The Therapeutic Potential of Propranolol and Other Beta-Blockers in Hyperthyroidism
Abstract
1. Introduction
2. β-Blockers’ Characterisation
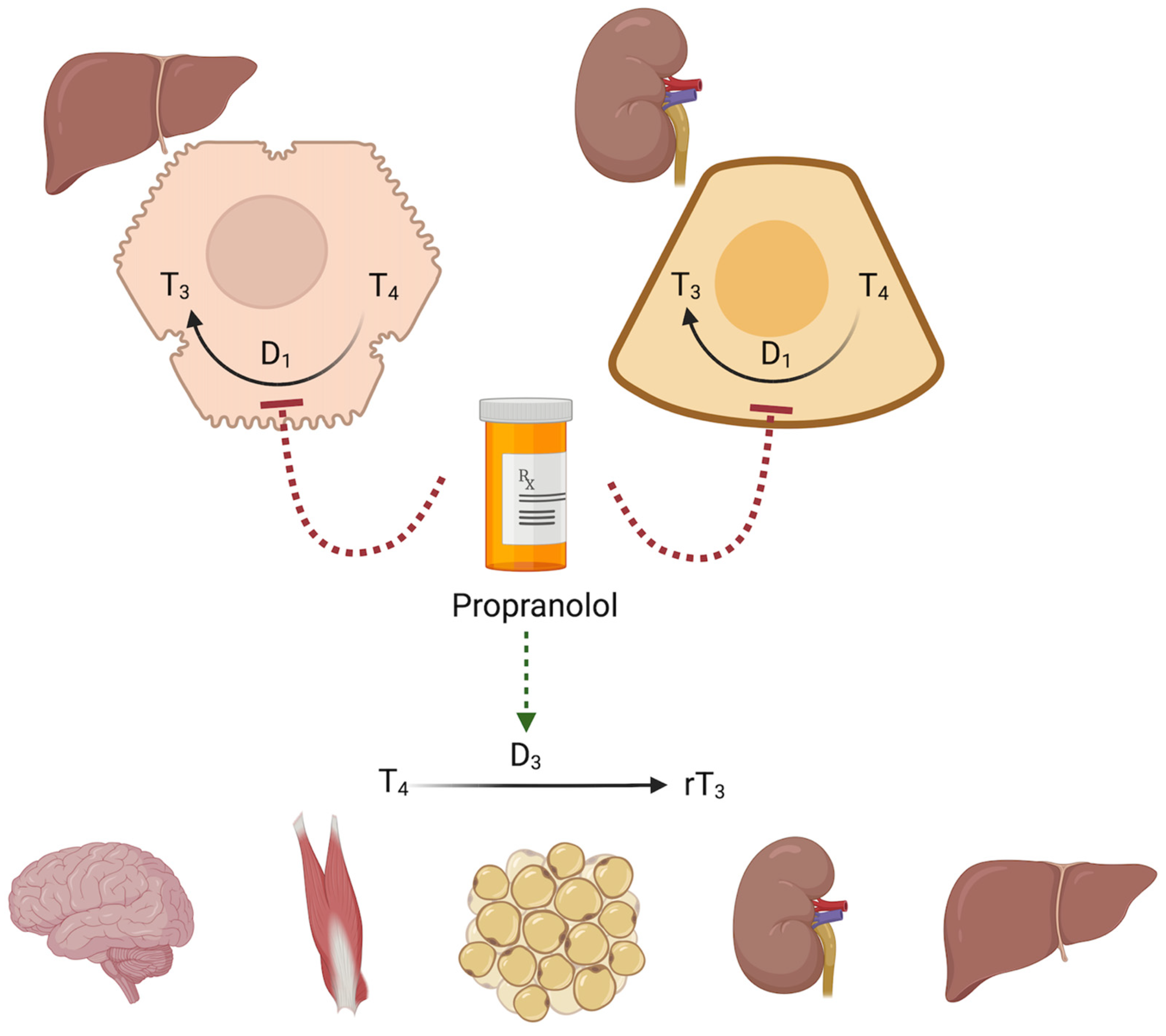
3. β-Blockers and Thyroid Hormone Metabolism
4. Hyperthyroidism
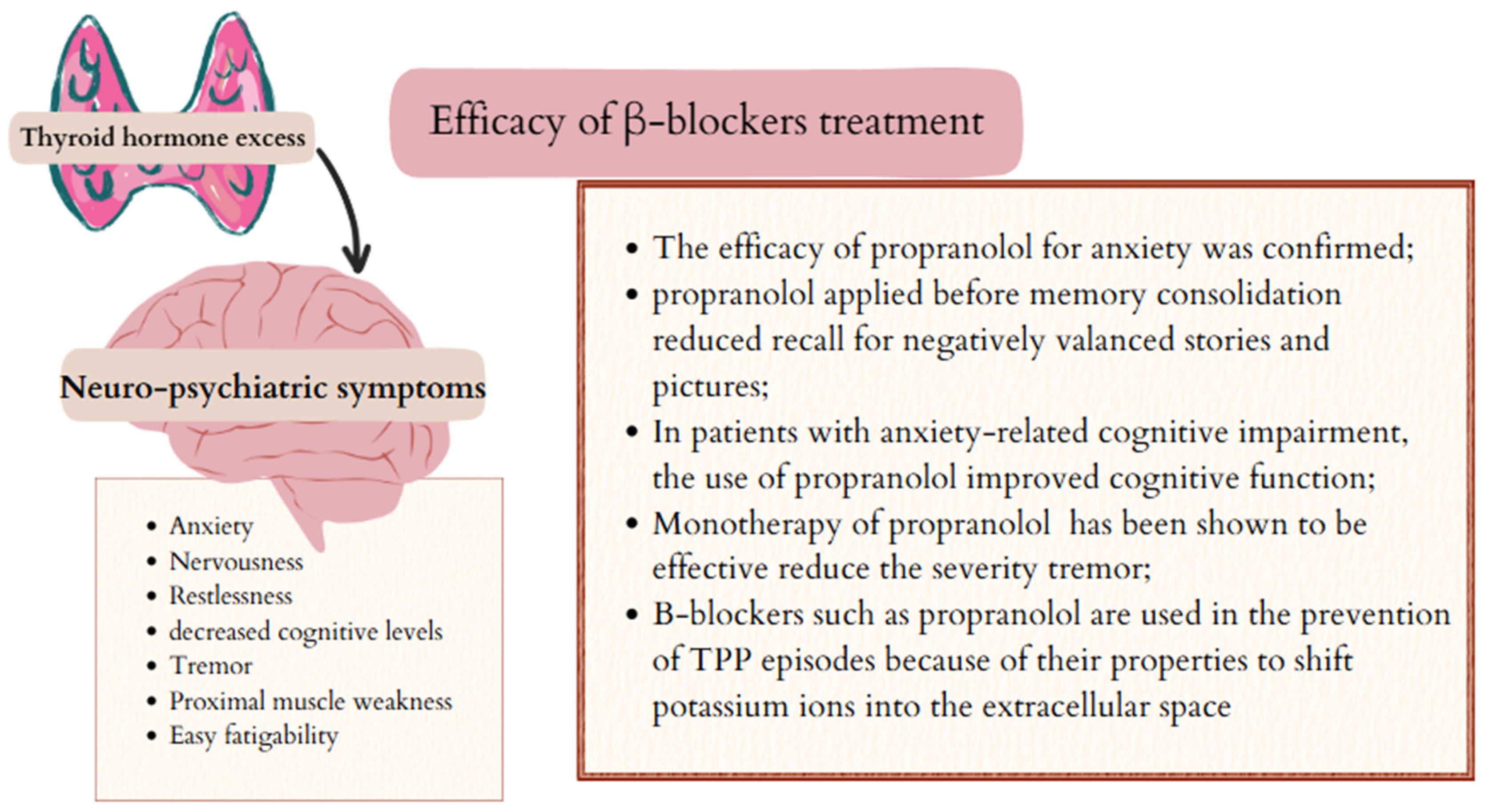
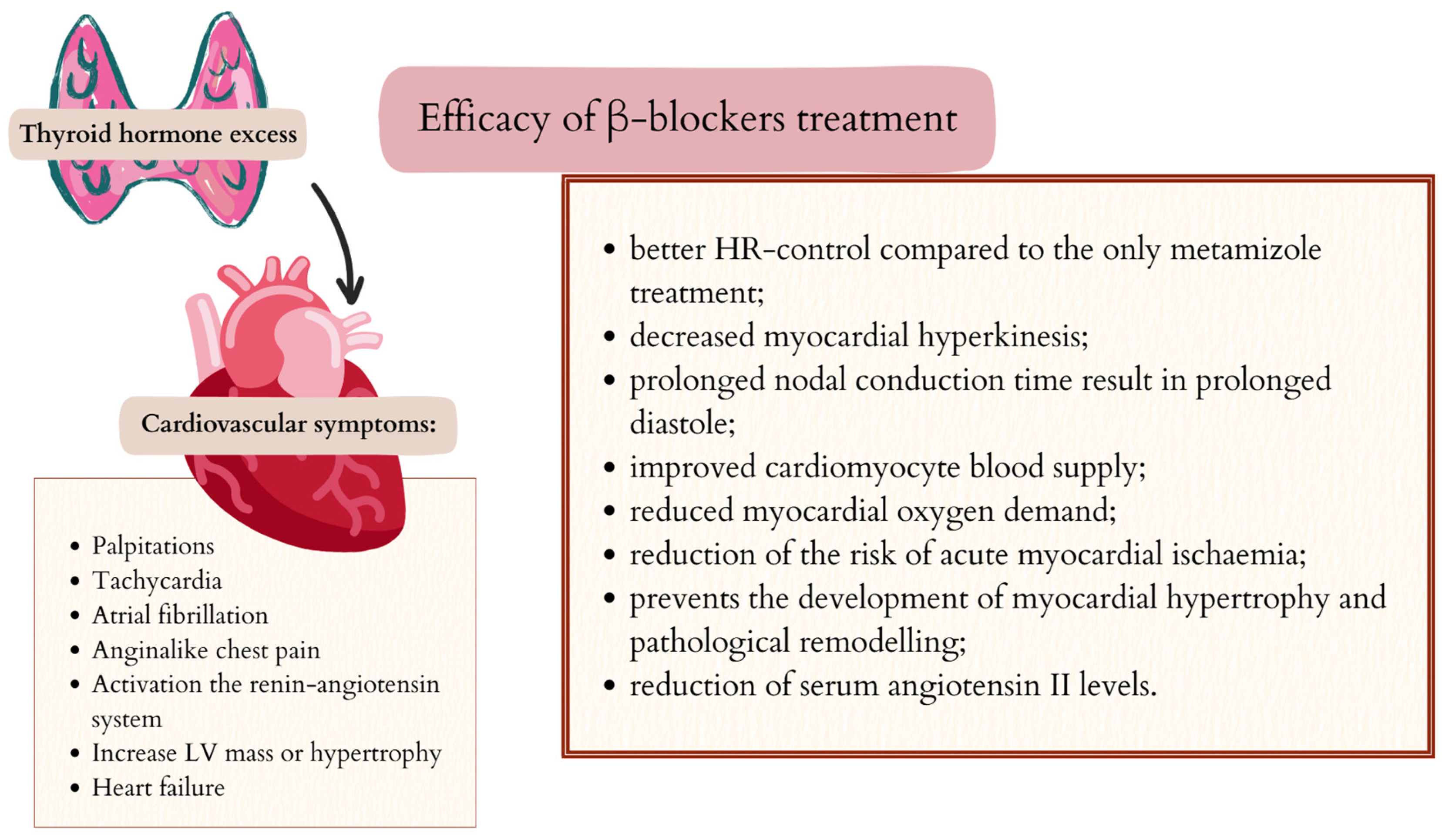
5. Efficacy of β-Blockers in Controlling Symptoms Caused by Excess Thyroid Hormones
5.1. Psychiatric and Neurological Symptoms and β-Blockers
5.2. Cardiovascular Symptoms and β-Blockers
5.3. Respiratory Symptoms and β-Blockers
6. Treatment with β-Blockers in Specific Clinical Situations Associated with Excess Thyroid Hormones
6.1. Levothyroxine Suppressive Therapy in Differentiated Thyroid Cancer
6.2. Pregnancy
6.3. Amiodarone-Induced Thyroid Disease
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farzam, K.; Jan, A. Beta blockers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Poirier, L.; Tobe, S.W. Contemporary use of β-blockers: Clinical relevance of subclassification. Can. J. Cardiol. 2014, 30, S9–S15. [Google Scholar] [CrossRef]
- Perez, D.M. The Adrenergic Receptors in the 21st Century; Humana Press: Totowa, NJ, USA, 2006; Volume 54, pp. 129–134. [Google Scholar]
- Xie, X.; Fan, X.; Fan, L.; Liu, X.; Zheng, Y.; Yu, Z. The effects of methimazole combined with propranolol on heart rate, bone metabolism, and thyroid hormone levels in patients with hyperthyroidism: A systematic review and a meta-analysis of case-control studies. Medicine 2024, 103, e40495. [Google Scholar] [CrossRef]
- Kim, S.M.; Briggs, J.P.; Schnermann, J. Convergence of major physiological stimuli for renin release on the Gs-alpha/cyclic adenosine monophosphate signaling pathway. Clin. Exp. Nephrol. 2012, 16, 17–24. [Google Scholar] [CrossRef]
- Danesh, A.; Gottschalk, P.C.H. Beta-Blockers for Migraine Prevention: A Review Article. Curr. Treat. Options Neurol. 2019, 21, 20. [Google Scholar] [CrossRef]
- Archer, C.; Wiles, N.; Kessler, D.; Turner, K.; Caldwell, D.M. Beta-blockers for the treatment of anxiety disorders: A systematic review and meta-analysis. J. Affect. Disord. 2025, 368, 90–99. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Circulation 2007,116,1725–1735; Erratum in Circulation 2008,117, e18. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Klein, I.; Roberts, M.; Greenhouse, J.; Levey, G.S. Graves’ disease: An analysis of thyroid hormone levels and hyperthyroid signs and symptoms. Am. J. Med. 1989, 87, 558–561. [Google Scholar] [CrossRef]
- Tagami, T.; Yambe, Y.; Tanaka, T.; Tanaka, T.; Ogo, A.; Yoshizumi, H.; Kaise, K.; Higashi, K.; Tanabe, M.; Shimazu, S.; et al. Short-term effects of beta-adrenergic antagonists and methimazole in new-onset thyrotoxicosis caused by Graves’ disease. Intern. Med. 2012, 51, 2285–2290. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Grześk, G.; Osiakl, J.; Wijata, A.; Mędlewska, M.; Gaborek, P.; Banach, J.; Wołowiec, A.; Głowacka, M. Beta-blockers in cardiac arrhythmias-Clinical pharmacologist’s point of view. Front. Pharmacol. 2023, 13, 1043714. [Google Scholar] [CrossRef]
- Barrese, V.; Taglialatela, M. New advances in beta-blocker therapy in heart failure. Front. Physiol. 2013, 4, 323. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, S.; Wang, Q.; Xiang, B.; Xu, Z.; Zhong, L.; Yang, K.; Lu, G.; Qiu, L. Intolerable side effects during propranolol therapy for infantile hemangioma: Frequency, risk factors and management. Sci. Rep. 2018, 8, 4264. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.; Thiagalingam, A.; Turner, C.; Lakkireddy, D.J.; Kovoor, P. The Use of Intravenous Sotalol in Cardiac Arrhythmias. Heart Lung Circ. 2018, 27, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 1 August 2025).
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Bailuni Neto, J.J.; Siqueira, B.L.; Machado, F.C.; Boros, G.A.B.; Akamine, M.A.V.; Cordeiro de Paula, L.J.; Rodrigues de Assis, A.C.; Soares, P.R.; Scudeler, T.L. BRASH Syndrome: A Case Report. Am. J. Case Rep. 2022, 23, e934600. [Google Scholar] [CrossRef]
- Buckley, M.M.; Goa, K.L.; Clissold, S.P. Ocular betaxolol. A review of its pharmacological properties, and therapeutic efficacy in glaucoma and ocular hypertension. Drugs 1990, 40, 75–90. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Scheer, F.A.; Morris, C.J.; Garcia, J.I.; Smales, C.; Kelly, E.E.; Marks, J.; Malhotra, A.; Shea, S.A. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: A randomized controlled trial. Sleep 2012, 35, 1395–1402. [Google Scholar] [CrossRef]
- Fonseca, V.A. Effects of beta-blockers on glucose and lipid metabolism. Curr. Med. Res. Opin. 2010, 26, 615–629. [Google Scholar] [CrossRef]
- Awad, V.M.; Sakhamuru, S.; Kambampati, S.; Wasim, S.; Malik, B.H. Mechanisms of Beta-Blocker Induced Psoriasis, and Psoriasis De Novo at the Cellular Level. Cureus 2020, 12, e8964. [Google Scholar] [CrossRef]
- Waqar, S.; Sarkar, P.K. Exacerbation of psoriasis with beta-blocker therapy. CMAJ 2009, 181, 60. [Google Scholar] [CrossRef]
- Yu, S.K.; Tait, G.; Karkouti, K.; Wijeysundera, D.; McCluskey, S.; Beattie, W.S. The safety of perioperative esmolol: A systematic review and meta-analysis of randomized controlled trials. Anesth. Analg. 2011, 112, 267–281. [Google Scholar] [CrossRef]
- Byrd, R.C.; Sung, R.J.; Marks, J.; Parmley, W.W. Safety and efficacy of esmolol (ASL-8052: An ultrashort-acting beta-adrenergic blocking agent) for control of ventricular rate in supraventricular tachycardias. J. Am. Coll. Cardiol. 1984, 3 Pt 1, 394–399. [Google Scholar] [CrossRef]
- Cork, R.C.; Kramer, T.H.; Dreischmeier, B.; Behr, S.; DiNardo, J.A. The effect of esmolol given during cardiopulmonary bypass. Anesth. Analg. 1995, 80, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Addison, D.; Kayani, W.; Misra, A.; Jneid, H.; Resar, J.; Lakkis, N.; Alam, M. Outcomes of beta blocker use in cocaine-associated chest pain: A meta-analysis. Emerg. Med. J. 2018, 35, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wiest, D.B.; Haney, J.S. Clinical pharmacokinetics and therapeutic efficacy of esmolol. Clin. Pharmacokinet. 2012, 51, 347–356. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Kuwabara, M.; Borghi, C. A Critical Review of Nebivolol and its Fixed-Dose Combinations in the Treatment of Hypertension. Drugs 2018, 78, 1783–1790. [Google Scholar] [CrossRef]
- Mangrella, M.; Rossi, F.; Fici, F.; Rossi, F. Pharmacology of nebivolol. Pharmacol. Res. 1998, 38, 419–431. [Google Scholar] [CrossRef]
- Dunn, C.J.; Lea, A.P.; Wagstaff, A.J. Carvedilol. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs 1997, 54, 161–185. [Google Scholar] [CrossRef]
- McTavish, D.; Campoli-Richards, D.; Sorkin, E.M. Carvedilol. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1993, 45, 232–258. [Google Scholar] [CrossRef]
- Koraćević, G.; Stojanović, M.; Kostić, T.; Lović, D.; Zdravković, M.; Koraćević, M.; Pavlović, D.; Mićić, S. Contraindications Differ Widely Among Beta Blockers and Ought to be Cited for an Individual Drug, Not for the Entire Class. Curr. Pharm. Des. 2021, 27, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W. Nebivolol: A third-generation beta-blocker for hypertension. Clin. Ther. 2009, 31, 447–462. [Google Scholar] [CrossRef]
- Ong, H.T.; Ong, L.M.; Kow, F.P. Beta-blockers for heart failure: An evidence based review answering practical therapeutic questions. Med. J. Malays. 2012, 67, 7–11. [Google Scholar]
- Pessina, A.C. Metabolic effects and safety profile of nebivolol. J. Cardiovasc. Pharmacol. 2001, 38 (Suppl. S3), S33–S35. [Google Scholar] [CrossRef]
- Osmonov, D.; Erdinler, I.; Ozcan, K.S.; Altay, S.; Turkkan, C.; Yildirim, E.; Hasdemir, H.; Alper, A.T.; Cakmak, N.; Satilmis, S.; et al. Management of patients with drug-induced atrioventricular block. Pacing Clin. Electrophysiol. 2012, 35, 804–810. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Ormiston, T.M.; Salpeter, E.E. Cardioselective beta-blockers in patients with reactive airway disease: A meta-analysis. Ann. Intern. Med. 2002, 137, 715–725. [Google Scholar] [CrossRef]
- Momčilović, S.; Jovanović, A.; Radojković, D.; Nikolić, V.N.; Janković, S.M.; Pešić, M.; Milovanović, J.R. Population pharmacokinetic analysis of bisoprolol in type 2 diabetic patients with hypertension. Eur. J. Clin. Pharmacol. 2020, 76, 1539–1546. [Google Scholar] [CrossRef]
- Koracevic, G.; Micic, S.; Stojanovic, M. By Discontinuing Beta-Blockers Before an Exercise Test, We may Precipitate a Rebound Phenomenon. Curr. Vasc. Pharmacol. 2021, 19, 624–633. [Google Scholar] [CrossRef]
- Ripley, T.L.; Saseen, J.J. β-blockers: A review of their pharmacological and physiological diversity in hypertension. Ann. Pharmacother. 2014, 48, 723–733. [Google Scholar] [CrossRef]
- Bensky, K.P.; Donahue-Spencer, L.; Hertz, G.E.; Anderson, M.T.; James, R. The dose-related effects of bolus esmolol on heart rate and blood pressure following laryngoscopy and intubation. AANA J. 2000, 68, 437–442. [Google Scholar]
- Sheppard, D.; DiStefano, S.; Byrd, R.C.; Eschenbacher, W.L.; Bell, V.; Steck, J.; Laddu, A. Effects of esmolol on airway function in patients with asthma. J. Clin. Pharmacol. 1986, 26, 169–174. [Google Scholar] [CrossRef]
- Özcan, K.S.; Güngör, B.; Osmonov, D.; Tekkeşinm, A.I.; Altay, S.; Ekmekçi, A.; Toprak, E.; Yildirim, E.; Çalik, N.; Alper, A.T.; et al. Management and outcome of topical beta-blocker-induced atrioventricular block. Cardiovasc. J. Afr. 2015, 26, 210–213. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Alburikan, K.; Metra, M.; Rodgers, J.E. Acute decompensated heart failure update. Curr. Cardiol. Rev. 2015, 11, 53–62. [Google Scholar] [CrossRef]
- Sen, A.; Fairbairn, T.; Levy, F. Best evidence topic report. Beta-Blockers in cocaine induced acute coronary syndrome. Emerg. Med. J. 2006, 23, 401–402. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Greger, K.C.; Lee, S.; Kahoud, R.J.; Li, J.T.; Lohse, C.M.; Campbell, R.L. Patients Taking β-Blockers Do Not Require Increased Doses of Epinephrine for Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1553–1558.e1. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, K.R.; Yang, P.C.; Singh, V.; Furutani, K.; Dawson, J.R.D.; Jeng, M.T.; Fettinger, J.C.; Bekker, S.; Ngo, V.A.; Noskov, S.Y.; et al. Molecular determinants of pro-arrhythmia proclivity of d- and l-sotalol via a multi-scale modeling pipeline. J. Mol. Cell Cardiol. 2021, 158, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Propranolol and thyroid hormone metabolism. Thyroid 1991, 1, 273–277. [Google Scholar] [CrossRef]
- Verhoeven, R.P.; Visser, T.J.; Doctor, R.; Hennemann, G.; Schalekamp, M.A. Plasma thyroxine, 3,3’,5-triiodothyronine and 3,3’,5’-triiodothyronine during beta-adrenergic blockade in hyperthyroidism. J. Clin. Endocrinol. Metab. 1977, 44, 1002–1005. [Google Scholar] [CrossRef]
- Obi, M.F.; Namireddy, V.; Garg, Y.; Sharma, M. Benefit and Preference of Propranolol Over Metoprolol in Thyrotoxicosis-Induced Atrial Fibrillation: A Case Report and Review of Literature. Cureus 2023, 15, e34474. [Google Scholar] [CrossRef]
- Cooper, D.S. Hyperthyroidism. Lancet 2003, 362, 459–468. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Ippolito, S.; Cusini, C.; Lasalvia, P.; Gianfagna, F.; Veronesi, G.; Gallo, D.; Masiello, E.; Premoli, P.; Sabatino, J.; Mercuriali, A.; et al. Change in newly diagnosed Graves’ disease phenotype between the twentieth and the twenty-first centuries: Meta-analysis and meta-regression. J. Endocrinol. Investig. 2021, 44, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Knudsen, N.; Pedersen, I.B.; Rasmussen, L.B.; Carlé, A.; Vejbjerg, P. The Danish investigation on iodine intake and thyroid disease, DanThyr: Status and perspectives. Eur. J. Endocrinol. 2006, 155, 219–228. [Google Scholar] [CrossRef]
- Cyna, W.; Wojciechowska, A.; Szybiak-Skora, W.; Lacka, K. The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis-Review. Biomedicines 2024, 12, 1788. [Google Scholar] [CrossRef]
- Miller, K.K.; Daniels, G.H. Association between lithium use and thyrotoxicosis caused by silent thyroiditis. Clin. Endocrinol. 2001, 55, 501–508. [Google Scholar] [CrossRef]
- Illouz, F.; Braun, D.; Briet, C.; Schweizer, U.; Rodien, P. Endocrine side-effects of anti-cancer drugs: Thyroid effects of tyrosine kinase inhibitors. Eur. J. Endocrinol. 2014, 171, R91–R99. [Google Scholar] [CrossRef]
- Cohen-Lehman, J.; Dahl, P.; Danzi, S.; Klein, I. Effects of amiodarone therapy on thyroid function. Nat. Rev. Endocrinol. 2010, 6, 34–41. [Google Scholar] [CrossRef]
- Tondi Resta, I.; Sande, C.M.; LiVolsi, V.A. Neoplasms in Struma Ovarii: A Review. Endocr. Pathol. 2023, 34, 455–460. [Google Scholar] [CrossRef]
- Shigemasa, C.; Abe, K.; Taniguchi, S.; Mitani, Y.; Ueda, Y.; Adachi, T.; Urabe, K.; Tanaka, T.; Yoshida, A.; Mashiba, H. Lower serum free thyroxine (T4) levels in painless thyroiditis compared with Graves’ disease despite similar serum total T4 levels. J. Clin. Endocrinol. Metab. 1987, 65, 359–363. [Google Scholar] [CrossRef]
- Carle, A.; Knudsen, N.; Pedersen, I.B.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. Determinants of serum T4 and T3 at the time of diagnosis in nosological types of thyrotoxicosis: A population-based study. Eur. J. Endocrinol. 2013, 169, 537–545. [Google Scholar] [CrossRef][Green Version]
- Devereaux, D.; Tewelde, S.Z. Hyperthyroidism and Thyrotoxicosis. Emerg. Med. Clin. N. Am. 2014, 32, 277–292. [Google Scholar] [CrossRef]
- Łacka, K.; Fraczek, M.M. Classification and etiology of hyperthyroidism. Pol. Merkur. Lek. 2014, 36, 206–211. [Google Scholar]
- Boelaert, K.; Torlinska, B.; Holder, R.L.; Franklyn, J.A. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: A large cross-sectional study. J. Clin. Endocrinol. Metab. 2010, 95, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Kudrjavcev, T. Neurologic complications of thyroid dysfunction. Adv. Neurol. 1978, 19, 619–636. [Google Scholar] [PubMed]
- Szeleszczuk, Ł.; Frączkowski, D. Propranolol versus Other Selected Drugs in the Treatment of Various Types of Anxiety or Stress, with Particular Reference to Stage Fright and Post-Traumatic Stress Disorder. Int. J. Mol. Sci. 2022, 23, 10099. [Google Scholar] [CrossRef]
- Steenen, S.A.; van Wijk, A.J.; van der Heijden, G.J.M.G.; van Westrhenen, R.; de Lange, J.; de Jongh, A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2016, 30, 128–139. [Google Scholar] [CrossRef]
- Turner, P.; Granville-Grossman, K.L. Effect of adrenergic receptor blockade of the tachycardia of thyrotoxicosis and anxiety state. Lancet 1965, 2, 1316–1318. [Google Scholar] [CrossRef]
- Lonergan, M.H.; Olivera-Figueroa, L.A.; Pitman, R.K.; Brunet, A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J. Psychiatry Neurosci. 2013, 38, 222–231. [Google Scholar] [CrossRef]
- Jaracz, J.; Kucharska, A.; Rajewska-Rager, A.; Lacka, K. Cognitive functions and mood during chronic thyrotropin-suppressive therapy with L-thyroxine in patients with differentiated thyroid carcinoma. J. Endocrinol. Investig. 2012, 35, 760–765. [Google Scholar] [CrossRef]
- Faigel, H.C. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin. Pediatr. 1991, 30, 441–445. [Google Scholar] [CrossRef]
- Cojocariu, S.A.; Maștaleru, A.; Sascău, R.A.; Stătescu, C.; Mitu, F.; Leon-Constantin, M.M. Neuropsychiatric Consequences of Lipophilic Beta-Blockers. Medicina 2021, 57, 155. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G. Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Haubenberger, D.; Hallett, M. Essential Tremor. N. Engl. J. Med. 2018, 378, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.C. Propranolol therapy for essential tremor of the head. Neurology 1984, 34, 1077–1079. [Google Scholar] [CrossRef]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Barahona, M.J.; Vinagre, I.; Sojo, L.; Cubero, J.M.; Pérez, A. Thyrotoxic periodic paralysis: A case report and literature review. Clin. Med. Res. 2009, 7, 96–98. [Google Scholar] [CrossRef]
- Magsino, C.H., Jr.; Ryan, A.J., Jr. Thyrotoxic periodic paralysis. South Med. J. 2000, 93, 996–1003. [Google Scholar] [CrossRef]
- Tagami, T.; Usui, T.; Shimatsu, A.; Naruse, M. Toxic thyroid adenoma presenting as hypokalemic periodic paralysis. Endocr. J. 2007, 54, 797–803. [Google Scholar] [CrossRef]
- Napoli, R.; Biondi, B.; Guardasole, V.; Matarazzo, M.; Pardo, F.; Angelini, V.; Fazio, S.; Saccà, L. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation 2001, 104, 3076–3080. [Google Scholar] [CrossRef]
- Toft, A.D.; Boon, N.A. Thyroid disease and the heart. Heart 2000, 84, 455–460. [Google Scholar] [CrossRef]
- Fadel, B.M.; Ellahham, S.; Ringel, M.D.; Lindsay, J., Jr.; Wartofsky, L.; Burman, K.D. Hyperthyroid heart disease. Clin. Cardiol. 2000, 23, 402–408. [Google Scholar] [CrossRef]
- Osuna, P.M.; Udovcic, M.; Sharma, M.D. Hyperthyroidism and the Heart. Methodist. Debakey Cardiovasc. J. 2017, 13, 60–63. [Google Scholar] [CrossRef]
- Raguthu, C.C.; Gajjela, H.; Kela, I.; Kakarala, C.L.; Hassan, M.; Belavadi, R.; Gudigopuram, S.V.R.; Sange, I. Cardiovascular Involvement in Thyrotoxicosis Resulting in Heart Failure: The Risk Factors and Hemodynamic Implications. Cureus 2022, 14, e21213. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Fisichella, A.; Lauretani, F.; Ticinesi, A.; Ceresini, G.; Cappola, A.; Ferrucci, L.; Ceda, G.P. The Role of the Multiple Hormonal Dysregulation in the Onset of “Anemia of Aging”: Focus on Testosterone, IGF-1, and Thyroid Hormones. Int. J. Endocrinol. 2015, 2015, 292574. [Google Scholar] [CrossRef]
- Dillmann, W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail. Rev. 2010, 15, 125–132. [Google Scholar] [CrossRef]
- Yue, W.S.; Chong, B.H.; Zhang, X.H.; Liao, S.Y.; Jim, M.H.; Kung, A.W.; Tse, H.F.; Siu, C.W. Hyperthyroidism-induced left ventricular diastolic dysfunction: Implication in hyperthyroidism-related heart failure. Clin. Endocrinol. 2011, 74, 636–643. [Google Scholar] [CrossRef]
- Nanchen, D.; Gussekloo, J.; Westendorp, R.G.; Stott, D.J.; Jukema, J.W.; Trompet, S.; Ford, I.; Welsh, P.; Sattar, N.; Macfarlane, P.W.; et al. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2012, 97, 852–861. [Google Scholar] [CrossRef]
- Siu, C.W.; Yeung, C.Y.; Lau, C.P.; Kung, A.W.; Tse, H.F. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart 2007, 93, 483–487. [Google Scholar] [CrossRef]
- Roffi, M.; Cattaneo, F.; Topol, E.J. Thyrotoxicosis and the cardiovascular system: Subtle but serious effects. Cleve Clin. J. Med. 2003, 70, 57–63. [Google Scholar] [CrossRef]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef]
- Ogrodowczyk, M.; Dettlaff, K.; Jelinska, A. Beta-Blockers: Current State of Knowledge and Perspectives. Mini Rev. Med. Chem. 2016, 16, 40–54. [Google Scholar] [CrossRef]
- Blumenfeld, J.D.; Sealey, J.E.; Mann, S.J.; Bragat, A.; Marion, R.; Pecker, M.S.; Sotelo, J.; August, P.; Pickering, T.G.; Laragh, J.H. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am. J. Hypertens. 1999, 12, 451–459. [Google Scholar] [CrossRef]
- Biondi, B.; Fazio, S.; Cuocolo, A.; Sabatini, D.; Nicolai, E.; Lombardi, G.; Salvatore, M.; Saccà, L. Impaired cardiac reserve and exercise capacity in patients receiving long-term thyrotropin suppressive therapy with levothyroxine. J. Clin. Endocrinol. Metab. 1996, 81, 4224–4228. [Google Scholar] [CrossRef] [PubMed]
- Vickers, P.; Garg, K.M.; Arya, R.; Godha, U.; Mathur, P.; Jain, S. The role of selective beta-1 blocker in the preoperative preparation of thyrotoxicosis: A comparative study with propranalol. Int. Surg. 1990, 75, 179–183. [Google Scholar] [PubMed]
- Palmieri, E.A.; Fazio, S.; Palmieri, V.; Lombardi, G.; Biondi, B. Myocardial contractility and total arterial stiffness in patients with overt hyperthyroidism: Acute effects of beta1-adrenergic blockade. Eur. J. Endocrinol. 2004, 150, 757–762. [Google Scholar] [CrossRef]
- Weare-Regales, N.; Chiarella, S.E.; Cardet, J.C.; Prakash, Y.S.; Lockey, R.F. Hormonal Effects on Asthma, Rhinitis, and Eczema. J. Allergy Clin. Immunol. Pract. 2022, 10, 2066–2073. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Li, X.; He, Y.; Liu, X. The associations between asthma and common comorbidities: A comprehensive Mendelian randomization study. Front. Med. 2023, 10, 1251827. [Google Scholar] [CrossRef]
- Song, X.; Yang, K.; Chen, G.; Duan, W.; Yao, D.; Li, S.; Yuan, G.; Liu, L. Characteristics and Risk Factors of Pulmonary Hypertension in Patients With Hyperthyroidism. Endocr. Pract. 2021, 27, 918–924. [Google Scholar] [CrossRef]
- Badagliacca, R.; Mercurio, V.; Romeo, E.; Correale, M.; Masarone, D.; Papa, S.; Tocchetti, C.G.; Agostoni, P.; Members of the Study Group on Right and Left Heart Failure of the Italian Society of Cardiology. Beta-blockers in pulmonary arterial hypertension: Time for a second thought? Vascul. Pharmacol. 2022, 144, 106974. [Google Scholar] [CrossRef]
- Alaa, M.; Abdellatif, M.; Tavares-Silva, M.; Oliveira-Pinto, J.; Lopes, L.; Leite, S.; Leite-Moreira, A.F.; Lourenço, A.P. Right ventricular end-diastolic stiffness heralds right ventricular failure in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1004–H1013. [Google Scholar] [CrossRef]
- Fowler, E.D.; Drinkhill, M.J.; Stones, R.; White, E. Diastolic dysfunction in pulmonary artery hypertension: Creatine kinase and the potential therapeutic benefit of beta-blockers. Clin. Exp. Pharmacol. Physiol. 2018, 45, 384–389. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Krysińska, I.; Lacka, K.; Stawny, B.; Rólski, M.; Jarzab, B.; Włoch, J.; Gembicki, M. A study of thyroglobulin concentration in the thyroid and serum of patients with different thyroid disorders. Arch. Immunol. Ther. Exp. 2002, 50, 143–148. [Google Scholar]
- Lacka, K.; Maciejewski, A.; Tyburski, P.; Manuszewska-Jopek, E.; Majewski, P.; Więckowska, B. Rationale for Testing TP53 Mutations in Thyroid Cancer-Original Data and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 1035. [Google Scholar] [CrossRef]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Grani, G.; Zatelli, M.C.; Alfò, M.; Montesano, T.; Torlontano, M.; Morelli, S.; Deandrea, M.; Antonelli, A.; Francese, C.; Ceresini, G.; et al. Real-World Performance of the American Thyroid Association Risk Estimates in Predicting 1-Year Differentiated Thyroid Cancer Outcomes: A Prospective Multicenter Study of 2000 Patients. Thyroid 2021, 31, 264–271. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Jarząb, B.; Dedecjus, M.; Słowińska-Klencka, D.; Lewiński, A.; Adamczewski, Z.; Anielski, R.; Bagłaj, M.; Bałdys-Waligórska, A.; Barczyński, M.; Bednarczuk, T.; et al. Guidelines of Polish National Societies Diagnostics and Treatment of Thyroid Carcinoma. 2018 Update. Endokrynol. Pol. 2018, 69, 34–74. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 2010, 20, 135–146. [Google Scholar] [CrossRef]
- Jarząb, B.; Dedecjus, M.; Lewiński, A.; Adamczewski, Z.; Bakuła-Zalewska, E.; Bałdys-Waligórska, A.; Barczyński, M.; Biskup-Frużyńska, M.; Bobek-Billewicz, B.; Bossowski, A.; et al. Diagnosis and treatment of thyroid cancer in adult patients—Recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update. Endokrynol. Pol. 2022, 73, 173–300. [Google Scholar] [CrossRef]
- Samuels, M.H. Subclinical thyroid disease in the elderly. Thyroid 1998, 8, 803–813. [Google Scholar] [CrossRef]
- Sawin, C.T.; Geller, A.; Wolf, P.A.; Belanger, A.J.; Baker, E.; Bacharach, P.; Wilson, P.W.; Benjamin, E.J.; D’Agostino, R.B. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N. Engl. J. Med. 1994, 331, 1249–1252. [Google Scholar] [CrossRef]
- Cappola, A.R.; Fried, L.P.; Arnold, A.M.; Danese, M.D.; Kuller, L.H.; Burke, G.L.; Tracy, R.P.; Ladenson, P.W. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006, 295, 1033–1041. [Google Scholar] [CrossRef]
- Haentjens, P.; Van Meerhaeghe, A.; Poppe, K.; Velkeniers, B. Subclinical thyroid dysfunction and mortality: An estimate of relative and absolute excess all-cause mortality based on time-to-event data from cohort studies. Eur. J. Endocrinol. 2008, 159, 329–341. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Gomez-Bueno, M.; Barrios, V.; Caballero, C.; García-Robles, R.; Sancho, J.; Escobar-Morreale, H.F. Chronic thyrotropin-suppressive therapy with levothyroxine and short-term overt hypothyroidism after thyroxine withdrawal are associated with undesirable cardiovascular effects in patients with differentiated thyroid carcinoma. Endocr.-Relat. Cancer 2004, 11, 345–356. [Google Scholar] [CrossRef][Green Version]
- Gullu, S.; Altuntas, F.; Dincer, I.; Erol, C.; Kamel, N. Effects of TSH-suppressive therapy on cardiac morphology and function: Beneficial effects of the addition of beta-blockade on diastolic dysfunction. Eur. J. Endocrinol. 2004, 150, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Biondi, B.; Carella, C.; Sabatini, D.; Cittadini, A.; Panza, N.; Lombardi, G.; Saccà, L. Diastolic dysfunction in patients on thyroid-stimulating hormone suppressive therapy with levothyroxine: Beneficial effect of beta-blockade. J. Clin. Endocrinol. Metab. 1995, 80, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Glinoer, D. Thyroid hyperfunction during pregnancy. Thyroid 1998, 8, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Kobaly, K.; Mandel, S.J. Hyperthyroidism and Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 533–545. [Google Scholar] [CrossRef]
- Glinoer, D.; De Nayer, P.; Robyn, C.; Lejeune, B.; Kinthaert, J.; Meuris, S. Serum levels of intact human chorionic gonadotropin (HCG) and its free alpha and beta subunits, in relation to maternal thyroid stimulation during normal pregnancy. J. Endocrinol. Investig. 1993, 16, 881–888. [Google Scholar] [CrossRef]
- Amino, N.; Tanizawa, O.; Mori, H.; Iwatani, Y.; Yamada, T.; Kurachi, K.; Kumahara, Y.; Miyai, K. Aggravation of thyrotoxicosis in early pregnancy and after delivery in Graves’ disease. J. Clin. Endocrinol. Metab. 1982, 55, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wing, D.A.; Millar, L.K.; Koonings, P.P.; Montoro, M.N.; Mestman, J.H. A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am. J. Obstet. Gynecol. 1994, 170, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Andersen, S.L. Therapy of endocrine disease: Antithyroid drug use in early pregnancy and birth defects: Time windows of relative safety and high risk? Eur. J. Endocrinol. 2014, 171, R13–R20. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Birth defects after early pregnancy use of antithyroid drugs: A Danish nationwide study. J. Clin. Endocrinol. Metab. 2013, 98, 4373–4381. [Google Scholar] [CrossRef]
- Gladstone, G.R.; Hordof, A.; Gersony, W.M. Propranolol administration during pregnancy: Effects on the fetus. J. Pediatr. 1975, 86, 962–964. [Google Scholar] [CrossRef]
- Habib, A.; McCarthy, J.S. Effects on the neonate of propranolol administered during pregnancy. J. Pediatr. 1977, 91, 808–811. [Google Scholar] [CrossRef]
- Pruyn, S.C.; Phelan, J.P.; Buchanan, G.C. Long-term propranolol therapy in pregnancy: Maternal and fetal outcome. Am. J. Obstet. Gynecol. 1979, 135, 485–489. [Google Scholar] [CrossRef]
- Ferrero, S.; Colombo, B.M.; Ragni, N. Maternal arrhythmias during pregnancy. Arch. Gynecol. Obstet. 2004, 269, 244–253. [Google Scholar] [CrossRef]
- Masiukiewicz, U.S.; Burrow, G.N. Hyperthyroidism in pregnancy: Diagnosis and treatment. Thyroid 1999, 9, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Inai, K.; Shimada, E.; Shinohara, T. α/β- and β-Blocker Exposure in Pregnancy and the Risk of Neonatal Hypoglycemia and Small for Gestational Age. Circ. J. 2023, 87, 569–577. [Google Scholar] [CrossRef]
- Sherif, I.H.; Oyan, W.T.; Bosairi, S.; Carrascal, S.M. Treatment of hyperthyroidism in pregnancy. Acta Obstet. Gynecol. Scand. 1991, 70, 461–463. [Google Scholar] [CrossRef]
- Sandström, B. Antihypertensive treatment with the adrenergic beta-receptor blocker metoprolol during pregnancy. Gynecol. Obstet. Investig. 1978, 9, 195–204. [Google Scholar] [CrossRef]
- Rubin, P.C.; Butters, L.; Clark, D.M. Placebo-controlled trial of atenolol in treatment of pregnancy-associated hypertension. Lancet 1983, 1, 431–434. [Google Scholar]
- Katsi, V.; Papakonstantinou, I.P.; Papazachou, O.; Makris, T.; Tsioufis, K. Beta-Blockers in Pregnancy: Clinical Update. Curr. Hypertens. Rep. 2023, 25, 13–24. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, J.W.; Xu, L.J.; Chen, M.; Wan, L. Risk of congenital malformations in offspring of women using β-blockers during early pregnancy: An updated meta-analysis of observational studies. Br. J. Clin. Pharmacol. 2021, 87, 806–815. [Google Scholar] [CrossRef]
- Ramlakhan, K.P.; Roos-Hesselink, J.W.; Basso, T.; Greenslade, J.; Flint, R.B.; Krieger, E.V.; Shotan, A.; Budts, W.; De Backer, J.; Hall, R.; et al. Perinatal outcomes after in-utero exposure to beta-blockers in women with heart disease: Data from the ESC EORP registry of pregnancy and cardiac disease (ROPAC). Int. J. Cardiol. 2024, 410, 132234. [Google Scholar] [CrossRef]
- Martinez, A.; Lakkimsetti, M.; Maharjan, S.; Aslam, M.A.; Basnyat, A.; Kafley, S.; Reddy, S.S.; Ahmed, S.S.; Razzaq, W.; Adusumilli, S.; et al. Beta-Blockers and Their Current Role in Maternal and Neonatal Health: A Narrative Review of the Literature. Cureus 2023, 15, e44043. [Google Scholar] [CrossRef]
- Szałek, E.; Tomczak, H.; Seremak-Mrozikiewicz, A.; Bartkowiak-Wieczorek, J.; Grześkowiak, E. Optimization of antibiotic therapy in pregnancy—Clinical implications. Ginekol. Pol. 2012, 83, 462–468. [Google Scholar]
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Talajic, M.; Fermini, B.; Roy, D. Amiodarone: Pharmacology, clinical actions and relationships between them. J. Cardiovasc. Electrophysiol. 1992, 3, 266–280. [Google Scholar] [CrossRef]
- Bogazzi, F.; Bartalena, L.; Gasperi, M.; Braverman, L.E.; Martino, E. The various effects of amiodarone on thyroid function. Thyroid 2001, 11, 511–519. [Google Scholar] [CrossRef]
- Martino, E.; Bartalena, L.; Bogazzi, F.; Braverman, L.E. The effects of amiodarone on the thyroid. Endocr. Rev. 2001, 22, 240–254. [Google Scholar] [CrossRef]
- Han, T.S.; Williams, G.R.; Vanderpump, M.P. Benzofuran derivatives and the thyroid. Clin. Endocrinol. 2009, 70, 2–13. [Google Scholar] [CrossRef]
- Martino, E.; Aghini-Lombardi, F.; Mariotti, S.; Bartalena, L.; Lenziardi, M.; Ceccarelli, C.; Bambini, G.; Safran, M.; Braverman, L.E.; Pinchera, A. Amiodarone iodine-induced hypothyroidism: Risk factors and follow-up in 28 cases. Clin. Endocrinol. 1987, 26, 227–237. [Google Scholar] [CrossRef]
- Bogazzi, F.; Bartalena, L.; Cosci, C.; Brogioni, S.; Dell’Unto, E.; Grasso, L.; Aghini-Lombardi, F.; Rossi, G.; Pinchera, A.; Braverman, L.E.; et al. Treatment of type II amiodarone-induced thyrotoxicosis by either iopanoic acid or glucocorticoids: A prospective, randomized study. J. Clin. Endocrinol. Metab. 2003, 88, 1999–2002. [Google Scholar] [CrossRef]
- Anfinsen, O.G.; Lima, K. Amiodarone-induced thyrotoxicosis. Tidsskr. Nor. Laegeforen. 2021, 141, 10.4045. [Google Scholar] [CrossRef]
- Narayana, S.K.; Woods, D.R.; Boos, C.J. Management of amiodarone-related thyroid problems. Ther. Adv. Endocrinol. Metab. 2011, 2, 115–126. [Google Scholar] [CrossRef]
- Schubert, L.; Bricaire, L.; Groussin, L. Amiodarone-induced thyrotoxicosis. Ann. Endocrinol. 2021, 82, 163–166. [Google Scholar] [CrossRef]
- Macchia, P.E.; Feingold, K.R. Amiodarone induced thyrotoxicosis. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]

| β-Blocker | Receptors | Lipophilicity | T4→T3 Inhibitor | Additional Effect |
|---|---|---|---|---|
| Propranolol | β1, β2 | High | Yes | |
| Sotalol | β1, β2 | Low | No | K+ channel blocker |
| Acebutolol | β1 | Medium | No | ISA |
| Atenolol | β1 | Low | No | Hydrophilic |
| Betaxolol | β1 | Medium | No | |
| Bisoprolol | β1 | Medium | No | |
| Celiprolol | β1 | Medium | No | ISA |
| Esmolol | β1 | Low | No | Short period of action |
| Metoprolol | β1 | Medium | No | |
| Nebivolol | β1 | High | No | NO action |
| Carvedilol | β1, α1 | High | No | Antioxidant |
| Low-Risk <5% Risk of Recurrence | Intermediate-Risk 10–20% Risk of Recurrence | High-Risk 30–55% Risk of Recurrence |
|---|---|---|
| 0.1–0.5 mlU/L if thyroglobin is detectable 0.5–2.0 mlU/L if thyroglobin is undetectable or lobectomy is performed | 0.1–0.5 mlU/L only in high-risk patients or those who do not demonstrate excellent treatment response | <0.1 mlU/L only in high-risk patients or in patients who do not demonstrate excellent treatment response; in patients with persistent, clinically apparent DTC symptoms; with incomplete biochemical response according to the ATA—patients with no structural disease, but elevated stimulated Tg levels (>10 ng/mL) and/or elevated Tg levels on thyroxine suppression (>1 ng/mL), or an increased level of anti-Tg antibodies; those who are at high risk of recurrence and have no contraindications to suppressive therapy, or the benefits of therapy outweigh the risks of suppressive therapy; for those who are receiving complete suppressive therapy, the addition of a β-antagonist or angiotensin-converting enzyme inhibitor should be considered to prevent myocardial hypertrophy. |
| Author, Year | β-Blockers with Dose | Heart Parameter | Results | p |
|---|---|---|---|---|
| Gullu, 2005 [121] | Atenolol 50 mg/day | Isovolumetric relaxation time (IVR) | IVR decreased (92 +/− 10 vs. 101 +/− 9 ms) | <0.05 |
| Left ventricular mass index (LVMI) | LVMI decreased (96 +/− 17 vs. 88 +/− 16 g/m2) | NS a | ||
| Diastolic diameters, early (VE) and late (VA) | VE and VA improved VE (0.72 +/− 0.12 vs. 0.79 +/− 0.2 m/s) VA (0.72 +/− 0.23 vs. 0.69 +/− 0.12 m/s) | NS | ||
| Biondi, 1996 [97] | Bisoprolol (4.25 +/− 0.4 mg/day) | Left ventricular ejection fraction (LVEF) | LVEF improved (63 +/− 2% to 53 +/− 2%) | <0.01 |
| Fazio, 1995 [122] | Bisoprolol (4.25 +/− 1.2 mg/day) | Left ventricular mass index (LVMI) | LVMI decreased (80 +/− 18 vs. 95 +/− 19) | <0.001 |
| β-Blocker | FDA Category | Breastfeeding Recommendation |
|---|---|---|
| Propranolol | C | No Human Data—Potential Toxicity |
| Sotalol | B | No Human Data—Potential Toxicity |
| Acebutolol | B/D II–III trimester | No Human Data—Potential Toxicity |
| Atenolol | D | No Human Data—Potential Toxicity |
| Betaxolol | C | No Human Data—Potential Toxicity |
| Bisoprolol | C | No Human Data—Potential Toxicity |
| Celiprolol | NA | N/A |
| Esmolol | C | No Human Data—Probably Compatible |
| Metoprolol | C | No Human Data—Potential Toxicity |
| Nebivolol | C | No Human Data—Potential Toxicity |
| Carvedilol | C | No Human Data—Probably Compatible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szybiak-Skora, W.; Miedziaszczyk, M.; Szałek, E.; Lacka, K. The Therapeutic Potential of Propranolol and Other Beta-Blockers in Hyperthyroidism. Int. J. Mol. Sci. 2025, 26, 8322. https://doi.org/10.3390/ijms26178322
Szybiak-Skora W, Miedziaszczyk M, Szałek E, Lacka K. The Therapeutic Potential of Propranolol and Other Beta-Blockers in Hyperthyroidism. International Journal of Molecular Sciences. 2025; 26(17):8322. https://doi.org/10.3390/ijms26178322
Chicago/Turabian StyleSzybiak-Skora, Weronika, Miłosz Miedziaszczyk, Edyta Szałek, and Katarzyna Lacka. 2025. "The Therapeutic Potential of Propranolol and Other Beta-Blockers in Hyperthyroidism" International Journal of Molecular Sciences 26, no. 17: 8322. https://doi.org/10.3390/ijms26178322
APA StyleSzybiak-Skora, W., Miedziaszczyk, M., Szałek, E., & Lacka, K. (2025). The Therapeutic Potential of Propranolol and Other Beta-Blockers in Hyperthyroidism. International Journal of Molecular Sciences, 26(17), 8322. https://doi.org/10.3390/ijms26178322







