Genome-Wide Identification of Solute Carrier Family 12 and Functional Characterization of Its Role in Saline–Alkaline Stress Acclimation in the Ridgetail White Shrimp Exopalaemon carinicauda
Abstract
1. Introduction
2. Results
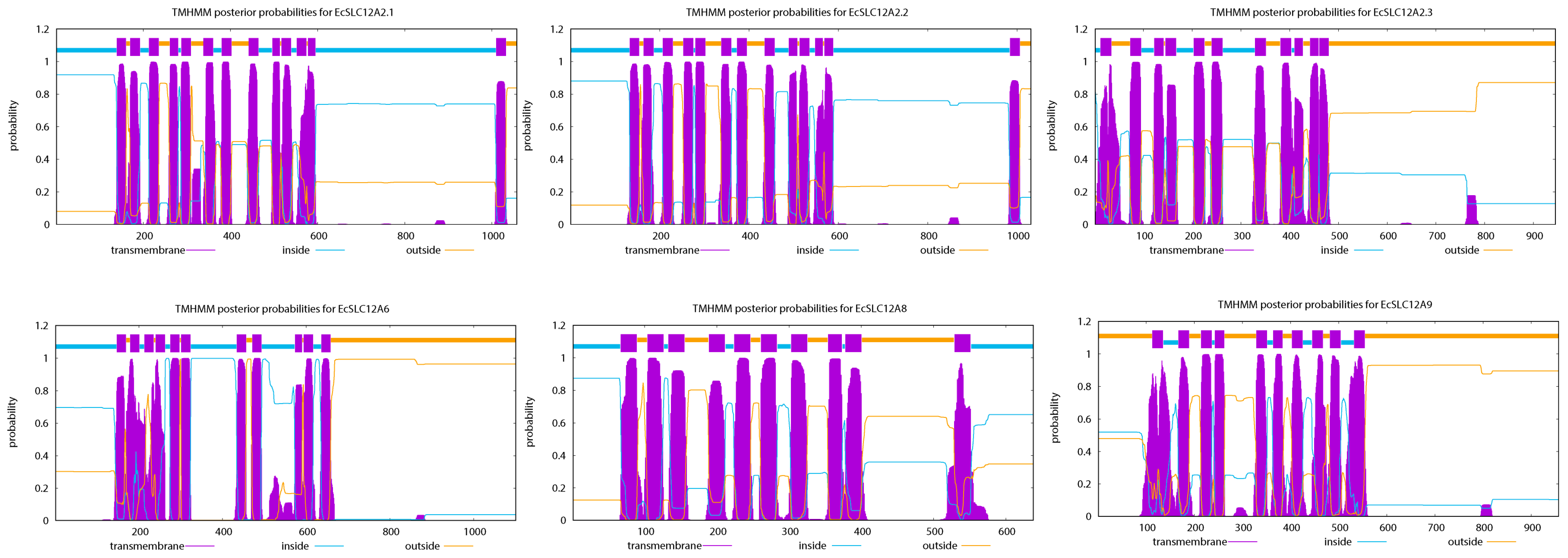
2.1. Identification of the EcSLC12 Gene Family
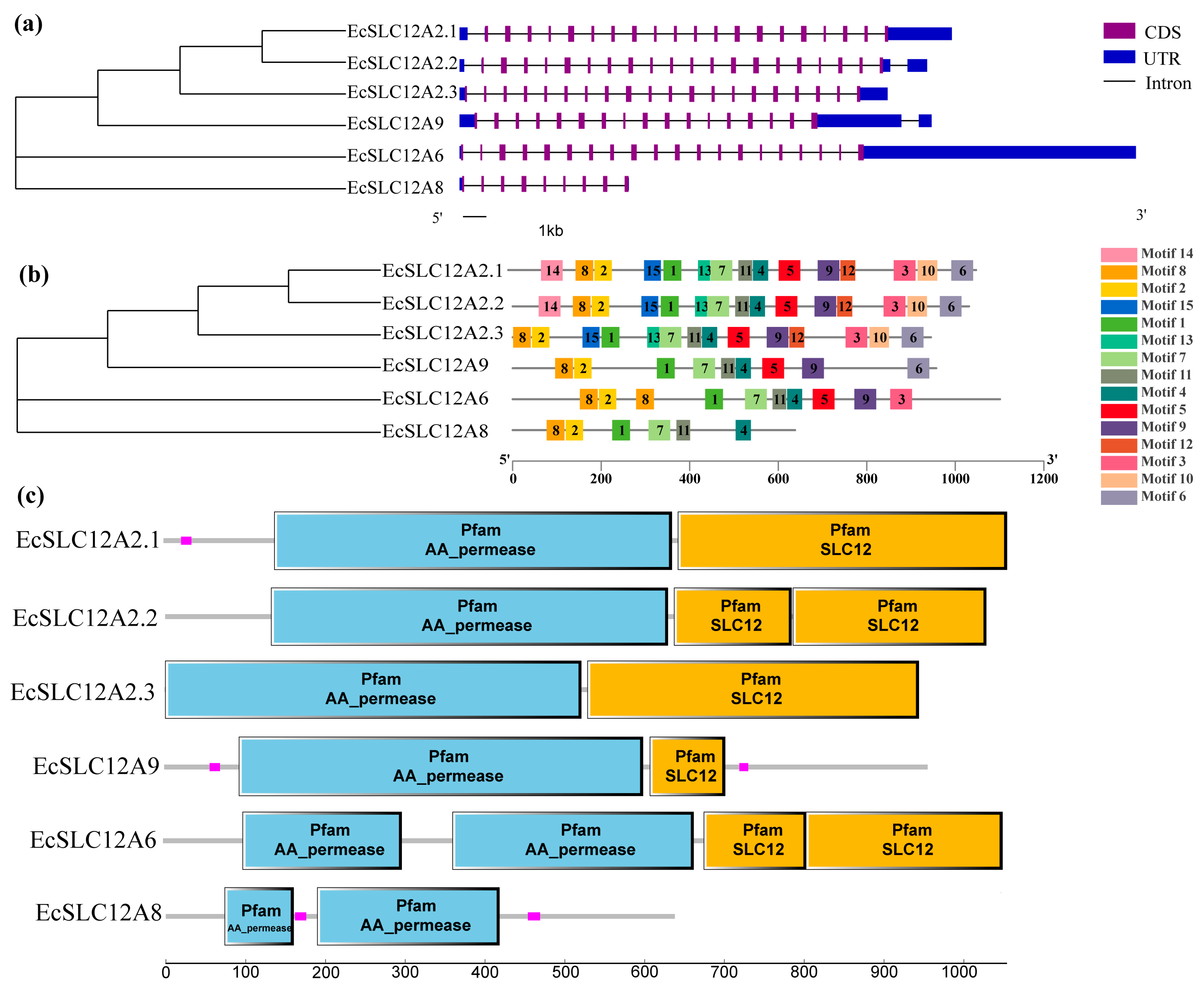
2.2. Gene Structures, Conserved Motifs, and Domains of the EcSLC12 Gene Family
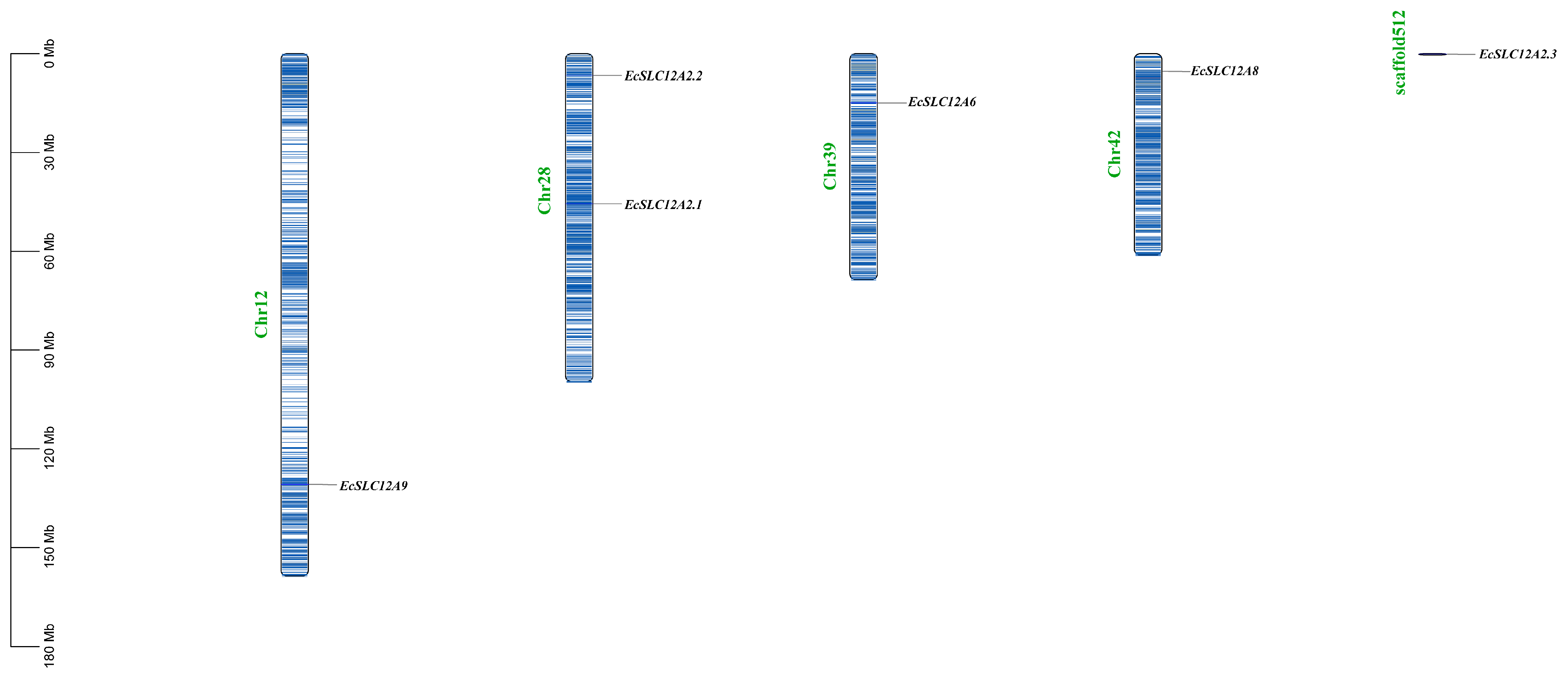
2.3. Chromosome Location and Phylogenetics of the EcSLC12 Gene Family
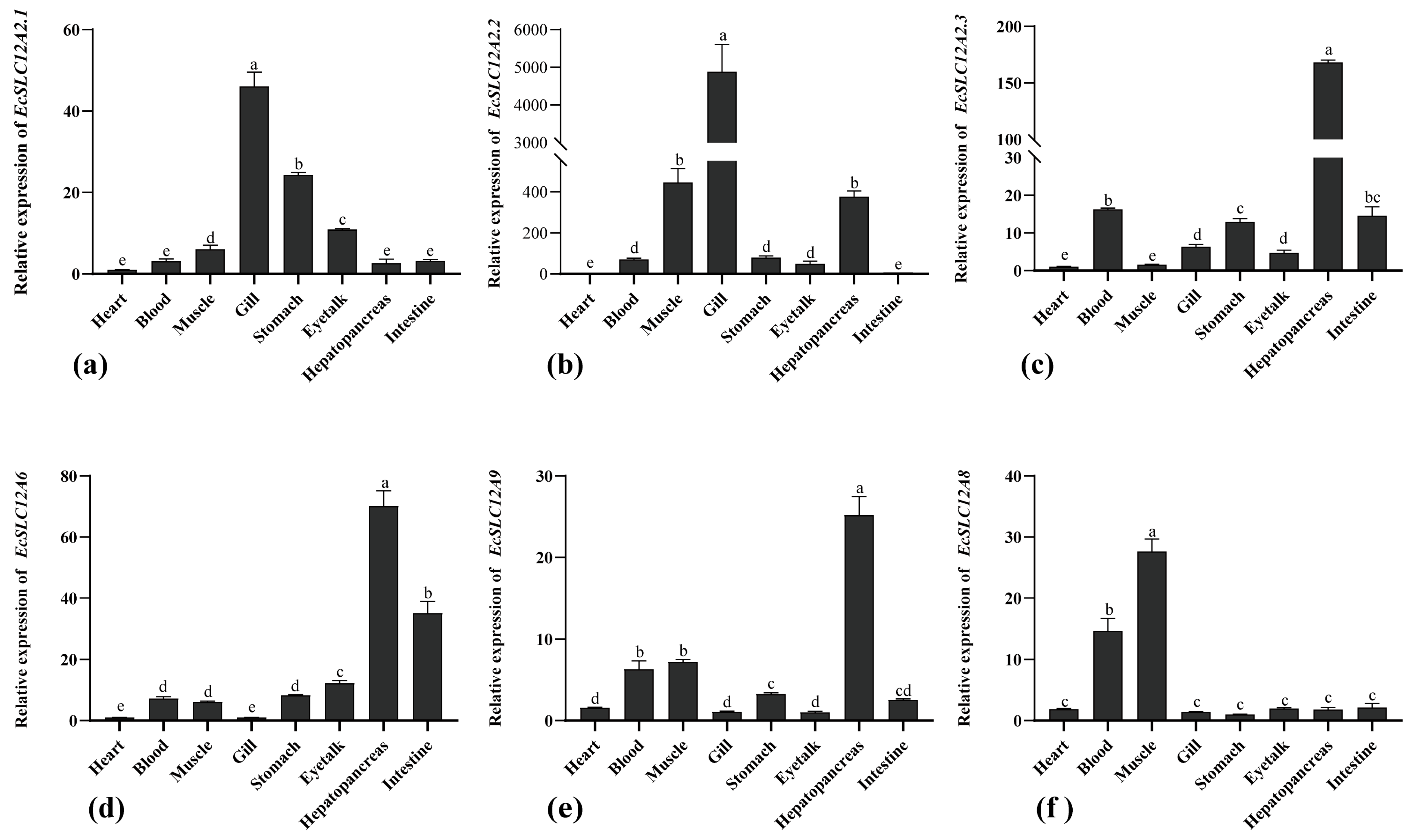
2.4. Expression of the EcSLC12 Genes in Various Tissues
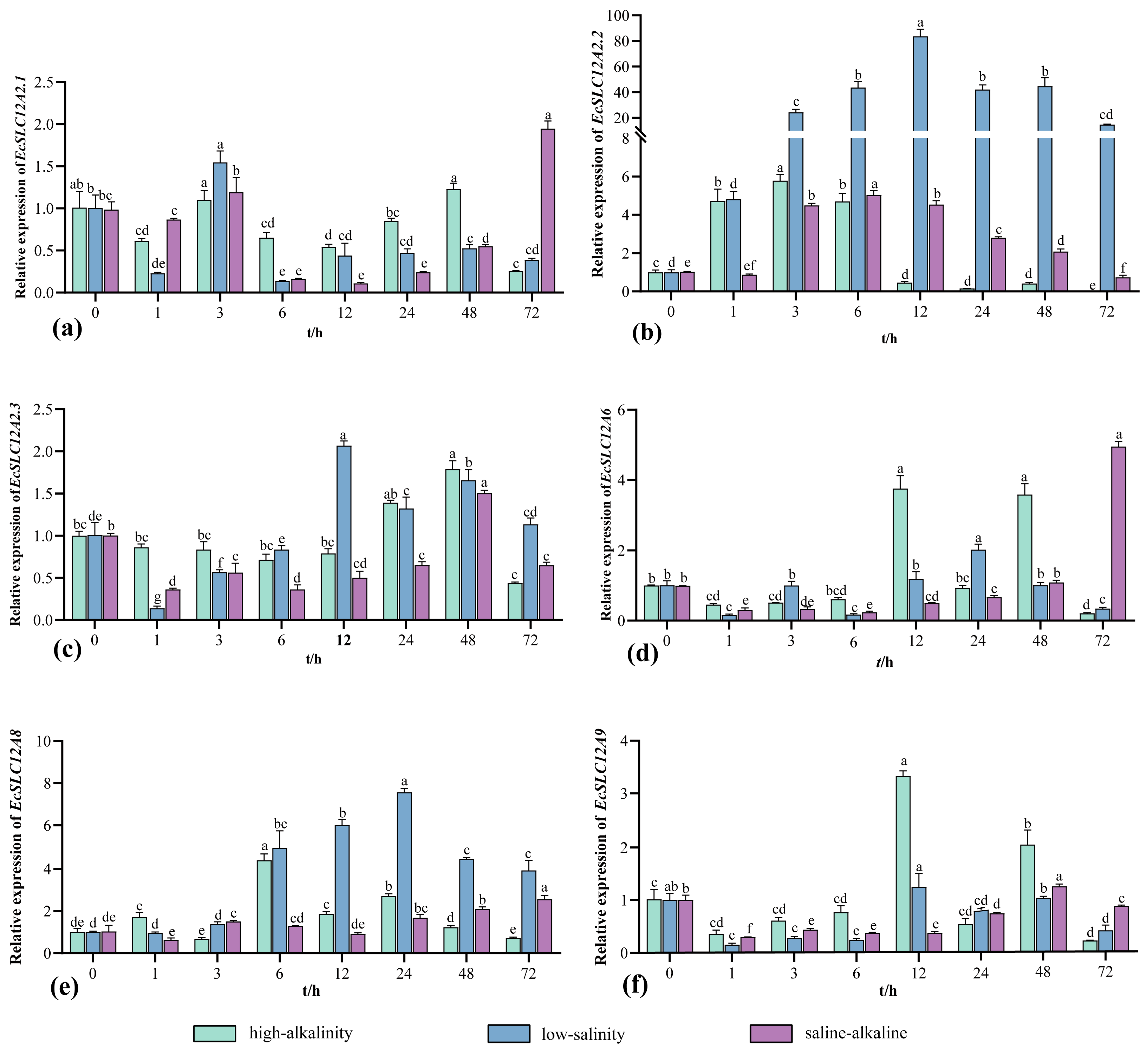
2.5. Analysis of the Expression Pattern of EcSLC12 Genes Under Varying Stress Conditions
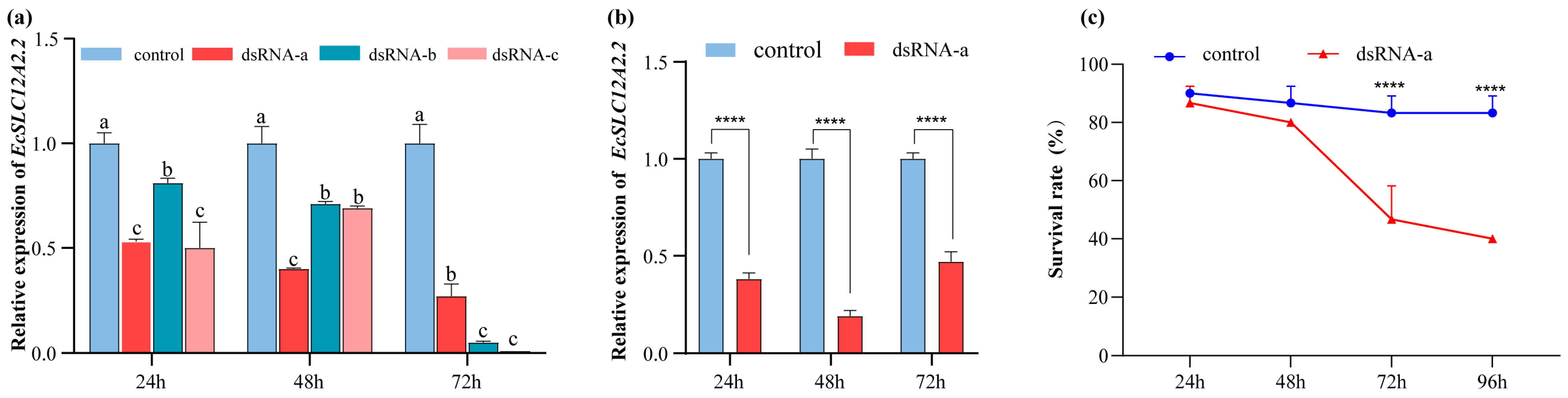
2.6. Saline–Alkaline Stress Analysis After EcSLC12A2.2 Silencing
3. Discussion
3.1. Identification and Evolutionary Analysis of the SLC12 Family in E. carinicauda
3.2. Functional Study of the EcSLC12 Family in Acclimating to Saline–Alkaline Environments
4. Materials and Methods
4.1. Identification and Bioinformatic Analysis of the SLC12 Family in E. carinicauda
4.1.1. Database Resources
4.1.2. Identification and Sequence Analysis of SLC12 Family in E. carinicauda
4.1.3. Phylogenetic Analysis of the SLC12 Family
4.1.4. Gene Structure Analysis, Conserved Motif/Domain, and Chromosomal Localization Identification
4.2. Functional Characterization of the SLC12 Family in E. carinicauda Under Differential Salinity and Alkalinity Conditions
4.2.1. Expression Pattern of the EcSLC12 Genes in Various Tissues and Stress Conditions
4.2.2. RNAi of EcSLC12A2.2
4.2.3. RNA Extraction and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Z.; Wu, D.; Zhang, Y.; Li, J.; Xu, Q.; Wang, L. Carbonate alkalinity and dietary protein levels affected growth performance, intestinal immune responses, and intestinal microflora in Songpu mirror carp (Cyprinus carpio Songpu). Aquaculture 2021, 545, 737135. [Google Scholar] [CrossRef]
- Hu, H.L.; Lai, Q.F.; Yao, Z.L. Healthy Aquaculture Technology Model for Saline-alkaline Water; China Agriculture Press: Beijing, China, 2021; pp. 1–3. ISBN 9787109290310. [Google Scholar]
- Niu, M.; Wang, H.; Shi, C.; Mu, C.; Wang, C.; Ye, Y. Saline-alkaline stress induced metabolomic changes and survival recovery by trimethylamine N-oxide in the mud crab (Scylla paramamosain). Aquaculture 2025, 599, 742097, Erratum in Aquaculture 2025, 599, 742194. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Luo, L.; Zhang, R.; Guo, K.; Zhao, Z. Untargeted LC-MS metabolomics reveals the metabolic responses in the Eriocheir sinensis gills exposed to salinity and alkalinity stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 281, 109908. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, W.; Yuan, F.; Liu, Q.; Cheng, H.; Jin, X.; Sun, Y. Integration of microbiomics and metabolomics reveals energy metabolism imbalance in crucian carp (Carassius auratus) under saline-alkaline exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 291, 110145. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in gill of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 2024, 586, 740766. [Google Scholar] [CrossRef]
- Chang, Y.M.; Zhao, X.F.; Liew, H.J.; Sun, B.; Wang, S.Y.; Luo, L.; Zhang, L.M.; Liang, L.Q. Effects of bicarbonate stress on serum ions and gill transporters in alkali and freshwater forms of Amur Ide (Leuciscus waleckii). Front Physiol. 2021, 12, 676096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Jin, R.Z.; Shi, C.; Ye, Y.F.; Chen, S.J.; Mu, C.K.; Li, R.H.; Liu, L.; Wang, X.P.; Zhou, Y.Y.; et al. Effects of trimethylamine N-oxide supplementation on low-salinity tolerance of the mud crab relative to Na/K-ATPase activity and haemolymph osmolality. Aquaculture 2025, 598, 742096. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; Breves, J.P. Molecular mechanisms of Cl- transport in fishes: New insights and their evolutionary context. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 207–216. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Feng, B.; Zhang, M.; Gong, J.; Chen, H.; Munganga, B.P.; Tao, X.; Feng, J. Temporal Transcriptomic Profiling Reveals Dynamic Changes in Gene Expression of Giant Freshwater Prawn upon Acute Saline-Alkaline Stresses. Mar. Biotechnol. 2024, 26, 511–525. [Google Scholar] [CrossRef]
- Adhikari, S.; Chaurasia, V.S.; Naqvi, A.A.; Pillai, B.R. Survival and growth of Macrobrachium rosenbergii (de Man) juvenile in relation to calcium hardness and bicarbonate alkalinity. Turk. J. Fish. Aquat. Sci. 2007, 7, 23–26. [Google Scholar]
- Li, L.; Luo, W.; Chen, P.; Wang, Y.; Liu, D.; Lan, Y.; Chen, X.; Zhou, L.; Yang, S.; Du, Z. Study on the physiological responses and tolerance mechanisms to subchronic carbonate alkalinity exposure in the gills of Paramisgurnus dabryanus. Ecotoxicol. Environ. Saf. 2024, 287, 117319. [Google Scholar] [CrossRef]
- Ma, J.L.; Bai, Y.L.; Liang, Y.D.; Zhang, T.; You, L.; Xu, D.P. Integrated analysis of lncRNA-miRNA-mRNA regulatory network revealing molecular mechanisms of immune response and ion transport in Eriocheir sinensis under alkalinity stress. Comp. Biochem. Physiol. Part C Toxicol. 2025, 297, 110262. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.J.; Wei, X.F.; Geng, C.Y.; Liu, W.Z.; Han, L.; Yuan, F.Y.; Wang, P.; Sun, Y.C. Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites 2023, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, Z.G.; Li, M.S.; Luo, L.; Wang, S.H.; Guo, K.; Xu, W. Effects of saline-alkali stress on the tissue structure, antioxidation, immunocompetence and metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef]
- Hebert, S.C.; Mount, D.B.; Gamba, G. Molecular physiology of cation-coupled Cl− cotransport: The SLC12 family. Pflugers Arch. 2004, 447, 580–593. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Pisella, L.I.; Medina, I.; Nothwang, H.G. Molecular cloning and biochemical characterization of two cation chloride cotransporter subfamily members of Hydra vulgaris. PLoS ONE 2017, 12, e0179968. [Google Scholar] [CrossRef]
- Zhang, S.; Meor Azlan, N.F.; Josiah, S.S.; Zhou, J.; Zhou, X.; Jie, L.; Zhang, Y.; Dai, C.; Liang, D.; Li, P.; et al. The role of SLC12A family of cation-chloride cotransporters and drug discovery methodologies. J. Pharm. Anal. 2023, 13, 1471–1495. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R.; Caceres, P.S.; Ortiz, P.A. Molecular regulation of NKCC2 in the thick ascending limb. Am. J. Physiol. Renal. Physiol. 2011, 301, F1143–F1159. [Google Scholar] [CrossRef]
- Gamba, G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 2005, 85, 423–493. [Google Scholar] [CrossRef]
- Kang, C.K.; Tsai, H.J.; Liu, C.C.; Lee, T.H.; Hwang, P.P. Salinity-dependent expression of a Na+, K+, 2Cl− cotransporter in gills of the brackish medaka Oryzias dancena: A molecular correlate for hyposmoregulatory endurance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Masroor, W.; Farcy, E.; Blondeau-Bidet, E.; Venn, A.; Tambutte, E.; Lorin-Nebel, C. Effect of salinity and temperature on the expression of genes involved in branchial ion transport processes in European sea bass. J. Therm. Biol. 2019, 85, 102422. [Google Scholar] [CrossRef]
- Maraschi, A.C.; Faria, S.C.; McNamara, J.C. Salt transport by the gill Na+-K+-2Cl− symporter in palaemonid shrimps: Exploring physiological, molecular and evolutionary landscapes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110968. [Google Scholar] [CrossRef]
- Li, X.; Dai, X. Characterization and functional analysis of Litopenaeus vannamei Na+/K+/2Cl− cotransporter 1 under nitrite stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 298, 111749. [Google Scholar] [CrossRef]
- Wang, J.W.; Qin, K.X.; Wang, C.L.; Mu, C.K.; Wang, H. Analysis based on osmoregulatory enzymes and LC-MS technology for the adaptation of antennal gland of mud crabs (Scylla paramamosain) to acute chloride type low-salt saline-alkali water stress. Aquac. Rep. 2024, 39, 12394. [Google Scholar] [CrossRef]
- Xu, W.J.; Xie, J.J.; Shi, H.; Li, C.W. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 2010, 300, 25–31. [Google Scholar] [CrossRef]
- Ge, Q.Q.; Li, J.; Wang, J.J.; Li, Z.D.; Li, J.T. Characterization, functional analysis, and expression levels of three carbonic anhydrases in response to pH and saline-alkaline stresses in the ridgetail white prawn Exopalaemon carinicauda. Cell Stress. Chaperones 2019, 24, 503–515. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, J.J.; Wang, C.W.; Li, W.Y.; Ge, Q.Q.; Qin, Z.; Li, J.; Li, J.T. The Responses of the Ovary and Eyestalk in Exopalaemon carinicauda under Low Salinity Stress. Fishes 2022, 7, 365. [Google Scholar] [CrossRef]
- Wang, J.J.; Lv, J.J.; Shi, M.; Ge, Q.Q.; Wang, Q.; He, Y.Y.; Li, J.; Li, J.T. Chromosome-level genome assembly of ridgetail white shrimp Exopalaemon carinicauda. Sci. Data 2024, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.J.; Ackels, T.; Kriebel, A.; Kriebel, K.; Mey, J.; Kuenzel, T.; Wagner, H. Expression patterns of chloride transporters in the auditory brainstem of developing chicken. Hearing Res. 2020, 393, 108013. [Google Scholar] [CrossRef]
- Xu, B.P.; Tu, D.D.; Yan, M.C.; Shu, M.A.; Shao, Q.J. Molecular characterization of a cDNA encoding Na+/K+/2Cl− cotransporter in the gill of mud crab (Scylla paramamosain) during the molt cycle: Implication of its function in osmoregulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 115–125. [Google Scholar] [CrossRef]
- Blondeau-Bidet, E.; Hiroi, J.; Lorin-Nebel, C. Ion uptake pathways in European sea bass Dicentrarchus labrax. Gene 2019, 692, 126–137. [Google Scholar] [CrossRef]
- Takabe, S.; Inokuchi, M.; Yamaguchi, Y.; Hyodo, S. Distribution and dynamics of branchial ionocytes in houndshark reared in full-strength and diluted seawater environments. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 198, 22–32. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Xia, S.; Geng, Y.; Ge, G.; Dong, Y.; Shen, T.; Liu, Z.; Zhou, L.; Sun, X.; Wu, B. Identification of SLC12 Gene Family in Sinonovacula constricta and Its Expression Patterns under Acute Salt Stress. J. Jilin Agric. Univ. 2025, 47, 507–515. [Google Scholar] [CrossRef]
- Gagnon, K.B.; Di Fulvio, M. A molecular analysis of the Na+-independent cation chloride cotransporters. Cell. Physiol. Biochem. 2013, 32, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tian, E.; Turner, R.J.; Ten Hagen, K.G. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. Am. J. Physiol.-Cell Physiol. 2010, 298, C26–C37. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M. SLC12 Genes in Planarians: A Study of Evolutionary Conservation and Functional Roles in Ion Transportation. Master’s Thesis, Southern Illinois University, Edwardsville, IL, USA, 2024. [Google Scholar]
- Rogers, R.L.; Grizzard, S.L.; Titus-McQuillan, J.E.; Bockrath, K.; Patel, S.; Wares, J.P.; Garner, J.T.; Moore, C.C. Gene family amplification facilitates adaptation in freshwater unionid bivalve Megalonaias nervosa. Mol. Ecol. 2021, 30, 1155–1173. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Ohno, S. Evolution by Gene Duplication; Springer: New York, NY, USA, 1970. [Google Scholar]
- Vandepoele, K.; De Vos, W.; Taylor, J.S.; Meyer, A.; Van de Peer, Y. Major events in the genome evolution of vertebrates: Paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 1638–1643. [Google Scholar] [CrossRef]
- Moreno, E.; Pacheco-Alvarez, D.; Chávez-Canales, M.; Elizalde, S.; Leyva-Ríos, K.; Gamba, G. Structure-function relationships in the sodium chloride cotransporter. Front. Physiol. 2023, 14, 1118706. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Zhang, X.Y.; Wen, H.S.; Qi, X.; Fan, H.Y.; Tian, Y.; Liu, Y.; Li, Y. Spotted sea bass (Lateolabrax maculatus) cftr, nkcc1a, nkcc1b and nkcc2: Genome-wide identification, characterization and expression analysis under salinity stress. J. Ocean. Univ. China 2019, 18, 1470–1480. [Google Scholar] [CrossRef]
- Zhou, T.; Yue, C.P.; Huang, J.Y.; Cui, J.Q.; Liu, Y.; Wang, W.M.; Tian, C.; Hua, Y.P. Genome-wide identification of the amino acid permease genes and molecular characterization of their transcriptional responses to various nutrient stresses in allotetraploid rapeseed. BMC Plant Biol. 2020, 20, 151. [Google Scholar] [CrossRef]
- Trejo, F.; Elizalde, S.; Mercado, A.; Gamba, G.; de losHeros, P. SLC12A cryo-EM: Analysis of relevant ion binding sites, structural domains, and amino acids. Am. J. Physiol.-Cell Physiol. 2023, 325, C921–C939. [Google Scholar] [CrossRef]
- Cutler, C.P.; Cramb, G. Two isoforms of the Na+/K+/2Cl− cotransporter are expressed in the European eel (Anguilla anguilla). Biochim. Biophys. Acta 2002, 1566, 92–103. [Google Scholar] [CrossRef]
- Watanabe, S.; Mekuchi, M.; Ideuchi, H.; Kim, Y.K.; Kaneko, T. Electroneutral cation-Cl- cotransporters NKCC2β and NCCβ expressed in the intestinal tract of Japanese eel Anguilla japonica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.J.P.; Weihrauch, D. Exploring the versatility of the perfused crustacean gill as a model for transbranchial transport processes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 254, 110572. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, J.; Li, J.; Li, J. Effect of high alkalinity on shrimp gills: Histopathological alterations and cell specific responses. Ecotoxicol. Environ. Saf. 2023, 256, 114902. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, D.; Liu, P.; Li, J. Effects of salinity acclimation and eyestalk ablation on Na+, K+, 2Cl− cotransporter gene expression in the gill of Portunus trituberculatus: A molecular correlate for salt-tolerant trait. Cell Stress. Chaperones 2016, 21, 829–836. [Google Scholar] [CrossRef]
- Moshtaghi, A.; Rahi, M.L.; Nguyen, V.T.; Mather, P.B.; Hurwood, D.A. A transcriptomic scan for potential candidate genes involved in osmoregulation in an obligate freshwater palaemonid prawn (Macrobrachium australiense). PeerJ 2016, 4, e2520. [Google Scholar] [CrossRef]
- Lema, S.C.; Carvalho, P.G.; Egelston, J.N.; Kelly, J.T.; McCormick, S.D. Dynamics of Gene Expression Responses for Ion Transport Proteins and Aquaporins in the Gill of a Euryhaline Pupfish during Freshwater and High-Salinity Acclimation. Physiol. Biochem. Zool. 2018, 91, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Lin, L.Y.; Lee, T.H. Na+;, K+, 2Cl−-cotransporter: A novel marker for identifying freshwater- and seawater-type mitochondria-rich cells in gills of the euryhaline tilapia, Oreochromis mossambicus. Zool. Stud. 2003, 42, 186–192. [Google Scholar]
- Lorin-Nebel, C.; Boulo, V.; Bodinier, C.; Charmantier, G. The Na+/K+/2Cl− cotransporter in the sea bass Dicentrarchus labrax during ontogeny: Involvement in osmoregulation. J. Exp. Biol. 2006, 209, 4908–4922. [Google Scholar] [CrossRef]
- Takei, Y.; Hiroi, J.; Takahashi, H.; Sakamoto, T. Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R778–R792. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Zhou, L.; Zeng, X.; Zhang, Z.; Zou, P.F.; Huang, W.; Wang, Y. Effects of low-salinity acclimation on the Na+/K+ ATPase activity and expression of osmoregulatory-related genes in large yellow croaker (Larimichthys crocea). Aquac. Rep. 2022, 26, 101326. [Google Scholar] [CrossRef]
- Inokuchi, M.; Hiroi, J.; Watanabe, S.; Lee, K.M.; Kaneko, T. Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 151–158. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.; Wei, H.; Lu, J.; Mu, C.; Wang, C. Transcriptomic analysis of adaptive mechanisms in response to sudden salinity drop in the mud crab, Scylla paramamosain. BMC Genom. 2018, 19, 421. [Google Scholar] [CrossRef]
- Jaffer, Y.D.; Bhat, I.A.; Mir, I.N.; Bhat, R.A.H.; Sidiq, M.J.; Jana, P. Adaptation of cultured decapod crustaceans to changing salinities: Physiological responses, molecular mechanisms and disease implications. Rev. Aquacult. 2024, 16, 1520–1543. [Google Scholar] [CrossRef]
- Yao, Z.L.; Lai, Q.F.; Zhou, K.; Rizalita, R.-E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, R.; Kang, Y.; Mao, X.; Shi, X.; Guo, J.; Wang, Z. Analysis of circRNA expression profile of Litopenaeus vannamei under pH and alkalinity interactive stress and verification of novel_circ_021024 and novel_circ_004981 regulating stress compounds metabolism. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 55, 101469. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, P.; Li, Z.; Zhao, L.; Lai, X.; Gao, H.; Li, J.; Yan, B. Cloning, expression and stability analysis of the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Exopalaemon carinicauda. J. Fish. Sci. China 2017, 24, 1003–1012. [Google Scholar] [CrossRef]

| Name | aa Number | MW (kDa) | pI | Instability Index | Aliphatic Index | GRAVY | Subcellular Location |
|---|---|---|---|---|---|---|---|
| EcSLC12A2.1 | 1057 | 117.19 | 5.68 | 42.72 | 98.7 | 0.091 | Cell membrane |
| EcSLC12A2.2 | 1029 | 113.51 | 6.2 | 41.46 | 99.52 | 0.152 | Cell membrane |
| EcSLC12A2.3 | 945 | 103.83 | 5.89 | 36.44 | 102.99 | 0.171 | Endoplasmic reticulum |
| EcSLC12A6 | 1052 | 116.58 | 8.21 | 35.62 | 100.67 | 0.155 | Cell membrane |
| EcSLC12A8 | 638 | 68.76 | 5.98 | 35.40 | 98.97 | 0.202 | Cell membrane |
| EcSLC12A9 | 957 | 105.4 | 6.35 | 35.08 | 104.11 | 0.262 | Cell membrane |
| Primer | Primer Sequence (5′–3′) | Size (bp) |
|---|---|---|
| For qRT-PCR | ||
| EcSLC12A2.2-F | CGTTGCACCGCAAGTTATGG | 105 |
| EcSLC12A2.2-R | CAGAAGCGTCAAACCTCCGTCATC | |
| EcSLC12A2.1-F | TTCACCAGGAAACAACCCAAAGG | 84 |
| EcSLC12A2.1-R | CAGGATATAAGGAAGCAGCAGAGTC | |
| EcSLC12A2.3-F | CGAAGGCAGCAACCAGAACATC | 83 |
| EcSLC12A2.3-R | AGAGATTAGCACCCGCAACAATG | |
| EcSLC12A6-F | TTGCTAAGAACTTGACTGAGGGAATC | 110 |
| EcSLC12A6-R | GTGACTGACGCCAACCATACG | |
| EcSLC12A8-F | GCTCTGGTGGTGACAGTGATG | 110 |
| EcSLC12A8-R | CAGGGATTCGCCTTTGG | |
| EcSLC12A9-F | CACAGGCTTCTCATTGACAACATTG | 89 |
| EcSLC12A9-R | GCTTGGTCGTCGTCATCATCTAC | |
| Ecβ-actin-F | AACTTTCAACACCCCAGCCA | 96 |
| Ecβ-actin-R | TCTCCAGAGTCCAGCACGAT | |
| For RNAi | ||
| dsRNA-a-F | GATCACTAATACGACTCACTATAGGGTGTTGACGTTGAAAACCCAA | 300 |
| dsRNA-a-R | GATCACTAATACGACTCACTATAGGGCTTATCCACTGGTGGCTGGT | |
| dsRNA-b-F | GATCACTAATACGACTCACTATAGGGTTTGCGTTATTGGTATGGCA | 486 |
| dsRNA-b-R | GATCACTAATACGACTCACTATAGGGGGAGCAGACACCAAAGAAGC | |
| dsRNA-c-F | GATCACTAATACGACTCACTATAGGGATGATGAGATTGCCAAAGGC | 561 |
| dsRNA-c-R | GATCACTAATACGACTCACTATAGGGGGAGCAGACACCAAAGAAGC | |
| GFPT7-F | GATCACTAATACGACTCACTATAGGGATGGTGAGCAAGGGGGAGGA | 741 |
| GFPT7-R | GATCACTAATACGACTCACTATAGGGTTACTTGTACAGCTCGTCCA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Wang, J.; Yan, K.; Yu, Z.; Li, J. Genome-Wide Identification of Solute Carrier Family 12 and Functional Characterization of Its Role in Saline–Alkaline Stress Acclimation in the Ridgetail White Shrimp Exopalaemon carinicauda. Int. J. Mol. Sci. 2025, 26, 8339. https://doi.org/10.3390/ijms26178339
Tang S, Wang J, Yan K, Yu Z, Li J. Genome-Wide Identification of Solute Carrier Family 12 and Functional Characterization of Its Role in Saline–Alkaline Stress Acclimation in the Ridgetail White Shrimp Exopalaemon carinicauda. International Journal of Molecular Sciences. 2025; 26(17):8339. https://doi.org/10.3390/ijms26178339
Chicago/Turabian StyleTang, Shuai, Jiajia Wang, Kuo Yan, Zhixin Yu, and Jitao Li. 2025. "Genome-Wide Identification of Solute Carrier Family 12 and Functional Characterization of Its Role in Saline–Alkaline Stress Acclimation in the Ridgetail White Shrimp Exopalaemon carinicauda" International Journal of Molecular Sciences 26, no. 17: 8339. https://doi.org/10.3390/ijms26178339
APA StyleTang, S., Wang, J., Yan, K., Yu, Z., & Li, J. (2025). Genome-Wide Identification of Solute Carrier Family 12 and Functional Characterization of Its Role in Saline–Alkaline Stress Acclimation in the Ridgetail White Shrimp Exopalaemon carinicauda. International Journal of Molecular Sciences, 26(17), 8339. https://doi.org/10.3390/ijms26178339






