Steam-Processed Stauntonia hexaphylla (Thunb.) Decne Fruit Stimulates Osteoblast Differentiation in MC3T3-E1 Cells and Inhibits Osteoclastogenesis in RAW 264.7 Cells
Abstract
1. Introduction
2. Results
2.1. The Effect of Steaming of SHF on the Properties and Extract Yield
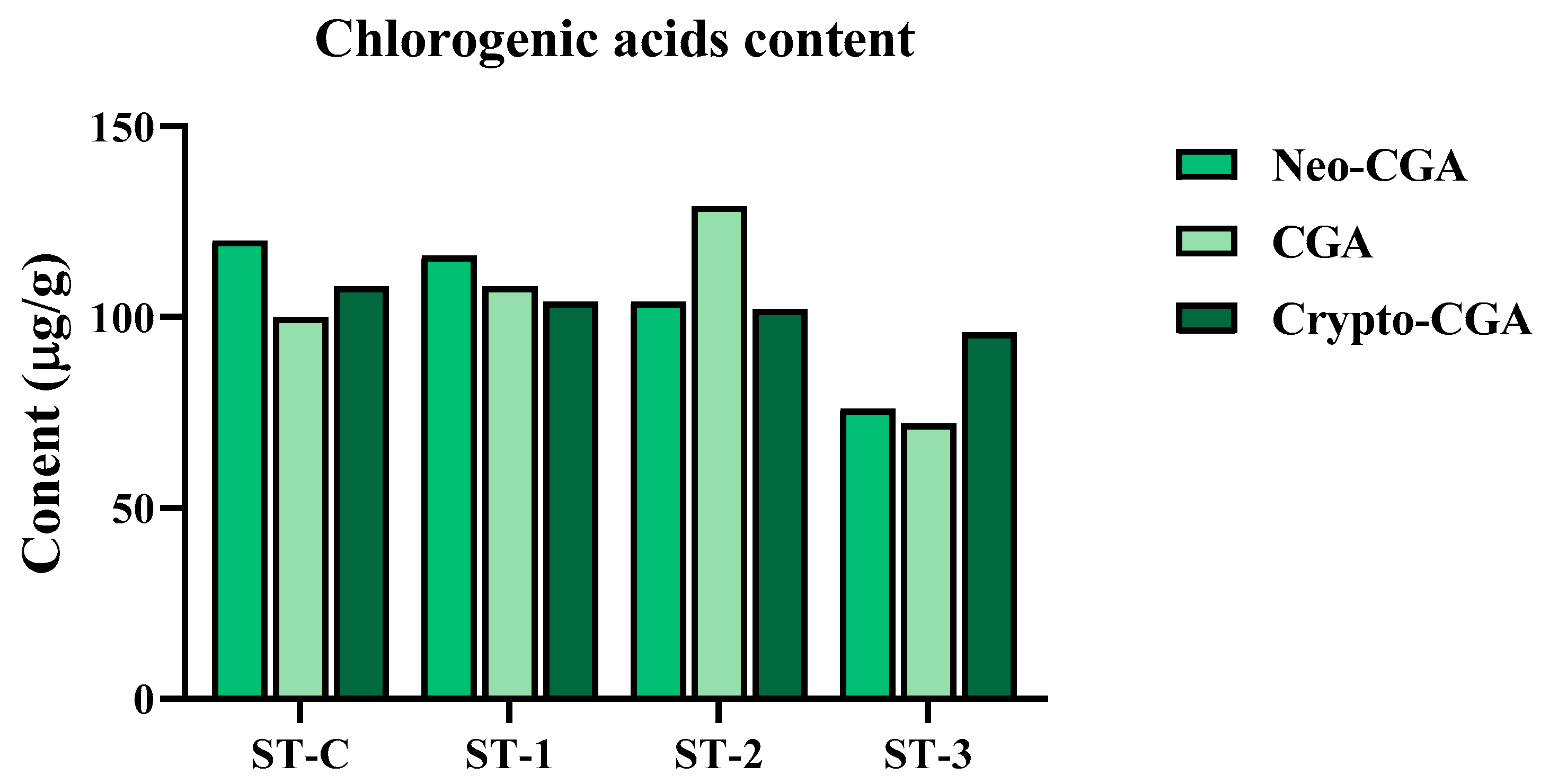
2.2. The Effect of Steaming of SHF on Phenolic Metabolite Content and Free Radical Scavenging Ability
2.3. The Effect of Steaming of SHF on Cell Proliferation in MC3T3-E1 Cells
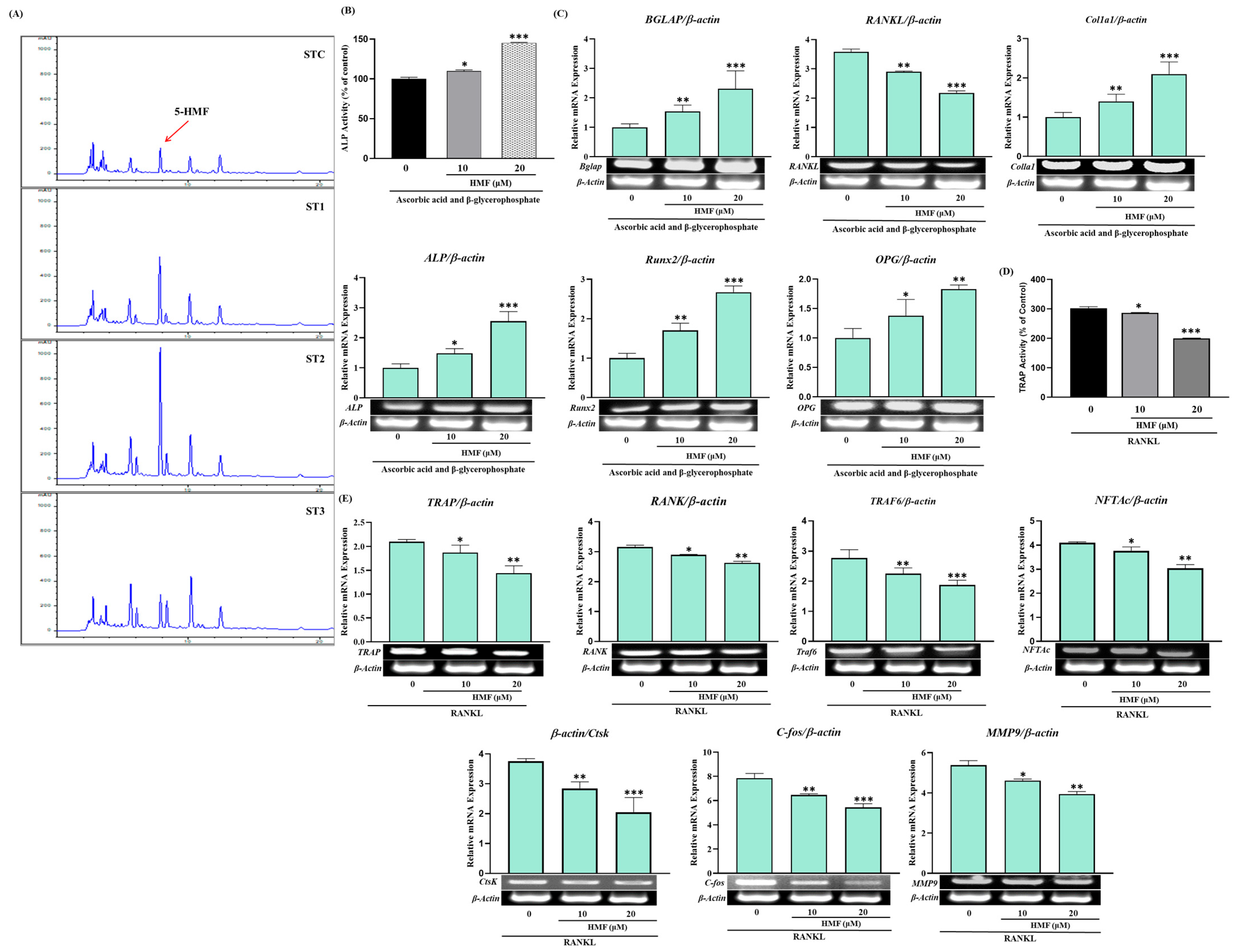
2.4. The Effect of Steaming of SHF on ALP Activity in MC3T3-E1 Cells
2.5. Effect of Steaming of SHF on Mineralization and Calcium Deposition
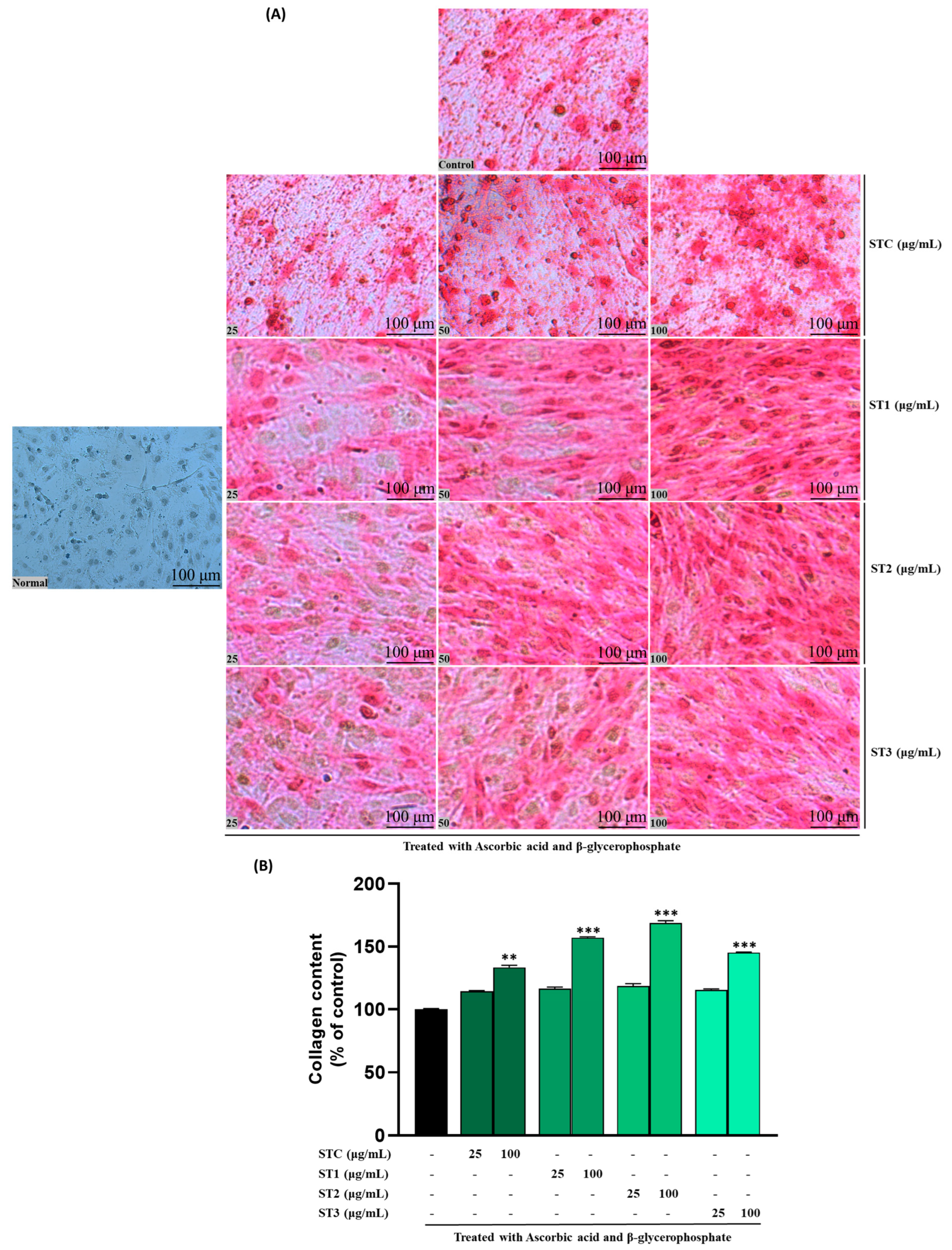
2.6. Effect of Steaming of SHF on Collagen Content
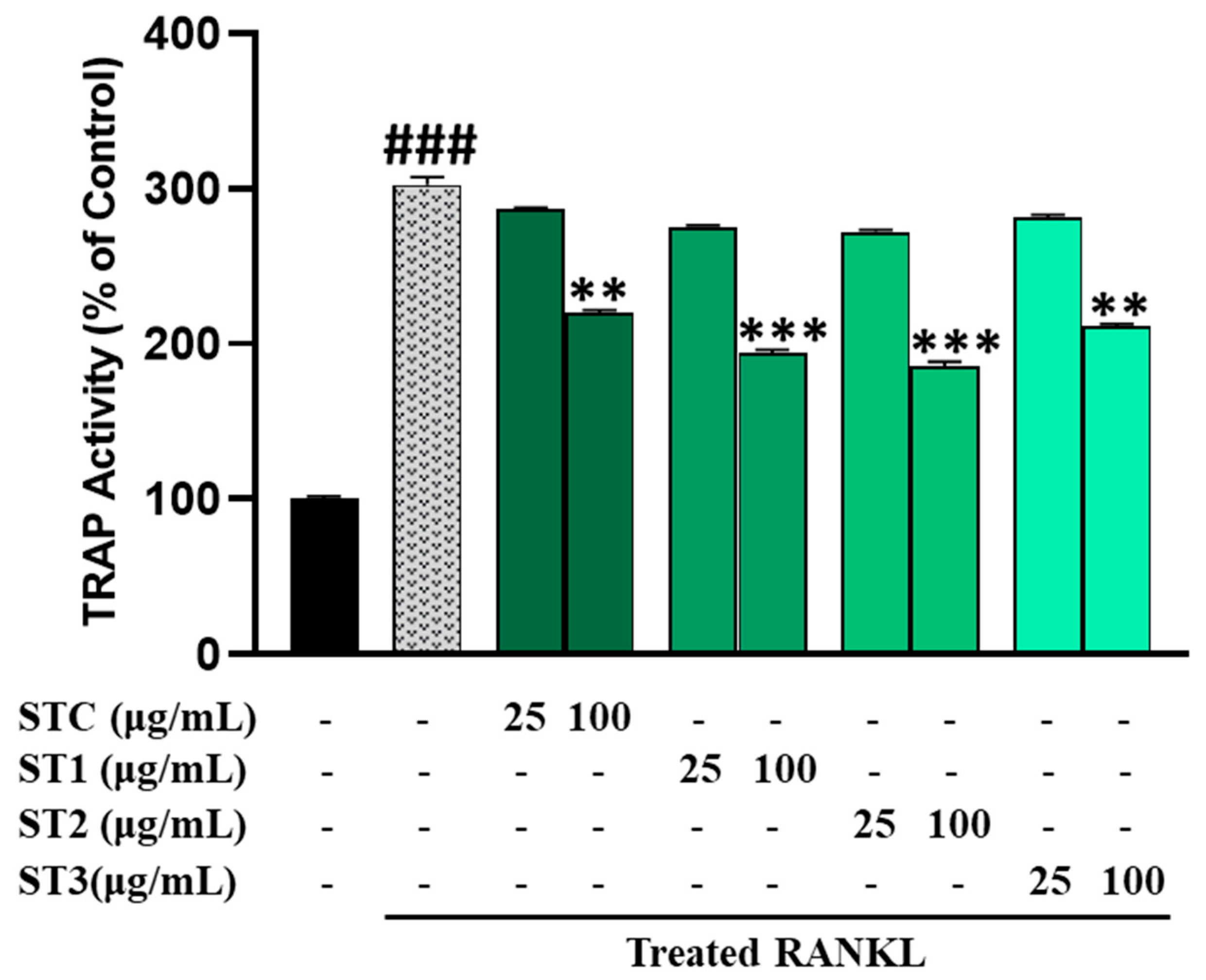
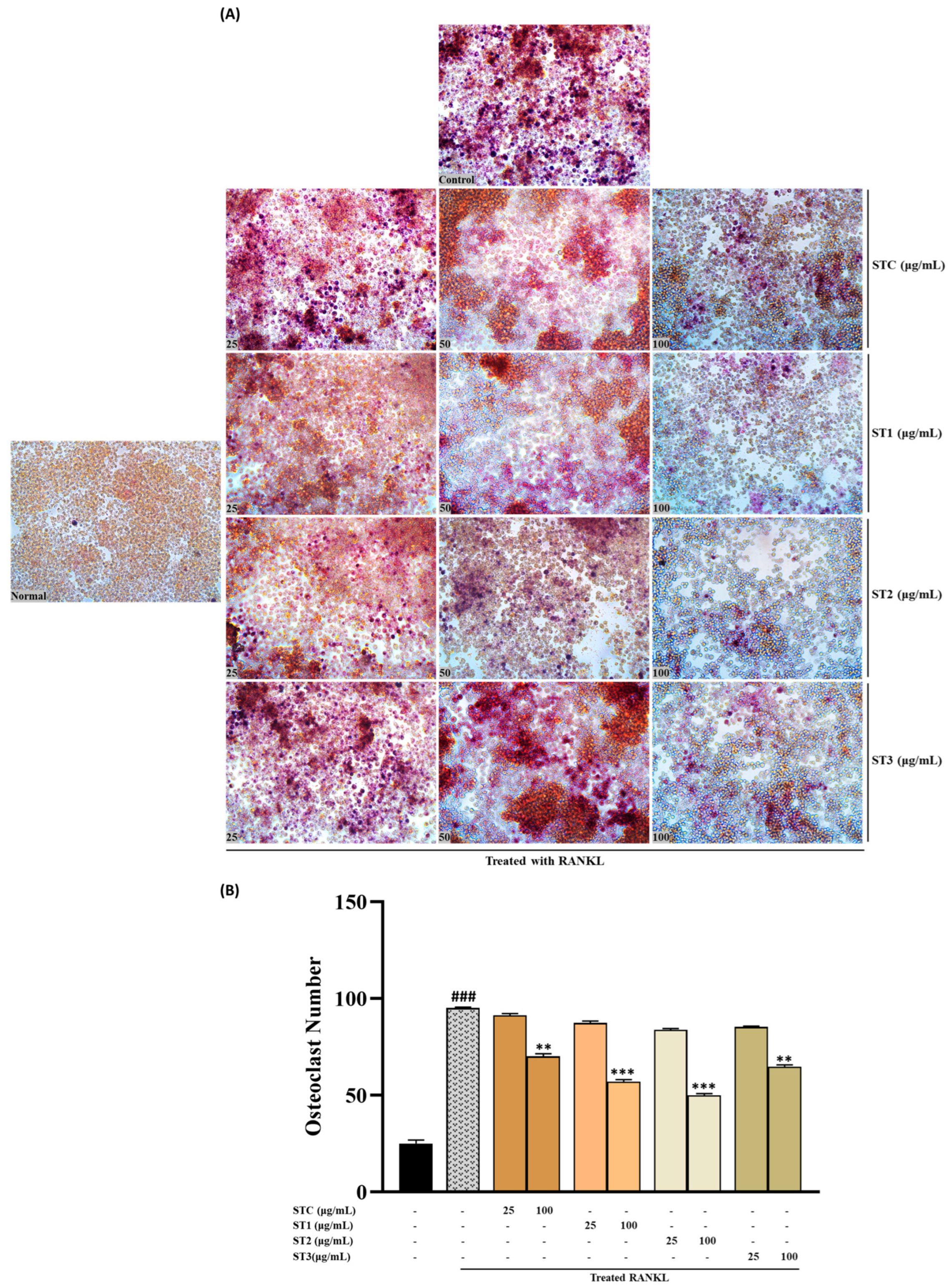
2.7. The Effect of Steaming of SHF on TRAP in RAW 264.7 Cells
2.8. The Effect of Steaming of SHF on the Expression of Genes Related to the Differentiation and Proliferation of Osteoblasts and Osteoclasts

2.9. Osteotropic Effect of 5-HMF, a Metabolite Increased in SHF by Steaming Processes
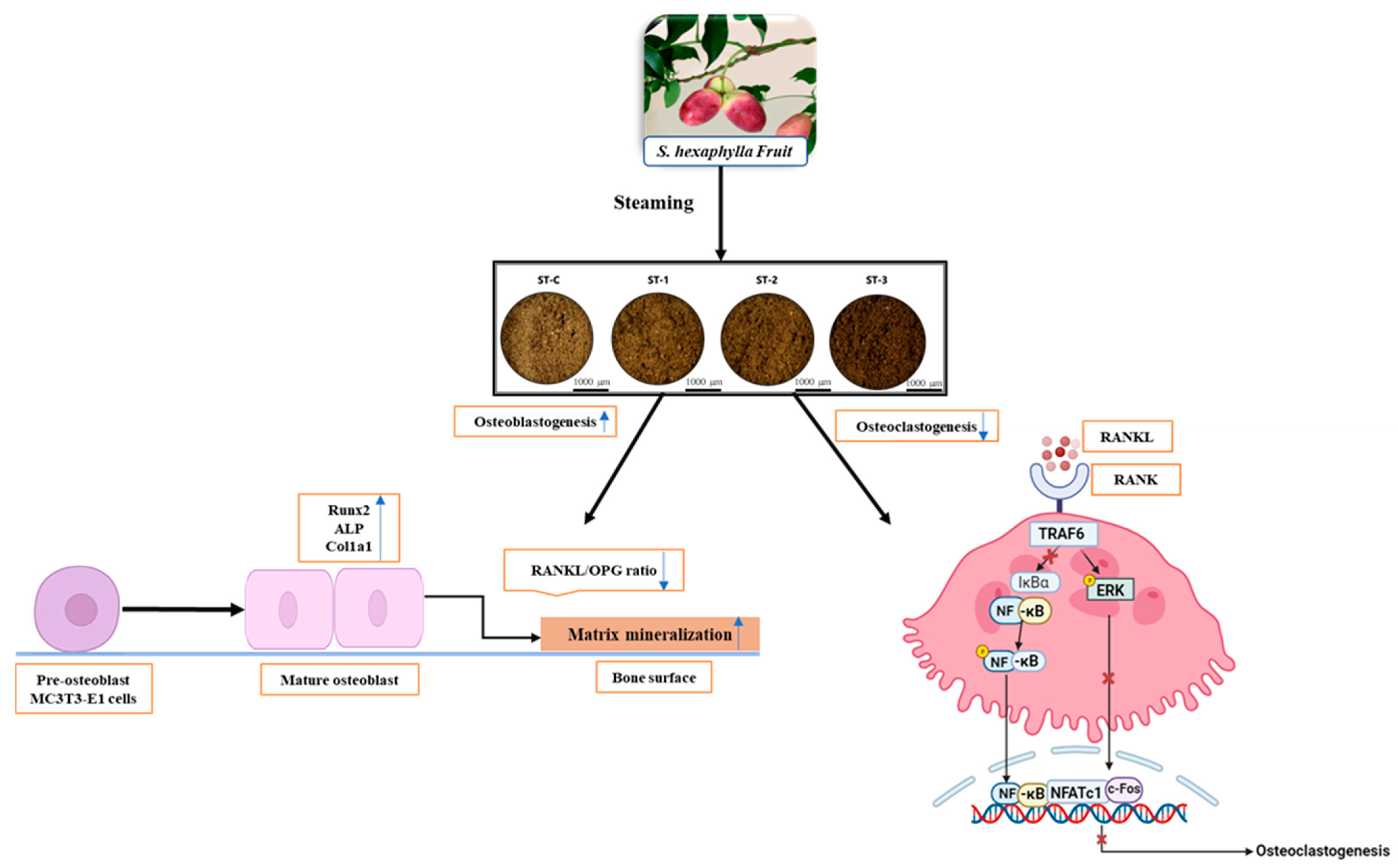
3. Discussion
4. Materials and Methods
4.1. SHF Preparation and Steaming
4.2. Isolation of 5-HMF and Identification
4.3. HPLC Analysis
4.4. Phytochemical Analysis
4.4.1. Determination of Polyphenol Content
4.4.2. Determination of Flavonoids
4.4.3. Measurement of Free Radical Scavenging Ability
4.5. Pharmacological Efficacy Analysis
4.5.1. Cell Viability Assay
4.5.2. Osteoblast Cell Culture and Differentiation
4.5.3. Alkaline Phosphatase (ALP) Activity Assay
4.5.4. Alizarin Red Staining
4.5.5. Collagen Content
4.5.6. Osteoclast Cell Culture and Differentiation
4.5.7. TRAP Activity Assay
4.5.8. RNA Isolation and Real-Time Reverse Transcription-PCR (qRT-PCR) Analysis
4.5.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HMF | 5-Hydroxymethylfurfural |
| SHF | Stauntonia hexaphylla (Thunb.) Decne fruit |
| Prep-HPLC | Preparative HPLC |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| RANKL | Receptor activator of nuclear factor kappa beta |
| CGAs | Chlorogenic acids |
| TRAP | Tartrate-resistant acid phosphatase |
| ALP | Alkaline phosphatase |
| ST-C | Unsteamed S. hexaphylla fruit extract |
| ST-1 | Once steamed S. hexaphylla fruit extract |
| ST-2 | Twice steamed S. hexaphylla fruit extract |
| ST-3 | Three times steamed S. hexaphylla fruit extract |
| DM | Differentiation medium |
| ELISA | Enzyme-linked immunosorbent assay |
| pNPP | p-Nitrophenyl phosphate |
| OD | Optical density |
| BCA | Bicinchoninic acid |
References
- Kim, J.H.; Kim, M.; Jung, H.S.; Sohn, Y. Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation. Int. J. Mol. Med. 2019, 44, 913–926. [Google Scholar] [CrossRef]
- Gkastaris, K.; Goulis, D.G.; Potoupnis, M.; Anastasilakis, A.D.; Kapetanos, G. Obesity, osteoporosis and bone metabolism. J. Musculoskelet. Neuronal Interact. 2020, 20, 372. [Google Scholar] [PubMed]
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch. Osteoporos. 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Skjødt, M.K.; Frost, M.; Abrahamsen, B. Side effects of drugs for osteoporosis and metastatic bone disease. Br. J. Clin. Pharmacol. 2019, 85, 1063–1071. [Google Scholar] [CrossRef]
- Ramesh, H.; Navabalan, V.; Natarajan, A. Bisphosphonates-what’s new? Int. J. Basic Clin. Pharmacol. 2022, 11, 664. [Google Scholar] [CrossRef]
- Raut, N.; Wicks, S.M.; Lawal, T.O.; Mahady, G.B. Epigenetic regulation of bone remodeling by natural compounds. Pharmacol. Res. 2019, 147, 104350. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef]
- Jagga, S.; Sharma, A.R.; Kim, E.J.; Nam, J.-S. Isoflavone-enriched whole soy milk powder stimulates osteoblast differentiation. J. Food Sci. Technol. 2021, 58, 595–603. [Google Scholar] [CrossRef]
- Lin, K.-M.; Lu, C.-L.; Hung, K.-C.; Wu, P.-C.; Pan, C.-F.; Wu, C.-J.; Syu, R.-S.; Chen, J.-S.; Hsiao, P.-J.; Lu, K.-C. The paradoxical role of uric acid in osteoporosis. Nutrients 2019, 11, 2111. [Google Scholar] [CrossRef]
- Akter, R.; Awais, M.; Morshed, M.N.; Kim, J.H.; Kong, B.M.; Lee, D.W.; Choi, S.K.; Lee, C.S.; Ahn, J.C.; Yang, D.C. Synergistic Effect of Stauntonia hexaphylla (Thunb.) Decne Fruit and Leaf on RAW 264.7 Osteoclast and MC3T3-E1 Osteoblast Differentiation. Biomolecules 2025, 15, 844. [Google Scholar] [CrossRef]
- Vinh, L.B.; Jo, S.J.; Nguyen Viet, P.; Gao, D.; Cho, K.W.; Koh, E.-J.; Park, S.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. The chemical constituents of ethanolic extract from Stauntonia hexaphylla leaves and their anti-inflammatory effects. Nat. Prod. Res. 2021, 35, 1852–1855. [Google Scholar] [CrossRef]
- Lee, G.; Shin, J.; Jo, A.; Lm, S.; Kim, M.-R.; Shoi, Y.; Yun, H.; Bae, D.; Kim, J.; Choi, C.-Y. Antipostmenopausal effects of Stauntonia hexaphylla and Vaccinium bracteatum fruit combination in estrogen-deficient rats. Food Nutr. Res. 2020, 64, 5233. [Google Scholar] [CrossRef]
- Lee, G.; Kim, J.; Kang, H.; Bae, D.; Choi, C.-Y. Antioxidant Activities and Hepato-protective Effects of Stauntonia hexaphylla Fruit Extract Against H2O2-induced Oxidative Stress and Acetaminophen-induced Toxicity. J. Life Sci. 2018, 28, 708–717. [Google Scholar]
- Oh, J.; Han, Y.; Kim, J.; Park, C.; Oh, D.; Yun, H.; Lee, G.; Kim, J.; Choi, C.; Lee, Y. Anti-fatigue activity of a mixture of Stauntonia hexaphylla (Thunb.) decaisne and Vaccinium bracteatum Thunb. fruit extract. Prev. Nutr. Food Sci. 2020, 25, 380. [Google Scholar] [CrossRef]
- Park, Y.-J.; Park, Y.-S.; Towantakavanit, K.; Park, J.-O.; Kim, Y.-M.; Jung, K.-J.; Cho, J.-Y.; Lee, K.-D.; Heo, B.-G. Chemical components and biological activity of Stauntonia hexaphylla. Korean J. Plant Resour. 2009, 22, 403–411. [Google Scholar]
- Karunasagara, S.; Hong, G.-L.; Jung, D.-Y.; Kim, K.-H.; Cho, K.; Jung, J.-Y. Protective effects of combination of Stauntonia hexaphylla and Cornus officinalis on testosterone-induced benign prostatic hyperplasia through inhibition of 5α-reductase type 2 and induced cell apoptosis. PLoS ONE 2020, 15, e0236879. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Mujumdar, A.S.; Zhang, M. New development in radio frequency heating for fresh food processing: A review. Food Eng. Rev. 2019, 11, 29–43. [Google Scholar] [CrossRef]
- Park, E.-S.; Song, G.-H.; Kim, S.-H.; Lee, S.-M.; Kim, Y.-G.; Lim, Y.-l.; Kang, S.A.; Park, K.-Y. Rumex crispus and Cordyceps militaris mixture ameliorates production of pro-inflammatory cytokines induced by lipopolysaccharide in C57BL/6 mice splenocytes. Prev. Nutr. Food Sci. 2018, 23, 374. [Google Scholar] [CrossRef]
- Hong, Q.; Chen, G.; Wang, Z.; Chen, X.; Kan, J. Effects of different thermal processing methods on bioactive components, phenolic compounds, and antioxidant activities of Qingke (highland hull-less barley). Food Sci. Hum. Wellness 2023, 12, 119–129. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, F.; Abbasi, A.M.; Chang, X.; Guo, X.J.M. Effect of steaming processing on phenolic profiles and cellular antioxidant activities of Castanea mollissima. Molecules 2019, 24, 703. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, H.; Kalra, S. Alkaline Phosphatase: The Poor Man’s iPTH-An Affordable Approach to Bone Health Assessment. JPMA. J. Pak. Med. Assoc. 2024, 74, 1374–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.; Nie, J.; Liu, Z.; Chen, F.; Li, A.; Pei, D. Journey of Mineral precursors in Bone mineralization: Evolution and inspiration for Biomimetic Design. Small 2024, 20, 2207951. [Google Scholar] [CrossRef]
- Chawla, V.; Bundel, P.; Singh, Y. ALP-Mimetic Cyclic Peptide Nanotubes: A Multifunctional Strategy for Osteogenesis and Bone Regeneration. Biomacromolecules 2025, 26, 1686–1700. [Google Scholar] [CrossRef]
- Rathod, B.; Desai, S.; Samvelyan, H.J.; Bock, L.; Wu, J.; Ohlsson, C.; Palmquist, A.; Alm, J.J.; Newton, P.T.; Andersson, G. Tartrate-resistant acid phosphatase (TRAP/ACP5) promotes bone length, regulates cortical and trabecular bone mass, and maintains growth plate architecture and width in a sex-and site-specific manner in mice. Bone 2024, 188, 117223. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative stress and natural antioxidants in osteoporosis: Novel preventive and therapeutic approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Akter, R.; Boopathi, V.; Awais, M.; Park, J.; Kong, B.M.; Oh, S.-W.; Oh, J.-H.; Ahn, J.C.; Yang, D.C. In Silico and In Vitro Evaluation of Antiobesogenic and Osteoprotective Effect of Pomegranate Juice Fermented by Tannin Acyl Hydrolase and Lactobacillus vespulae DCY75 via the Wnt/β-Catenin Pathway. ACS Food Sci. Technol. 2023, 3, 1975–1987. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; Mark, K.v.d.; Sakane, C.; Kaneko, H. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef]
- Zhang, P.; Ye, J.; Dai, J.; Wang, Y.; Chen, G.; Hu, J.; Hu, Q.; Fei, J. Gallic acid inhibits osteoclastogenesis and prevents ovariectomy-induced bone loss. Front. Endocrinol. 2022, 13, 963237. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chen, K.-T.; Lin, J.-A.; Chen, Y.-T.; Chen, Y.-A.; Wu, J.-T.; Hsieh, C.-W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Awais, M.; Akter, R.; Boopathi, V.; Ahn, J.C.; Lee, J.H.; Mathiyalagan, R.; Kwak, G.-Y.; Rauf, M.; Yang, D.C.; Lee, G.S. Discrimination of Dendropanax morbifera via HPLC fingerprinting and SNP analysis and its impact on obesity by modulating adipogenesis-and thermogenesis-related genes. Front. Nutr. 2023, 10, 1168095. [Google Scholar] [CrossRef]
- Akter, R.; Chan Ahn, J.; Nahar, J.; Awais, M.; Ramadhania, Z.M.; Oh, S.-W.; Oh, J.-H.; Kong, B.M.; Rupa, E.J.; Lee, D.W. Pomegranate juice fermented by tannin acyl hydrolase and Lactobacillus vespulae DCY75 enhance estrogen receptor expression and anti-inflammatory effect. Front. Pharmacol. 2022, 13, 1010103. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Akter, R.; Son, J.S.; Ahn, J.C.; Morshed, M.N.; Lee, G.J.; Kim, M.J.; An, J.T.; Kong, B.M.; Song, J.-H.; Yang, D.C. Korean Black Goat Extract Exerts Estrogen-like Osteoprotective Effects by Stimulating Osteoblast Differentiation in MC3T3-E1 Cells and Suppressing Osteoclastogenesis in RAW 264.7 Cells. Int. J. Mol. Sci. 2024, 25, 7247. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awais, M.; Akter, R.; Morshed, M.N.; Kim, J.H.; Kong, B.M.; Lee, D.W.; Choi, S.K.; Lee, C.S.; Ahn, J.C.; Yang, D.C.; et al. Steam-Processed Stauntonia hexaphylla (Thunb.) Decne Fruit Stimulates Osteoblast Differentiation in MC3T3-E1 Cells and Inhibits Osteoclastogenesis in RAW 264.7 Cells. Int. J. Mol. Sci. 2025, 26, 8411. https://doi.org/10.3390/ijms26178411
Awais M, Akter R, Morshed MN, Kim JH, Kong BM, Lee DW, Choi SK, Lee CS, Ahn JC, Yang DC, et al. Steam-Processed Stauntonia hexaphylla (Thunb.) Decne Fruit Stimulates Osteoblast Differentiation in MC3T3-E1 Cells and Inhibits Osteoclastogenesis in RAW 264.7 Cells. International Journal of Molecular Sciences. 2025; 26(17):8411. https://doi.org/10.3390/ijms26178411
Chicago/Turabian StyleAwais, Muhammad, Reshmi Akter, Md Niaj Morshed, Jong Hak Kim, Byoung Man Kong, Dong Wook Lee, Sung Keun Choi, Chang Soon Lee, Jong Chan Ahn, Deok Chun Yang, and et al. 2025. "Steam-Processed Stauntonia hexaphylla (Thunb.) Decne Fruit Stimulates Osteoblast Differentiation in MC3T3-E1 Cells and Inhibits Osteoclastogenesis in RAW 264.7 Cells" International Journal of Molecular Sciences 26, no. 17: 8411. https://doi.org/10.3390/ijms26178411
APA StyleAwais, M., Akter, R., Morshed, M. N., Kim, J. H., Kong, B. M., Lee, D. W., Choi, S. K., Lee, C. S., Ahn, J. C., Yang, D. C., & Lee, J. M. (2025). Steam-Processed Stauntonia hexaphylla (Thunb.) Decne Fruit Stimulates Osteoblast Differentiation in MC3T3-E1 Cells and Inhibits Osteoclastogenesis in RAW 264.7 Cells. International Journal of Molecular Sciences, 26(17), 8411. https://doi.org/10.3390/ijms26178411





