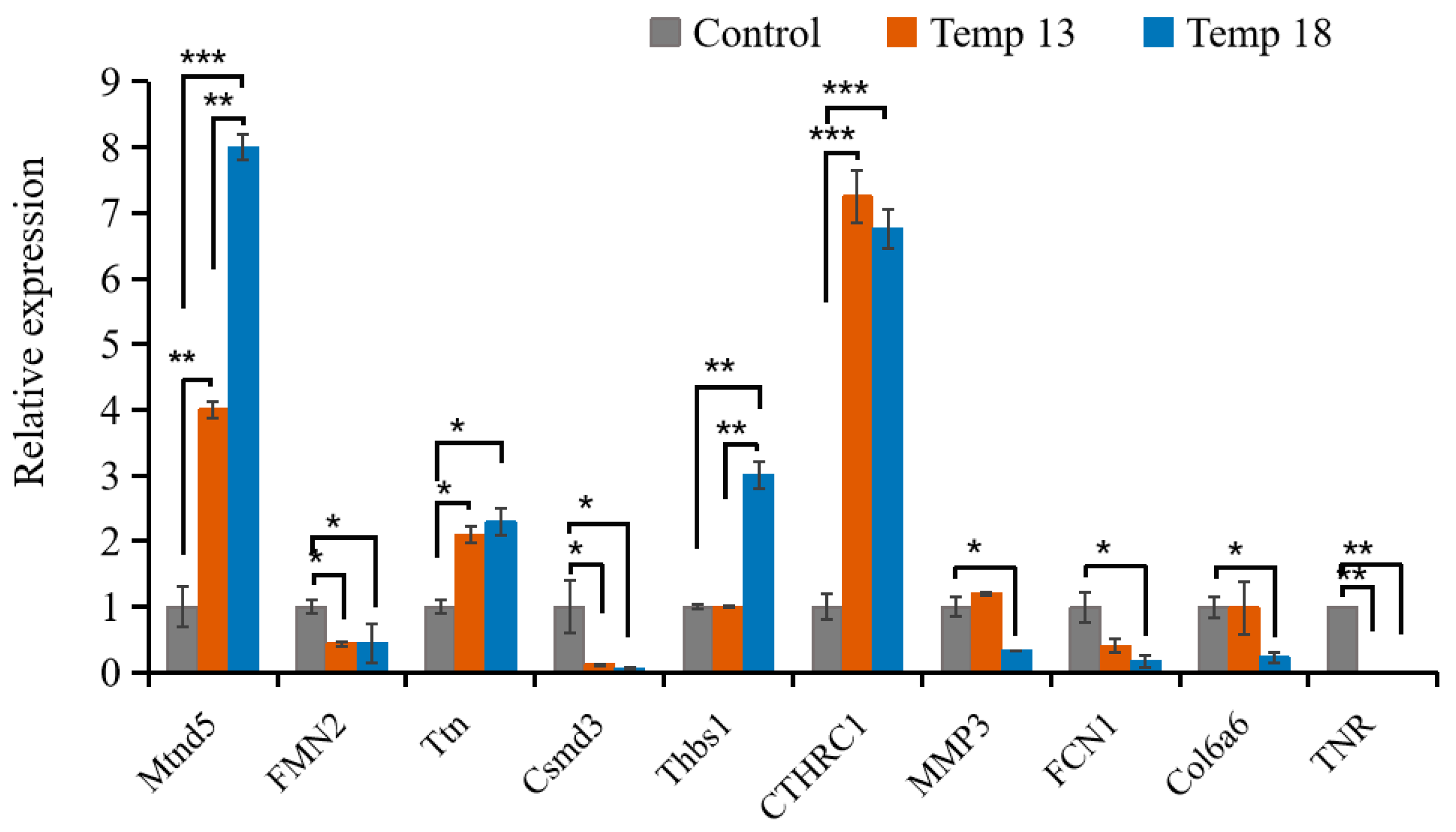
2.2.4. Validation of the RNA-Seq Results Using RT-PCR Methods
3. Discussion
3.1. Thermal Stress Disrupts Adhesion Through Collagen Degradation
3.2. Metabolic and Reproductive Trade-Offs Under Heat Stress
4. Materials and Methods
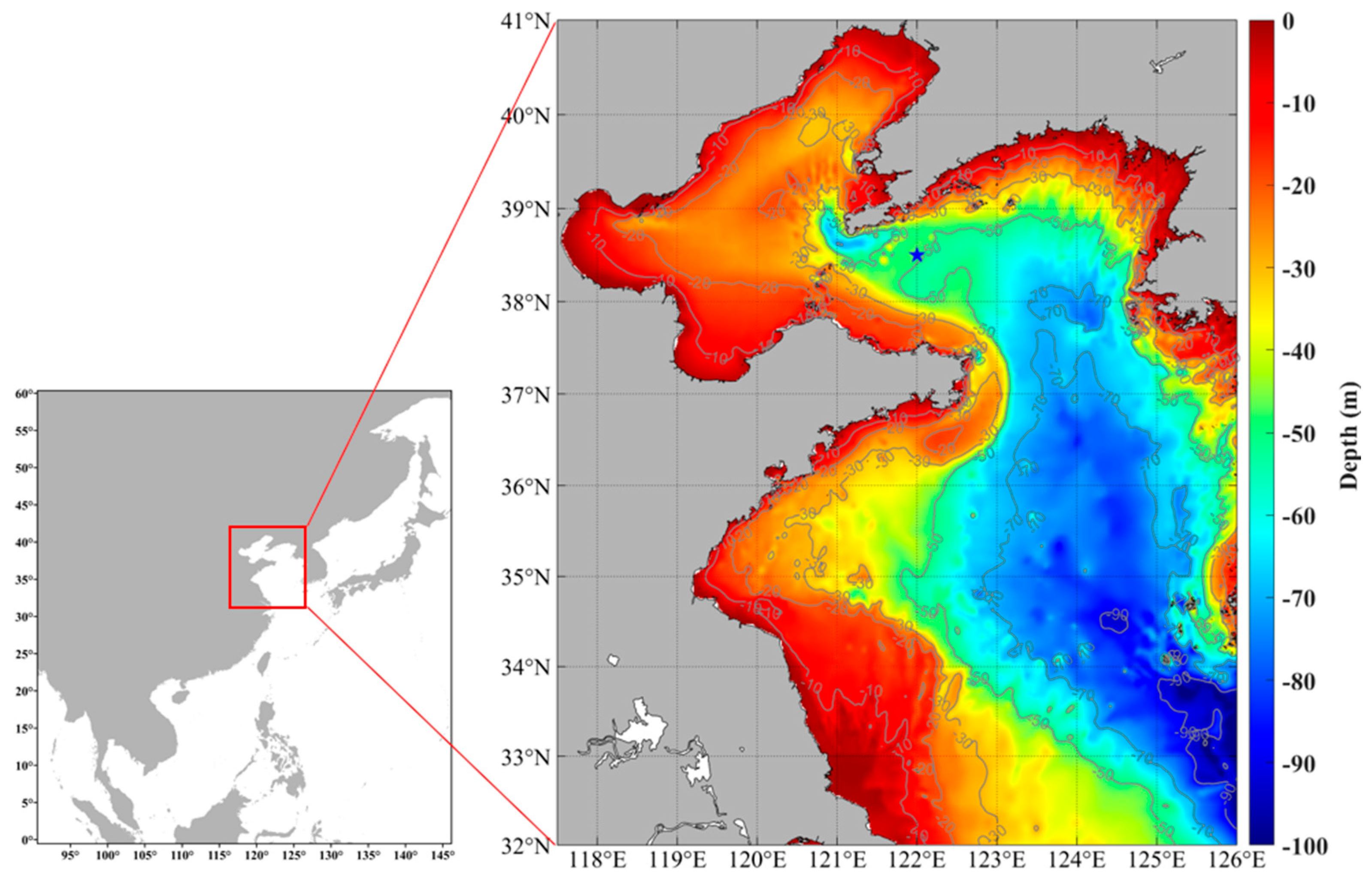
4.1. Sample Collection and Experimental Design
4.1.1. Temperature Acclimation Before the Behavioral Experiments
4.1.2. Behavioral Analysis of the Substrate Selectivity and Attachment Ability of M. senile
4.2. Transcriptomic Experiments and Analysis
4.2.1. Acquisition of Sequencing Samples for Whole-Tissue Transcriptome Analysis
4.2.2. RNA Extraction and Quality Control
4.2.3. cDNA Library Construction and Transcriptome Sequencing
4.2.4. Splicing and Annotation of Transcripts
4.2.5. Gene Annotation and Gene Enrichment Analysis
4.3. Data Validation by Quantitative Real-Time PCR (RT-PCR)
4.4. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jurgens, L. Poleward range expansion of a non-indigenous bryozoan and new occurrences of exotic ascidians in southeast Alaska. BioInvasions Rec. 2018, 7, 357–366. [Google Scholar] [CrossRef]
- Glon, H.; Daly, M.; Carlton, J.T.; Flenniken, M.M.; Currimjee, Z. Mediators of invasions in the sea: Life history strategies and dispersal vectors facilitating global sea anemone introductions. Biol. Invasions 2020, 22, 3195–3222. [Google Scholar] [CrossRef]
- Gomes, D.G.E.; Ruzicka, J.J.; Crozier, L.G.; Huff, D.D.; Brodeur, R.D.; Stewart, J.D. Marine heatwaves disrupt ecosystem structure and function via altered food webs and energy flux. Nat. Commun. 2024, 15, 1988. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Rawindran, H.; Alam, M.M.; Leong, W.H.; Sahrin, N.T.; Ng, H.-S.; Chan, Y.J.; Abdelfattah, E.A.; Lim, J.W.; Aliyu, U.S.; et al. Mitigating persistent organic pollutants from marine plastics through enhanced recycling: A review. Environ. Res. 2024, 240, 117533. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Teng, G.; Jin, X.; Fu, C.; Guan, L.; Jin, Y.; Chen, Y.; Yang, T.; Ding, Q.; Shan, X. Is seafloor litter contributing to sea anemone blooms? Sci. Total Environ. 2021, 759, 143479. [Google Scholar] [CrossRef] [PubMed]
- Ramalhosa, P.; Monteiro, J.G.; Rech, S.; Gestoso, I.; Álvarez, S.; Gizzi, F.; Parretti, P.; Castro, N.; Almeida, S.; Jiménez, J.L.; et al. The role of marine debris as a vector, dispersal agent, and substrate for non-indigenous species on Oceanic Islands (Northeast Atlantic). Mar. Pollut. Bull. 2025, 214, 117732. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.H. Temperature-dependent growth and fission rate plasticity drive seasonal and geographic changes in body size in a clonal sea anemone. Am. Nat. 2018, 191, 210–219. [Google Scholar] [CrossRef]
- Ryan, W.H.; Adams, L.; Bonthond, G.; Mieszkowska, N.; Pack, K.E.; Krueger-Hadfield, S.A. Environmental regulation of individual body size contributes to geographic variation in clonal life cycle expression. Mar. Biol. 2019, 166, 157. [Google Scholar] [CrossRef]
- Bucklin, A. Growth and asexual reproduction of the sea anemone Metridium: Comparative laboratory studies of three species. J. Exp. Mar. Biol. Ecol. 1987, 110, 41–52. [Google Scholar] [CrossRef]
- Martin, J.P.; Garese, A.; Sar, A.M.; Acuña, F.H. Fouling community dominated by Metridium senile (Cnidaria, Anthozoa, Actiniaria) in Bahía San Julián (Southern Patagonia, Argentina). Sci. Mar. 2015, 79, 211–221. [Google Scholar] [CrossRef]
- Gimenez, L.H.; Battini, N.; González-Muñoz, R.; Glon, H. Invader in disguise for decades: The plumose sea anemone Metridium senile in the Southwestern Atlantic Ocean. Biol. Invasions 2023, 25, 2159–2173. [Google Scholar] [CrossRef]
- Molinet, C.; Häussermann, V.; Astorga, M.; Barahona, N.; Espinoza, K.; Diaz, M.; Díaz, P.; Henríquez, J.; Matamala, T.; Soto, D. Population expansion of the invasive sea anemone Metridium senile in the spatial mesoscale of a sea urchin bed in north-western Patagonia. Biol. Invasions 2023, 25, 1101–1118. [Google Scholar] [CrossRef]
- Teng, G.; Shan, X.; Jin, X. Cascade effects of seafloor litter on benthic ecosystems in the northern Yellow Sea. Front. Mar. Sci. 2023, 9, 1044232. [Google Scholar] [CrossRef]
- Glon, H.; Haruka, Y.; Daly, M.; Nakaoka, M. Temperature and salinity survival limits of the fluffy sea anemone, Metridium senile (L.) in Japan. Hydrobiologia 2019, 830, 303–315. [Google Scholar] [CrossRef]
- Li, Y.; Xu, K.D. Species diversity and faunal characteristics of the order Actiniaria (Cnidaria: Anthozoa) in the seas of China. Oceanol. Limnol. Sin. 2020, 51, 434–443. [Google Scholar] [CrossRef]
- Su, J.L.; Huang, D.J. On the current field associated with the yellow sea cold water mass. Oceanol. Limnol. Sin. Suppl. 1995, 26, 1–7. [Google Scholar]
- Li, H.; Zhai, F.G.; Dong, Y.J.; Liu, Z.; Gu, Y.; Bai, P. Interannual-decadal variations in the Yellow Sea Cold Water Mass in summer during 1958—2016 using an eddy-resolving hindcast simulation based on OFES2. Cont. Shelf Res. 2024, 275, 105223. [Google Scholar] [CrossRef]
- Li, Y.; Ren, G.Y.; Wang, Q.Y.; Mu, L.; Niu, Q.; Su, H. Record-breaking marine heatwave in northern Yellow Sea during summer 2018: Characteristics, drivers and ecological impact. Sci. Total Environ. 2023, 904, 166385. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.N.; Svane, I. Effects of substratum instability on locomotion and pedal laceration in Metridium senile (Anthozoa: Actiniaria). Mar. Ecol. Prog. Ser. 1995, 124, 171–180. [Google Scholar] [CrossRef]
- Wahl, M. Metridium senile: Dispersion and small scale colonization by the combined strategy of locomotion and asexual reproduction (laceration). Mar. Ecol. Prog. Ser. 1985, 26, 271–277. [Google Scholar] [CrossRef]
- Wahl, M. The recolonization potential of Metridium senile in an area previously depopulated by oxygen deficiency. Oecologia 1985, 67, 255–259. [Google Scholar] [CrossRef]
- Pinochet, J.; Urbina, M.A.; Lagos, M.E. Marine invertebrate larvae love plastics: Habitat selection and settlement on artificial substrates. Environ. Pollut. 2020, 257, 113571. [Google Scholar] [CrossRef]
- Pesheva, P.; Probstmeier, R. The yin and yang of tenascin-R in CNS development and pathology. Prog. Neurobiol. 2000, 61, 465–493. [Google Scholar] [CrossRef]
- Pinzón, J.H.; Kamel, B.; Burge, C.A.; Harvell, C.D.; Medina, M.; Weil, E.; Mydlarz, L.D. Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R. Soc. Open Sci. 2015, 2, 140214. [Google Scholar] [CrossRef]
- Cao, M.; Ke, D.; Zhou, H. The role and molecular mechanism of CTHRC1 in fibrosis. Life Sci. 2024, 350, 122745. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.J.; Muscatine, L. Cellular mechanisms underlying temperature-induced bleaching in the tropical sea anemone Aiptasia pulchella. J. Exp. Biol. 2001, 204, 3443–3456. [Google Scholar] [CrossRef]
- Misaka, T.; Yoshihisa, A.; Takeishi, Y. Titin in muscular dystrophy and cardiomyopathy: Urinary titin as a novel marker. Clin. Chim. Acta 2019, 495, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, B.; Lin, F.; Hui, Q.; Tao, K.; Kontos, C.K. Effect of THBS1 on the Biological Function of Hypertrophic Scar Fibroblasts. BioMed Res. Int. 2020, 2020, 8605407. [Google Scholar] [CrossRef] [PubMed]
- Juárez, O.E.; Fabiola, L.I.C.; Leyva-Valencia, I.; López-Landavery, E.; García-Esquivel, Z.; Díaz, F.; Re-Araujo, D.; Vadopalas, B.; Galindo-Sánchez, C.E. Transcriptomic and metabolic response to chronic and acute heat exposure of juvenile geoduck clams Panopea globosa. Mar. Genom. 2018, 42, 1–13. [Google Scholar] [CrossRef]
- Zoysa, M.D.; Whang, I.; Lee, Y.; Lee, S.; Lee, J.-S.; Lee, J. Transcriptional analysis of antioxidant and immune defense genes in disk abalone (Haliotis discus discus) during thermal, low salinity and hypoxic stress. Comp. Biochem. Physiol. B 2009, 154, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Amadoa, J.; Silvaa, A.X.; Manzi, C.; Nespolo, R.F.; Cárdenas, L. Differential expression of stress candidate genes for thermal tolerance in the sea urchin Loxechinus albus. J. Therm. Biol. 2017, 68, 104–109. [Google Scholar] [CrossRef]
- Belin, B.J.; Lee, T.; Mullins, R.D. Correction: DNA damage induces nuclear actin filament assembly by Formin -2 and Spire-½ that promotes efficient DNA repair. Elife 2015, 4, e11935. [Google Scholar] [CrossRef]
- He, X.; Brakebusch, C. Regulation of Precise DNA Repair by Nuclear Actin Polymerization: A Chance for Improving Gene Therapy? Cells 2024, 13, 1093. [Google Scholar] [CrossRef]
- Tobias, L.; Hiroshi, W.; Oleg, S.; Lindgens, D.; Gee, L.; Law, L.; Schmidt, H.A.; Özbek, S.; Bode, H.; Holstein, T.W. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev. Biol. 2009, 330, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Shum, C.W.Y.; Nong, W.; So, W.L.; Li, Y.; Qu, Z.; Yip, H.Y.; Swale, T.; Ang, P.O.; Chan, K.M.; Chan, T.F.; et al. Genome of the sea anemone Exaiptasia pallida and transcriptome profiles during tentacle regeneration. Front. Cell Dev. Biol. 2022, 10, 1558. [Google Scholar] [CrossRef]
- Al-Shaer, L.; Leach, W.; Baban, N.; Yagodich, M.; Gibson, M.C.; Layden, M.J. Environmental and molecular regulation of asexual reproduction in the sea anemone Nematostella vectensis. R. Soc. Open Sci. 2023, 10, 230152. [Google Scholar] [CrossRef]
- Trevino, M.; Harmon, S.; Burton, P. Wnt Signaling Promotes Oral Fates During Regeneration and Embryogenesis in the Cnidarian Nematostella vectensis. Dev. Biol. 2011, 356, 252–254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, G.; Chen, W.; Shan, X.; Zhu, Q.; Jin, X. Heat Stress and Anthropogenic Substrates: Molecular and Behavioral Adaptation of Metridium senile in Human-Modified Marine Environments. Int. J. Mol. Sci. 2025, 26, 8415. https://doi.org/10.3390/ijms26178415
Teng G, Chen W, Shan X, Zhu Q, Jin X. Heat Stress and Anthropogenic Substrates: Molecular and Behavioral Adaptation of Metridium senile in Human-Modified Marine Environments. International Journal of Molecular Sciences. 2025; 26(17):8415. https://doi.org/10.3390/ijms26178415
Chicago/Turabian StyleTeng, Guangliang, Wen Chen, Xiujuan Shan, Qing Zhu, and Xianshi Jin. 2025. "Heat Stress and Anthropogenic Substrates: Molecular and Behavioral Adaptation of Metridium senile in Human-Modified Marine Environments" International Journal of Molecular Sciences 26, no. 17: 8415. https://doi.org/10.3390/ijms26178415
APA StyleTeng, G., Chen, W., Shan, X., Zhu, Q., & Jin, X. (2025). Heat Stress and Anthropogenic Substrates: Molecular and Behavioral Adaptation of Metridium senile in Human-Modified Marine Environments. International Journal of Molecular Sciences, 26(17), 8415. https://doi.org/10.3390/ijms26178415




