Effect of Singlet Oxygen on the Stomatal and Cell Wall of Rice Seedling Under Different Stresses
Abstract
1. Introduction
2. Results
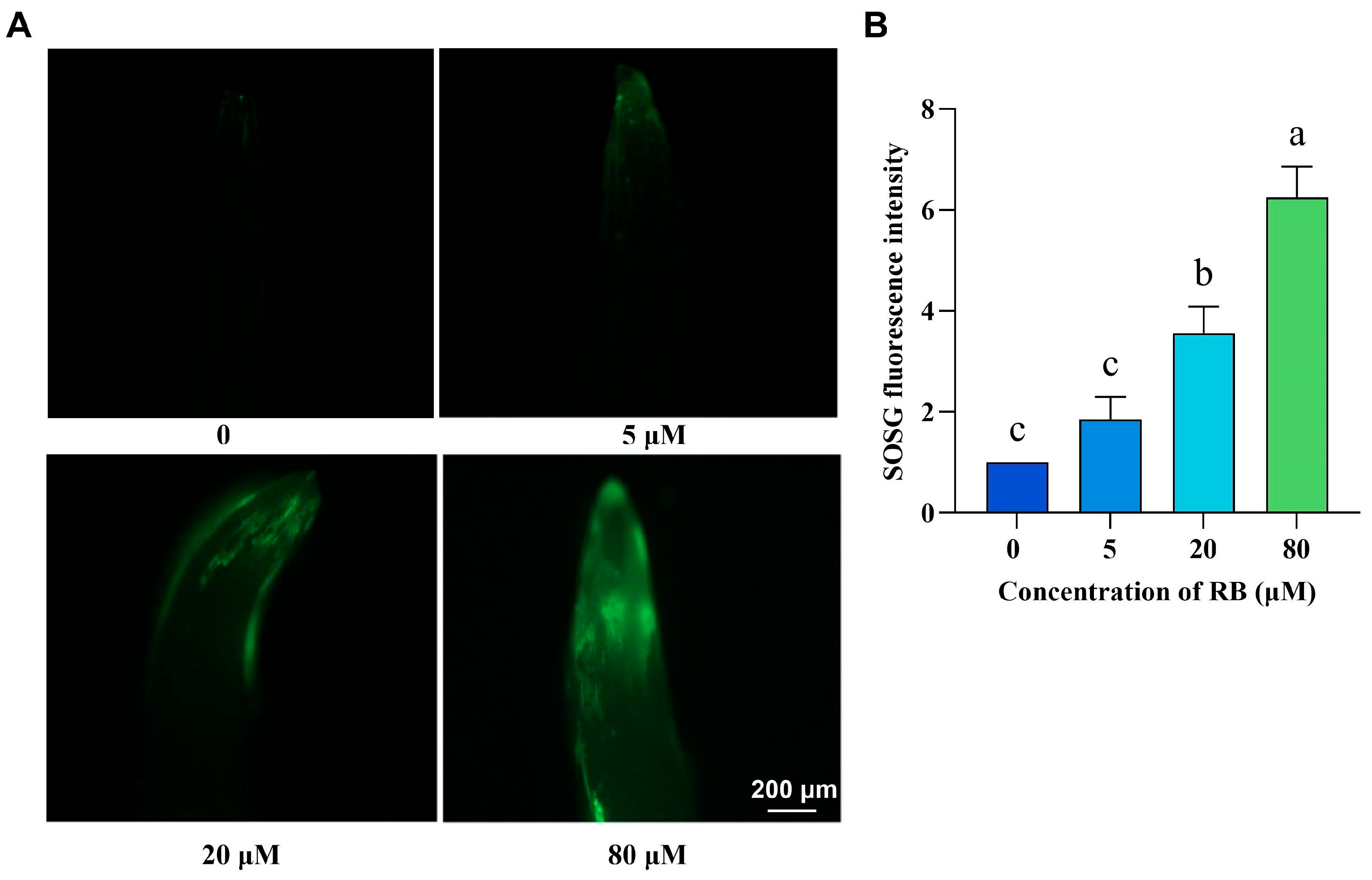
2.1. Exogenous RB Treatment Promotes the Production of Singlet Oxygen in Rice
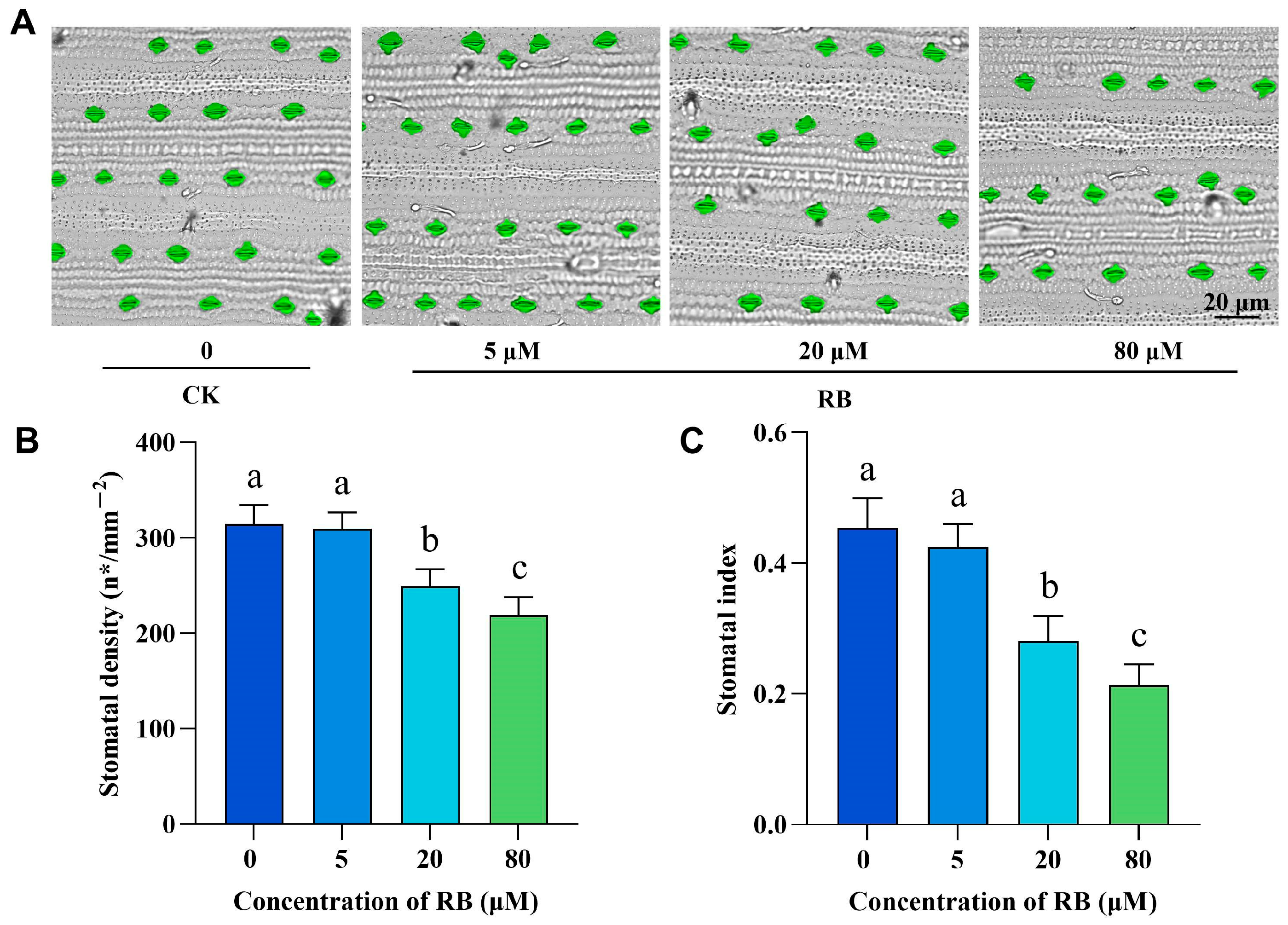
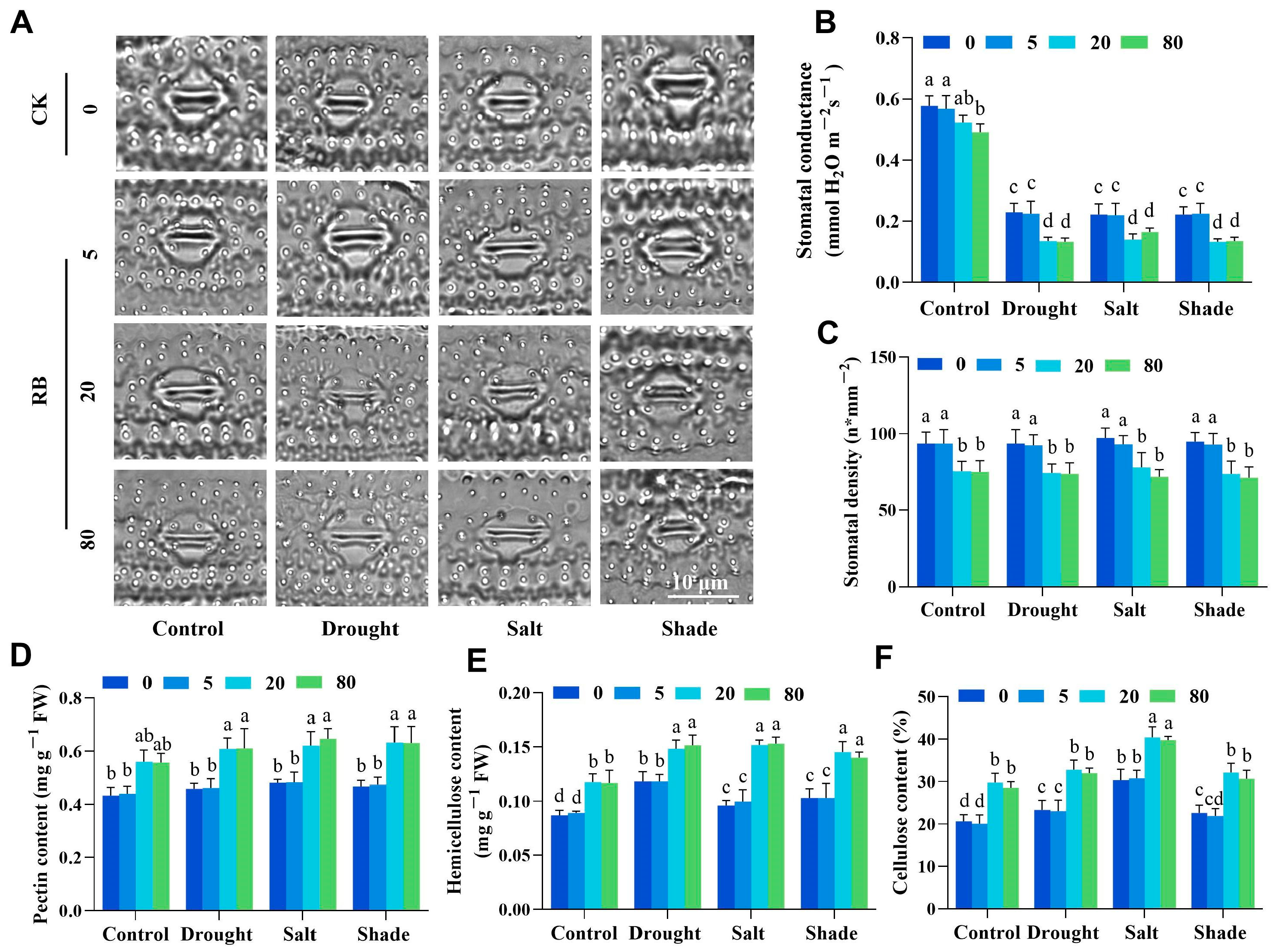
2.2. Singlet Oxygen Induces Stomatal Density Reduction and Cell Wall Thickening in Rice Seedlings
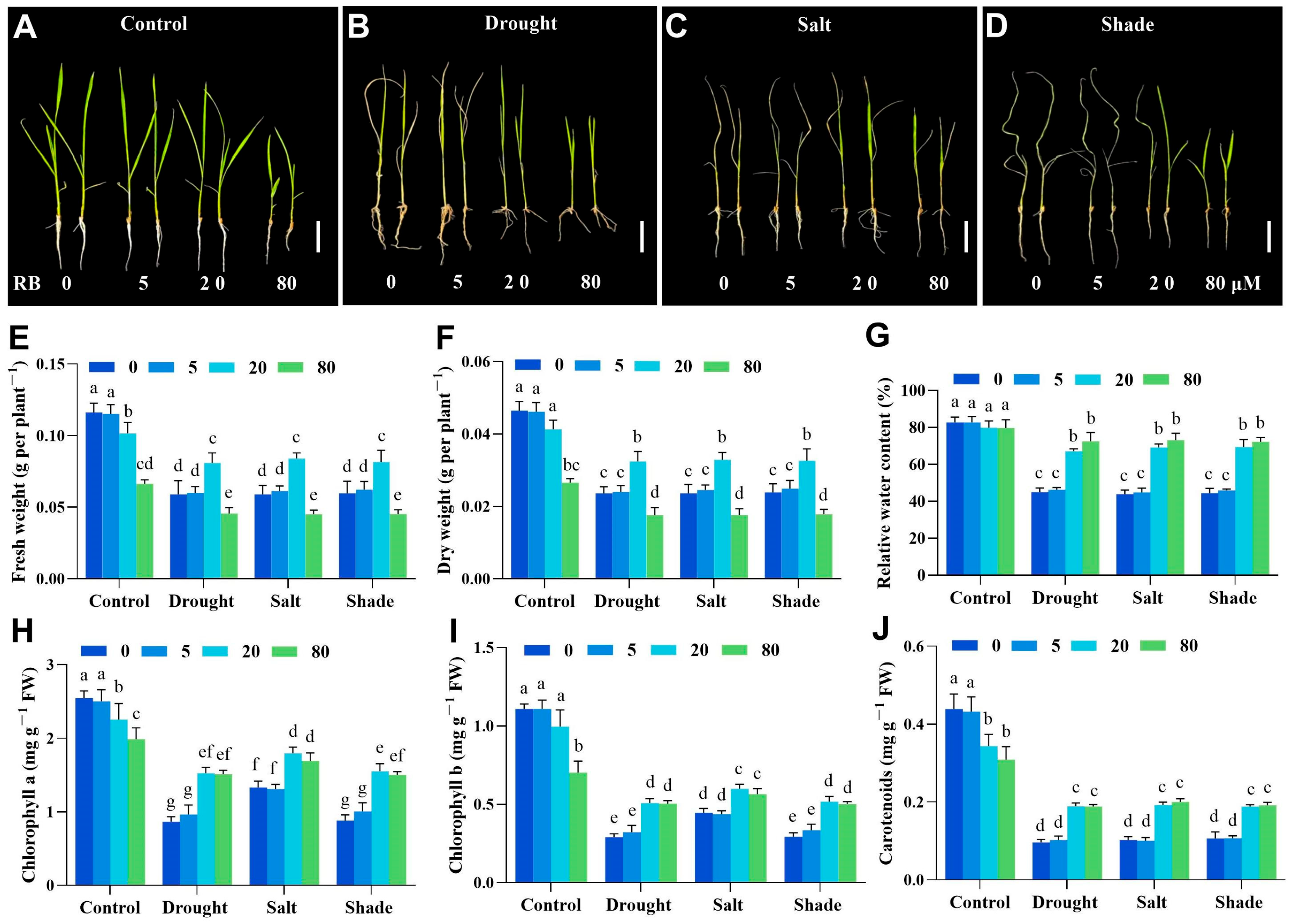
2.3. Moderate Singlet Oxygen Signals Enhance Rice Tolerance to Abiotic Stress
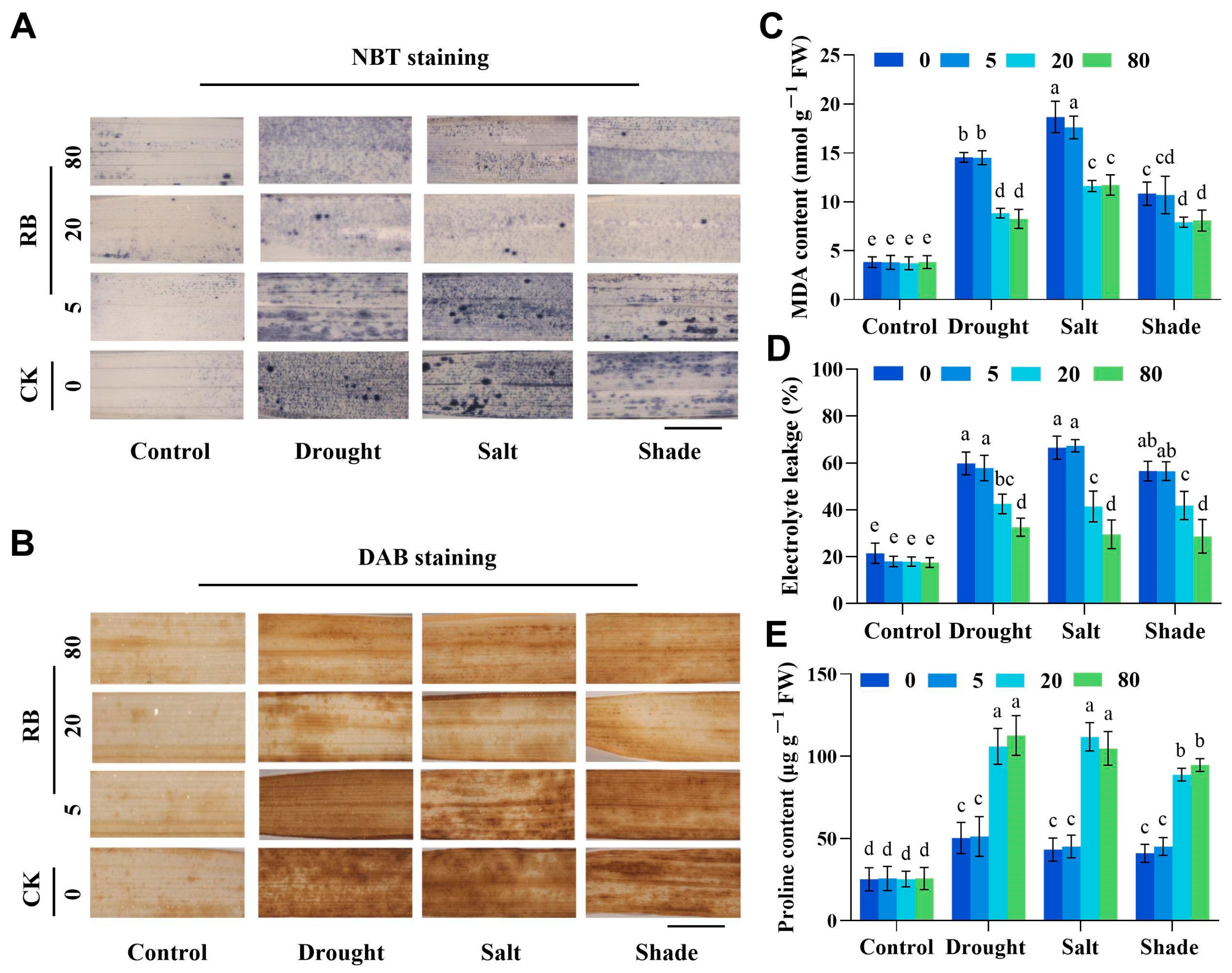
2.4. Moderate Singlet Oxygen Alleviates Oxidative Damage in Rice Under Different Stresses
2.5. Singlet Oxygen Signaling Mediates Stomatal and Cell Wall Adaptations Under Abiotic Stresses
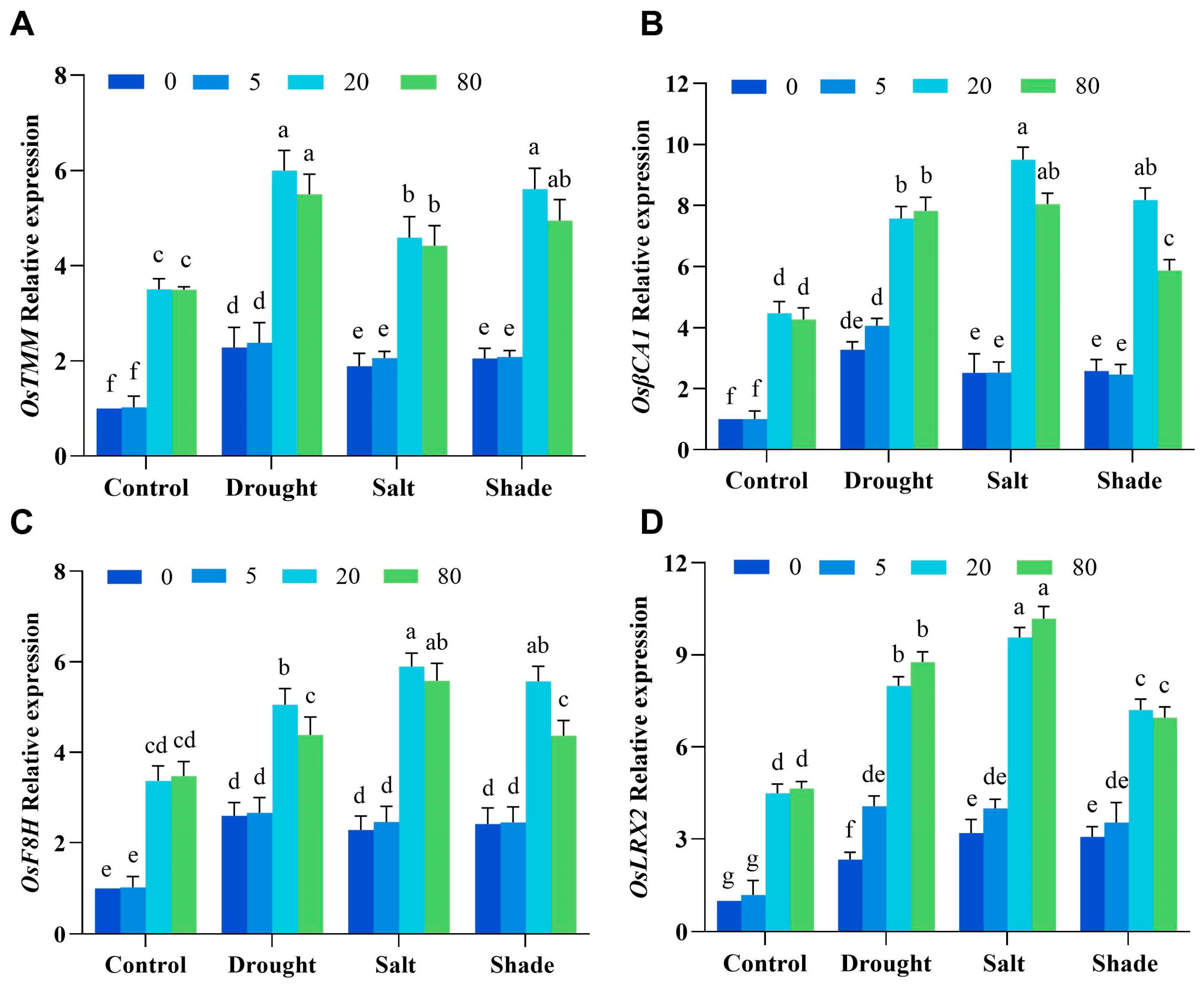
2.6. Singlet Oxygen Regulates Stomatal Development and Cell Wall Synthesis Genes to Enhance Abiotic Stress Tolerance
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Determination of Photosynthetic Pigment Content
4.3. Stomatal Count
4.4. Microscopy Analysis of Root Cell Walls
4.5. Pectin and Cellulose Content Determination
4.6. ROS Staining and Quantification of Oxidative Damages
4.7. Determination of Antioxidant Enzyme Activities
4.8. Quantitative Real-Time PCR Analysis
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylides, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960–968. [Google Scholar] [CrossRef]
- Chan, K.X.; Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N.; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl. Acad. Sci. USA 2016, 113, E4567–E4576. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity response in chloroplasts: Insights from gene characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Xie, H.-T.; Wan, Z.-Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet oxygen in plants: Generation, detection, and signaling roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Hatz, S.; Lambert, J.D.; Ogilby, P.R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sci. 2007, 6, 1106–1116. [Google Scholar] [CrossRef]
- Fischer, B.B.; Krieger-Liszkay, A.; Hideg, É.; Šnyrychová, I.; Wiesendanger, M.; Eggen, R.I. Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett. 2007, 581, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fluhr, R. Singlet oxygen plays an essential role in the root’s response to osmotic stress. Plant Physiol. 2018, 177, 1717–1727. [Google Scholar] [CrossRef]
- Laloi, C.; Havaux, M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015, 6, 39. [Google Scholar] [CrossRef]
- op den Camp, R.G.; Przybyla, D.; Ochsenbein, C.; Laloi, C.; Kim, C.; Danon, A.; Wagner, D.; Hideg, É.; Göbel, C.; Feussner, I. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 2003, 15, 2320–2332. [Google Scholar] [CrossRef]
- Meskauskiene, R.; Nater, M.; Goslings, D.; Kessler, F.; op den Camp, R.; Apel, K. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 12826–12831. [Google Scholar] [CrossRef]
- Wagner, D.; Przybyla, D.; op den Camp, R.; Kim, C.; Landgraf, F.; Lee, K.P.; Würsch, M.; Laloi, C.; Nater, M.; Hideg, E. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Apel, K. Dose-dependent effects of 1O2 in chloroplasts are determined by its timing and localization of production. J. Exp. Bot. 2019, 70, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Carmieli, R.; Mor, A.; Fluhr, R. Singlet oxygen-induced membrane disruption and serpin-protease balance in vacuolar-driven cell death. Plant Physiol. 2016, 171, 1616–1625. [Google Scholar] [CrossRef]
- Koh, E.; Cohen, D.; Brandis, A.; Fluhr, R. Attenuation of cytosolic translation by RNA oxidation is involved in singlet oxygen-mediated transcriptomic responses. Plant Cell Environ. 2021, 44, 3597–3615. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Ma, T.; Li, J.; Yuan, J.; Xu, Y.-C.; Sun, R.; Zhang, X.; Jing, Y.; Guo, Y.-L. Arabidopsis EXECUTER1 interacts with WRKY transcription factors to mediate plastid-to-nucleus singlet oxygen signaling. Plant Cell 2023, 35, 827–851. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Fu, Y.-F.; Yang, X.-Y.; Yuan, M.; Zheng, X.-J.; Luo, X.-F.; Zhang, M.-Y.; Xie, L.-B.; Shu, K.; Reinbothe, S.; et al. Singlet oxygen induces cell wall thickening and stomatal density reducing by transcriptome reprogramming. J. Biol. Chem. 2023, 299, 105481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Fu, Y.-F.; Chen, G.-D.; Reinbothe, C.; Reinbothe, S.; Yuan, S. The interplay of singlet oxygen and ABI4 in plant growth regulation. Trends Plant Sci. 2025, 30, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Caine, R.S.; Harrison, E.L.; Sloan, J.; Flis, P.M.; Fischer, S.; Khan, M.S.; Nguyen, P.T.; Nguyen, L.T.; Gray, J.E.; Croft, H. The influences of stomatal size and density on rice abiotic stress resilience. New Phytol. 2023, 237, 2180–2195. [Google Scholar] [CrossRef]
- Luo, L.; Cui, Y.; Ouyang, N.; Huang, S.; Gong, X.; Wei, L.; Zou, B.; Hua, J.; Lu, S. Tolerance to multiple abiotic stresses is mediated by interacting CNGC proteins that regulate Ca2+ influx and stomatal movement in rice. J. Integr. Plant Biol. 2025, 67, 226–242. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Karavolias, N.G.; Patel-Tupper, D.; Seong, K.; Tjahjadi, M.; Gueorguieva, G.-A.; Tanaka, J.; Gallegos Cruz, A.; Lieberman, S.; Litvak, L.; Dahlbeck, D. Paralog editing tunes rice stomatal density to maintain photosynthesis and improve drought tolerance. Plant Physiol. 2023, 192, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Deng, S.; Zhu, M.; Fan, X.; Xu, M.; Ye, J. ZmDRR206 functions in maintaining cell wall integrity during maize seedling growth and defense response to external stresses. Crop J. 2023, 11, 1649–1664. [Google Scholar] [CrossRef]
- Jia, S.; Wang, C.; Sun, W.; Yan, X.; Wang, W.; Xu, B.; Guo, G.; Bi, C. OsWRKY12 negatively regulates the drought-stress tolerance and secondary cell wall biosynthesis by targeting different downstream transcription factor genes in rice. Plant Physiol. Biochem. 2024, 212, 108794. [Google Scholar] [CrossRef]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, J.; Zhang, W.; Guan, H.; Zheng, D.; Xiong, H.; Jia, L.; Hu, Y.; Zhou, H.; Wen, Y. ZmLBD5, a class-II LBD gene, negatively regulates drought tolerance by impairing abscisic acid synthesis. Plant J. 2022, 112, 1364–1376. [Google Scholar] [CrossRef]
- Xie, W.; Ke, Y.; Cao, J.; Wang, S.; Yuan, M. Knock out of transcription factor WRKY53 thickens sclerenchyma cell walls, confers bacterial blight resistance. Plant Physiol. 2021, 187, 1746–1761. [Google Scholar] [CrossRef]
- Zhong, R.; Pena, M.J.; Zhou, G.-K.; Nairn, C.J.; Wood-Jones, A.; Richardson, E.A.; Morrison, W.H., III; Darvill, A.G.; York, W.S.; Ye, Z.-H. Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell 2005, 17, 3390–3408. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Steiner, M.; Ryser, U.; Keller, B.; Ringli, C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003, 35, 71–81. [Google Scholar] [CrossRef]
- Yang, M.; Sack, F.D. The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 1995, 7, 2227–2239. [Google Scholar]
- Hu, H.; Rappel, W.-J.; Occhipinti, R.; Ries, A.; Böhmer, M.; You, L.; Xiao, C.; Engineer, C.B.; Boron, W.F.; Schroeder, J.I. Distinct cellular locations of carbonic anhydrases mediate carbon dioxide control of stomatal movements. Plant Physiol. 2015, 169, 1168–1178. [Google Scholar] [CrossRef]
- Chen, T.; Wu, H.; Wu, J.; Fan, X.; Li, X.; Lin, Y. Absence of OsβCA1 causes a CO2 deficit and affects leaf photosynthesis and the stomatal response to CO2 in rice. Plant J. 2017, 90, 344–357. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, Z.; Zhou, Q.; Chen, C.; Hao, Y.; Wang, Z.; Zhu, J.-K.; Guo, H.; Huang, A.C. NAD+ deficiency primes defense metabolism via 1O2-escalated jasmonate biosynthesis in plants. Nat. Commun. 2024, 15, 6652. [Google Scholar] [CrossRef]
- Lemke, M.D.; Woodson, J.D. A genetic screen for dominant chloroplast reactive oxygen species signaling mutants reveals life stage-specific singlet oxygen signaling networks. Front. Plant Sci. 2024, 14, 1331346. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Xiao, C.; Yan, Z.; Ning, N.; Chen, G.; Zhang, J.; Hu, H. Pectin methylesterase inhibitor 18 functions in stomatal dynamics and stomatal dimension. Plant Physiol. 2023, 192, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Panter, P.E.; Seifert, J.; Dale, M.; Pridgeon, A.J.; Hulme, R.; Ramsay, N.; Contera, S.; Knight, H. Cell wall fucosylation in Arabidopsis influences control of leaf water loss and alters stomatal development and mechanical properties. J. Exp. Bot. 2023, 74, 2680–2691. [Google Scholar] [CrossRef] [PubMed]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Hoch, D.; Chen, T.; Sharabi, L.; Dezorella, N.; Itkin, M.; Feiguelman, G.; Malitsky, S.; Fluhr, R. Osmotic stress in roots drives lipoxygenase-dependent plastid remodeling through singlet oxygen production. Plant Physiol. 2025, 197, kiae589. [Google Scholar] [CrossRef]
- Yang, Y.; Karthikeyan, A.; Yin, J.; Jin, T.; Ren, R.; Fang, F.; Cai, H.; Liu, M.; Wang, D.; Li, K. The E3 ligase GmPUB21 negatively regulates drought and salinity stress response in soybean. Int. J. Mol. Sci. 2022, 23, 6893. [Google Scholar] [CrossRef]
- Mishra, S.; Sahu, G.; Shaw, B.P. Insight into the cellular and physiological regulatory modulations of Class-I TCP9 to enhance drought and salinity stress tolerance in cowpea. Physiol. Plant. 2022, 174, e13542. [Google Scholar] [CrossRef]
- Djemal, R.; Khoudi, H. The barley SHN1-type transcription factor HvSHN1 imparts heat, drought and salt tolerances in transgenic tobacco. Plant Physiol. Biochem. 2021, 164, 44–53. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, L.; Yuan, Y.; Hu, S.; Guo, F.; Zhao, H.; Yun, Z.; Wang, Y.; Wang, M.; Ni, D. Ectopic overexpression of histone H3K4 methyltransferase CsSDG36 from tea plant decreases hyperosmotic stress tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 5064. [Google Scholar] [CrossRef]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and screening of agro-physiological indices for salinity stress tolerance in wheat at the seedling stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Illescas-Miranda, J.; Martín-Forero, A.F.; de Marcos, A.; Barón, M.; Fenoll, C.; Mena, M. An extremely low stomatal density mutant overcomes cooling limitations at supra-optimal temperature by adjusting stomatal size and leaf thickness. Front. Plant Sci. 2022, 13, 919299. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.R.; Caine, R.S.; Gray, J.E. Pores for thought: Can genetic manipulation of stomatal density protect future rice yields? Front. Plant Sci. 2020, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Zekri, M.A.; Lang, I. Lack of trichomes and variation in stomata properties influence the quantum efficiency of photosynthesis in Arabidopsis. Environ. Exp. Bot. 2024, 227, 105948. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, J.; Zhou, Y.; Li, Y.; He, H.; Yang, Y.; Wang, X.; Lian, X.; Dong, X.; Ma, Z. DSD1/ZmICEb regulates stomatal development and drought tolerance in maize. J. Integr. Plant Biol. 2025, 67, 1487–1500. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Plant cell wall as a key player during resistant and susceptible plant-virus interactions. Front Microbiol. 2021, 12, 656809. [Google Scholar] [CrossRef]
- Khasin, M.; Bernhardson, L.F.; O’Neill, P.M.; Palmer, N.A.; Scully, E.D.; Sattler, S.E.; Funnell-Harris, D.L. Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biol. 2021, 21, 391. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, D.; Cao, L.; Zhang, W.; Zheng, H.; Liu, Z.; Han, S.; Dong, Y.; Zhu, F.; Liu, H. Functions and regulatory framework of ZmNST3 in maize under lodging and drought stress. Plant Cell Environ. 2020, 43, 2272–2286. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, M.; Cao, L.; Dang, Z.; Ruan, N.; Wang, Y.; Huang, Y.; Wu, J.; Zhang, M.; Xu, Z. OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ. 2022, 45, 2492–2507. [Google Scholar] [CrossRef]
- Gigli-Bisceglia, N.; van Zelm, E.; Huo, W.; Lamers, J.; Testerink, C. Arabidopsis root responses to salinity depend on pectin modification and cell wall sensing. Development 2022, 149, dev200363. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.; Luo, J.; Li, J.; Kováč, J.; Li, B.; Li, Q.; Wu, K.; Liang, Y. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lee, B.H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.K. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef]
- Voiniciuc, C. It’s time to go glyco in cell wall bioengineering. Curr. Opin. Plant Biol. 2023, 71, 102313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, L.; Qin, S.; Kuang, Z.; Wan, M.; Li, Z.; Li, L. Heterogeneity in mechanical properties of plant cell walls. Plants 2024, 13, 3561. [Google Scholar] [CrossRef]
- Thompson, D.S.; Islam, A. Plant cell wall hydration and plant physiology: An exploration of the consequences of direct effects of water deficit on the plant cell wall. Plants 2021, 10, 1263. [Google Scholar] [CrossRef]
- Burguete, M.I.; Galindo, F.; Gavara, R.; Luis, S.V.; Moreno, M.; Thomas, P.; Russell, D.A. Singlet oxygen generation using a porous monolithic polymer supported photosensitizer: Potential application to the photodynamic destruction of melanoma cells. Photochem. Photobiol. Sci. 2009, 8, 37–44. [Google Scholar] [CrossRef]
- Li, Z.; Mo, W.; Jia, L.; Xu, Y.-C.; Tang, W.; Yang, W.; Guo, Y.-L.; Lin, R. Rice FLUORESCENT1 is involved in the regulation of chlorophyll. Plant Cell Physiol. 2019, 60, 2307–2318. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–593. [Google Scholar] [CrossRef]
- Kusumi, K.; Hashimura, A.; Yamamoto, Y.; Negi, J.; Iba, K. Contribution of the S-type anion channel SLAC1 to stomatal control and its dependence on developmental stage in rice. Plant Cell Physiol. 2017, 58, 2085–2094. [Google Scholar] [CrossRef]
- Huang, L.; Chen, L.; Wang, L.; Yang, Y.; Rao, Y.; Ren, D.; Dai, L.; Gao, Y.; Zou, W.; Lu, X. A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice. Plant J. 2019, 98, 884–897. [Google Scholar] [CrossRef]
- Liang, X.; Qian, R.; Ou, Y.; Wang, D.; Lin, X.; Sun, C. Lipid peroxide-derived short-chain aldehydes promote programmed cell death in wheat roots under aluminum stress. J. Hazard. Mater. 2023, 443, 130142. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, T.; Zheng, Y.; Cosgrove, D.J.; Anderson, C.T. Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol. 2016, 170, 234–249. [Google Scholar] [CrossRef]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.N.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S. Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J. Exp. Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Dong, Y.-Y.; Feng, L.-Y.; Deng, Z.-L.; Xu, Q.; Tao, Q.; Wang, C.-Q.; Chen, Y.-E.; Yuan, M.; Yuan, S. Selenium enhances cadmium accumulation capability in two mustard family species—Brassica napus and B. juncea. Plants 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-E.; Cui, J.-M.; Yang, J.-C.; Zhang, Z.-W.; Yuan, M.; Song, C.; Yang, H.; Liu, H.-M.; Wang, C.-Q.; Zhang, H.-Y. Biomonitoring heavy metal contaminations by moss visible parameters. J. Hazard. Mater. 2015, 296, 201–209. [Google Scholar] [CrossRef]
- Xu, J.; Jansma, S.Y.; Wolters-Arts, M.; de Groot, P.F.; Jansen, M.J.; Rieu, I. Long-term mild heat causes post-mitotic pollen abortion through a local effect on flowers. Front. Plant Sci. 2022, 13, 925754. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, K.; Wang, A.; Lian, C.; Shen, Z. Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere 2010, 78, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; Shakiba, M.R.; Mahboob, S.A.; Alyari, H.; Toorchi, M. Water stress, antioxidant enzyme activity and lipid peroxidation in wheat seedling. J. Food Agric. Environ. 2007, 5, 149–153. [Google Scholar]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Zhang, Z.-W.; Yang, X.-Y.; Xie, L.-B.; Chen, L.-P.; Chen, Y.-E.; Yuan, M.; Chen, G.-D.; Yuan, S. Effect of Singlet Oxygen on the Stomatal and Cell Wall of Rice Seedling Under Different Stresses. Int. J. Mol. Sci. 2025, 26, 8382. https://doi.org/10.3390/ijms26178382
Xiao Y, Zhang Z-W, Yang X-Y, Xie L-B, Chen L-P, Chen Y-E, Yuan M, Chen G-D, Yuan S. Effect of Singlet Oxygen on the Stomatal and Cell Wall of Rice Seedling Under Different Stresses. International Journal of Molecular Sciences. 2025; 26(17):8382. https://doi.org/10.3390/ijms26178382
Chicago/Turabian StyleXiao, Yao, Zhong-Wei Zhang, Xin-Yue Yang, Lin-Bei Xie, Li-Ping Chen, Yang-Er Chen, Ming Yuan, Guang-Deng Chen, and Shu Yuan. 2025. "Effect of Singlet Oxygen on the Stomatal and Cell Wall of Rice Seedling Under Different Stresses" International Journal of Molecular Sciences 26, no. 17: 8382. https://doi.org/10.3390/ijms26178382
APA StyleXiao, Y., Zhang, Z.-W., Yang, X.-Y., Xie, L.-B., Chen, L.-P., Chen, Y.-E., Yuan, M., Chen, G.-D., & Yuan, S. (2025). Effect of Singlet Oxygen on the Stomatal and Cell Wall of Rice Seedling Under Different Stresses. International Journal of Molecular Sciences, 26(17), 8382. https://doi.org/10.3390/ijms26178382





