Loss of Zonula Occludens-1 (ZO-1) Enhances Angiogenic Signaling in Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
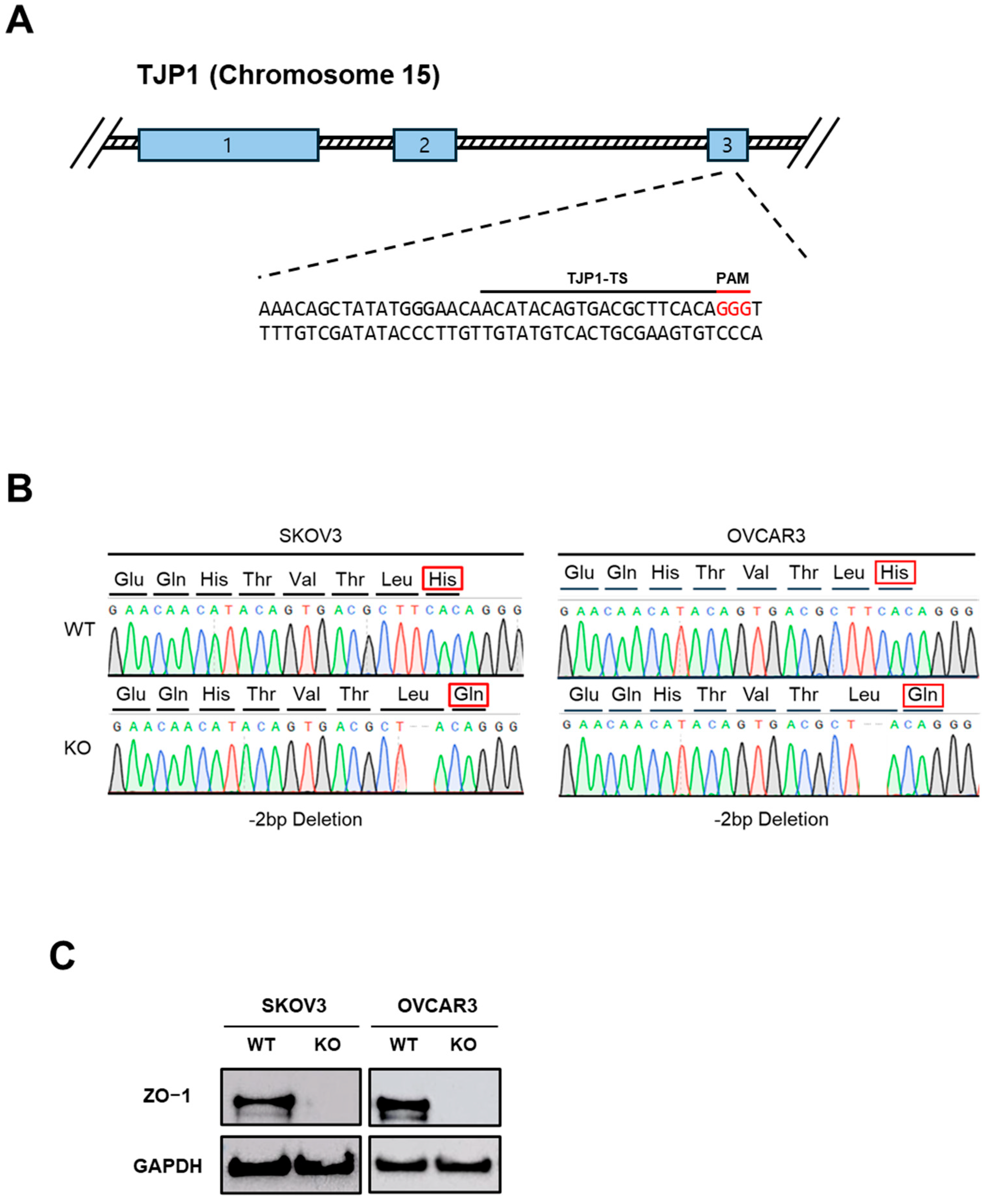
2.1. Establishment of ZO-1-Deficient Ovarian Cancer Cell Lines
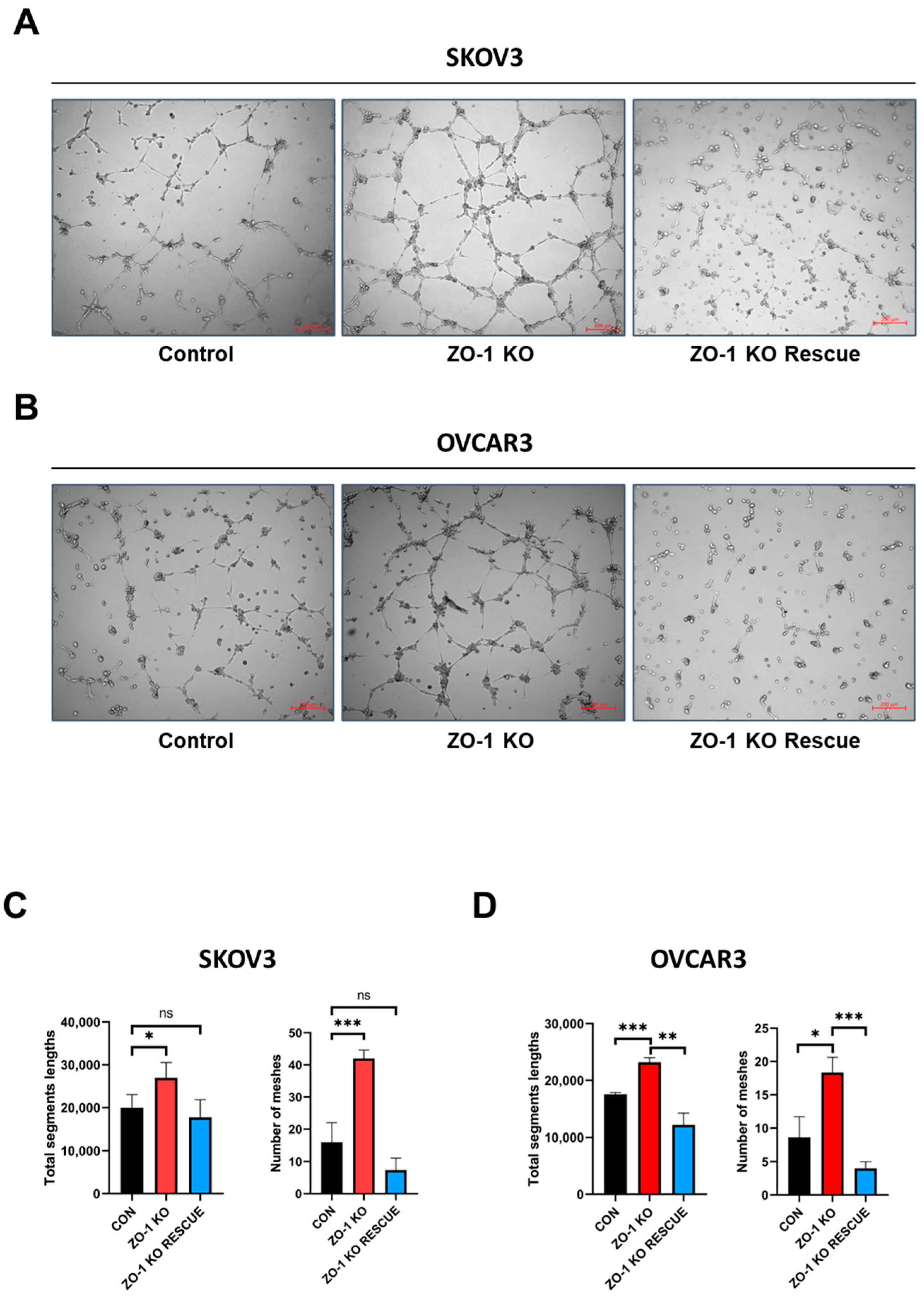
2.2. Enhanced in Vitro Tumor Angiogenesis Following ZO-1 Knockout
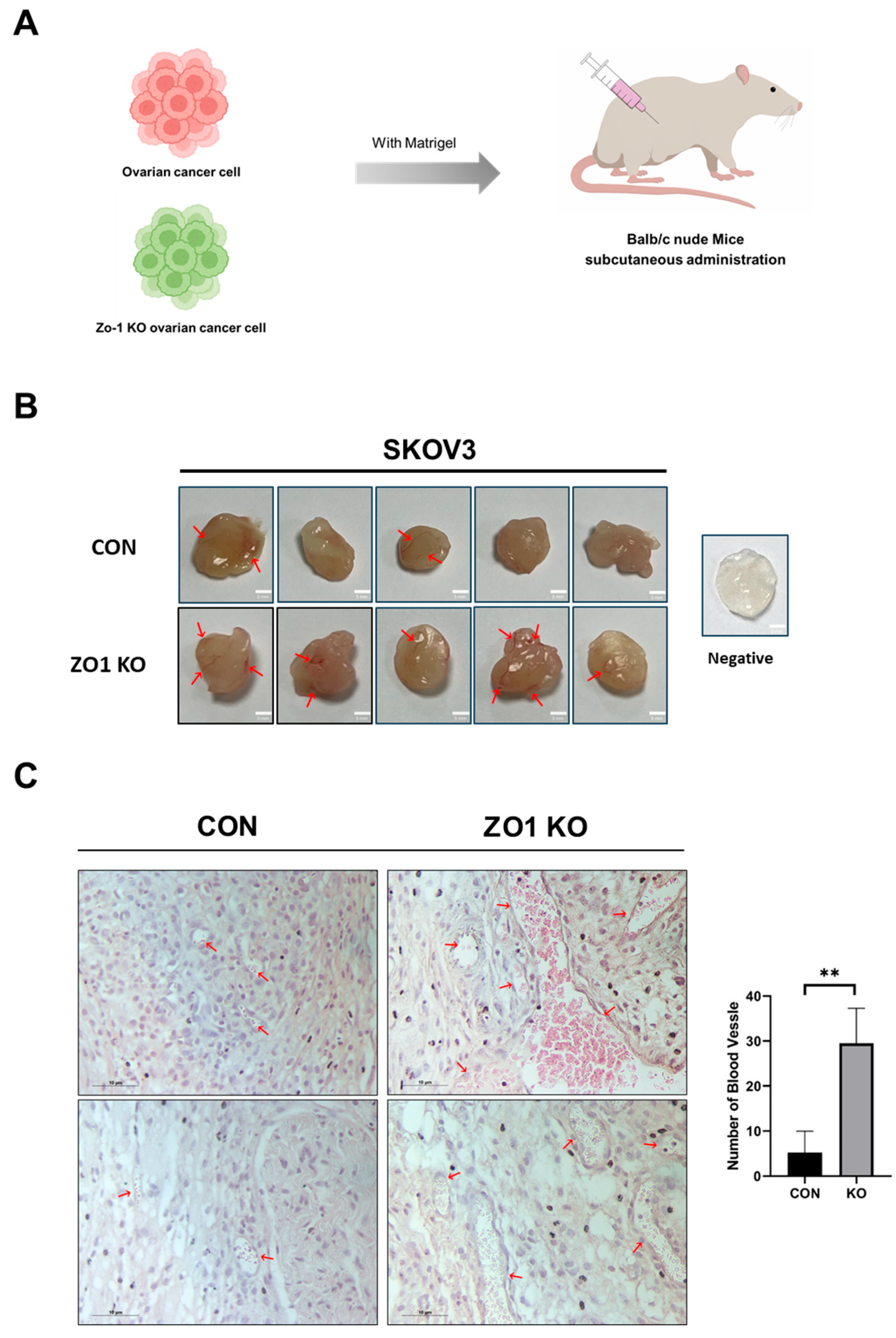
2.3. ZO-1 Loss Induces Angiogenesis in the Matrigel Plug Assay
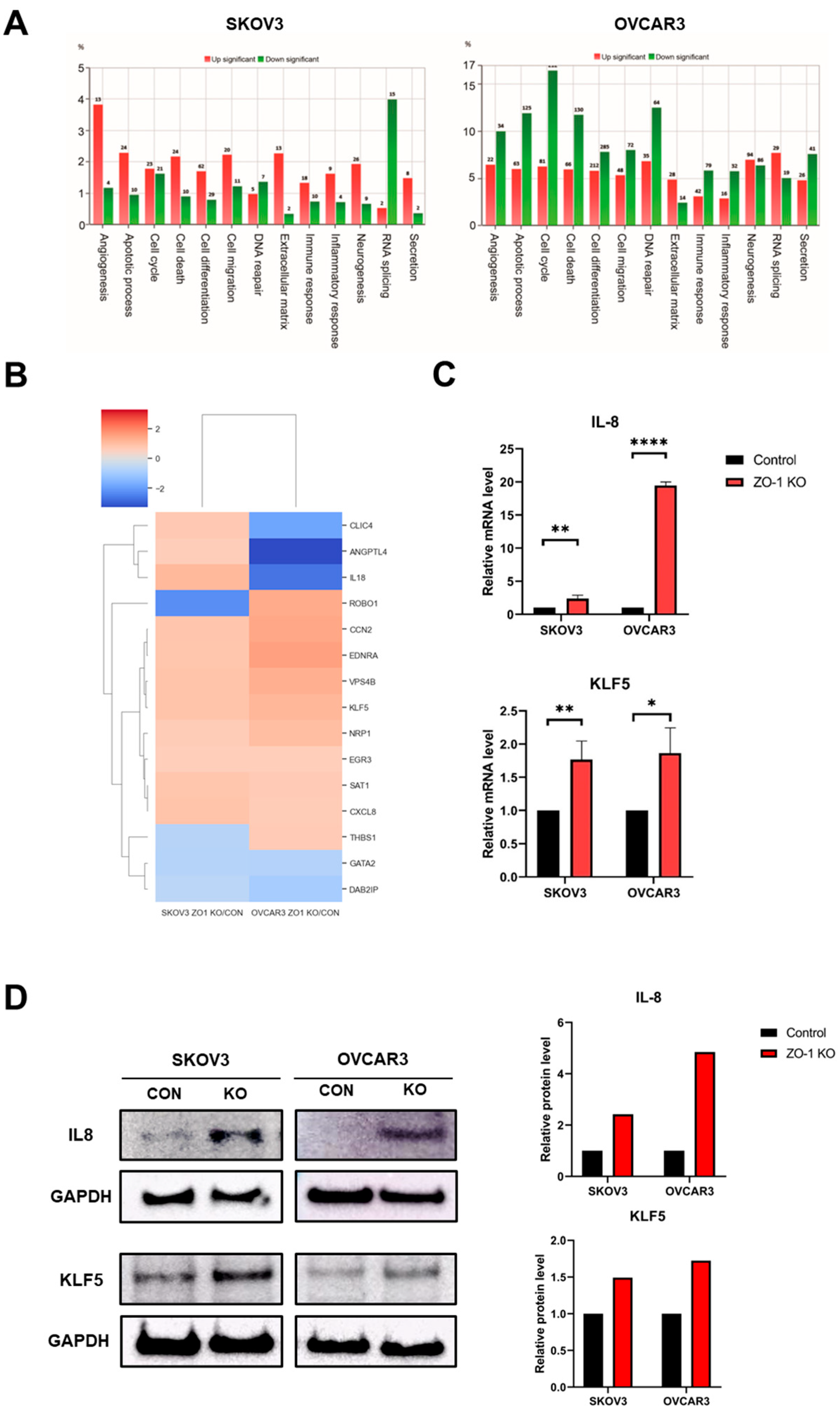
2.4. Regulation of ZO-1–Dependent Angiogenesis by IL-8 and KLF5
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blot Analysis
4.3. Generation of ZO-1 Knockdown Cells
4.4. Tube Formation Assay
4.5. Matrigel Plug Assay
4.6. Collection of Conditioned Media
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. mRNA Sequencing and Analysis
4.10. Plasmid Construct for Over-Expression
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | conditioned media |
| DEGs | differentially expressed genes |
| EMT | epithelial–mesenchymal transition |
| JAMs | junctional adhesion molecules |
| KO | knockout |
| Tjp | tight junction protein |
| ZO | zonula occludens |
References
- Ko, E.J.; Kim, D.Y.; Kim, M.H.; An, H.; Kim, J.; Jeong, J.Y.; Song, K.S.; Cha, H.J. Functional Analysis of Membrane-Associated Scaffolding Tight Junction (TJ) Proteins in Tumorigenic Characteristics of B16-F10 Mouse Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 833. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Nehme, Z.; Roehlen, N.; Dhawan, P.; Baumert, T.F. Tight Junction Protein Signaling and Cancer Biology. Cells 2023, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. A short guide to the tight junction. J. Cell Sci. 2024, 137, jcs261776. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: A scaffolding and signalling center. Biochim. Biophys. Acta 2008, 1778, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.K.; Vairappan, B. Role of zonula occludens in gastrointestinal and liver cancers. World J. Clin. Cases 2022, 10, 3647–3661. [Google Scholar] [CrossRef]
- Paris, L.; Tonutti, L.; Vannini, C.; Bazzoni, G. Structural organization of the tight junctions. Biochim. Biophys. Acta 2008, 1778, 646–659. [Google Scholar] [CrossRef]
- Neyrinck-Leglantier, D.; Lesage, J.; Blacher, S.; Bonnomet, A.; Hunziker, W.; Noël, A.; Dormoy, V.; Nawrocki-Raby, B.; Gilles, C.; Polette, M. ZO-1 Intracellular Localization Organizes Immune Response in Non-Small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 749364. [Google Scholar] [CrossRef]
- Kleeff, J.; Shi, X.; Bode, H.P.; Hoover, K.; Shrikhande, S.; Bryant, P.J.; Korc, M.; Büchler, M.W.; Friess, H. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas 2001, 23, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, J.; Xie, K. Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis. Int. J. Biol. Sci. 2023, 19, 3804–3815. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhang, H.; Tu, F.; Qiang, Y.; Nie, C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol. Lett. 2019, 17, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Terashima, M.; Satoh, J.; Soeta, N.; Saze, Z.; Kashimura, S.; Ohsuka, F.; Hoshino, Y.; Kogure, M.; Gotoh, M. Expression of tight-junction-associated proteins in human gastric cancer: Downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer 2009, 12, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.M.; Zheng, W.; Allard, F.D.; Yee, E.U.; et al. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin. Cancer Res. 2018, 24, 3186–3196. [Google Scholar] [CrossRef]
- Orbán, E.; Szabó, E.; Lotz, G.; Kupcsulik, P.; Páska, C.; Schaff, Z.; Kiss, A. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol. Oncol. Res. 2008, 14, 299–306. [Google Scholar] [CrossRef]
- El Bakkouri, Y.; Chidiac, R.; Delisle, C.; Corriveau, J.; Cagnone, G.; Gaonac’h-Lovejoy, V.; Chin, A.; Lécuyer, É.; Angers, S.; Joyal, J.S.; et al. ZO-1 interacts with YB-1 in endothelial cells to regulate stress granule formation during angiogenesis. Nat. Commun. 2024, 15, 4405. [Google Scholar] [CrossRef]
- Choe, S.; Jeon, M.; Yoon, H. Advanced Therapeutic Approaches for Metastatic Ovarian Cancer. Cancers 2025, 17, 788. [Google Scholar] [CrossRef]
- Sambasivan, S. Epithelial ovarian cancer: Review article. Cancer Treat. Res. Commun. 2022, 33, 100629. [Google Scholar] [CrossRef]
- Batlle, R.; Andrés, E.; Gonzalez, L.; Llonch, E.; Igea, A.; Gutierrez-Prat, N.; Berenguer-Llergo, A.; Nebreda, A.R. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38α through TGF-β and JNK signaling. Nat. Commun. 2019, 10, 3071. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.Q.; He, J.Z.; Xian, S.P.; Yang, P.F.; Mai, Z.Y.; Li, M.; Liu, Y.; Zhang, X.D. Occludin facilitates tumour angiogenesis in bladder cancer by regulating IL8/STAT3 through STAT4. J. Cell Mol. Med. 2022, 26, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, H.; Ren, H.; Cao, J.; Shao, Y.; Liu, G.; Lu, P. Inhibition of Experimental Corneal Neovascularization by the Tight Junction Protein ZO-1. J. Ocul. Pharmacol. Ther. 2024, 40, 379–388. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, K.; Chen, Y.; Zhou, J.; Du, C.; Shi, Q.; Xu, S.; Jia, J.; Tang, X.; Li, F.; et al. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget 2015, 6, 43791–43805. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Ochi, N.; Sawai, H.; Yasuda, A.; Takahashi, H.; Funahashi, H.; Takeyama, H.; Tong, Z.; Guha, S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int. J. Cancer 2009, 124, 853–861. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Lopes-Bastos, B.M.; Jiang, W.G.; Cai, J. Tumour-Endothelial Cell Communications: Important and Indispensable Mediators of Tumour Angiogenesis. Anticancer Res. 2016, 36, 1119–1126. [Google Scholar]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Shahzad, M.M.; Arevalo, J.M.; Armaiz-Pena, G.N.; Lu, C.; Stone, R.L.; Moreno-Smith, M.; Nishimura, M.; Lee, J.W.; Jennings, N.B.; Bottsford-Miller, J.; et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J. Biol. Chem. 2010, 285, 35462–35470, Erratum in J. Biol. Chem. 2018, 293, 10041. [Google Scholar] [CrossRef]
- Li, Y.; Kong, R.; Chen, H.; Zhao, Z.; Li, L.; Li, J.; Hu, J.; Zhang, G.; Pan, S.; Wang, Y.; et al. Overexpression of KLF5 is associated with poor survival and G1/S progression in pancreatic cancer. Aging 2019, 11, 5035–5057. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, C.; Ju, Z.; Jiao, D.; Hu, D.; Qi, L.; Liu, S.; Wu, X.; Zhao, C. Krüppel-like factors in tumors: Key regulators and therapeutic avenues. Front. Oncol. 2023, 13, 1080720. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef]
- Kavanagh, E.; Buchert, M.; Tsapara, A.; Choquet, A.; Balda, M.S.; Hollande, F.; Matter, K. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J. Cell Sci. 2006, 119 Pt 24, 5098–5105. [Google Scholar] [CrossRef][Green Version]
- Si, W.; Liu, J.; Wang, Y.; Mao, Y.; Zhang, Y.; Xu, S.; Guo, K.; Zhang, Y.; Hu, Y.; Zhang, F. IL-8 promotes lens capsular residual cells migration by down-regulates expression of E-cadherin and ZO-1 via the CXCR1/2-NF-κB-RhoA signal pathway. Int. Immunopharmacol. 2024, 142 Pt A, 113074. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Lesage, J.; Suarez-Carmona, M.; Neyrinck-Leglantier, D.; Grelet, S.; Blacher, S.; Hunziker, W.; Birembaut, P.; Noël, A.; Nawrocki-Raby, B.; Gilles, C.; et al. Zonula occludens-1/NF-κB/CXCL8: A new regulatory axis for tumor angiogenesis. Faseb J. 2017, 31, 1678–1688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.S.; Lee, S.I.; Choi, Y.R.; Kim, J.; Eun, J.W.; Song, K.S.; Jeong, J.Y. GNAQ-Regulated ZO-1 and ZO-2 Act as Tumor Suppressors by Modulating EMT Potential and Tumor-Repressive Microenvironment in Lung Cancer. Int. J. Mol. Sci. 2023, 24, 8801. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight Junctions and the Tumor Microenvironment. Curr. Pathobiol. Rep. 2016, 4, 135–145. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Kim, K.H.; Kim, M.-H.; An, H.; Kim, D.-Y.; Eo, W.K.; Lee, J.Y.; Kim, H.; Kim, H.; Cha, H.-J. Loss of Zonula Occludens-1 (ZO-1) Enhances Angiogenic Signaling in Ovarian Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8389. https://doi.org/10.3390/ijms26178389
Choi S, Kim KH, Kim M-H, An H, Kim D-Y, Eo WK, Lee JY, Kim H, Kim H, Cha H-J. Loss of Zonula Occludens-1 (ZO-1) Enhances Angiogenic Signaling in Ovarian Cancer Cells. International Journal of Molecular Sciences. 2025; 26(17):8389. https://doi.org/10.3390/ijms26178389
Chicago/Turabian StyleChoi, Seongsoo, Ki Hyung Kim, Min-Hye Kim, HyoJin An, Do-Ye Kim, Wan Kyu Eo, Ji Young Lee, Hongbae Kim, Heungyeol Kim, and Hee-Jae Cha. 2025. "Loss of Zonula Occludens-1 (ZO-1) Enhances Angiogenic Signaling in Ovarian Cancer Cells" International Journal of Molecular Sciences 26, no. 17: 8389. https://doi.org/10.3390/ijms26178389
APA StyleChoi, S., Kim, K. H., Kim, M.-H., An, H., Kim, D.-Y., Eo, W. K., Lee, J. Y., Kim, H., Kim, H., & Cha, H.-J. (2025). Loss of Zonula Occludens-1 (ZO-1) Enhances Angiogenic Signaling in Ovarian Cancer Cells. International Journal of Molecular Sciences, 26(17), 8389. https://doi.org/10.3390/ijms26178389





