Tibolone Improves Motor Recovery and Regulates Neuroinflammation and Gliosis in a Model of Traumatic Spinal Cord Injury
Abstract
1. Introduction
2. Results
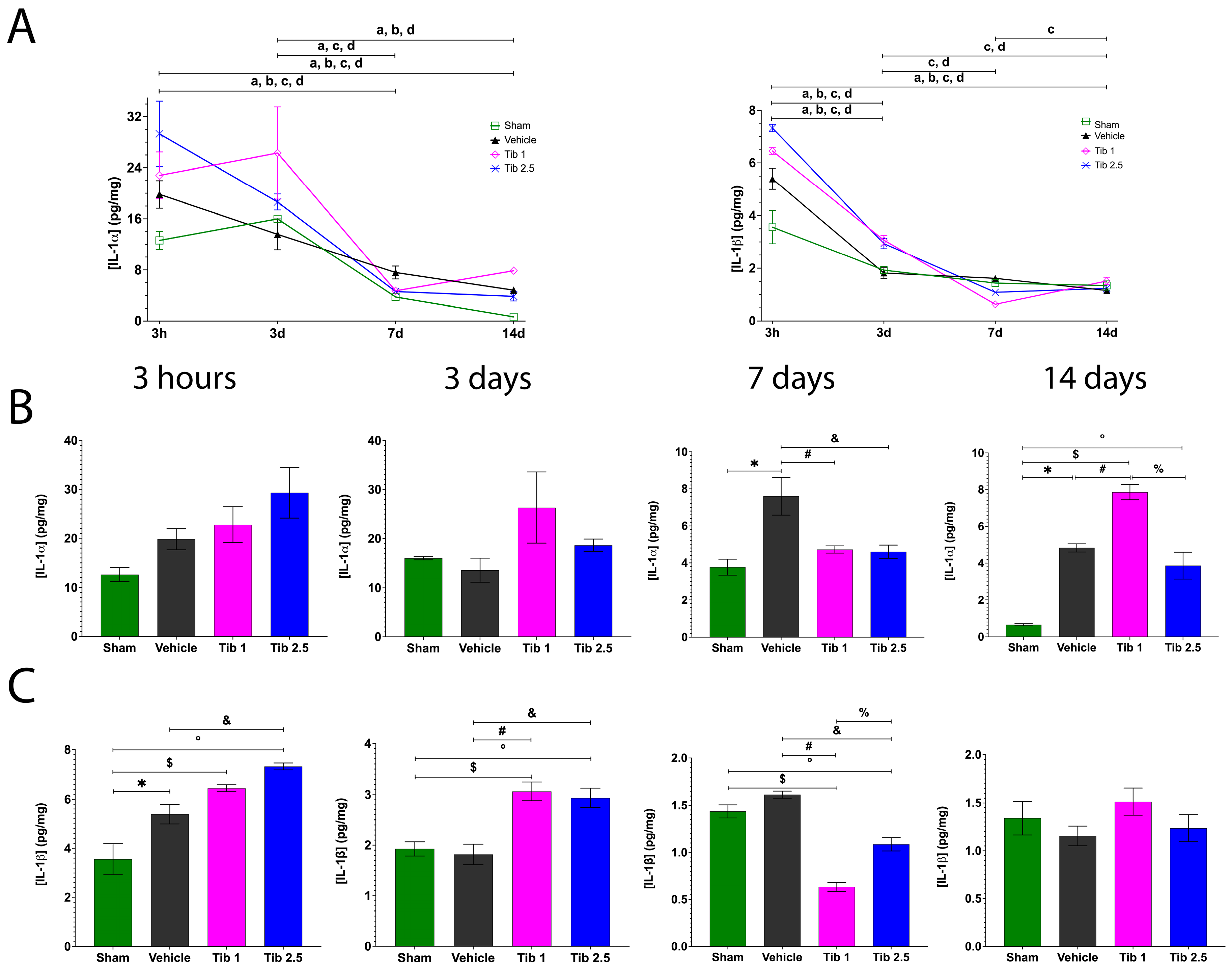
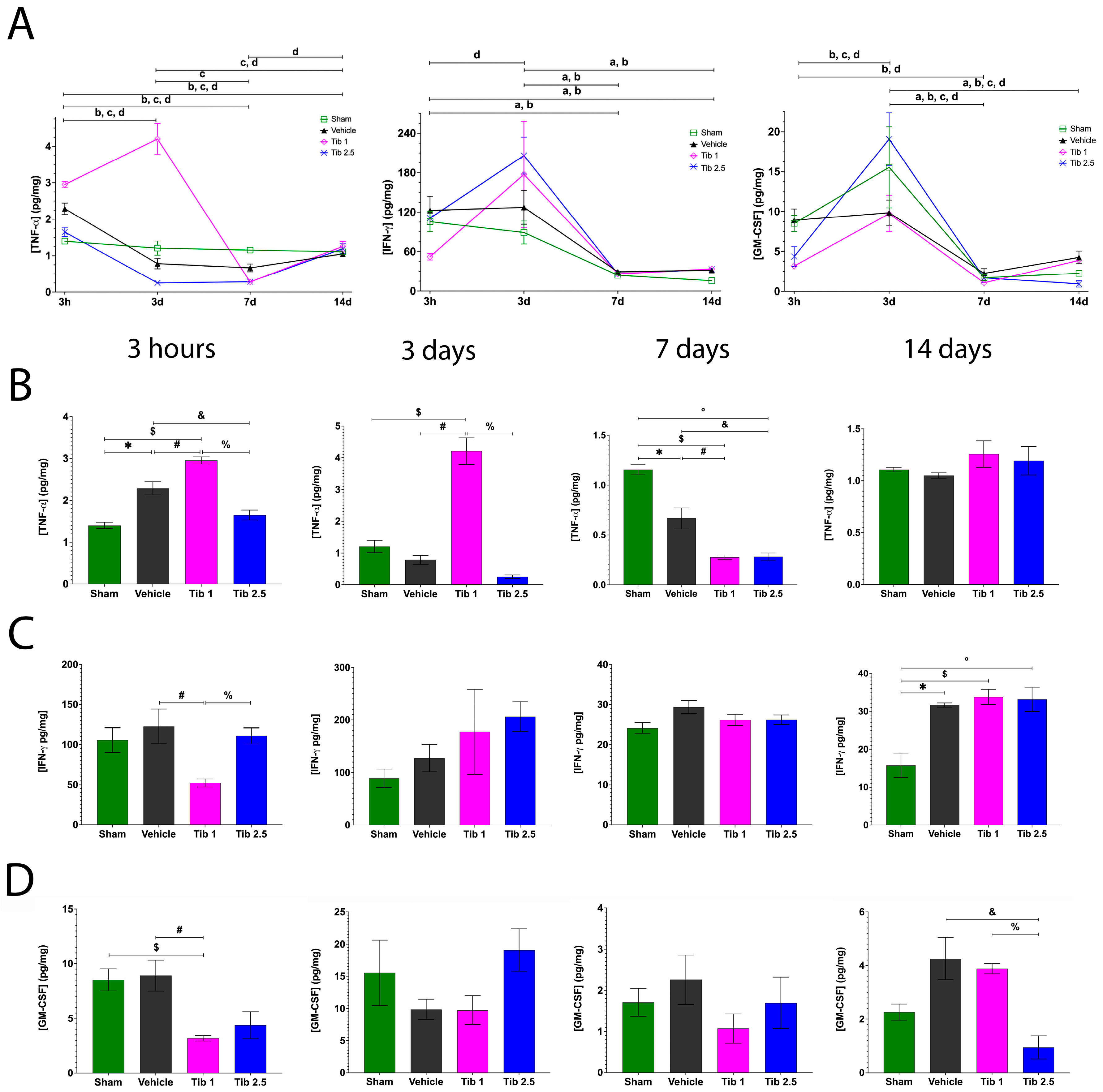
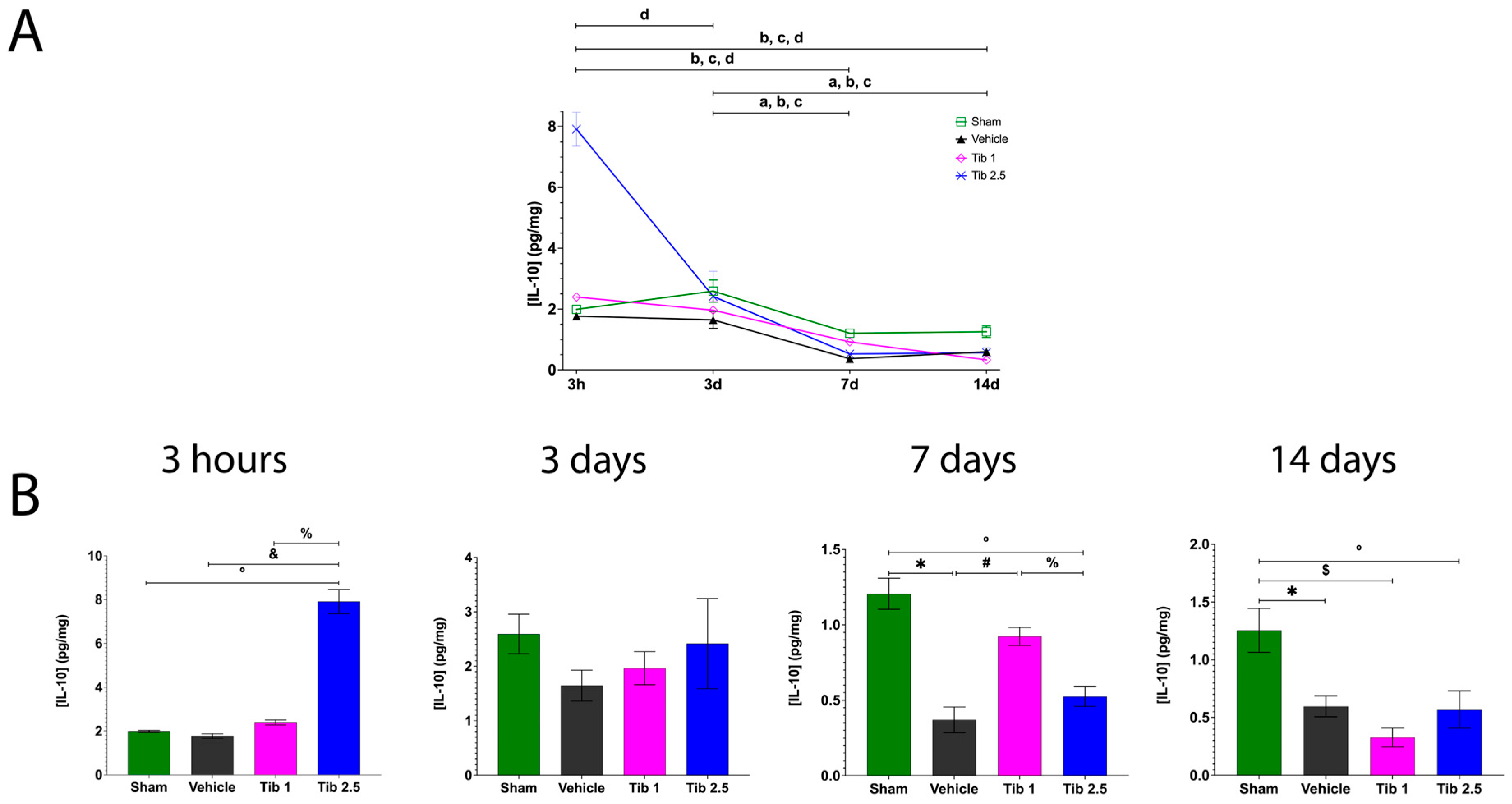
2.1. Tibolone Regulates Inflammation in Spinal Cord Injury
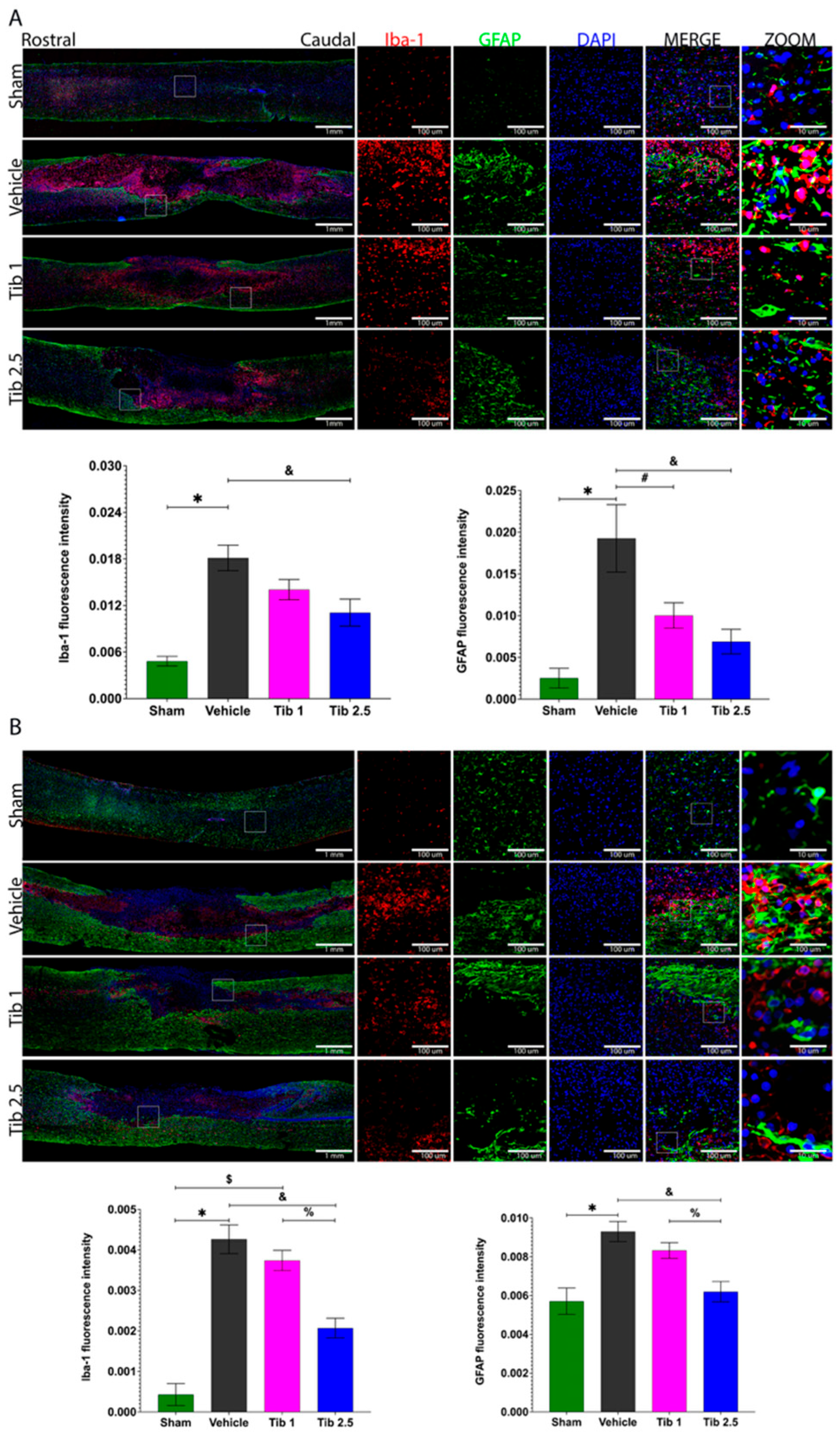
2.2. Tibolone Regulates Gliosis in Spinal Cord Injury
2.3. Tibolone Promotes Tissue Preservation
2.4. Tibolone Administration Improves Motor Function Recovery
3. Discussion
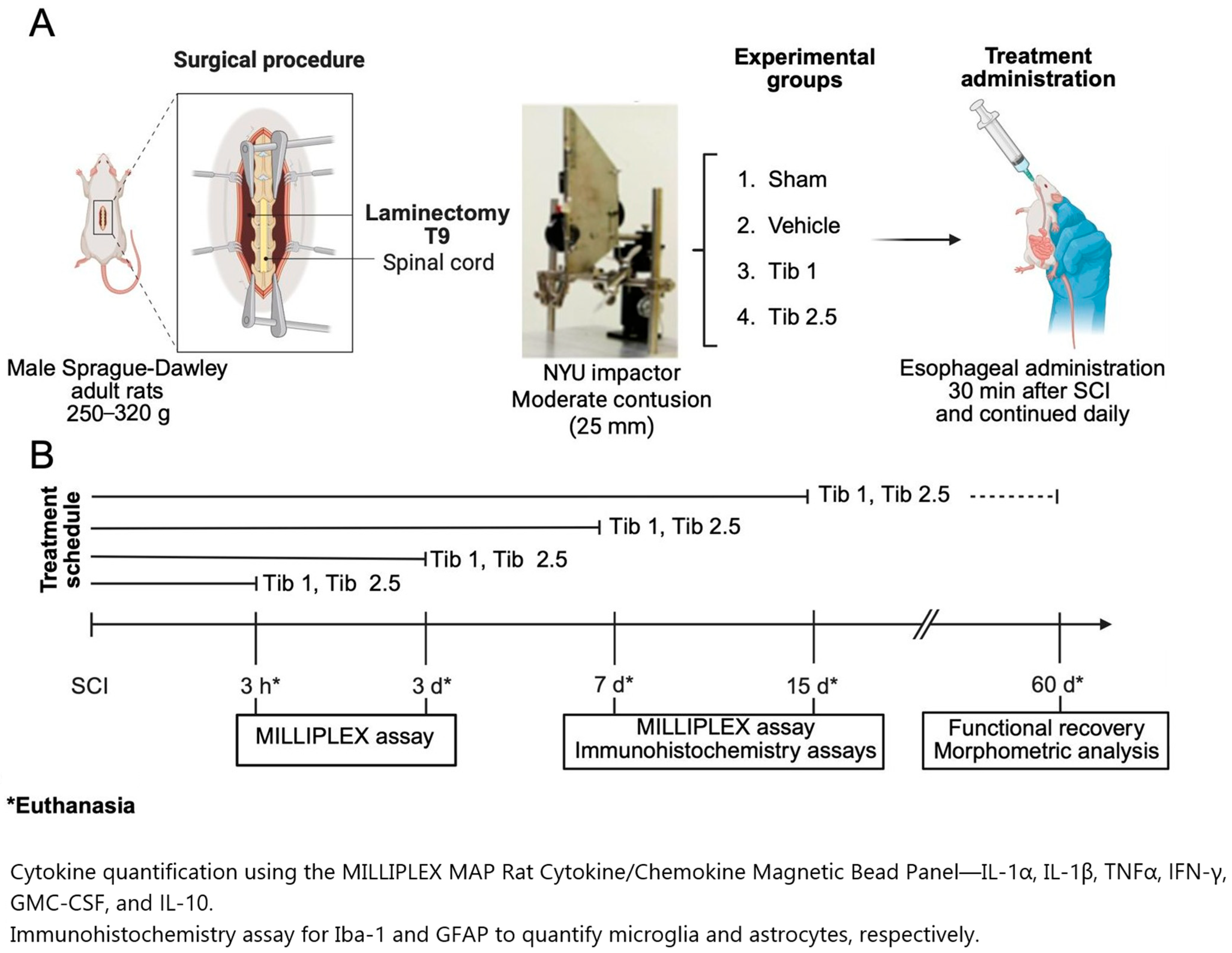
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Treatments
4.4. Cytokine Concentrations
4.5. Immunohistochemistry
4.6. Morphometric Analysis
4.7. Functional Recovery
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| DPN | diarylpropionitrile |
| GDNF | glial cell line-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| Iba1 | ionized calcium binding adaptor molecule 1 |
| IFN-γ | interferon-gamma |
| IGF-1 | insulin-like growth factor 1 |
| IL-1α | interleukin-1α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor κB |
| PPT | propyl pyrazole triol |
| SCI | spinal cord injury |
| Tib | tibolone |
| TNFα | tumor necrosis factor-alpha |
References
- National Spinal Cord Injury Statistical Center (NSCISC). Available online: https://sites.uab.edu/nscisc/ (accessed on 15 November 2024).
- Devivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Divanoglou, A.; Westgren, N.; Bjelak, S.; Levi, R. Medical conditions and outcomes at 1 year after acute traumatic spinal cord injury in a Greek and a Swedish region: A prospective, population-based study. Spinal Cord 2010, 48, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Golestani, A.; Shobeiri, P.; Sadeghi-Naini, M.; Jazayeri, S.B.; Maroufi, S.F.; Ghodsi, Z.; Dabbagh Ohadi, M.A.; Mohammadi, E.; Rahimi-Movaghar, V.; Ghodsi, S.M. Epidemiology of traumatic spinal cord injury in developing countries from 2009 to 2020: A systematic review and meta-analysis. Neuroepidemiology 2022, 56, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.W.; Gao, F.; Li, J.; Li, J.J. Epidemiological features of traumatic spinal cord injury in Beijing, China. J. Spinal Cord Med. 2022, 45, 214–220. [Google Scholar] [CrossRef]
- Bloom, O.; Herman, P.E.; Spungen, A.M. Systemic inflammation in traumatic spinal cord injury. Exp. Neurol. 2020, 325, 113143. [Google Scholar] [CrossRef] [PubMed]
- Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A target for treatment in spinal cord injury. Cells 2022, 11, 2692. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflammation 2021, 18, 284. [Google Scholar] [CrossRef]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef]
- Thompson, C.D.; Zurko, J.C.; Hanna, B.F.; Hellenbrand, D.J.; Hanna, A. The therapeutic role of interleukin-10 after spinal cord injury. J. Neurotrauma 2013, 30, 1311–1324. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Reichl, K.A.; Travis, B.J.; Filipp, M.E.; Khalil, A.S.; Pulito, D.J.; Gavigan, A.V.; Maginot, E.R.; Arnold, M.T.; Adler, A.G.; et al. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J. Neuroinflamm. 2019, 16, 93. [Google Scholar] [CrossRef]
- Bethea, J.R.; Nagashima, H.; Acosta, M.C.; Briceno, C.; Gomez, F.; Marcillo, A.E.; Loor, K.; Green, J.; Dietrich, W.D. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma 1999, 16, 851–863. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.Y.; Mun, C.H.; Suh, M.; Lee, J.E. Agmatine modulates the phenotype of macrophage acute phase after spinal cord injury in rats. Exp. Neurobiol. 2017, 26, 278–286. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Saxena, T.; Deng, B.; Stelzner, D.; Hasenwinkel, J.; Chaiken, J. Raman spectroscopic investigation of spinal cord injury in a rat model. J. Biomed. Opt. 2011, 16, 027003. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Guerra-Araiza, C.; Orozco-Suárez, S.; Salgado-Ceballos, H.; Feria-Romero, I.A.; Gallardo, J.M.; Orozco-Barrios, C.E. The importance of natural antioxidants in the treatment of spinal cord injury in animal models: An overview. Oxidative Med. Cell. Longev. 2019, 2019, 3642491. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, R.J. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J. Neurosurg. 2000, 93 (Suppl. 1), 1–7. [Google Scholar] [CrossRef]
- Pointillart, V.; Petitjean, M.E.; Wiart, L.; Vital, J.M.; Lassié, P.; Thicoipé, M.; Dabadie, P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000, 38, 71–76. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low-dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Kachadroka, S.; Hall, A.M.; Niedzielko, T.L.; Chongthammakun, S.; Floyd, C.L. Effect of endogenous androgens on 17β-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 2010, 27, 611–626. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Post-injury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef]
- Benyi, E.; Kieler, H.; Linder, M.; Ritzén, M.; Carlstedt-Duke, J.; Tuvemo, T.; Westphal, O.; Sävendahl, L. Risks of malignant and non-malignant tumours in tall women treated with high-dose oestrogen during adolescence. Horm. Res. Paediatr. 2014, 82, 89–96. [Google Scholar] [CrossRef]
- Wibowo, E.; Schellhammer, P.; Wassersug, R.J. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J. Urol. 2011, 185, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; Charpeaud, T.; Logan, S.; Hannaert, P.; Garay, R.G.; Llorca, P.M.; Shorey, S. Pharmacotherapeutic approaches to treating depression during the perimenopause. Expert Opin. Pharmacother. 2019, 20, 1837–1845. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Segura-Uribe, J.J.; Farfán-García, E.D.; Guerra-Araiza, C. Effects of tibolone on the central nervous system: Clinical and experimental approaches. BioMed Res. Int. 2017, 2017, 8630764. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Ferraboschi, P.; Franchini, L.; Nishino, H.; Takayasu, J.; Tokuda, H. Anti-tumor-promoting activity of tibolone and its metabolites. Arzneimittelforschung 2008, 58, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Farfán-García, E.D.; Castillo-Hernández, M.C.; Pinto-Almazán, R.; Rivas-Arancibia, S.; Gallardo, J.M.; Guerra-Araiza, C. Tibolone prevents oxidation and ameliorates cholinergic deficit induced by ozone exposure in the male rat hippocampus. Neurochem. Res. 2014, 39, 1776–1786. [Google Scholar] [CrossRef]
- Neri-Gómez, T.; Espinosa-Raya, J.; Díaz-Cintra, S.; Segura-Uribe, J.; Orozco-Suárez, S.; Gallardo, J.M.; Guerra-Araiza, C. Tibolone modulates neuronal plasticity through regulating Tau, GSK3β/Akt/PI3K pathway and CDK5 p35/p25 complexes in the hippocampus of aged male mice. Neural Regen. Res. 2017, 12, 588–595. [Google Scholar] [CrossRef]
- Hidalgo-Lanussa, O.; Ávila-Rodriguez, M.; Baez-Jurado, E.; Zamudio, J.; Echeverria, V.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone reduces oxidative damage and inflammation in microglia stimulated with palmitic acid through mechanisms involving estrogen receptor beta. Mol. Neurobiol. 2018, 55, 5462–5477. [Google Scholar] [CrossRef]
- Sánchez-Torres, S.; Orozco-Barrios, C.; Salgado-Ceballos, H.; Segura-Uribe, J.J.; Guerra-Araiza, C.; León-Cholula, Á.; Morán, J.; Coyoy-Salgado, A. Tibolone improves locomotor function in a rat model of spinal cord injury by modulating apoptosis and autophagy. Int. J. Mol. Sci. 2023, 24, 15285. [Google Scholar] [CrossRef]
- Estrada-Cruz, N.A.; Almanza-Pérez, J.C.; Fortis-Barrera, Á.; Gallardo, J.M.; Manuel-Apolinar, L.; Segura-Uribe, J.J.; Orozco-Suárez, S.; Coyoy-Salgado, A.; Guerra-Araiza, C. Acute administration of tibolone prevents oxidative stress in ovariectomized rats fed high-fat-and-fructose diet. Exp. Clin. Endocrinol. Diabetes 2019, 127, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Campos, V.; Díaz-Ruiz, A.; Padilla-Gómez, E.; Aguilar Zavala, H.; Ríos, C.; Díaz Cintra, S. Effect of tibolone on dendritic spine density in the rat hippocampus. Neurologia 2015, 30, 401–406. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef]
- Bautista-Poblet, G.; Coyoy-Salgado, A.; Bonilla-Jaime, H.; Salgado-Ceballos, H.; Castillo-García, E.L.; Sánchez-Torres, S.; Castillo-Mendieta, T.; Segura-Uribe, J.J.; Pinto-Almazán, R.; Guerra-Araiza, C. Acute administration of tibolone reduces oxidative stress and inflammation in a rat model of traumatic spinal cord injury. Neurol. Res. 2025; 1–14, Online ahead of print. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barrera, R.; Flores-Romero, A.; García, E.; Fernández-Presas, A.M.; Incontri-Abraham, D.; Navarro-Torres, L.; García-Sánchez, J.; Juárez-Vignon Whaley, J.J.; Madrazo, I.; Ibarra, A. Immunization with neural-derived peptides increases neurogenesis in rats with chronic spinal cord injury. CNS Neurosci. Ther. 2020, 26, 650–658. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zai, L.J.; Wrathall, J.R. Cell proliferation and replacement following contusive spinal cord injury. Glia 2005, 50, 247–257. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- Blank, E.W.; Wong, P.Y.; Lakshmanaswamy, R.; Guzman, R.; Nandi, S. Both ovarian hormones estrogen and progesterone are necessary for hormonal mammary carcinogenesis in ovariectomized ACI rats. Proc. Natl. Acad. Sci. USA 2008, 105, 3527–3532. [Google Scholar] [CrossRef]
- Yarrow, J.F.; Conover, C.F.; Beggs, L.A.; Beck, D.T.; Otzel, D.M.; Balaez, A.; Combs, S.M.; Miller, J.R.; Ye, F.; Aguirre, J.I.; et al. Testosterone dose-dependently prevents bone and muscle loss in rodents after spinal cord injury. J. Neurotrauma 2014, 31, 834–845. [Google Scholar] [CrossRef]
- Cox, A.; Varma, A.; Barry, J.; Vertegel, A.; Banik, N. Nanoparticle estrogen in rat spinal cord injury elicits rapid anti-inflammatory effects in plasma, cerebrospinal fluid, and tissue. J. Neurotrauma 2015, 32, 1413–1421. [Google Scholar] [CrossRef]
- Ray, S.K.; Samantaray, S.; Banik, N.L. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen. Res. 2016, 11, 1418–1419. [Google Scholar] [CrossRef]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and breast cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef]
- Sawada, H.; Ibi, M.; Kihara, T.; Urushitani, M.; Akaike, A.; Shimohama, S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J. Neurosci. Res. 1998, 54, 707–719. [Google Scholar] [CrossRef]
- Myers, R.E.; Anderson, L.I.; Dluzen, D.E. Estrogen, but not testosterone, attenuates methamphetamine-evoked dopamine output from superfused striatal tissue of female and male mice. Neuropharmacology 2003, 44, 624–632. [Google Scholar] [CrossRef]
- Fee, D.B.; Swartz, K.R.; Joy, K.M.; Roberts, K.N.; Scheff, N.N.; Scheff, S.W. Effects of progesterone on experimental spinal cord injury. Brain Res. 2007, 1137, 146–152. [Google Scholar] [CrossRef]
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective effects of testosterone on muscle and motoneurons morphology following spinal cord injury. J. Comp. Neurol. 2012, 520, 2683–2696. [Google Scholar] [CrossRef]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as hormonal therapy and neuroprotective agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Kloosterboer, H.J. Tissue-selectivity: The mechanism of action of tibolone. Maturitas 2004, 48 (Suppl. 1), 30–40. [Google Scholar] [CrossRef]
- de Gooyer, M.E.; Deckers, G.H.; Schoonen, W.G.; Verheul, H.A.; Kloosterboer, H.J. Receptor profiling and endocrine interactions of tibolone. Steroids 2003, 68, 21–30. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Wingrave, J.M.; Matzelle, D.D.; Wilford, G.G.; Ray, S.K.; Banik, N.L. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005, 82, 283–293. [Google Scholar] [CrossRef]
- Samantaray, S.; Smith, J.A.; Das, A.; Matzelle, D.D.; Varma, A.K.; Ray, S.K.; Banik, N.L. Low-dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: Effect of dosing, route of administration, and therapy delay. Neurochem. Res. 2011, 36, 1809–1816. [Google Scholar] [CrossRef]
- Letaif, O.B.; Cristante, A.F.; de Barros Filho, T.E.P.; Ferreira, R.; dos Santos, G.B.; da Rocha, I.D.; Marcon, R.M. Effects of estrogen on functional and neurological recovery after spinal cord injury: An experimental study with rats. Clinics 2015, 70, 700–705. [Google Scholar] [CrossRef]
- Akuzawa, S.; Kazui, T.; Shi, E.; Yamashita, K.; Bashar, A.H.; Terada, H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J. Vasc. Surg. 2008, 48, 694–700. [Google Scholar] [CrossRef]
- Sato, A.; Ohtaki, H.; Tsumuraya, T.; Song, D.; Ohara, K.; Asano, M.; Iwakura, Y.; Atsumi, T.; Shioda, S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J. Neuroinflamm. 2012, 9, 65. [Google Scholar] [CrossRef]
- Boato, F.; Rosenberger, K.; Nelissen, S.; Geboes, L.; Peters, E.M.; Nitsch, R.; Hendrix, S. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J. Neuroinflamm. 2013, 10, 6. [Google Scholar] [CrossRef]
- Liu, S.; Xu, G.Y.; Johnson, K.M.; Echetebu, C.; Ye, Z.S.; Hulsebosch, C.E.; McAdoo, D.J. Regulation of interleukin-1beta by the interleukin-1 receptor antagonist in the glutamate-injured spinal cord: Endogenous neuroprotection. Brain Res. 2008, 1231, 63–74. [Google Scholar] [CrossRef]
- Ritz, M.F.; Hausmann, O.N. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008, 1203, 177–188. [Google Scholar] [CrossRef]
- Kaneko, N.; Kudo, K.; Mabuchi, T.; Takemoto, K.; Fujimaki, K.; Wati, H.; Iguchi, H.; Tezuka, H.; Kanba, S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology 2006, 31, 2619–2626. [Google Scholar] [CrossRef]
- Liu, X.; Quan, N. Microglia and CNS interleukin-1: Beyond immunological concepts. Front. Neurol. 2018, 9, 8. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Styren, S.D.; O’Malley, M.E.; Goss, J.R.; Kochanek, P.; Marion, D.; Evans, C.H.; Robbins, P.D. Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Ann. Neurol. 1996, 39, 123–127. [Google Scholar] [CrossRef]
- Lukacova, N.; Kisucka, A.; Kiss Bimbova, K.; Bacova, M.; Ileninova, M.; Kuruc, T.; Galik, J. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int. J. Mol. Sci. 2021, 22, 13577. [Google Scholar] [CrossRef]
- Sharma, H.S. A combination of tumor necrosis factor-alpha and neuronal nitric oxide synthase antibodies applied topically over the traumatized spinal cord enhances neuroprotection and functional recovery in the rat. Ann. N. Y. Acad. Sci. 2010, 1199, 175–185. [Google Scholar] [CrossRef]
- Cantarella, G.; Di Benedetto, G.; Scollo, M.; Paterniti, I.; Cuzzocrea, S.; Bosco, P.; Nocentini, G.; Riccardi, C.; Bernardini, R. Neutralization of tumor necrosis factor-related apoptosis-inducing ligand reduces spinal cord injury damage in mice. Neuropsychopharmacology 2010, 35, 1302–1314. [Google Scholar] [CrossRef]
- Yune, T.Y.; Chang, M.J.; Kim, S.J.; Lee, Y.B.; Shin, S.W.; Rhim, H.; Kim, Y.C.; Shin, M.L.; Oh, Y.J.; Han, C.T.; et al. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J. Neurotrauma 2003, 20, 207–219. [Google Scholar] [CrossRef]
- Sharma, H.S.; Winkler, T.; Stålberg, E.; Gordh, T.; Alm, P.; Westman, J. Topical application of TNF-alpha antiserum attenuates spinal cord trauma-induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir. Suppl. 2003, 86, 407–413. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Christensen, R.N.; Gensel, J.C.; Miller, B.A.; Sun, F.; Beattie, E.C.; Bresnahan, J.C.; Beattie, M.S. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008, 28, 11391–11400. [Google Scholar] [CrossRef]
- Labombarda, F.; Jure, I.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J. Steroid Biochem. Mol. Biol. 2015, 154, 274–284. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; Kubo, T.; Chan, C.C.; Koda, M.; Okawa, A.; Takahashi, K.; Yamazaki, M. Interferon-γ decreases chondroitin sulfate proteoglycan expression and enhances hindlimb function after spinal cord injury in mice. J. Neurotrauma 2010, 27, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Roselli, F.; Chandrasekar, A.; Morganti-Kossmann, M.C. Interferons in traumatic brain and spinal cord injury: Current evidence for translational application. Front. Neurol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Sun, G.; Yang, S.; Cao, G.; Wang, Q.; Hao, J.; Wen, Q.; Li, Z.; So, K.F.; Liu, Z.; Zhou, S.; et al. γδ T cells provide the early source of IFN-γ to aggravate lesions in spinal cord injury. J. Exp. Med. 2018, 215, 521–535. [Google Scholar] [CrossRef]
- Ha, Y.; Kim, Y.S.; Cho, J.M.; Yoon, S.H.; Park, S.R.; Yoon, D.H.; Kim, E.Y.; Park, H.C. Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J. Neurosurg. Spine 2005, 2, 55–61. [Google Scholar] [CrossRef]
- Huang, X.; Kim, J.M.; Kong, T.H.; Park, S.R.; Ha, Y.; Kim, M.H.; Park, H.; Yoon, S.H.; Park, H.C.; Park, J.O.; et al. GM-CSF inhibits glial scar formation and shows long-term protective effect after spinal cord injury. J. Neurol. Sci. 2009, 277, 87–97. [Google Scholar] [CrossRef]
- Chung, J.; Kim, M.H.; Yoon, Y.J.; Kim, K.H.; Park, S.R.; Choi, B.H. Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on glial scar formation after spinal cord injury in rats. J. Neurosurg. Spine 2014, 21, 966–973. [Google Scholar] [CrossRef]
- Bouhy, D.; Malgrange, B.; Multon, S.; Poirrier, A.L.; Scholtes, F.; Schoenen, J.; Franzen, R. Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 2006, 20, 1239–1241. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Mandelin, J.; Ceponis, A.; Miller, N.E.; Hukkanen, M.; Ma, G.F.; Konttinen, Y.T. Regulation of macrophage activation. Cell. Mol. Life Sci. 2003, 60, 2334–2346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, B.; Li, H.; Wang, Y.; Zhou, Y.; Wang, W.; Song, T.; Du, N.; Gu, X.; Luo, Y.; et al. SOCS3 attenuates GM-CSF/IFN-γ-mediated inflammation during spontaneous spinal cord regeneration. Neurosci. Bull. 2020, 36, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Sonobe, Y.; Kawanokuchi, J.; Doi, Y.; Noda, M.; Takeuchi, H.; Mizuno, T.; Suzumura, A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J. Neuroinflamm. 2012, 9, 268. [Google Scholar] [CrossRef]
- Sisson, S.D.; Dinarello, C.A. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood 1988, 72, 1368–1374. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Insolera, R.; Fink, D.J.; Mata, M. IL-10 promotes neuronal survival following spinal cord injury. Exp. Neurol. 2009, 220, 183–190. [Google Scholar] [CrossRef]
- Pearse, D.D.; Marcillo, A.E.; Oudega, M.; Lynch, M.P.; Wood, P.M.; Bunge, M.B. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: Does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma 2004, 21, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, J.A.; Yu, C.G.; Easton, J.M.; Bethea, J.R.; Yezierski, R.P. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp. Neurol. 2001, 168, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Harte, S.E.; Morrow, T.J.; Wang, S.; Mata, M.; Fink, D.J. Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury. Neurorehabilit. Neural Repair 2012, 26, 889–897. [Google Scholar] [CrossRef][Green Version]
- Yu, C.G.; Fairbanks, C.A.; Wilcox, G.L.; Yezierski, R.P. Effects of agmatine, interleukin-10, and cyclosporin on spontaneous pain behavior after excitotoxic spinal cord injury in rats. J. Pain 2003, 4, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.A.; Messinger, J.; Peduzzi, J.D.; Ansardi, D.C.; Morrow, C.D. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology 2005, 336, 173–183. [Google Scholar] [CrossRef]
- Margul, D.J.; Park, J.; Boehler, R.M.; Smith, D.R.; Johnson, M.A.; McCreedy, D.A.; He, T.; Ataliwala, A.; Kukushliev, T.V.; Liang, J.; et al. Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioeng. Transl. Med. 2016, 1, 136–148. [Google Scholar] [CrossRef]
- Brewer, K.L.; Bethea, J.R.; Yezierski, R.P. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp. Neurol. 1999, 159, 484–493. [Google Scholar] [CrossRef]
- Abraham, K.E.; McMillen, D.; Brewer, K.L. The effects of endogenous interleukin-10 on gray matter damage and the development of pain behaviors following excitotoxic spinal cord injury in the mouse. Neuroscience 2004, 124, 945–952. [Google Scholar] [CrossRef]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di Paola, R.; Caminiti, R.; Bramanti, P.; Cappelani, A.; Cuzzocrea, S. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J. Neurochem. 2009, 108, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Sabirzhanov, B.; Li, Y.; Coll-Miro, M.; Matyas, J.J.; He, J.; Kumar, A.; Ward, N.; Yu, J.; Faden, A.I.; Wu, J. Inhibition of NOX2 signaling limits pain-related behavior and improves motor function in male mice after spinal cord injury: Participation of IL-10/miR-155 pathways. Brain Behav. Immun. 2019, 80, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, P.; Zhou, T.; Jiang, W.; Wu, H.; Zhang, S.; Deng, L.; Wang, H. Neuroprotective effects of interleukin 10 in spinal cord injury. Front. Mol. Neurosci. 2023, 16, 1214294. [Google Scholar] [CrossRef]
- Sandrow-Feinberg, H.R.; Houlé, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef]
- Tan, M.; Feng, Z.; Chen, H.; Min, L.; Wen, H.; Liu, H.; Hou, J. Transcranial direct current stimulation regulates phenotypic transformation of microglia to relieve neuropathic pain induced by spinal cord injury. Front. Behav. Neurosci. 2023, 17, 1147693. [Google Scholar] [CrossRef]
- Montoto-Meijide, R.; Meijide-Faílde, R.; Díaz-Prado, S.M.; Montoto-Marqués, A. Mesenchymal stem cell therapy in traumatic spinal cord injury: A systematic review. Int. J. Mol. Sci. 2023, 24, 11719. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Ávila Rodriguez, M.; Garcia-Segura, L.M.; Cabezas, R.; Torrente, D.; Capani, F.; Gonzalez, J.; Barreto, G.E. Tibolone protects T98G cells from glucose deprivation. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 294–303, Corrigendum in J. Steroid Biochem. Mol. Biol. 2023, 232, 106292. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.; Garcia-Segura, L.M.; Hidalgo-Lanussa, O.; Baez, E.; Gonzalez, J.; Barreto, G.E. Tibolone protects astrocytic cells from glucose deprivation through a mechanism involving estrogen receptor beta and the upregulation of neuroglobin expression. Mol. Cell. Endocrinol. 2016, 433, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Castrillo, A.; Yanguas-Casás, N.; Arevalo, M.A.; Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. The synthetic steroid tibolone decreases reactive gliosis and neuronal death in the cerebral cortex of female mice after a stab wound injury. Mol. Neurobiol. 2018, 55, 8651–8667. [Google Scholar] [CrossRef]
- Villapol, S.; Byrnes, K.R.; Symes, A.J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front. Neurol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Azcoitia, I.; Sierra, A.; Garcia-Segura, L.M. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia 1999, 26, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Naftolin, F.; Hutchison, J.B.; Azcoitia, I.; Chowen, J.A. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J. Neurobiol. 1999, 40, 574–584. [Google Scholar] [CrossRef]
- Cardona-Gómez, G.P.; DonCarlos, L.; Garcia-Segura, L.M. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience 2000, 99, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ovejero, D.; Azcoitia, I.; Doncarlos, L.L.; Melcangi, R.C.; Garcia-Segura, L.M. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res. Rev. 2005, 48, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Romeo, H.E.; Micevych, P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: Localization of ERbeta and IGF-1R in substantia nigra. J. Comp. Neurol. 2007, 503, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Karolczak, M.; Krust, A.; Chambon, P.; Beyer, C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia 2005, 50, 270–275. [Google Scholar] [CrossRef]
- Bondar, G.; Kuo, J.; Hamid, N.; Micevych, P. Estradiol-induced estrogen receptor-alpha trafficking. J. Neurosci. 2009, 29, 15323–15330. [Google Scholar] [CrossRef]
- Micevych, P.; Bondar, G.; Kuo, J. Estrogen actions on neuroendocrine glia. Neuroendocrinology 2010, 91, 211–222. [Google Scholar] [CrossRef]
- De Marinis, E.; Acaz-Fonseca, E.; Arevalo, M.A.; Ascenzi, P.; Fiocchetti, M.; Marino, M.; Garcia-Segura, L.M. 17β-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor β-mediated neuroglobin up-regulation. J. Neuroendocrinol. 2013, 25, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Tuszynski, M.H. Reactive astrocytes express estrogen receptors in the injured primate brain. J. Comp. Neurol. 2001, 433, 115–123. [Google Scholar] [CrossRef]
- García-Ovejero, D.; Veiga, S.; García-Segura, L.M.; Doncarlos, L.L. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002, 450, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Yokoi, T.; Nakajima, R.; Hattori, H.; Matsuoka, O.; Yamano, T. Time course changes of estrogen receptor alpha expression in the adult rat hippocampus after kainic acid-induced status epilepticus. Acta Neuropathol. 2005, 110, 411–416. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, C.S.; Kim, J.; Kim, W.H.; Chung, Y.H.; Shin, D.H. Reactive astrocytes expressing intense estrogen receptor-alpha immunoreactivities have much elongated cytoplasmic processes: An autopsy case of human cerebellar tissue with multiple genitourinary and gastrointestinal anomalies. J. Korean Med. Sci. 2007, 22, 936–941. [Google Scholar] [CrossRef]
- Sakuma, S.; Tokuhara, D.; Hattori, H.; Matsuoka, O.; Yamano, T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology 2009, 29, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.B.; Zhao, C.; Deighton-Collins, S.; Kleerekoper, M.; Benjamins, J.A.; Skafar, D.F. Agonist activity of the 3-hydroxy metabolites of tibolone through the oestrogen receptor in the mouse N20.1 oligodendrocyte cell line and normal human astrocytes. J. Neuroendocrinol. 2007, 19, 958–965. [Google Scholar] [CrossRef]
- Spence, R.D.; Hamby, M.E.; Umeda, E.; Itoh, N.; Du, S.; Wisdom, A.J.; Cao, Y.; Bondar, G.; Lam, J.; Ao, Y.; et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 8867–8872. [Google Scholar] [CrossRef]
- Cerciat, M.; Unkila, M.; Garcia-Segura, L.M.; Arevalo, M.A. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 2010, 58, 93–102. [Google Scholar] [CrossRef]
- Lee, E.S.; Sidoryk, M.; Jiang, H.; Yin, Z.; Aschner, M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J. Neurochem. 2009, 110, 530–544. [Google Scholar] [CrossRef]
- Lee, E.; Sidoryk-Wegrzynowicz, M.; Farina, M.; Rocha, J.B.; Aschner, M. Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes. Neurotox. Res. 2013, 23, 124–130. [Google Scholar] [CrossRef][Green Version]
- Sato, K.; Kuriwaki, J.; Takahashi, K.; Saito, Y.; Oka, J.; Otani, Y.; Sha, Y.; Nakazawa, K.; Sekino, Y.; Ohwada, T. Discovery of a Tamoxifen-related compound that suppresses glial l-glutamate transport activity without interaction with estrogen receptors. ACS Chem. Neurosci. 2012, 3, 105–113. [Google Scholar] [CrossRef][Green Version]
- Karki, P.; Webb, A.; Smith, K.; Lee, K.; Son, D.S.; Aschner, M.; Lee, E. cAMP response element-binding protein (CREB) and nuclear factor κB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J. Biol. Chem. 2013, 288, 28975–28986. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Wade, F.M.; Mahesh, V.B.; Brann, D.W. Astrocyte-derived transforming growth factor-b mediates the neuroprotective effects of 17β-estradiol: Involvement of nonclassical genomic signaling pathways. Endocrinology 2005, 146, 2749–2759. [Google Scholar] [CrossRef]
- Guptarak, J.; Wiktorowicz, J.E.; Sadygov, R.G.; Zivadinovi, D.; Paulucci-Holthauzen, A.A.; Vergara, L.; Nesic-Taylor, D.O. The cancer drug tamoxifen: A potential therapeutic treatment for spinal cord injury. J. Neurotrauma 2014, 31, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Kloosterboer, H.J. Tibolone: A steroid with a tissue-specific mode of action. J. Steroid Biochem. Mol. Biol. 2001, 76, 231–238. [Google Scholar] [CrossRef]
- Acaz-Fonseca, E.; Avila-Rodriguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog. Neurobiol. 2016, 144, 5–26. [Google Scholar] [CrossRef]
- Habib, P.; Beyer, C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015, 146, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Santos-Galindo, M.; Acaz-Fonseca, E.; Azcoitia, I.; Garcia-Segura, L.M. Gonadal hormones and the control of reactive gliosis. Horm. Behav. 2013, 63, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Finley, S.K.; Kritzer, M.F. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J. Neurobiol. 1999, 40, 446–457. [Google Scholar] [CrossRef]
- DonCarlos, L.L.; Sarkey, S.; Lorenz, B.; Azcoitia, I.; Garcia-Ovejero, D.; Huppenbauer, C.; Garcia-Segura, L.M. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience 2006, 138, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Veiga, S.; Azcoitia, I.; Garcia-Segura, L.M.; Garcia-Ovejero, D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 2007, 25, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.M.; Wright, D.K.; Sun, M.; Semple, B.D.; Ozturk, E.; Stein, D.G.; O’Brien, T.J.; Shultz, S.R. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J. Neuroinflamm. 2015, 12, 238. [Google Scholar] [CrossRef]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef]
- Liu, M.; Hurn, P.D.; Roselli, C.E.; Alkayed, N.J. Role of P450 aromatase in sex specific astrocytic cell death. J. Cereb. Blood Flow Metab. 2007, 27, 135–141. [Google Scholar] [CrossRef]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef]
- Heyer, A.; Hasselblatt, M.; von Ahsen, N.; Häfner, H.; Sirén, A.L.; Ehrenreich, H. In vitro gender differences in neuronal survival on hypoxia and in 17beta-estradiol-mediated neuroprotection. J. Cereb. Blood Flow Metab. 2005, 25, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.N.; Dorsa, D.M. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience 2010, 170, 1261–1269. [Google Scholar] [CrossRef]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef]
- Gillies, G.E.; McArthur, S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson’s disease: A contribution to sex-specific neuroprotection by estrogens. Horm. Behav. 2010, 57, 23–34. [Google Scholar] [CrossRef]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Male/female differences in neuroprotection and neuromodulation of brain dopamine. Front. Endocrinol. 2011, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.H.; Warden, S.; Lenz, K.M. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav. Immun. 2017, 64, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Lapato, A.; Chen, Y.; Vandenbark, A.A.; Saugstad, J.A.; Offner, H. Role for microglia in sex differences after ischemic stroke: Importance of M2. Metab. Brain Dis. 2015, 30, 1515–1529. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Bergeon Burns, C.M.; Wellman, C.L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 2016, 52, 88–97. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Collins, K.E.; Patel, R.; Wellman, C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS ONE 2017, 12, e0187631. [Google Scholar] [CrossRef]
- Yanguas-Casas, N.; Crespo-Castrillo, A.; de Ceballos, M.L.; Chowen, J.A.; Azcoitia, I.; Arevalo, M.A.; Garcia-Segura, L.M. Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia 2018, 66, 522–537. [Google Scholar] [CrossRef]
- Beggs, S.; Trang, T.; Salter, M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 2012, 15, 1068–1073. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.; Beggs, S.; Salter, M.W. Molecules in pain and sex: A developing story. Mol. Brain 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef]
- Farooque, M.; Suo, Z.; Arnold, P. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 2006, 44, 182–187. [Google Scholar] [CrossRef]
- Emamhadi, M.; Soltani, B.; Babaei, P.; Mashhadinezhad, H.; Ghadarjani, S. Influence of sexuality in functional recovery after spinal cord injury in rats. Arch. Bone Jt. Surg. 2016, 4, 56–59. [Google Scholar] [CrossRef]
- Walker, C.L.; Fry, C.M.E.; Wang, J.; Du, X.; Zuzzio, K.; Liu, N.K.; Walker, M.J.; Xu, X.M. Functional and histological gender comparison of age-matched rats after moderate thoracic contusive spinal cord injury. J. Neurotrauma 2019, 36, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Gruner, J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 1992, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Vialle, L.R.G.; Grochocki, L.R.; Nohama, P. NYU device: Automation of the Weight-drop rod. In World Congress on Medical Physics and Biomedical Engineering 2006; IFMBE Proceedings; Magjarevic, R., Nagel, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 14, pp. 728–730. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Das, A.; Samantaray, S.; Smith, J.A.; Banik, N.L.; Haque, A.; Ray, S.K. Molecular mechanisms of estrogen for neuroprotection in spinal cord injury and traumatic brain injury. Rev. Neurosci. 2016, 27, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, J.W.; Singh, M.; Brock, C.; Etgen, A.M. Neuroprotection and estrogen receptors. Neuroendocrinology 2012, 96, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Coyoy-Salgado, A.; Segura-Uribe, J.; Salgado-Ceballos, H.; Castillo-Mendieta, T.; Sánchez-Torres, S.; Freyermuth-Trujillo, X.; Orozco-Barrios, C.; Orozco-Suarez, S.; Feria-Romero, I.; Pinto-Almazán, R.; et al. Evaluating sex steroid hormone neuroprotection in spinal cord injury in animal models: Is it promising in the clinic? Biomedicines 2024, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freyermuth-Trujillo, X.; Sánchez-Torres, S.; Orozco-Barrios, C.E.; Salgado-Ceballos, H.; Segura-Uribe, J.J.; Guerra-Araiza, C.; León-Cholula, Á.; Arrieta-Cruz, I.; Morán, J.; Coyoy-Salgado, A. Tibolone Improves Motor Recovery and Regulates Neuroinflammation and Gliosis in a Model of Traumatic Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 8327. https://doi.org/10.3390/ijms26178327
Freyermuth-Trujillo X, Sánchez-Torres S, Orozco-Barrios CE, Salgado-Ceballos H, Segura-Uribe JJ, Guerra-Araiza C, León-Cholula Á, Arrieta-Cruz I, Morán J, Coyoy-Salgado A. Tibolone Improves Motor Recovery and Regulates Neuroinflammation and Gliosis in a Model of Traumatic Spinal Cord Injury. International Journal of Molecular Sciences. 2025; 26(17):8327. https://doi.org/10.3390/ijms26178327
Chicago/Turabian StyleFreyermuth-Trujillo, Ximena, Stephanie Sánchez-Torres, Carlos E. Orozco-Barrios, Hermelinda Salgado-Ceballos, Julia J. Segura-Uribe, Christian Guerra-Araiza, Ángel León-Cholula, Isabel Arrieta-Cruz, Julio Morán, and Angélica Coyoy-Salgado. 2025. "Tibolone Improves Motor Recovery and Regulates Neuroinflammation and Gliosis in a Model of Traumatic Spinal Cord Injury" International Journal of Molecular Sciences 26, no. 17: 8327. https://doi.org/10.3390/ijms26178327
APA StyleFreyermuth-Trujillo, X., Sánchez-Torres, S., Orozco-Barrios, C. E., Salgado-Ceballos, H., Segura-Uribe, J. J., Guerra-Araiza, C., León-Cholula, Á., Arrieta-Cruz, I., Morán, J., & Coyoy-Salgado, A. (2025). Tibolone Improves Motor Recovery and Regulates Neuroinflammation and Gliosis in a Model of Traumatic Spinal Cord Injury. International Journal of Molecular Sciences, 26(17), 8327. https://doi.org/10.3390/ijms26178327





