Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials
Abstract
1. Introduction
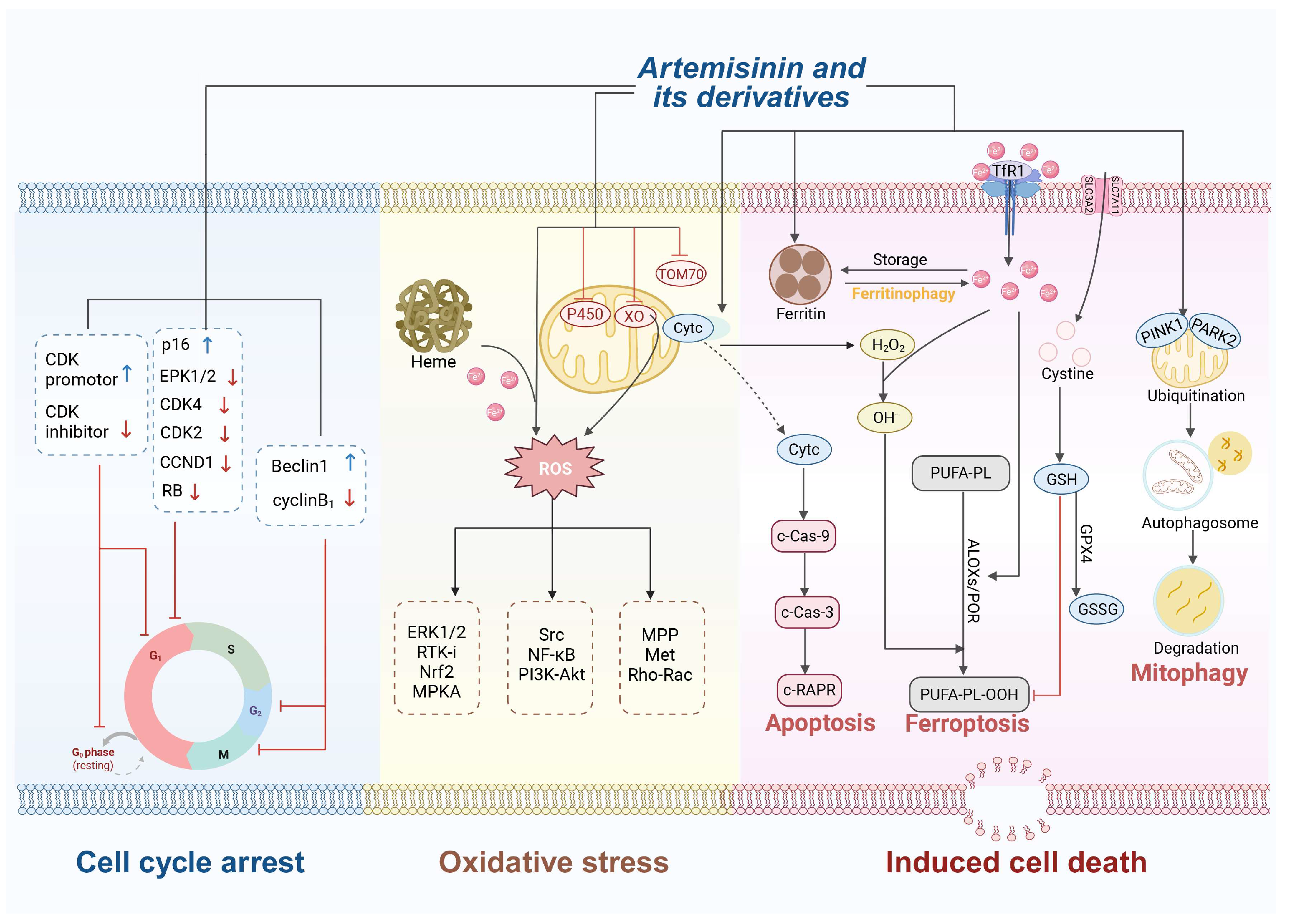
2. Antitumor Activity of Artemisinin and Its Derivatives
2.1. Mechanisms of Antitumor Activity
2.1.1. Oxidative Stress
2.1.2. Cell Cycle Arrest
2.1.3. Induction of Cell Death
Apoptosis
Ferroptosis
Autophagy
Anti-Angiogenesis
2.2. Applications in Distinct Malignancies
2.2.1. Lung Cancer
2.2.2. Hepatocellular Carcinoma
2.2.3. Breast Cancer
2.2.4. Colorectal Cancer
2.2.5. Other Tumors
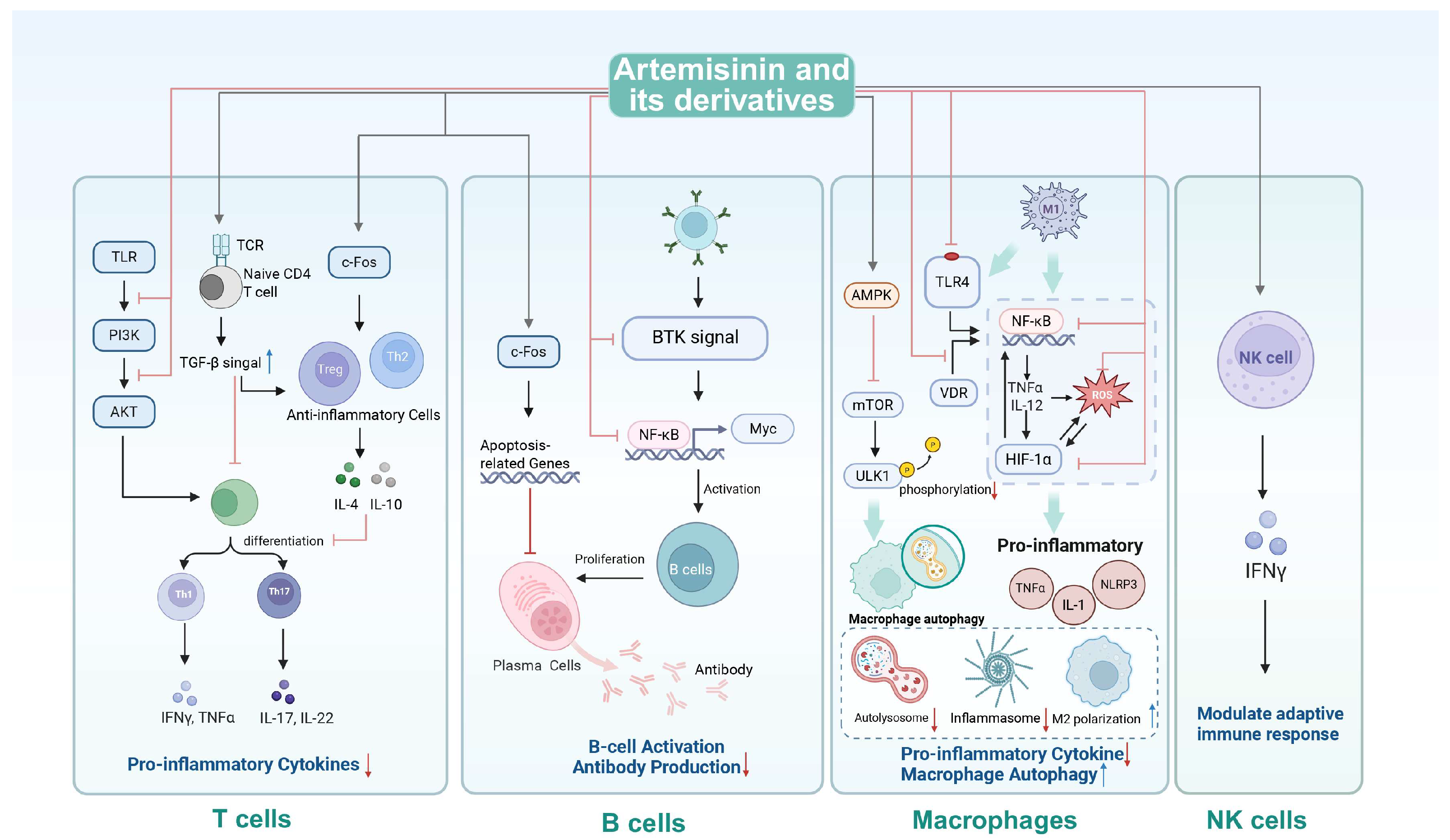
3. Immunomodulatory Activity of Artemisinin and Its Derivatives
3.1. Mechanisms of Immunomodulatory Activity
3.1.1. T-Cell Regulation
3.1.2. B-Cell Regulation
3.1.3. Macrophage Modulation
3.1.4. Natural Killer Cell Potentiation
3.2. Applications in Immune-Mediated Disorders
3.2.1. Systemic Lupus Erythematosus
3.2.2. Rheumatoid Arthritis
3.2.3. Psoriasis
3.2.4. Multiple Sclerosis
3.2.5. Other Immune Disorders
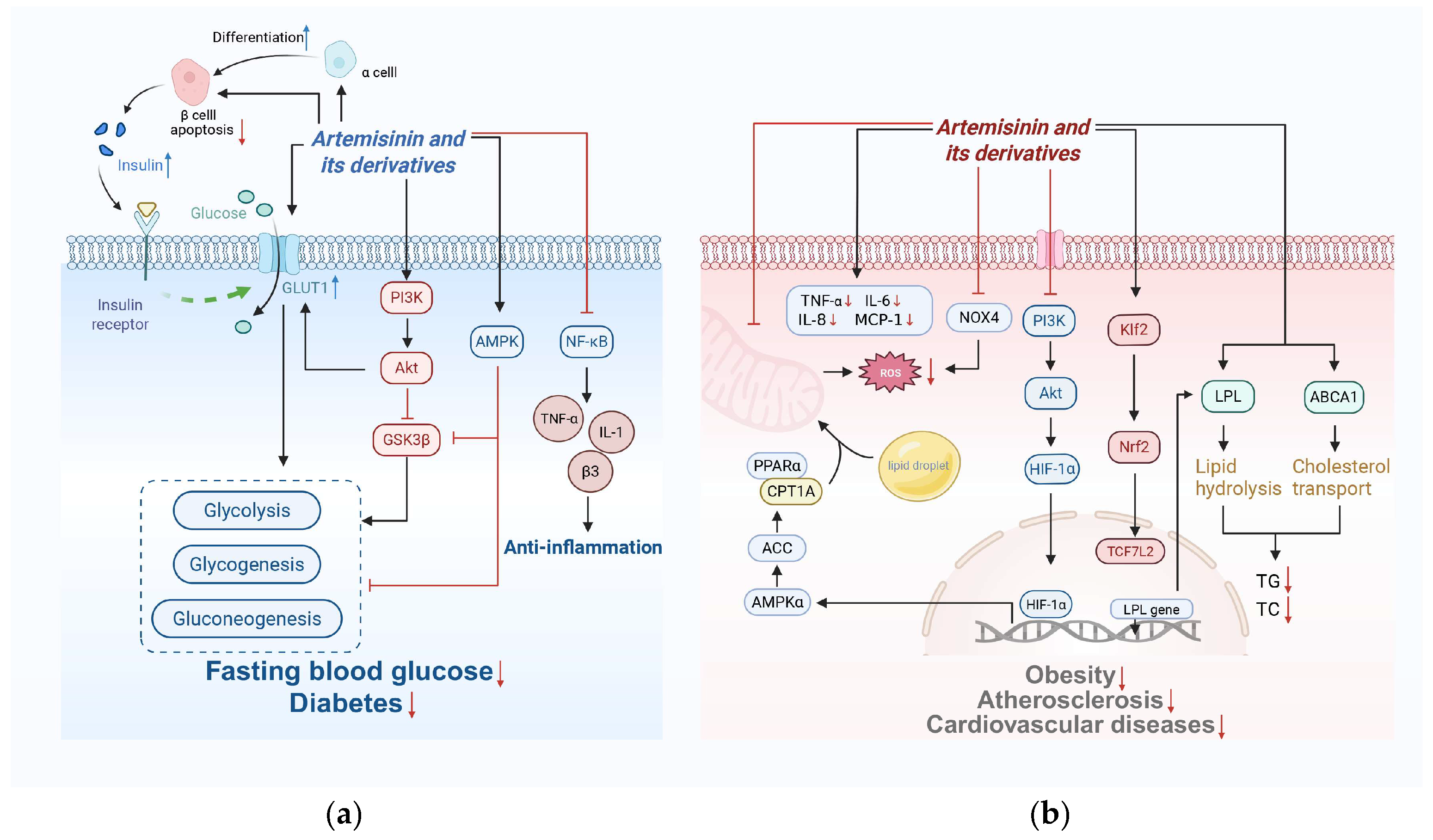
4. Metabolic Regulation of Artemisinin and Its Derivatives
4.1. Glucose Metabolism
4.2. Lipid Metabolism
4.3. Regulation of Oxidative Stress and Metabolism Interplay
5. The Trinity Modulation Network Among Antitumor Activity, Immunoregulation and Metabolic Homeostasis
5.1. Antitumor-Immunometabolic Synergy
5.2. Oxidative Stress Regulation in Tumor and Metabolic Diseases
5.3. Immune-Metabolic Crosstalk
6. Clinical Safety and Efficacy
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | Cyclin-dependent kinase |
| p16 | Cyclin-dependent kinase inhibitor 2A (CDKN2A) |
| ERK1/2 | Extracellular signal-regulated protein kinase 1/2 |
| CDK4 | Cyclin-dependent kinase 4 |
| CDK2 | Cyclin-dependent kinase 2 |
| RB | Retinoblastoma protein |
| TOM70 | Translocase of Outer Mitochondrial Membrane 70 |
| P450 | Cytochrome P450 |
| XO | Xanthine Oxidase |
| Cytc | Cytochrome c |
| ROS | Reactive Oxygen Species |
| RTK-i | Receptor Tyrosine Kinase inhibitor |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| MAPK | Mitogen-activated protein kinase |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PI3K/Akt | Phosphoinositide 3-kinase—Protein kinase B |
| MMP | Matrix metalloproteinases |
| Met | Hepatocyte Growth Factor Receptor |
| Rho | Ras homolog |
| Rac | Ras-related C3 botulinum toxin substrate |
| TfR1 | Transferrin Receptor 1 |
| SLC3A2 | Solute Carrier Family 3 Member 2 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| c-Casp9 | Cleaved Caspase-9 |
| c-Casp3 | Cleaved Caspase-3 |
| c-PARP | Cleaved Poly (ADP-ribose) Polymerase |
| PUFA-PL | Polyunsaturated Fatty Acid-Phospholipid |
| ALOXs | Arachidonate lipoxygenases |
| POR | Cytochrome P450 oxidoreductase |
| PUFA-PL-OOH | Polyunsaturated Fatty Acid-Phospholipid Hydroperoxide |
| PINK1 | PTEN Induced Kinase 1 |
| PARK2 | Parkin RBR E3 Ubiquitin Protein Ligase |
| TLR | Toll-like Receptor |
| PI3K | Phosphoinositide 3-Kinase |
| AKT | Protein Kinase B (PKB) |
| c-Fos | Cellular FOS Proto-Oncogene |
| IFN-γ | Interferon-Gamma |
| TNF-α | Tumor Necrosis Factor-α |
| BTK signal | Bruton’s Tyrosine Kinase signaling |
| Myc | MYC Proto-Oncogene |
| AMPK | AMP-activated Protein Kinase |
| mTOR | Mechanistic Target of Rapamycin |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| VDR | Vitamin D Receptor |
| HIF-1α | Hypoxia-Inducible Factor 1-α |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| GLUT1 | Glucose Transporter 1 |
| GSK-3β | Glycogen Synthase Kinase 3 beta |
| β3 | Integrin beta-3 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| NOX4 | NADPH Oxidase 4 |
| PPARα | Peroxisome Proliferator-Activated Receptor α |
| Klf2 | Krüppel-Like Factor 2 |
| LPL | Lipoprotein Lipase |
| ABCA1 | ATP-Binding Cassette Subfamily A Member 1 |
| TG | Triglyceride |
| TC | Total Cholesterol |
References
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese herbal garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B. Biological Actions of Artemisinin: Insights from Medicinal Chemistry Studies. Molecules 2010, 15, 1378–1397. [Google Scholar] [CrossRef] [PubMed]
- Ridley, R.G.; Hudson, A.T. Chemotherapy of malaria. Curr. Opin. Infect. Dis. 1998, 11, 691–706. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Chen, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, P.; Zhan, Y. Therapeutic potential of artemisinin and its derivatives in managing kidney diseases. Front. Pharmacol. 2023, 14, 1097206. [Google Scholar] [CrossRef]
- Addissouky, T.A. Artemisinin and its derivatives throughout the therapeutic mechanisms and clinical potential. Discov. Chem. 2025, 2, 10. [Google Scholar] [CrossRef]
- Augustin, Y.; Staines, H.M.; Krishna, S. Artemisinins as a novel anti-cancer therapy: Targeting a global cancer pandemic through drug repurposing. Pharmacol. Ther. 2020, 216, 107706. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Chen, D.; Chen, L.; He, B.; Li, Y. A comprehensive overview of Artemisinin and its derivatives as anticancer agents. Eur. J. Med. Chem. 2023, 247, 115000. [Google Scholar] [CrossRef]
- Li, W.D.; Dong, Y.J.; Tu, Y.Y.; Lin, Z.B. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int. Immunopharmacol. 2006, 6, 1243–1250. [Google Scholar] [CrossRef]
- Singh, N.P.; Lai, H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001, 70, 49–56. [Google Scholar] [CrossRef]
- Elhassanny, A.E.M.; Soliman, E.; Marie, M.; McGuire, P.; Gul, W.; ElSohly, M.; Van Dross, R. Heme-Dependent ER Stress Apoptosis: A Mechanism for the Selective Toxicity of the Dihydroartemisinin, NSC735847, in Colorectal Cancer Cells. Front. Oncol. 2020, 10, 965. [Google Scholar] [CrossRef]
- McCorkle, J.R.; Ahn, R.; Cao, C.D.; Hill, K.S.; Dietrich, C.S.; Kolesar, J.M. Antineoplastic Drug Synergy of Artesunate with Navitoclax in Models of High-Grade Serous Ovarian Cancer. Cancers 2024, 16, 1321. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Wang, Q.; Wang, H.; Liu, H.; Han, D.; Ren, J. A metal-organic framework-based redox homeostasis disruptor selectively potentiate the cytotoxicity of dihydroartemisinin for cancer therapy. Nano Res. 2023, 16, 7489–7495. [Google Scholar] [CrossRef]
- Ba, Q.; Zhou, N.; Duan, J.; Chen, T.; Hao, M.; Yang, X.; Li, J.; Yin, J.; Chu, R.; Wang, H. Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS ONE 2012, 7, e42703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, H.J. [Dihydroartemisinin down-regulates the expression of transferrin receptor in myeloid leukemia cells]. Yao Xue Xue Bao 2008, 43, 576–583. [Google Scholar]
- Xu, C.; Xiao, L.; Zhang, X.; Zhuang, T.; Mu, L.; Yang, X. Synthesis and biological activities of novel mitochondria-targeted artemisinin ester derivatives. Bioorg. Med. Chem. Lett. 2021, 39, 127912. [Google Scholar] [CrossRef]
- Lin, L.; Lu, W.; Dai, T.; Chen, H.; Wang, T.; Yang, L.; Yang, X.; Liu, Y.; Sun, D. Novel artemisinin derivatives with potent anticancer activities and the anti-colorectal cancer effect by the mitochondria-mediated pathway. Bioorg. Chem. 2021, 106, 104496. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90(rsk)-CREB pathway. Stem Cell Res. Ther. 2019, 10, 312. [Google Scholar] [CrossRef]
- Li, L.G.; Hu, J.; Han, N.; Chen, N.N.; Yu, T.T.; Ren, T.; Xu, H.Z.; Peng, X.C.; Li, X.Y.; Ma, T.Q.; et al. Dihydroartemisinin-driven TOM70 inhibition leads to mitochondrial destabilization to induce pyroptosis against lung cancer. Phytother. Res. 2024, 38, 3856–3876. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim. Biophys. Acta 2008, 1780, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, E.A.C. Stressed tumor cell, chemosensitized cancer. Nat. Med. 2011, 17, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Jia, J.; Qin, Y.; Zhang, L.; Guo, C.; Wang, Y.; Yue, X.; Qian, J. Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol. Med. Rep. 2016, 13, 4461–4468. [Google Scholar] [CrossRef]
- Chen, G.; Gong, R.; Shi, X.; Yang, D.; Zhang, G.; Lu, A.; Yue, J.; Bian, Z. Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget 2016, 7, 50302–50314. [Google Scholar] [CrossRef]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 5519–5530. [Google Scholar] [CrossRef]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, W.; Li, B.; Yao, Q.; Dong, J.; Cen, Y.; Pan, X.; Li, J.; Zheng, J.; Pang, X.; et al. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int. Immunopharmacol. 2011, 11, 2039–2046. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Shepherd, T.G.; Hoskin, D.W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 2017, 56, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Basu, V.; Shabnam; Murghai, Y.; Ali, M.; Sahu, S.; Verma, B.K.; Seervi, M. ONC212, alone or in synergistic conjunction with Navitoclax (ABT-263), promotes cancer cell apoptosis via unconventional mitochondrial-independent caspase-3 activation. Cell Commun. Signal. 2024, 22, 441. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Gdynia, G.; Roth, W.; Bonavida, B.; Efferth, T. Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int. J. Oncol. 2009, 35, 149–158. [Google Scholar][Green Version]
- Chen, Y.; Tao, H.; Wang, F.; Wu, P.; Gao, J.; Zhang, X.; He, Z.; Zhou, Z.; Jia, Y. Artesunate synergistically promotes sorafenib-induced apoptosis and ferroptosis in non-Hodgkin lymphoma cells through inhibition of the STAT3 pathway. Oncol. Rep. 2023, 50, 147. [Google Scholar] [CrossRef]
- Nazmabadi, R.; Pooladi, M.; Amri, J.; Abbasi, Y.; Karami, H.; Darvish, M. Dihydroartemisinin Enhances the Therapeutic Efficacy of BH3 Mimetic Inhibitor in Acute Lymphoblastic Leukemia Cells via Inhibition of Mcl-1. Asian Pac. J. Cancer Prev. 2024, 25, 325–332. [Google Scholar] [CrossRef]
- Shi, S.; Luo, H.; Ji, Y.; Ouyang, H.; Wang, Z.; Wang, X.; Hu, R.; Wang, L.; Wang, Y.; Xia, J.; et al. Repurposing Dihydroartemisinin to Combat Oral Squamous Cell Carcinoma, Associated with Mitochondrial Dysfunction and Oxidative Stress. Oxid. Med. Cell. Longev. 2023, 2023, 9595201. [Google Scholar] [CrossRef]
- Beccafico, S.; Morozzi, G.; Marchetti, M.C.; Riccardi, C.; Sidoni, A.; Donato, R.; Sorci, G. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis 2015, 36, 1071–1083. [Google Scholar] [CrossRef]
- Cheng, R.; Li, C.; Li, C.; Wei, L.; Li, L.; Zhang, Y.; Yao, Y.; Gu, X.; Cai, W.; Yang, Z.; et al. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3400–3409. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Chen, G.Q.; Benthani, F.A.; Wu, J.; Liang, D.; Bian, Z.X.; Jiang, X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020, 27, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, N.; Yang, T.; Yan, J.; Wang, W.; Li, X. The Potential Mechanisms by which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 1458143. [Google Scholar] [CrossRef] [PubMed]
- Markowitsch, S.D.; Schupp, P.; Lauckner, J.; Vakhrusheva, O.; Slade, K.S.; Mager, R.; Efferth, T.; Haferkamp, A.; Juengel, E. Artesunate Inhibits Growth of Sunitinib-Resistant Renal Cell Carcinoma Cells through Cell Cycle Arrest and Induction of Ferroptosis. Cancers 2020, 12, 3150. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, J.; Lin, R.; Lu, S.; Rong, L.; Xue, Y.; Fang, Y. Understanding the unique mechanism of ferroptosis: A promising therapeutic target. Front. Cell Dev. Biol. 2023, 11, 1329147. [Google Scholar] [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Q.; Huo, C.; Li, Y.; He, L.; Ran, B.; Chen, J.; Li, Y.; Liu, W. Ferroptosis: A Novel Mechanism of Artemisinin and its Derivatives in Cancer Therapy. Curr. Med. Chem. 2021, 28, 329–345. [Google Scholar] [CrossRef]
- Estrada-Cárdenas, P.; Camacho-Jiménez, L.; Peregrino-Uriarte, A.B.; Contreras-Vergara, C.A.; Hernandez-López, J.; Yepiz-Plascencia, G. p53 knock-down and hypoxia affects glutathione peroxidase 4 antioxidant response in hepatopancreas of the white shrimp Litopenaeus vannamei. Biochimie 2022, 199, 1–11. [Google Scholar] [CrossRef]
- Qian, B.; Che, L.; Du, Z.B.; Guo, N.J.; Wu, X.M.; Yang, L.; Zheng, Z.X.; Gao, Y.L.; Wang, M.Z.; Chen, X.X.; et al. Protein phosphatase 2A-B55β mediated mitochondrial p-GPX4 dephosphorylation promoted sorafenib-induced ferroptosis in hepatocellular carcinoma via regulating p53 retrograde signaling. Theranostics 2023, 13, 4288–4302. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef]
- Ren, M.; Liang, S.; Lin, S.; Huang, R.; Chen, Y.; Zhang, Y.; Xu, Y. Design, synthesis and biological evaluation of artesunate-Se derivatives as anticancer agents by inducing GPX4-mediated ferroptosis. Bioorg. Chem. 2024, 152, 107733. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Ren, M.; Wang, Z. Ferroptosis: A New Research Direction of Artemisinin and Its Derivatives in Anti-Cancer Treatment. Am. J. Chin. Med. 2024, 52, 161–181. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, D.; Jin, C.; Li, Z.; Sun, S.; Xia, S.; Zhang, Y.; Zhang, Z.; Zhang, F.; Xu, X.; et al. Dihydroartemisinin Induces Ferroptosis in HCC by Promoting the Formation of PEBP1/15-LO. Oxid. Med. Cell. Longev. 2021, 2021, 3456725. [Google Scholar] [CrossRef]
- Norell, P.N.; Campisi, D.; Mohan, J.; Wollert, T. Biogenesis of omegasomes and autophagosomes in mammalian autophagy. Biochem. Soc. Trans. 2024, 52, 2145–2155. [Google Scholar] [CrossRef]
- Li, B.; Bu, S.; Sun, J.; Guo, Y.; Lai, D. Artemisinin derivatives inhibit epithelial ovarian cancer cells via autophagy-mediated cell cycle arrest. Acta Biochim. Biophys. Sin. 2018, 50, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, C.; Tan, Y.H.; Zuo, S.Q.; Deng, F.M.; Sun, J.; Liu, Y.L. Dihydroartemisinin eliminates senescent cells by promoting autophagy-dependent ferroptosis via AMPK/mTOR signaling pathway. Cell Biol. Int. 2024, 48, 726–736. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, Y.; Kang, N.; Liu, Y. A new perspective on antiangiogenic antibody drug resistance: Biomarkers, mechanisms, and strategies in malignancies. Drug Dev. Res. 2024, 85, e22257. [Google Scholar] [CrossRef] [PubMed]
- Soomro, S.; Langenberg, T.; Mahringer, A.; Konkimalla, V.B.; Horwedel, C.; Holenya, P.; Brand, A.; Cetin, C.; Fricker, G.; Dewerchin, M.; et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 2011, 15, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Xu, X.Y.; Zhu, Y.Z.; Yao, N.N.; Liu, Y.C.; Gao, X.D.; Zhang, Q.; Luo, W.J. Artesunate inhibits vasculogenic mimicry in choroidal melanoma through HIF-1 α/VEGF/PDGF pathway. Acta Histochem. 2024, 126, 152174. [Google Scholar] [CrossRef]
- Rao, Q.; Yu, H.; Li, R.; He, B.; Wang, Y.; Guo, X.; Zhao, G.; Wu, F. Dihydroartemisinin inhibits angiogenesis in breast cancer via regulating VEGF and MMP-2/-9. Fundam. Clin. Pharmacol. 2024, 38, 113–125. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Chen, Q.; Ying, S.; Qin, J.; Zhang, L. Optimization of treatment strategies for elderly patients with advanced non-small cell lung cancer. Front. Oncol. 2024, 14, 1384906. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Liu, J.; Liang, D.; Shi, J.; Li, S.; Jin, J.; He, Y. Small cell lung cancer (SCLC) incidence and trends vary by gender, geography, age, and subcategory based on population and hospital cancer registries in Hebei, China (2008–2017). Thorac. Cancer 2020, 11, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, G.; Zhang, S.; Wang, D.; Saravana Prabha, P.; Zuo, Z. Antitumor Research on Artemisinin and Its Bioactive Derivatives. Nat. Prod. Bioprospect. 2018, 8, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, I.; Haider, S.; Khan, S.; Ahmed, S.; Hussain, A.; Alajmi, M.F.; Chakrabarty, A.; Afzal, M.; Imtaiyaz Hassan, M. Thymoquinone, artemisinin, and thymol attenuate proliferation of lung cancer cells as Sphingosine kinase 1 inhibitors. Biomed. Pharmacother. 2024, 177, 117123. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, J.; Yao, X.; Hou, G. Network pharmacological mechanism and molecular experimental validation of artemisinin in the treatment of lung adenocarcinoma. Toxicol. Appl. Pharmacol. 2025, 495, 117226. [Google Scholar] [CrossRef]
- Ni, L.; Zhu, X.; Zhao, Q.; Shen, Y.; Tao, L.; Zhang, J.; Lin, H.; Zhuge, W.; Cho, Y.C.; Cui, R.; et al. Dihydroartemisinin, a potential PTGS1 inhibitor, potentiated cisplatin-induced cell death in non-small cell lung cancer through activating ROS-mediated multiple signaling pathways. Neoplasia 2024, 51, 100991. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Bai, C.; Hu, Y.; Tang, J.; Zhang, Y.; Chen, J.; Zhong, Z.; He, Y.; Hu, K.; et al. Dihydroartemisinin inhibits tumor progress via blocking ROR1-induced STAT3-activation in non-small cell lung cancer. Int. Immunopharmacol. 2024, 133, 112157. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Cheng, A.; Liu, Y.; Guo, J.; Li, X.; Chen, M.; Hu, D.; Wu, J. Artesunate-binding FABP5 promotes apoptosis in lung cancer cells via the PPARgamma-SCD pathway. Int. Immunopharmacol. 2024, 143 Pt 1, 113381. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, M.J.; Lu, X.; Zhang, J.; Liu, Q.X.; Zhou, D.; Dai, J.G.; Zheng, H. Artesunate inhibits epithelial-mesenchymal transition in non-small-cell lung cancer (NSCLC) cells by down-regulating the expression of BTBD7. Bioengineered 2020, 11, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Eresen, A.; Yang, J.; Scotti, A.; Cai, K.; Yaghmai, V.; Zhang, Z. Combination of natural killer cell-based immunotherapy and irreversible electroporation for the treatment of hepatocellular carcinoma. Ann. Transl. Med. 2021, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gong, Y.; Lu, J.; Yang, Y.; Zhang, Y.; Xiong, Y.; Shi, X. Dihydroartemisinin breaks the positive feedback loop of YAP1 and GLUT1-mediated aerobic glycolysis to boost the CD8+ effector T cells in hepatocellular carcinoma. Biochem. Pharmacol. 2024, 225, 116294. [Google Scholar] [CrossRef]
- Ji, J.; Cheng, Z.; Zhang, J.; Wu, J.; Xu, X.; Guo, C.; Feng, J. Dihydroartemisinin induces ferroptosis of hepatocellular carcinoma via inhibiting ATF4-xCT pathway. J. Cell. Mol. Med. 2024, 28, e18335. [Google Scholar] [CrossRef]
- Nandi, D.; Cheema, P.S.; Singal, A.; Bharti, H.; Nag, A. Artemisinin Mediates Its Tumor-Suppressive Activity in Hepatocellular Carcinoma Through Targeted Inhibition of FoxM1. Front. Oncol. 2021, 11, 751271. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, W.; Liu, Y.; Yu, L.; Mao, X.; Guo, X.; Jiang, F.; Guo, Q.; Lin, N.; Zhang, Y. Artesunate Sensitizes human hepatocellular carcinoma to sorafenib via exacerbating AFAP1L2-SRC-FUNDC1 axis-dependent mitophagy. Autophagy 2024, 20, 541–556. [Google Scholar] [CrossRef]
- Wang, F.; Meszoely, I.; Pal, T.; Mayer, I.A.; Bailey, C.E.; Zheng, W.; Shu, X.O. Radiotherapy after breast-conserving surgery for elderly patients with early-stage breast cancer: A national registry-based study. Int. J. Cancer 2021, 148, 857–867. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Zhang, G.; Li, J.; Liu, Y.; Wang, M.; Ren, X. Nano Strategies for Artemisinin Derivatives to Enhance Reverse Efficiency of Multidrug Resistance in Breast Cancer. Curr. Pharm. Des. 2023, 29, 3458–3466. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, S.; Zeng, F.; Pan, D.; Cai, L.; Zhou, Y.; Wang, H.; Qin, G.; Zhang, C.; Chen, W. Dihydroartemisinin enhances the radiosensitivity of breast cancer by targeting ferroptosis signaling pathway through hsa_circ_0001610. Eur. J. Pharmacol. 2024, 983, 176943. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Y.; Wang, Z.F. Antitumor effects of artesunate on human breast carcinoma MCF-7 cells and IGF-IR expression in nude mice xenografts. Chin. J. Cancer Res. 2014, 26, 200–207. [Google Scholar] [PubMed]
- Gu, L.; Zhang, J.; Liu, D.; Chen, J.; Liu, S.; Peng, Q.; Tian, Y.; Du, M.; Zhang, J.; Xiao, W.; et al. Development of artesunate intelligent prodrug liposomes based on mitochondrial targeting strategy. J. Nanobiotechnol. 2022, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Brenner, H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yadav, T.T.; Khair, R.R.; Peters, G.J.; Yergeri, M.C. Combination Therapies of Artemisinin and its Derivatives as a Viable Approach for Future Cancer Treatment. Curr. Pharm. Des. 2019, 25, 3323–3338. [Google Scholar] [CrossRef]
- Geng, Y.; Li, W.; Wong, N.K.; Xue, F.; Li, Q.; Zhang, Y.; Xu, J.; Deng, Z.; Zhou, Y. Discovery of Artemisinins as Microsomal Prostaglandins Synthase-2 Inhibitors for the Treatment of Colorectal Cancer via Chemoproteomics. J. Med. Chem. 2024, 67, 2083–2094. [Google Scholar] [CrossRef]
- Dai, X.; Chen, W.; Qiao, Y.; Chen, X.; Chen, Y.; Zhang, K.; Zhang, Q.; Duan, X.; Li, X.; Zhao, J.; et al. Dihydroartemisinin inhibits the development of colorectal cancer by GSK-3beta/TCF7/MMP9 pathway and synergies with capecitabine. Cancer Lett. 2024, 582, 216596. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, F.; Zhu, X.; Liu, X.; Li, H.; Hou, L.; Ma, X.; Li, F.; Liu, H. Artesunate disrupts ribosome RNA biogenesis and inhibits ovarian cancer growth by targeting FANCA. Phytomedicine 2025, 136, 156333. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Chen, L.; Chen, H.; Qi, H.; Zheng, Y.; Du, Y.; Zhang, L.; Wang, T.; Li, Q. Dihydroartemisinin suppresses glioma growth by repressing ERRalpha-mediated mitochondrial biogenesis. Mol. Cell. Biochem. 2024, 479, 2809–2825. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.; Efferth, T.; Kaina, B. Artesunate in glioblastoma therapy: Case reports and review of clinical studies. Phytomedicine 2024, 123, 155274. [Google Scholar] [CrossRef]
- Yang, J.; Xia, T.; Zhou, S.; Liu, S.; Pan, T.; Li, Y.; Luo, Z. Anticancer Effect of Dihydroartemisinin via Dual Control of ROS-induced Apoptosis and Protective Autophagy in Prostate Cancer 22Rv1 Cells. Curr. Pharm. Biotechnol. 2024, 25, 1321–1332. [Google Scholar] [CrossRef]
- Zhou, Z.; Farhan, M.; Xing, X.; Zhou, W.; Lin, R.; Zeng, S.; Li, M.; Zheng, W. Artemisinin Suppressed Melanoma Recurrence and Metastasis after Radical Surgery through the KIT/PI3K/AKT Pathway. Int. J. Biol. Sci. 2025, 21, 75–94. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Gamper, A.M.; Kumar, D.; Agrawal, A.K. Oral delivery of dihydroartemisinin for the treatment of melanoma via bovine milk exosomes. Drug Deliv. Transl. Res. 2025, 15, 2862–2877. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, H.; Lai, W.; Hu, C.; Wang, Q.; Yuan, C.; Luo, C.; Yang, M.; Hu, M.; Zhang, R.; et al. Artesunate induces melanoma cell ferroptosis and augments antitumor immunity through targeting Ido1. Cell Commun. Signal. 2024, 22, 378. [Google Scholar] [CrossRef]
- RoyMahapatra, D.; Singh, R.; Sk, U.H.; Manna, P.P. Engineered Artesunate-Naphthalimide Hybrid Dual Drug for Synergistic Multimodal Therapy against Experimental Murine Lymphoma. Mol. Pharm. 2024, 21, 1090–1107. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; Zhou, H.; Huang, C.; Liu, G.; Zhao, X.; Cheng, Z.; You, X. Dihydroartemisinin inhibited vasculogenic mimicry in gastric cancer through the FGF2/FGFR1 signaling pathway. Phytomedicine 2024, 134, 155962. [Google Scholar] [CrossRef]
- Xu, D.L.; Fan, K.; Zhang, H.; Tang, L.X.; Wang, Y.; Xiang, Z.; Shi, A.M.; Qu, Y.C.; Su, C.J.; Pan, J. Anti-proliferation and apoptosis-inducing effects of dihydroartemisinin on SH-SY5Y cells and metabolomic analysis. Transl. Pediatr. 2022, 11, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Kleinschmidt, M.C.; Barth, S.; Rothweiler, F.; Geiler, J.; Breitling, R.; Mayer, B.; Deubzer, H.; Witt, O.; Kreuter, J.; et al. Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem. Pharmacol. 2010, 79, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.Q.; Chen, G.; Ye, M.; Jia, B. Artemether Regulates Chemosensitivity to Doxorubicin via Regulation of B7-H3 in Human Neuroblastoma Cells. Med. Sci. Monit. 2017, 23, 4252–4259. [Google Scholar] [CrossRef]
- Yu, X.H.; Wu, J.B.; Fan, H.Y.; Dai, L.; Xian, H.C.; Chen, B.J.; Liao, P.; Huang, M.C.; Pang, X.; Zhang, M.; et al. Artemisinin suppressed tumour growth and induced vascular normalisation in oral squamous cell carcinoma via inhibition of macrophage migration inhibitory factor. Oral Dis. 2024, 30, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, C.; Li, Z.; Lv, P.; An, X.; Peng, X.; Li, Y.; Jiang, X.; Mao, X.; Chen, D.; et al. Dihydroartemisinin inhibits HNSCC invasion and migration by controlling miR-195-5p expression. Heliyon 2024, 10, e32522. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Vidoni, C.; Ferraresi, A.; Loilome, W.; Yongvanit, P.; Namwat, N.; Isidoro, C. Dihydroartemisinin induces apoptosis and autophagy-dependent cell death in cholangiocarcinoma through a DAPK1-BECLIN1 pathway. Mol. Carcinog. 2018, 57, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, Y.; Liang, J.; Li, Q.; Hu, H.; Zhou, Y.; Zhang, B. Dihydroartemisinin Potentiates VEGFR-TKIs Antitumorigenic Effect on Osteosarcoma by Regulating Loxl2/VEGFA Expression and Lipid Metabolism Pathway. J. Cancer 2023, 14, 809–820. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, J.; Li, L.; Dong, Q.; Shi, Q.; Zuo, G.; Zhou, L.; Weng, Y.; Tang, M.; et al. Dihydroartemisinin inhibits tumor growth of human osteosarcoma cells by suppressing Wnt/beta-catenin signaling. Oncol. Rep. 2013, 30, 1723–1730. [Google Scholar] [CrossRef]
- Luo, J.; Odaka, Y.; Huang, Z.; Cheng, B.; Liu, W.; Li, L.; Shang, C.; Zhang, C.; Wu, Y.; Luo, Y.; et al. Dihydroartemisinin Inhibits mTORC1 Signaling by Activating the AMPK Pathway in Rhabdomyosarcoma Tumor Cells. Cells 2021, 10, 1363. [Google Scholar] [CrossRef]
- Youns, M.; Efferth, T.; Reichling, J.; Fellenberg, K.; Bauer, A.; Hoheisel, J.D. Gene expression profiling identifies novel key players involved in the cytotoxic effect of Artesunate on pancreatic cancer cells. Biochem. Pharmacol. 2009, 78, 273–283. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Wang, M.; Cao, X.; Qi, J.; Wang, D.; Gong, A.; Zhu, H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des. Dev. Ther. 2019, 13, 2135–2144. [Google Scholar] [CrossRef]
- Botrous, S.; Elmaghraby, A.; Achy, S.E.; Mustafa, Y.; Abdel-Rahman, S. Artemisinin pre-treatment fore cisplatin dosage enhances high grade urothelial carcinoma treatment in male albino mice via reverse gene expression modulation of FGFR3, HRAS, P53 and KDM6A. BMC Cancer 2024, 24, 971. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, L.; Yan, G.; Du, S.; Liu, J.; Shi, Y. Susceptibility of cervical cancer to dihydroartemisinin-induced ferritinophagy-dependent ferroptosis. Front. Mol. Biosci. 2023, 10, 1156062. [Google Scholar] [CrossRef]
- Hu, C.J.; Zhou, L.; Cai, Y. Dihydroartemisinin induces apoptosis of cervical cancer cells via upregulation of RKIP and downregulation of bcl-2. Cancer Biol. Ther. 2014, 15, 279–288. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Shi, X.; Li, S.; Tang, P.M.; Li, Z.; Li, H.; Wei, C. Antimalarial Dihydroartemisinin triggers autophagy within HeLa cells of human cervical cancer through Bcl-2 phosphorylation at Ser70. Phytomedicine 2019, 52, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Disbrow, G.L.; Baege, A.C.; Kierpiec, K.A.; Yuan, H.; Centeno, J.A.; Thibodeaux, C.A.; Hartmann, D.; Schlegel, R. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 2005, 65, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Liu, Z.N.; Ye, J.; Sha, M.; Qian, H.; Bu, X.H.; Luan, Z.Y.; Xu, X.L.; Huang, A.H.; Yuan, D.L.; et al. Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol. Int. 2014, 38, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Thanaketpaisarn, O.; Waiwut, P.; Sakurai, H.; Saiki, I. Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-kappaB and PI3K/Akt signaling pathways. Int. J. Oncol. 2011, 39, 279–285. [Google Scholar]
- Yang, C.; Wei, W.; Hu, F.; Zhao, X.; Yang, H.; Song, X.; Sun, Z. Dihydroartemisinin suppresses the tumorigenesis of esophageal carcinoma by elevating DAB2IP expression in a NFIC-dependent manner. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 8117–8128. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, Y.; Huang, Z.; Ke, N.; Zheng, Y.; Zhuang, W.; Zhang, Y.; Yin, X.; Tu, M.; Chen, J.; et al. Artesunate-loaded solid lipid nanoparticles resist esophageal squamous cell carcinoma by inducing Ferroptosis through inhibiting the AKT/mTOR signaling. Cell. Signal. 2024, 117, 111108. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Zhao, Z.; Liu, Y.; Zhao, Z.; Fu, K.; Li, B.; Jin, J. Artemisinin suppresses aerobic glycolysis in thyroid cancer cells by downregulating HIF-1a, which is increased by the XIST/miR-93/HIF-1a pathway. PLoS ONE 2023, 18, e0284242. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Min, K.-J.; Kwon, T.K. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Mol. Cell. Biochem. 2017, 435, 15–24. [Google Scholar] [CrossRef]
- Huang, C.F.; Lin, S.S.; Liao, P.H.; Young, S.C.; Yang, C.C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell. Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Langroudi, L.; Hassan, Z.M.; Ebtekar, M.; Mahdavi, M.; Pakravan, N.; Noori, S. A comparison of low-dose cyclophosphamide treatment with artemisinin treatment in reducing the number of regulatory T cells in murine breast cancer model. Int. Immunopharmacol. 2010, 10, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.I.; Azambuja, J.H.; Olalla Saad, S.T. Artesunate strongly modulates myeloid and regulatory T cells to prevent LPS-induced systemic inflammation. Biomed. Pharmacother. 2021, 143, 112211. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Tang, W.; Shi, L.P.; Wan, J.; Zhou, R.; Ni, J.; Fu, Y.F.; Yang, Y.F.; Li, Y.; Zuo, J.P. Investigation of the immunosuppressive activity of artemether on T-cell activation and proliferation. Br. J. Pharmacol. 2007, 150, 652–661. [Google Scholar] [CrossRef]
- Hou, L.F.; He, S.J.; Wang, J.X.; Yang, Y.; Zhu, F.H.; Zhou, Y.; He, P.L.; Zhang, Y.; Yang, Y.F.; Li, Y.; et al. SM934, a water-soluble derivative of arteminisin, exerts immunosuppressive functions in vitro and in vivo. Int. Immunopharmacol. 2009, 9, 1509–1517. [Google Scholar] [CrossRef]
- Li, Y.; Shan, Y.; Xu, L.; Chen, W.; Li, Y. Dihydroartemisinin ameliorates experimental autoimmune myasthenia gravis by regulating CD4(+) T cells and modulating gut microbiota. Int. Immunopharmacol. 2024, 139, 112699. [Google Scholar] [CrossRef]
- Zhu, S.; Cui, Y.; Hu, H.; Zhang, C.; Chen, K.; Shan, Z.; Teng, W.; Li, J. Dihydroartemisinin inhibits the development of autoimmune thyroiditis by modulating oxidative stress and immune imbalance. Free Radic. Biol. Med. 2025, 231, 57–67. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Lv, J.; Zhuang, W.; Xie, L.; Liu, G.; Saimaier, K.; Han, S.; Shi, C.; Hua, Q.; et al. TPN10475 Constrains Effector T Lymphocytes Activation and Attenuates Experimental Autoimmune Encephalomyelitis Pathogenesis by Facilitating TGF-β Signal Transduction. J. Neuroimmune Pharmacol. 2024, 19, 6. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, N.; Zhang, Y.; Liu, Y.; Su, Z.; Yuan, Q.; Sang, X.; Chen, R.; Feng, Y.; Chen, Q. Dihydroartemisinin imposes positive and negative regulation on Treg and plasma cells via direct interaction and activation of c-Fos. Commun. Biol. 2023, 6, 52. [Google Scholar] [CrossRef]
- Yang, Z.; Han, F.; Liao, T.; Zheng, H.; Luo, Z.; Ma, M.; He, J.; Li, L.; Ye, Y.; Zhang, R.; et al. Artemisinin Attenuates Transplant Rejection by Inhibiting Multiple Lymphocytes and Prolongs Cardiac Allograft Survival. Front. Immunol. 2021, 12, 634368. [Google Scholar] [CrossRef]
- Shi, X.; Liao, T.; Chen, Y.; Chen, J.; Liu, Y.; Zhao, J.; Dang, J.; Sun, Q.; Pan, Y. Dihydroartemisinin inhibits follicular helper T and B cells: Implications for systemic lupus erythematosus treatment. Arch. Pharm. Res. 2024, 47, 632–644. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, N.; Zhao, X.; Sang, X.; Yang, N.; Feng, Y.; Chen, R.; Chen, Q. Dihydroartemisinin regulates the immune system by promotion of CD8+ T lymphocytes and suppression of B cell responses. Sci. China Life Sci. 2020, 63, 737–749. [Google Scholar] [CrossRef]
- Zeng, X.Z.; Zhang, Y.Y.; Yang, Q.; Wang, S.; Zou, B.H.; Tan, Y.H.; Zou, M.; Liu, S.W.; Li, X.J. Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca(2+)-NFATc1 signaling pathway. Acta Pharmacol. Sin. 2020, 41, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, J.; Li, A.; Yang, X.; Sprangers, B.; Li, S. Artemisinin attenuates IgM xenoantibody production via inhibition of T cell-independent marginal zone B cell proliferation. J. Leukoc. Biol. 2021, 109, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, L.; Liu, X.; Wang, P.; Wei, S.; Huang, Y.; Gu, W.; Bo, R.; Liu, M.; Yu, J.; et al. Chitosan-coated artesunate protects against ulcerative colitis via STAT6-mediated macrophage M2 polarization and intestinal barrier protection. Int. J. Biol. Macromol. 2024, 254 Pt 1, 127680. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, C.; Deng, F.; Ouyang, F.; Qin, R.; Zhai, Z.; Wang, Y.; Zhang, Y.; Liao, M.; Pan, X.; et al. Artesunate protects against a mouse model of cerulein and lipopolysaccharide-induced acute pancreatitis by inhibiting TLR4-dependent autophagy. Int. J. Mol. Med. 2025, 55, 25. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Q.; Zhou, L.; Cao, Y.; Yang, J.; Li, J.; Liu, J.; Bi, J.; Liu, Y. An ROS-responsive artesunate prodrug nanosystem co-delivers dexamethasone for rheumatoid arthritis treatment through the HIF-1alpha/NF-kappaB cascade regulation of ROS scavenging and macrophage repolarization. Acta Biomater. 2022, 152, 406–424. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, K.; Yang, Y.; Wu, Z. Dihydroartemisinin ameliorates innate inflammatory response induced by Streptococcussuis-derived muramidase-released protein via inactivation of TLR4-dependent NF-kappaB signaling. J. Pharm. Anal. 2023, 13, 1183–1194. [Google Scholar] [CrossRef]
- Yang, F.M.; Fan, D.; Yang, X.Q.; Zhu, F.H.; Shao, M.J.; Li, Q.; Liu, Y.T.; Lin, Z.M.; Cao, S.Q.; Tang, W.; et al. The artemisinin analog SM934 alleviates dry eye disease in rodent models by regulating TLR4/NF-κB/NLRP3 signaling. Acta Pharmacol. Sin. 2021, 42, 593–603. [Google Scholar] [CrossRef]
- Huai, M.; Zeng, J.; Ge, W. Artemisinin ameliorates intestinal inflammation by skewing macrophages to the M2 phenotype and inhibiting epithelial-mesenchymal transition. Int. Immunopharmacol. 2021, 91, 107284. [Google Scholar] [CrossRef]
- Sun, W.; Han, X.; Wu, S.; Wu, J.; Yang, C.; Li, X. Unexpected mechanism of colitis amelioration by artesunate, a natural product from Artemisia annua L. Inflammopharmacology 2020, 28, 851–868. [Google Scholar] [CrossRef]
- Houh, Y.K.; Kim, K.E.; Park, S.; Hur, D.Y.; Kim, S.; Kim, D.; Bang, S.I.; Yang, Y.; Park, H.J.; Cho, D. The Effects of Artemisinin on the Cytolytic Activity of Natural Killer (NK) Cells. Int. J. Mol. Sci. 2017, 18, 1600. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Bi, J.; Wan, X. Artemisinin sensitizes tumor cells to NK cell-mediated cytolysis. Biochem. Biophys. Res. Commun. 2020, 524, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Izmirly, P.M.; Parton, H.; Wang, L.; McCune, W.J.; Lim, S.S.; Drenkard, C.; Ferucci, E.D.; Dall’Era, M.; Gordon, C.; Helmick, C.G.; et al. Prevalence of Systemic Lupus Erythematosus in the United States: Estimates From a Meta-Analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021, 73, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, T.; Wang, W.; Yang, B.; Cha, X. Dihydroartemisinin attenuated the symptoms of mice model of systemic lupus erythematosus by restoring the Treg/Th17 balance. Clin. Exp. Pharmacol. Physiol. 2021, 48, 626–633. [Google Scholar] [CrossRef]
- Zhou, F.; Li, G.; Tan, R.; Wu, G.; Deng, C. Artemisinins in autoimmune diseases: Effects and mechanisms in systemic lupus erythematosus and rheumatoid arthritis. Br. J. Pharmacol. 2025, 182, 3411–3427. [Google Scholar] [CrossRef]
- Hou, L.F.; He, S.J.; Li, X.; Yang, Y.; He, P.L.; Zhou, Y.; Zhu, F.H.; Yang, Y.F.; Li, Y.; Tang, W.; et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011, 63, 2445–2455. [Google Scholar] [CrossRef]
- Nieuwenhuis, W.P.; van Steenbergen, H.W.; Stomp, W.; Stijnen, T.; Huizinga, T.W.; Bloem, J.L.; van der Heijde, D.; Reijnierse, M.; van der Helm-van Mil, A.H. The Course of Bone Marrow Edema in Early Undifferentiated Arthritis and Rheumatoid Arthritis: A Longitudinal Magnetic Resonance Imaging Study at Bone Level. Arthritis Rheumatol. 2016, 68, 1080–1088. [Google Scholar] [CrossRef]
- Rani, R.; Raina, N.; Sharma, A.; Kumar, P.; Tulli, H.S.; Gupta, M. Advancement in nanotechnology for treatment of rheumatoid arthritis: Scope and potential applications. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2287–2310. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, Y.; Yao, C.; Liu, X.; Liu, J.; Yu, B. DC32, a Dihydroartemisinin Derivative, Ameliorates Collagen-Induced Arthritis Through an Nrf2-p62-Keap1 Feedback Loop. Front. Immunol. 2018, 9, 2762. [Google Scholar] [CrossRef]
- Chen, J.; Lin, X.; He, J.; Liu, D.; He, L.; Zhang, M.; Luan, H.; Hu, Y.; Tao, C.; Wang, Q. Artemisitene suppresses rheumatoid arthritis progression via modulating METTL3-mediated N6-methyladenosine modification of ICAM2 mRNA in fibroblast-like synoviocytes. Clin. Transl. Med. 2022, 12, e1148. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.M.; Yang, X.Q.; Zhu, F.H.; He, S.J.; Tang, W.; Zuo, J.P. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci. Rep. 2016, 6, 38115. [Google Scholar] [CrossRef]
- Liu, J.; Hong, X.; Lin, D.; Luo, X.; Zhu, M.; Mo, H. Artesunate influences Th17/Treg lymphocyte balance by modulating Treg apoptosis and Th17 proliferation in a murine model of rheumatoid arthritis. Exp. Ther. Med. 2017, 13, 2267–2273. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Liu, H.; Qiu, F.; Liang, C.L.; Zhang, Q.; Huang, R.Y.; Han, L.; Lu, C.; Dai, Z. Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8+ T-cell memory in wild-type and humanized mice. Theranostics 2020, 10, 10466–10482. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Xu, Y.; Xu, M.; Shi, Z.R.; Mai, S.Z.; Guo, Z.X.; Tang, Z.Q.; Luo, Y.J.; Guo, Q.; Xiong, H. Artesunate alleviates imiquimod-induced psoriasis-like dermatitis in BALB/c mice. Int. Immunopharmacol. 2019, 75, 105817. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, Z.; Jianchi, M.; Guo, Z.; Shi, Z.; Tang, Z.; Guo, Q.; Xiong, H. Artesunate alleviates psoriasis-like dermatitis by reducing interleukin-23 expression in tumor necrosis factor-alpha-induced HaCaT cells. Clin. Exp. Pharmacol. Physiol. 2023, 50, 903–913. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Khakzad, M.R.; Ganji, A.; Ariabod, V.; Farahani, I. Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis. Immunopharmacol. Immunotoxicol. 2017, 39, 348–353. [Google Scholar] [CrossRef]
- Thomé, R.; de Carvalho, A.C.; Alves da Costa, T.; Ishikawa, L.L.; Fraga-Silva, T.F.; Sartori, A.; de Oliveira, A.L.; Verinaud, L. Artesunate Ameliorates Experimental Autoimmune Encephalomyelitis by Inhibiting Leukocyte Migration to the Central Nervous System. CNS Neurosci. Ther. 2016, 22, 707–714. [Google Scholar] [CrossRef]
- Lv, J.; Zhuang, W.; Zhang, Y.; Xie, L.; Xiang, Z.; Zhao, Q.; Jiang, X.; Shen, J.; Du, C. 9,10-Anhydrodehydroartemisinin Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Th1 and Th17 Cell Differentiation. Inflammation 2021, 44, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, M.; Li, X.; Lv, J.; Zhuang, W.; Xie, L.; Liu, G.; Saimaier, K.; Han, S.; Shi, C.; et al. TPN10475 alleviates concanavalin A-induced autoimmune hepatitis by limiting T cell development and function through inhibition of PI3K-AKT pathway. Int. Immunopharmacol. 2023, 125 Pt A, 111110. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Han, S.; Zhuang, W.; Lv, J.; Han, M.; Xie, L.; Jiang, X.; Wang, C.; Saimaier, K.; et al. TPN10466 ameliorates Concanavalin A-induced autoimmune hepatitis in mice via inhibiting ERK/JNK/p38 signaling pathway. Eur. J. Immunol. 2023, 53, e2250100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Wang, Y.; Guo, Z.; Gu, A.D.; Dan, H.C.; Baldwin, A.S.; Hao, W.; Wan, Y.Y. Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J. Immunol. 2012, 189, 4417–4425. [Google Scholar] [CrossRef]
- Yu, R.; Jin, L.; Li, F.; Fujimoto, M.; Wei, Q.; Lin, Z.; Ren, X.; Jin, Q.; Li, H.; Meng, F.; et al. Dihydroartemisinin inhibits melanoma by regulating CTL/Treg anti-tumor immunity and STAT3-mediated apoptosis via IL-10 dependent manner. J. Dermatol. Sci. 2020, 99, 193–202. [Google Scholar] [CrossRef]
- Xiao, F.; Rui, K.; Han, M.; Zou, L.; Huang, E.; Tian, J.; Zhang, L.; Jiang, Q.; Wu, Y.; Lu, L. Artesunate suppresses Th17 response via inhibiting IRF4-mediated glycolysis and ameliorates Sjog̈ren’s syndrome. Signal Transduct. Target. Ther. 2022, 7, 274. [Google Scholar] [CrossRef]
- Cao, Q.; Du, H.; Fu, X.; Duan, N.; Liu, C.; Li, X. Artemisinin Attenuated Atherosclerosis in High-Fat Diet-Fed ApoE-/- Mice by Promoting Macrophage Autophagy Through the AMPK/mTOR/ULK1 Pathway. J. Cardiovasc. Pharmacol. 2020, 75, 321–332. [Google Scholar] [CrossRef]
- He, L.H.; Gao, J.H.; Yu, X.H.; Wen, F.J.; Luo, J.J.; Qin, Y.S.; Chen, M.X.; Zhang, D.W.; Wang, Z.B.; Tang, C.K. Artesunate inhibits atherosclerosis by upregulating vascular smooth muscle cells-derived LPL expression via the KLF2/NRF2/TCF7L2 pathway. Eur. J. Pharmacol. 2020, 884, 173408. [Google Scholar] [CrossRef]
- Huang, X.T.; Liu, W.; Zhou, Y.; Hao, C.X.; Zhou, Y.; Zhang, C.Y.; Sun, C.C.; Luo, Z.Q.; Tang, S.Y. Dihydroartemisinin attenuates lipopolysaccharide-induced acute lung injury in mice by suppressing NF-κB signaling in an Nrf2-dependent manner. Int. J. Mol. Med. 2019, 44, 2213–2222. [Google Scholar] [CrossRef]
- Jiang, M.; Zhong, G.; Zhu, Y.; Wang, L.; He, Y.; Sun, Q.; Wu, X.; You, X.; Gao, S.; Tang, D.; et al. Retardant effect of dihydroartemisinin on ulcerative colitis in a JAK2/STAT3-dependent manner. Acta Biochim. Biophys. Sin. 2021, 53, 1113–1123. [Google Scholar] [CrossRef]
- Hua, L.; Liang, S.; Zhou, Y.; Wu, X.; Cai, H.; Liu, Z.; Ou, Y.; Chen, Y.; Chen, X.; Yan, Y.; et al. Artemisinin-derived artemisitene blocks ROS-mediated NLRP3 inflammasome and alleviates ulcerative colitis. Int. Immunopharmacol. 2022, 113 Pt B, 109431. [Google Scholar] [CrossRef]
- Meng, Y.; Ma, N.; Lyu, H.; Wong, Y.K.; Zhang, X.; Zhu, Y.; Gao, P.; Sun, P.; Song, Y.; Lin, L.; et al. Recent pharmacological advances in the repurposing of artemisinin drugs. Med. Res. Rev. 2021, 41, 3156–3181. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hua, H.; Zhu, Z.; Wu, T.; Jia, Z.; Liu, Q. Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress. Apoptosis 2020, 25, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Du, M.; Liu, Q.; Gao, P.; Wang, J.; Liu, S.; Gu, L. Development of GLUT1-targeting alkyl glucoside-modified dihydroartemisinin liposomes for cancer therapy. Nanoscale 2020, 12, 21901–21912. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Y.; Yu, H.; Wei, P. Dihydroartemisinin prevents palmitate-induced β-cell apoptosis. Apoptosis 2021, 26, 147–149. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zheng, Y.; Chen, Z.; Xu, H.; Pan, S.; Liang, X.; Zhai, L.; Guan, Y.Q. Oral glucose-responsive nanoparticles loaded with artemisinin induce pancreatic beta-cell regeneration for the treatment of type 2 diabetes. J. Colloid. Interface Sci. 2025, 684 Pt 1, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Lyu, M.; Wong, Y.K.; Zhang, X.; Zhou, J.; Ma, N.; Wang, J. The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol. Ther. 2020, 216, 107696. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Pei, R.; Lei, W.; Zhao, M.; Zhang, J.; Tian, L.; Shang, J. Antidiabetic Effect of Artemether in Db/Db Mice Involves Regulation of AMPK and PI3K/Akt Pathways. Front. Endocrinol. 2020, 11, 568864. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Shen, S.; Li, X.; Liu, D.; Meng, Y.; Liu, Y.; Zhu, Y.; Zhang, J.; Luo, P.; Gu, L. Dihydroartemisinin Inhibits the Proliferation of Leukemia Cells K562 by Suppressing PKM2 and GLUT1 Mediated Aerobic Glycolysis. Drug Des. Dev. Ther. 2020, 14, 2091–2100. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, S.; Song, Y.; Ling, C. Artemisinin attenuates early renal damage on diabetic nephropathy rats through suppressing TGF-β1 regulator and activating the Nrf2 signaling pathway. Life Sci. 2020, 256, 117966. [Google Scholar] [CrossRef]
- Gao, T.; Yu, C.; Shi, X.; Hu, Y.; Chang, Y.; Zhang, J.; Wang, Y.; Zhai, Z.; Jia, X.; Mao, Y. Artemisinic acid attenuates osteoclast formation and titanium particle-induced osteolysis via inhibition of RANKL-induced ROS accumulation and MAPK and NF-κB signaling pathways. Front. Pharmacol. 2024, 15, 1345380. [Google Scholar] [CrossRef]
- Chen, Y.; Mi, Y.; Zhang, X.; Ma, Q.; Song, Y.; Zhang, L.; Wang, D.; Xing, J.; Hou, B.; Li, H.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef]
- Li, S.; Peng, T.; Zhao, X.; Silva, M.; Liu, L.; Zhou, W.; Chen, L.; Zheng, W. Artemether confers neuroprotection on cerebral ischemic injury through stimulation of the Erk1/2-P90rsk-CREB signaling pathway. Redox Biol. 2021, 46, 102069. [Google Scholar] [CrossRef]
- Peng, T.; Li, S.; Liu, L.; Yang, C.; Farhan, M.; Chen, L.; Su, Q.; Zheng, W. Artemisinin attenuated ischemic stroke induced cell apoptosis through activation of ERK1/2/CREB/BCL-2 signaling pathway in vitro and in vivo. Int. J. Biol. Sci. 2022, 18, 4578–4594. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Y.; Ge, H.; Wang, J.; Chen, X.; Lei, X.; Zhong, J.; Zhang, C.; Xian, J.; Lu, Y.; et al. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27(kip1) signaling pathway. Aging 2020, 12, 8029–8048. [Google Scholar] [CrossRef]
- Jiang, J.; Geng, G.; Yu, X.; Liu, H.; Gao, J.; An, H.; Cai, C.; Li, N.; Shen, D.; Wu, X.; et al. Repurposing the anti-malarial drug dihydroartemisinin suppresses metastasis of non-small-cell lung cancer via inhibiting NF-κB/GLUT1 axis. Oncotarget 2016, 7, 87271–87283. [Google Scholar] [CrossRef]
- Albasher, G.; Aljarba, N.; Al Sultan, N.; Alqahtani, W.S.; Alkahtani, S. Evaluation of the neuro-protective effect of Artemisia judaica extract in a murine diabetic model. J. Food Biochem. 2020, 44, e13337. [Google Scholar] [CrossRef] [PubMed]
- Farrag, E.A.E.; Hammad, M.O.; Safwat, S.M.; Hamed, S.; Hellal, D. Artemisinin attenuates type 2 diabetic cardiomyopathy in rats through modulation of AGE-RAGE/HMGB-1 signaling pathway. Sci. Rep. 2023, 13, 11043. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cen, Y.; Song, Y.; Li, P.; Qin, R.; Liu, C.; Zhao, Y.; Zheng, J.; Zhou, H. Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine 2016, 23, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.C. Artesunate inhibits adipogeneis in 3T3-L1 preadipocytes by reducing the expression and/or phosphorylation levels of C/EBP-alpha, PPAR-gamma, FAS, perilipin A, and STAT-3. Biochem. Biophys. Res. Commun. 2016, 474, 220–225. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, F.C.; Qian, S.W.; Li, X.; Cui, Z.M.; Dang, Y.J.; Tang, Q.Q. Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. 2016, 26, 1169–1172. [Google Scholar] [CrossRef]
- Guo, X.; Asthana, P.; Zhai, L.; Cheng, K.W.; Gurung, S.; Huang, J.; Wu, J.; Zhang, Y.; Mahato, A.K.; Saarma, M.; et al. Artesunate treats obesity in male mice and non-human primates through GDF15/GFRAL signalling axis. Nat. Commun. 2024, 15, 1034. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef]
- Hua, H.; Wu, M.; Wu, T.; Ji, Y.; Jin, L.; Du, Y.; Zhang, Y.; Huang, S.; Zhang, A.; Ding, G.; et al. Reduction of NADPH oxidase 4 in adipocytes contributes to the anti-obesity effect of dihydroartemisinin. Heliyon 2023, 9, e14028. [Google Scholar] [CrossRef]
- Tsui, K.H.; Wu, M.Y.; Lin, L.T.; Wen, Z.H.; Li, Y.H.; Chu, P.Y.; Li, C.J. Disruption of mitochondrial homeostasis with artemisinin unravels anti-angiogenesis effects via auto-paracrine mechanisms. Theranostics 2019, 9, 6631–6645. [Google Scholar] [CrossRef]
- Zhou, H.; You, P.; Liu, H.; Fan, J.; Tong, C.; Yang, A.; Jiang, Y.; Liu, B. Artemisinin and Procyanidins loaded multifunctional nanocomplexes alleviate atherosclerosis via simultaneously modulating lipid influx and cholesterol efflux. J. Control. Release 2022, 341, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, H.; Li, X. Artesunate attenuates atherosclerosis by inhibiting macrophage M1-like polarization and improving metabolism. Int. Immunopharmacol. 2022, 102, 108413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, H.; Liu, X.; Fu, X.; Li, X.; Cao, Q. Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J. Drug Target. 2020, 28, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chen, X.; Bai, Y.Z.; Gao, P.; Yang, Z.; Guo, Q.; Lu, Y.Y.; Zheng, J.; Liu, D.; Yang, J.; et al. Palmitoylation of PKCdelta by ZDHHC5 in hypothalamic microglia presents as a therapeutic target for fatty liver disease. Theranostics 2024, 14, 988–1009. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, J.; Gao, P.; Wang, L.; Xiang, L.; Lu, Z.; Cao, T.; Mou, A.; Zhang, X.; Jiang, X.; et al. Artemisinin alleviates obesity-related glomerulopathy by downregulating CYP24A1 expression. Diabetes Obes. Metab. 2024, 26, 767–771. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Tan, P.; Shi, H.; Huang, Z.; Cai, T.; Cheng, Y.; Du, Y.; Fu, W. Dihydroartemisinin alleviates steatosis and inflammation in nonalcoholic steatohepatitis by decreasing endoplasmic reticulum stress and oxidative stress. Bioorg. Chem. 2022, 122, 105737. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, Y.; Li, H.; Guan, H.; Xiao, H.; Li, Y. The Potential of Artemisinins as Novel Treatment for Thyroid Eye Disease by Inhibiting Adipogenesis in Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Yang, C.; Xiong, W.; Dong, J.; Zhao, X.; Liang, G.; Zheng, W. Artemisinin protected human bronchial epithelial cells from amiodarone-induced oxidative damage via 5’-AMP-activated protein kinase (AMPK) activation. Redox Rep. 2025, 30, 2447721. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, X.; Zhou, W.; Li, Q.; Lazarovici, P.; Zheng, W. Artemisinin conferred cytoprotection to human retinal pigment epithelial cells exposed to amiodarone-induced oxidative insult by activating the CaMKK2/AMPK/Nrf2 pathway. J. Transl. Med. 2024, 22, 844. [Google Scholar] [CrossRef]
- Yu, S.; Yang, N.; Li, H.; Hu, X.; Zhang, L.; Li, S. Artemether ameliorates acetaminophen-induced liver injury through Nrf2 pathway. Biomed. Pharmacother. 2024, 179, 117280. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, L.; Ma, S.; Sun, J.; Liu, J.; Gao, N.; Gou, Z.; Zhou, Y.; Lai, C.; Li, Y.; et al. Artemisinin attenuates perinatal inflammation and consequent oxidative stress in oligodendrocyte precursor cells by inhibiting IRAK-4 and IRAK-1. Int. Immunopharmacol. 2024, 142 Pt B, 113117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, J.J.; Du, S.Y.; Mu, L.S.; Fan, J.J.; Hu, J.C.; Ye, Y.; Ding, M.; Zhou, W.Y.; Yu, Q.H.; et al. Artemisinins ameliorate polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction. Science 2024, 384, eadk5382. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Y.; Han, J.; Sun, Y.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Artesunate Exerts Organ- and Tissue-Protective Effects by Regulating Oxidative Stress, Inflammation, Autophagy, Apoptosis, and Fibrosis: A Review of Evidence and Mechanisms. Antioxidants 2024, 13, 686. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Wu, T.; Feng, H.; He, M.; Yue, R.; Wu, S. Efficacy of artemisinin and its derivatives in animal models of type 2 diabetes mellitus: A systematic review and meta-analysis. Pharmacol. Res. 2022, 175, 105994. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Shui, J.C.; Zhang, B.X.; Chin, J.W.; Yue, R.S. The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus. Front. Pharmacol. 2020, 11, 585487. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ren, Y.; Ma, Y.; Liu, J.; Jiang, H.; Liu, C. Artesunate improves glucose and lipid metabolism in db/db mice by regulating the metabolic profile and the MAPK/PI3K/Akt signalling pathway. Phytomedicine 2024, 126, 155382. [Google Scholar] [CrossRef] [PubMed]
- Bantug, G.R.; Hess, C. The immunometabolic ecosystem in cancer. Nat. Immunol. 2023, 24, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Q.; Shan, Q.; Liang, T.; Forde, P.; Zheng, L. Clinical development of immuno-oncology therapeutics. Cancer Lett. 2025, 617, 217616. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Huang, C.; Xiao, X.; Zhong, Z.; Tang, J.; Lu, H.; Tang, Y.; Yang, J. Dihydroartemisinin and artesunate inhibit aerobic glycolysis via suppressing c-Myc signaling in non-small cell lung cancer. Biochem. Pharmacol. 2022, 198, 114941. [Google Scholar] [CrossRef]
- Chen, R. Dihydroartemisinin Inhibits M2-like Tumor-Associated Macrophage Polarization to Suppress the Invasion and Proliferation of Head and Neck Squamous Cell Carcinoma Cells and Its Underlying Mechanism. Ph.D. Thesis, Hebei Medical University, Shijiazhuang, China, 2020. [Google Scholar]
- Xia, L.; Qiu, Y.; Li, J.; Xu, M.; Dong, Z. The Potential Role of Artemisinins Against Neurodegenerative Diseases. Am. J. Chin. Med. 2024, 52, 1641–1660. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Tian, K.; Bai, X.; Wang, Y.; Wang, Y. Artesunate inhibits macrophage-like phenotype switching of vascular smooth muscle cells and attenuates vascular inflammatory injury in atherosclerosis via NLRP3. Biomed. Pharmacother. 2024, 172, 116255. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Firestone, G.L.; Sundar, S.N. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev. Mol. Med. 2009, 11, e32. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, X. Mitochondrial Reactive Oxygen Species (mROS) Generation and Cancer: Emerging Nanoparticle Therapeutic Approaches. Int. J. Nanomed. 2025, 20, 6085–6119. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef]
- Cluxton, D.; Petrasca, A.; Moran, B.; Fletcher, J.M. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front. Immunol. 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, L.; Nong, X. Artesunate targets cellular metabolism to regulate the Th17/Treg cell balance. Inflamm. Res. 2023, 72, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Su, Z.; Liu, D. Immunometabolism and immune response regulate macrophage function in atherosclerosis. Ageing Res. Rev. 2023, 90, 101993. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef] [PubMed]
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef]
- Svolacchia, F.; Brongo, S.; Catalano, A.; Ceccarini, A.; Svolacchia, L.; Santarsiere, A.; Scieuzo, C.; Salvia, R.; Finelli, F.; Milella, L.; et al. Natural Products for the Prevention, Treatment and Progression of Breast Cancer. Cancers 2023, 15, 2981. [Google Scholar] [CrossRef]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. EBioMedicine 2015, 2, 82–90. [Google Scholar] [CrossRef]
- Mungo, C.; Sorgi, K.; Misiko, B.; Cheserem, C.; Rahangdale, L.; Githongo, G.; Ogollah, C.; Omoto, J.; Plesa, M.; Zamboni, W. Phase I study on the pharmacokinetics of intravaginal, self-administered artesunate vaginal pessaries among women in Kenya. PLoS ONE 2025, 20, e0316334. [Google Scholar] [CrossRef]
- Wen, L.; Chan, B.C.; Qiu, M.H.; Leung, P.C.; Wong, C.K. Artemisinin and Its Derivatives as Potential Anticancer Agents. Molecules 2024, 29, 3886. [Google Scholar] [CrossRef]
- Mu, X.; Wang, C. Artemisinins—A Promising New Treatment for Systemic Lupus Erythematosus: A Descriptive Review. Curr. Rheumatol. Rep. 2018, 20, 55. [Google Scholar] [CrossRef]
- Tong, X.; Chen, L.; He, S.J.; Zuo, J.P. Artemisinin derivative SM934 in the treatment of autoimmune and inflammatory diseases: Therapeutic effects and molecular mechanisms. Acta Pharmacol. Sin. 2022, 43, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Nyaaba, N.; Andoh, N.E.; Amoh, G.; Amuzu, D.S.Y.; Ansong, M.; Ordóñez-Mena, J.M.; Hirst, J. Comparative efficacy and safety of the artemisinin derivatives compared to quinine for treating severe malaria in children and adults: A systematic update of literature and network meta-analysis. PLoS ONE 2022, 17, e0269391. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Wang, H.M.; Wang, Q.J.; Dong, Y.S.; Han, G.; Guan, Y.B.; Zhao, K.Y.; Qu, W.S.; Yuan, Y.; Gao, X.X.; et al. Subchronic toxicological study of two artemisinin derivatives in dogs. PLoS ONE 2014, 9, e94034. [Google Scholar] [CrossRef] [PubMed]
- Shakir, L.; Hussain, M.; Javeed, A.; Ashraf, M.; Riaz, A. Artemisinins and immune system. Eur. J. Pharmacol. 2011, 668, 6–14. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Goswami, A.; Saikia, P.P.; Barua, N.C.; Rao, P.G. Artemisinin and its derivatives: A novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454. [Google Scholar] [CrossRef]
- Lanna, E.G.; Siqueira, R.P.; Machado, M.G.C.; de Souza, A.; Trindade, I.C.; Branquinho, R.T.; Mosqueira, V.C.F. Lipid-based nanocarriers co-loaded with artemether and triglycerides of docosahexaenoic acid: Effects on human breast cancer cells. Biomed. Pharmacother. 2021, 134, 111114. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Chen, Q.; Han, S.; Shao, H.; Chen, P.; Jin, Q.; Yang, M.; Shangguan, F.; Fei, M.; et al. Artemisinin suppresses hepatocellular carcinoma cell growth, migration and invasion by targeting cellular bioenergetics and Hippo-YAP signaling. Arch. Toxicol. 2019, 93, 3367–3383. [Google Scholar] [CrossRef]
- Yao, Y.; Guo, Q.; Cao, Y.; Qiu, Y.; Tan, R.; Yu, Z.; Zhou, Y.; Lu, N. Artemisinin derivatives inactivate cancer-associated fibroblasts through suppressing TGF-β signaling in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 282. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.Y.; Zhu, Y.; Xie, S.Z.; Qin, L.X. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: Current status and prospectives. J. Hematol. Oncol. 2024, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Wang, Z.; Wang, J. Reconstruction of Immune Microenvironment and Signaling Pathways in Endometrioid Endometrial Adenocarcinoma During Formation of Lymphovascular Space Involvement and Lymph Node Metastasis. Front. Oncol. 2020, 10, 595082. [Google Scholar] [CrossRef]
- Steyn, J.D.; Wiesner, L.; du Plessis, L.H.; Grobler, A.F.; Smith, P.J.; Chan, W.C.; Haynes, R.K.; Kotzé, A.F. Absorption of the novel artemisinin derivatives artemisone and artemiside: Potential application of Pheroid™ technology. Int. J. Pharm. 2011, 414, 260–266. [Google Scholar] [CrossRef]
- Liu, R.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Nanoparticle Delivery of Artesunate Enhances the Anti-tumor Efficiency by Activating Mitochondria-Mediated Cell Apoptosis. Nanoscale Res. Lett. 2017, 12, 403. [Google Scholar] [CrossRef]
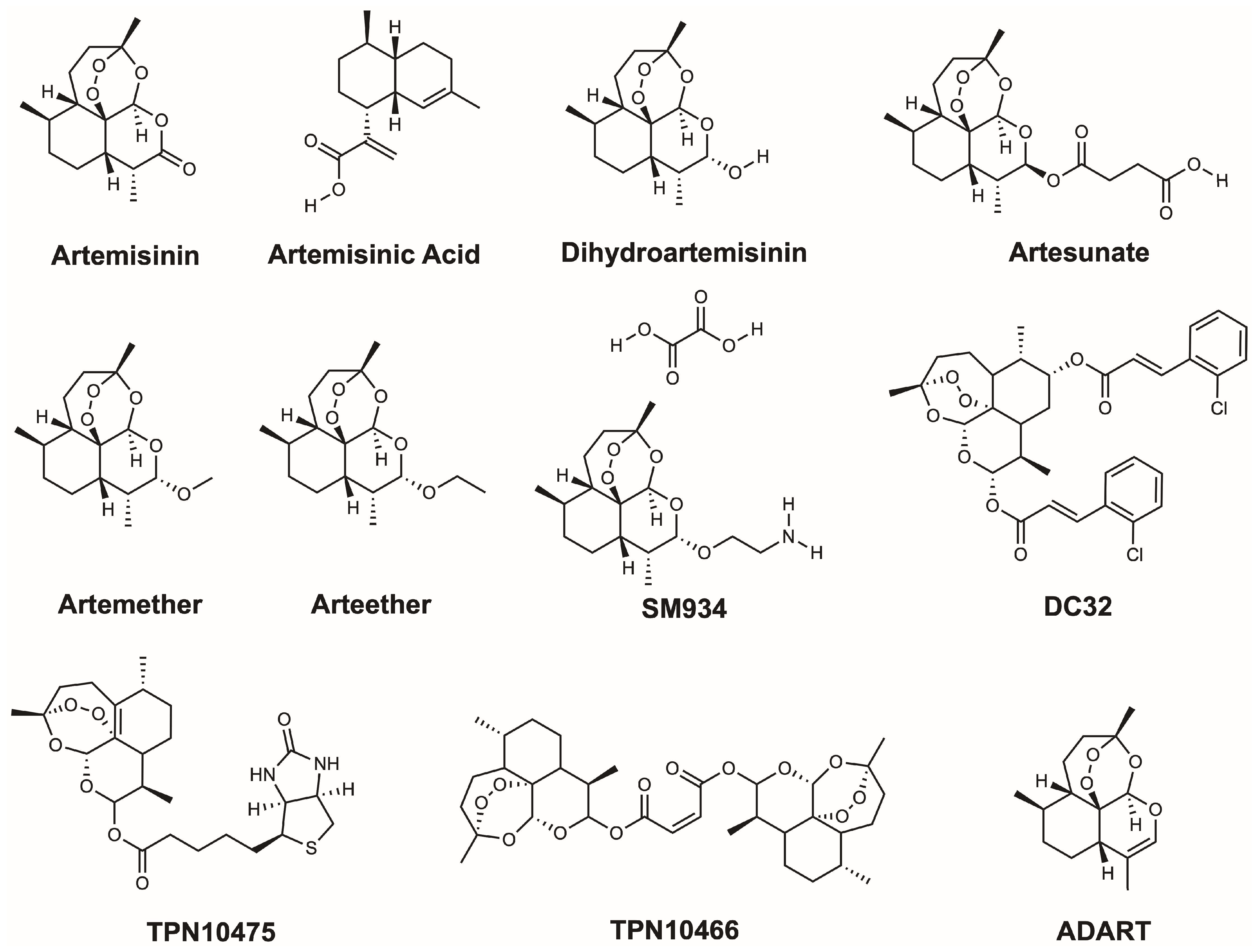
| Disease | Compound | Effect | Mechanism | Model | Reference |
|---|---|---|---|---|---|
| Leukemia | Dihydroartemisinin | inducing cell death | Mcl-1 ↓, Bcl-2 ↓, Bax ↑, P21 ↑ | in vitro | [36] |
| Ovarian cancer | Artesunate | reducing rRNA transcription, inhibiting cell proliferation and migration, and inducing apoptosis | FANCA-targeted, mTOR ↓, RPS 6 ↓ | in vitro and vivo | [90] |
| Glioblastoma | Dihydroartemisinin | blocking the cell cycle, inducing apoptosis | ERRα ↓, Caspase—3 ↑, Cleaved Caspase—9 ↑, PARP ↑ | in vitro and vivo | [91] |
| Artesunate | inducing oxidative stress, DNA damage, apoptosis, and ferroptosis | ATM/ATR axis ↑ | in vitro and vivo | [92] | |
| Prostate cancer | Dihydroartemisinin | inducing ROS-dependent mitochondrial apoptosis, autophagy | PI3K/AKT/mTOR pathway ↓ | in vitro | [93] |
| Melanoma | Artemisinin | suppressing postoperative recurrence and metastasis | KIT/PI3K/AKT pathway ↓, Cyclin D1 ↓, Bcl-2 ↓ | in vitro and vivo | [94] |
| Dihydroartemisinin | inducing ROS-dependent apoptosis, inhibiting cell migration | Bcl-2 ↓, Survivin ↓, Bax ↑, MMP—9 ↓ | in vitro and vivo | [95] | |
| Artesunate | inducing ferroptosis, activating CD8+ T-cell immunity | IDO1 ↓ | in vitro and vivo | [96] | |
| Lymphoma | Artesunate | inducing ROS-dependent apoptosis, ferroptosis, DNA damage, inhibiting angiogenesis, and activating antitumor immunity | Topo I ↓, Topo II ↓ | in vitro and vivo | [97] |
| Gastric cancer | Dihydroartemisinin | inhibiting vasculogenic mimicry | FGF2/FGFR1-MAPK/PI3K pathway ↓, VE-cadherin ↓, MMP-2 ↓, MMP-9 ↓ | in vitro and vivo | [98] |
| Neuroblastoma | Dihydroartemisinin | blocking the cell cycle, inducing mitochondrial apoptosis | PARP-1 ↑, caspase 3 ↑, γH2AX ↑ | in vitro | [99] |
| Artesunate | blocking the cell cycle, inducing mitochondrial apoptosis | activated caspase-3 ↑, sub-G1 fraction ↑ | in vitro | [100] | |
| Artemether | enhancing the caspase-dependent apoptotic effect of Doxorubicin | B7-H3 ↓, NF-κB pathway ↓ | in vitro | [101] | |
| Head and neck squamous cell carcinoma (HNSCC) | Artemisinin | inducing apoptosis, promoting vascular normalization, and enhancing antitumor immunity | MIF ↓, VEGF ↓, IL-8 ↓ | in vitro and vivo | [102] |
| Dihydroartemisinin | blocking EMT and invasive migration | miR-195-5p ↑, ZEB1/MMP-9 ↓ | in vitro | [103] | |
| Cholangiocarcinoma | Dihydroartemisinin | inducing apoptosis and autophagy | DAPK1-BECLIN1 pathway ↑ | in vitro | [104] |
| Osteosarcoma | Dihydroartemisinin | enhancing anti-angiogenesis | Loxl2 ↓, VEGFA ↓ | in vitro and vivo | [105] |
| blocking the cell cycle | Wnt/β-catenin pathway ↓, Cyclin D1 ↓, c-Myc ↓ | in vitro and vivo | [106] | ||
| Rhabdomyosarcoma | Dihydroartemisinin | inducing autophagy, blocking the cell cycle | AMPK pathway ↑, mTORC1 ↓ | in vitro and vivo | [107] |
| Artesunate | inducing mitochondrial apoptosis, inhibiting angiogenesis | ROS ↑, p38 MAPK ↑, VEGF ↓ | in vitro and vivo | [38] | |
| Pancreatic cancer | Artesunate | Inhibiting cell growth, inducing apoptosis | Top2A ↓, RRM2 ↓, PCNA ↓, NAG—1 ↑ | in vitro | [108] |
| inducing ferroptosis | GRP78-xCT axis ↓ | in vitro and vivo | [109] | ||
| Urothelial carcinoma | Artemisinin | inhibiting proliferation, enhancing the DNA-damaging effects of cisplatin | FGFR3 ↓, HRAS ↓, P53 ↑, KDM6A ↑ | in vitro and vivo | [110] |
| Cervical cancer | Dihydroartemisinin | inducing ferroptosis | GPX4 ↓, SLC7A11 ↓ | in vitro | [111] |
| inducing apoptosis | RKIP-NF-κB axis ↑, Bcl-2 ↓ | in vitro | [112] | ||
| inducing autophagy | Bcl-2 ↓ | in vitro and vivo | [113] | ||
| blocking the cell cycle | ROS ↑, caspase-9 ↑, PARP ↑ | in vitro and vivo | [114] | ||
| Artesunate | reversing immunosuppression | COX-2 ↓, PGE2 ↓, Foxp3 ↓ | in vitro and vivo | [115] | |
| enhancing the TRAIL-induced DR4/DR5-Caspase Apoptosis | NF-κB ↓, PI3K-Akt ↓ | in vitro | [116] | ||
| Esophageal carcinoma | Dihydroartemisinin | inhibiting proliferation, promoting apoptosis | DAB2IP ↑, RAS/ERK pathway ↓ | in vitro and vivo | [117] |
| Artesunate | inducing ferroptosis | AKT/mTOR-xCT/GPX4 axis ↓ | in vitro and vivo | [118] | |
| Thyroid cancer | Artemisinin | inhibiting proliferation, promoting apoptosis | XIST/miR-93/HIF-1α axis1 ↓ | in vitro and vivo | [119] |
| Renal carcinoma | Artesunate | inducing ROS-dependent necrotic apoptosis | RIP1-MLKL pathway ↑ | in vitro | [120] |
| Disease | Compound | Effect | Mechanism | Model | Reference |
|---|---|---|---|---|---|
| Autoimmune hepatitis | TPN10475 | reducing serum levels of alanine aminotransferase and aspartate aminotransferase, and inhibiting infiltrating inflammatory T cells | PI3K—AKT pathway ↓, INF-γ ↓, TNF-α ↓ | In vivo and in vitro | [166] |
| TPN10466 | reducing T-cell responses | ERK/JNK/p38 pathway ↓, TNF-α ↓, IL-6 ↓ | In vivo | [167] | |
| Autoimmune Encephalomyelitis | TPN10475 | increasing Treg, decreasing T cells, and inflammatory cell infiltration | TGF-βpathway ↑, Th1 ↓, Th17 ↓ | In vivo and in vitro | [128] |
| Dihydroartemisinin | increasing Treg and inhibiting Th cell differentiation | IFN-γ ↓, IL-4 ↓, mTOR pathway ↓, TGF-βR:Smad pathway ↑ | In vivo and in vitro | [168] | |
| Melanoma | Dihydroartemisinin | increasing CTL, inhibiting the immunosuppressive effect of Treg, and the invasion and migration of tumor cells | STAT3 pathway ↓, Treg ↓, IL-10 ↓ | In vivo and in vitro | [169] |
| Sjögren’s syndrome | Artesunate | reducing lymphocyte infiltration and immune inflammation | IRF4 ↓, Th17 ↓ | In vivo and in vitro | [170] |
| Atherosclerosis | Artemisinin | inhibiting foam macrophage transformation and enhancing macrophage autophagy | AMPK pathway ↑, mTOR ↓, ULK1 ↓, LC—3II ↑, P62 ↓ | In vivo | [171] |
| Artesunate | reducing the size of atheroplaque and lipid deposition | KLF2/NRF2/TCF7L2 pathway ↑, LPL ↑ | In vivo | [172] | |
| Acute lung injury | Dihydroartemisinin | inhibiting LPS-induced inflammatory cell infiltration, oxidative stress, and pro-inflammatory cytokine production | IL-1β ↓, TNF-α ↓, IL-6 ↓, NF-κB pathway ↓, Nrf2 pathway ↑ | In vivo and in vitro | [173] |
| Ulcerative colitis (UC) | Dihydroartemisinin | reducing inflammatory cell infiltration | JAK2/STAT3 pathway ↓, IL-6 ↓, IL-1β ↓, TNF-α ↓ | In vivo | [174] |
| Artemether | inhibiting ROS production and preventing the assembly and activation of NLRP3 inflammasomes | IL-1β ↓, IL-6 ↓, TNF-α ↓, mtROS ↓ | In vivo and in vitro | [175] | |
| Dry eye disease | SM934 | reducing inflammatory cell infiltration and inflammatory factor expression | IL-1β ↓, IL-6 ↓, TNF-α ↓, TLR4/NF-κB pathway ↓, NLRP3 ↓ | In vivo | [140] |
| Diseases | Compound | Effect | Mechanism | Progress | References |
|---|---|---|---|---|---|
| Type 2 diabetes | Artemisinin | reducing blood glucose, oxidative stress, and inflammation levels and reversing IR; increasing regeneration of pancreatic β-cell | Pax4 ↑, Arx ↑ | in vitro and in vivo | [181] |
| Artemether | promoting glycogen synthesis with antidiabetic effects | AMPK ↑, PI3K/Akt ↑ | in vivo | [183] | |
| Diabetic cardiomyopathy | Artemisinin | reducing oxidative stress, inflammation, and fibrosis | AGE-RAGE/HMGB-1 ↓ | in vivo | [193] |
| Leukemia | Dihydroartemisinin | inhibiting leukemia cell proliferation by regulating glycolysis and metabolism | PKM2 ↓, GLUT1 ↓ | in vitro | [184] |
| Non-small-cell lung cancer | Dihydroartemisinin | inhibiting glucose metabolism via NF-κB pathway inhibition | NF-κB↓, GLUT1↓ | in vitro and in vivo | [191] |
| Obesity | Artesunate | reducing food intake and promoting energy expenditure, leading to weight loss | GDF15/GFRAL ↑ | in vivo | [197] |
| Artesunate | inhibiting lipid accumulation and TG synthesis | C/EBP-a ↓, PPAR-γ ↓, FAS ↓, periilipin A ↓, STAT-3 ↓ | in vitro | [195] | |
| Atherosclerosis | Artemisinin | reducing ROS and NO; suppressing lipid influx; reducing the uptake and internalization of oxLDL | NF-κB/NLRP3 ↓, AMPK/mTOR ↑ | in vitro and in vivo | [202] |
| Artemisinin | suppressing inflammatory reaction | NF-κB/NLRP3 ↓, AMPK ↑ | in vivo | [204] | |
| Artesunate | reducing pro-inflammatory cytokine and downregulating the pro-inflammatory chemokines; attenuating the progression of atherosclerosis lesions | TNF-a ↓, IL-6 ↓, IL-8 ↓, MCP-1 ↓ | in vivo | [194] | |
| Fatty liver diseases | Artemether | inhibiting microglial activation in the hypothalamus; protecting the TRH neurons from inflammatory damage and promoting the release of TRH | palmitoylation of PKCδ ↓ | in vivo | [205] |
| Obesity-related glomerulopathy | Artemisinin | reducing the degradation of vitamin D; mitigating renal tubular damage and fibrosis | CYP24A1 ↓, vitamin D ↑ | in vivo | [206] |
| Nonalcoholic steatohepatitis (NASH) | Dihydroartemisinin | decreasing the synthesis of lipids; inhibiting OS and ERS; reducing liver steatosis | NF-κB ↓ | in vivo | [207] |
| Thyroid eye disease (TED) | Dihydroartemisinin, Artesunate | inhibiting adipogenesis; inhibiting cell proliferation and hyaluronan production | HAS2 ↓, IGF1R ↓, adipogenic markers ↓ | in vitro | [208] |
| Amiodarone-induced pulmonary toxicity | Artemisinin | regulating cellular energy homeostasis and mitigating oxidative stress; protecting against amiodarone-induced apoptosis and bronchial epithelial cell shedding | p-AMPK ↑, CaMKK2 ↑, Nrf2 ↑, SOD1 ↑ | in vitro and in vivo | [209] |
| Amiodarone-induced ocular toxicity | Artemisinin | reducing the cytotoxicity induced by amiodarone | LDH ↓, ROS ↓, MMP ↑, p-AMPK ↑, CaMKK2 ↑, Nrf2 ↑ | in vitro | [210] |
| APAP-induced acute liver injury (AILI) | Artemether | mitigating liver histological changes, including mitochondrial damage, hepatocyte necrosis, hepatocyte apoptosis, and inflammatory infiltration | Nrf2-HO-1/GPX4 ↑, ROS ↓ | in vivo | [211] |
| Abnormal white matter development (periventricular leukomalacia) | Artemisinin | reducing perinatal inflammation and inflammatory cytokine production | IRAK-4 ↓, IRAK-1 ↓, IL-1β ↓, IL-6 ↓, TNF-α ↓, ROS ↓, Nrf2 ↑ | in vitro | [212] |
| Periprosthetic osteolysis (PPO) | Artemisic acid | reducing osteoclast formation and alleviating titanium particle-induced calvarial osteolysis | ROS ↓, Nrf2 ↑, MAPK ↓, NF-κB ↓, NFATc1 ↓, c-Fos ↓ | in vitro and in vivo | [186] |
| Polycystic ovarian syndrome (PCOS) | Artemisinin | inhibiting ovarian androgen synthesis; mediating LONP1-CYP11A1 interaction; regulating mitochondrial function | CYP11A1 ↓ | in vitro and in vivo | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Shi, C.; Lu, J.; Zhu, Z.; Li, M.; Pan, Y.; Huang, X.; Zhang, L.; Liu, A. Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials. Int. J. Mol. Sci. 2025, 26, 8409. https://doi.org/10.3390/ijms26178409
Xia Y, Shi C, Lu J, Zhu Z, Li M, Pan Y, Huang X, Zhang L, Liu A. Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials. International Journal of Molecular Sciences. 2025; 26(17):8409. https://doi.org/10.3390/ijms26178409
Chicago/Turabian StyleXia, Yi, Chuanjing Shi, Jingze Lu, Zeyu Zhu, Mohan Li, Yinan Pan, Xinyan Huang, Lei Zhang, and Aifen Liu. 2025. "Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials" International Journal of Molecular Sciences 26, no. 17: 8409. https://doi.org/10.3390/ijms26178409
APA StyleXia, Y., Shi, C., Lu, J., Zhu, Z., Li, M., Pan, Y., Huang, X., Zhang, L., & Liu, A. (2025). Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials. International Journal of Molecular Sciences, 26(17), 8409. https://doi.org/10.3390/ijms26178409






