In Vitro Bioaccessibility and Speciation of Toxic and Nutritional Trace Elements in Brazil Nuts
Abstract
1. Introduction
2. Results and Discussion
2.1. Element and Radionuclide Analysis in Brazil Nuts
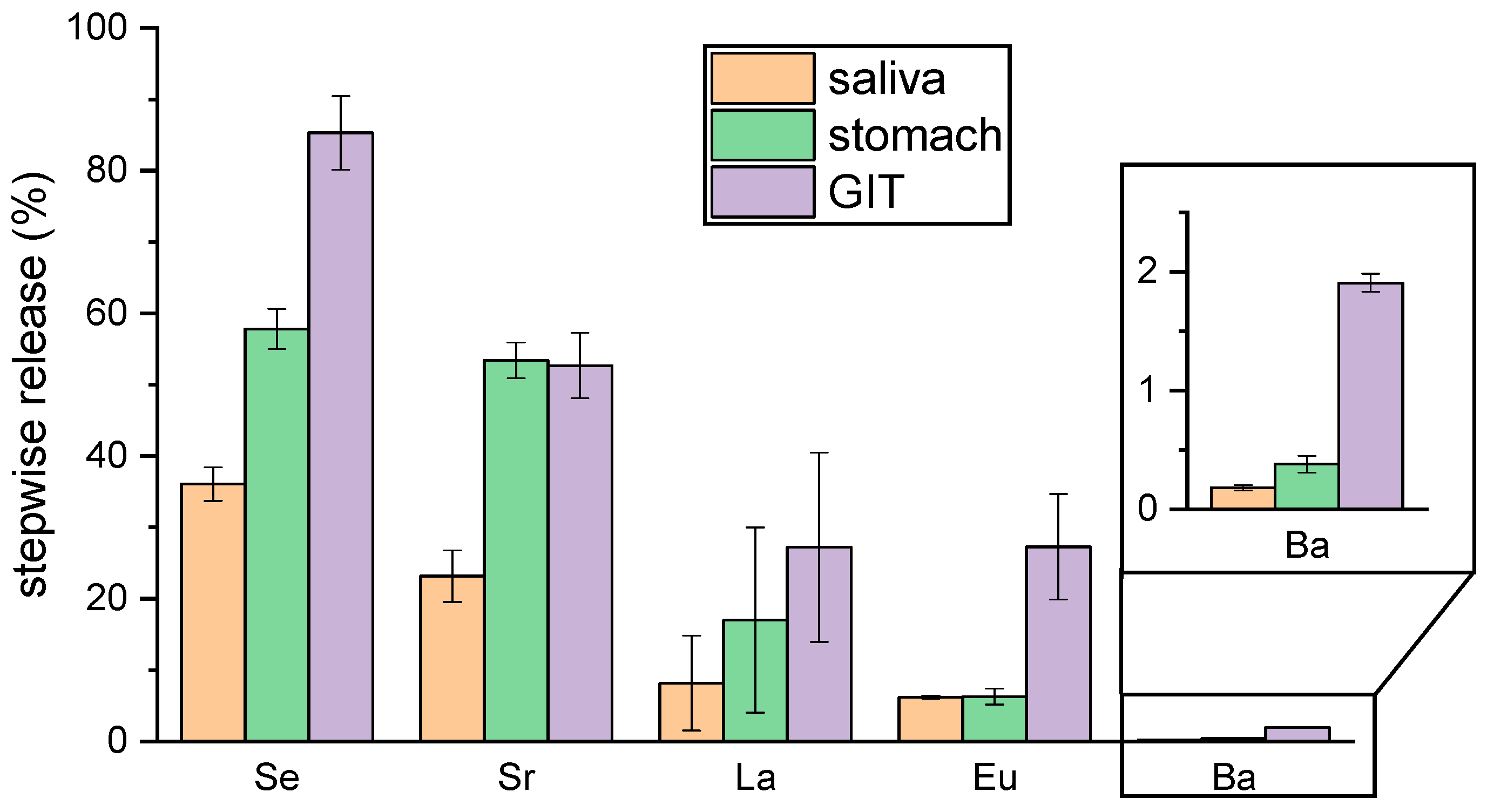
2.2. Bioaccessibility of Selected Elements and Radionuclides in Brazil Nut Flour
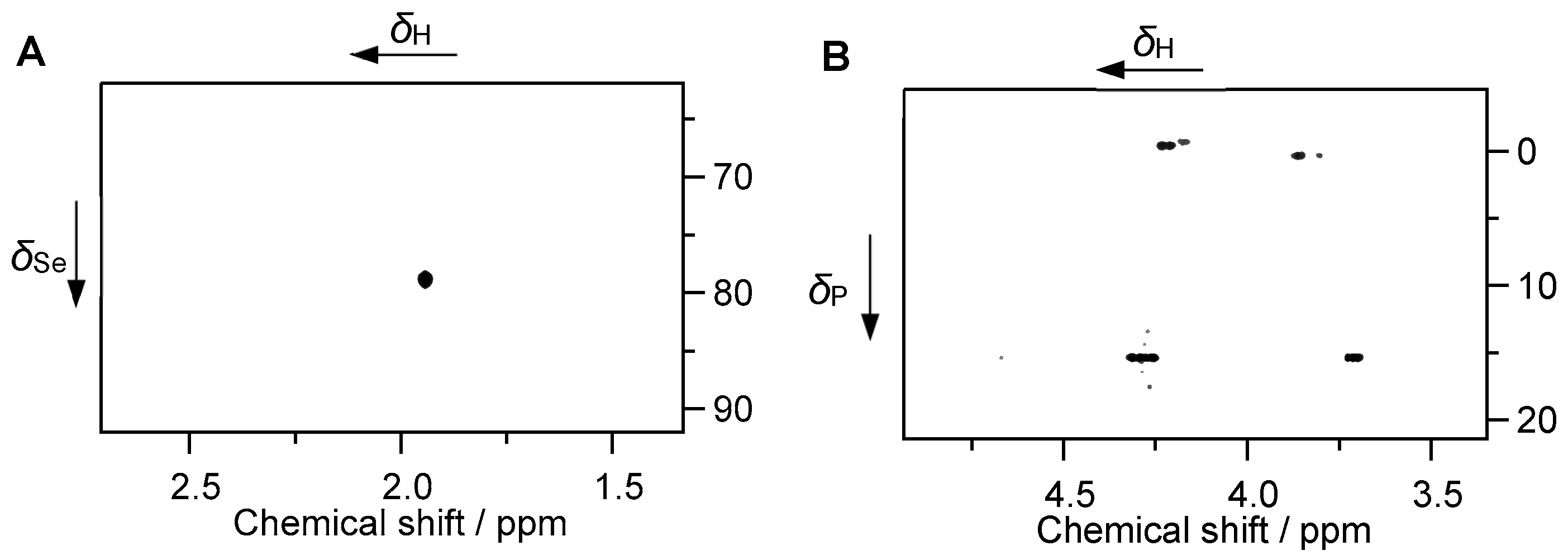
2.3. Speciation of Se and Determination of Selected Organic Species by NMR
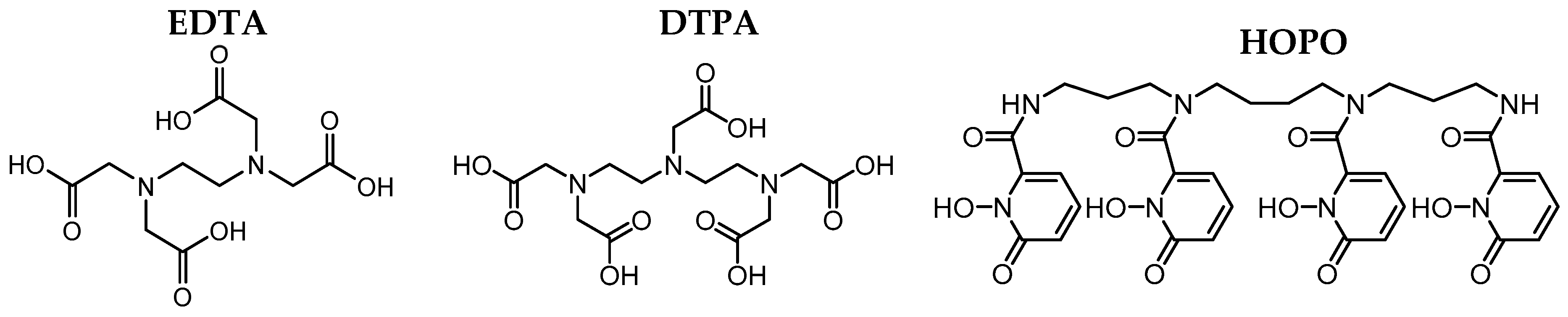
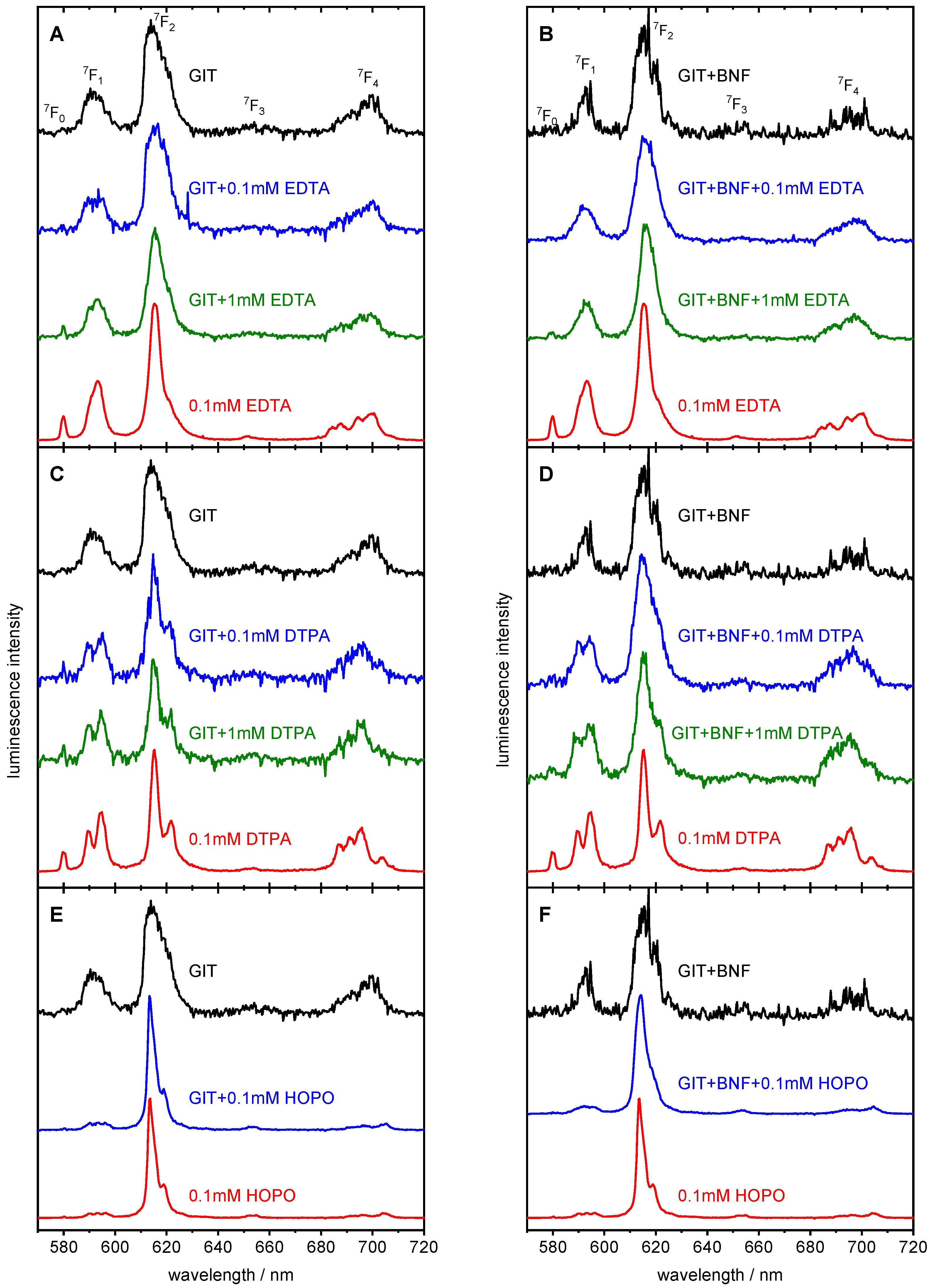
2.4. Speciation Determination of Eu in GIT by Luminescence Spectroscopy
3. Materials and Methods
3.1. Chemicals and Materials
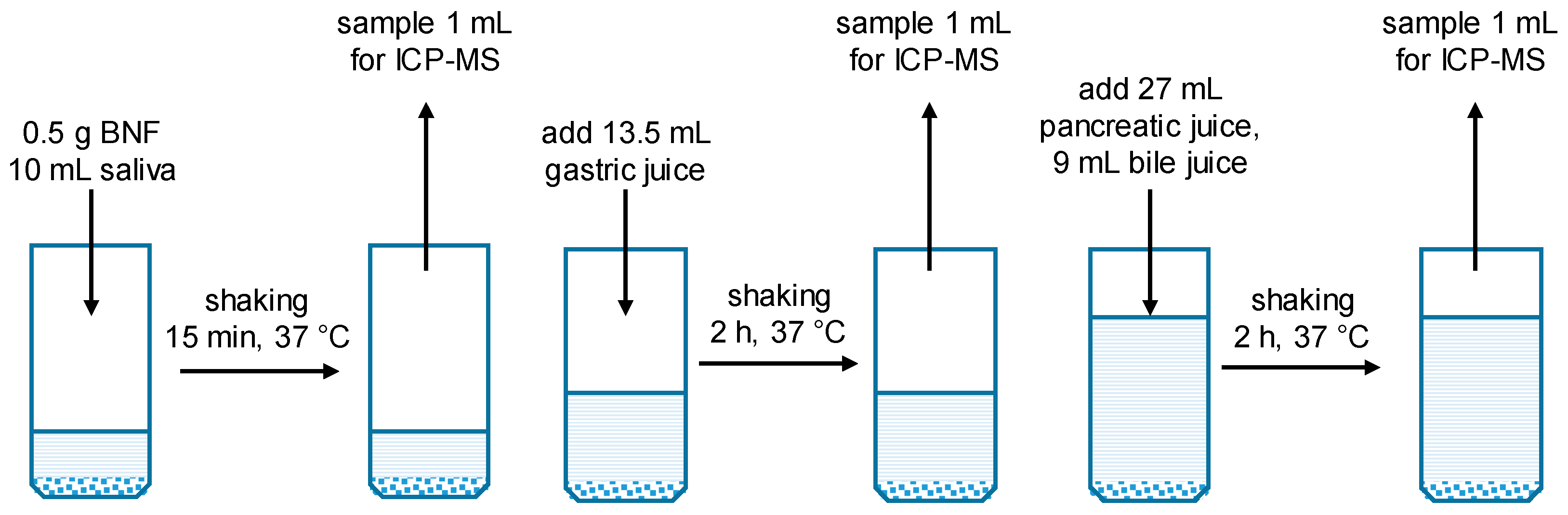
3.2. Simulation of Digestive Process
3.3. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
3.4. Gamma Spectrometry
3.5. Alpha Spectrometry
3.6. NMR Spectroscopy
3.7. Luminescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| An | Actinides |
| ATSDR | Agency for Toxic Substances and Disease Registry |
| BARGE | Bioaccessibility Research Group of Europe |
| BNF | Brazil nut flour |
| BW | Body weight |
| DA(s) | Decorporation agent(s) |
| DTPA | Diethylenetriaminepentaacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| EFSA | European Food Safety Authority |
| EPA | U.S. Environmental Protection Agency |
| GIT | Gastrointestinal tract |
| HMBC | Heteronuclear multiple-bond correlation |
| HOPO | 1,5,10,14-tetra(1-hydroxy-2-pyridon-6-oyl)-1,5,10,14-tetraazatetradecan3,4,3-LI(1,2-HOPO) |
| HPLC | High Performance Liquid Chromatography |
| HSQC | Heteronuclear single-quantum coherence |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| Ln | Lanthanides |
| NMR | Nuclear magnetic resonance |
| REE(s) | Rare earth element(s) |
| RN | Radionuclide |
| SF(0…4) | Soluble fraction(0…4) |
| TOCSY | Total Correlation Spectroscopy |
| TRLFS | Time-resolved laser-induced fluorescence spectroscopy |
| UBM | Unified bioaccessibility method |
| WHO | World Health Organization |
References
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT-Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Alcantara, D.B.; Dionísio, A.P.; Artur, A.G.; Silveira, B.K.S.; Lopes, A.F.; Guedes, J.A.C.; Luz, L.R.; Nascimento, R.F.; Lopes, G.S.; Hermsdorff, H.H.M.; et al. Selenium in Brazil nuts: An overview of agronomical aspects, recent trends in analytical chemistry, and health outcomes. Food Chem. 2022, 372, 17. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; Duran, N.M.; Lessa, J.H.D.; Ribeiro, P.G.; Wadt, L.H.D.; da Silva, K.E.; de Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; de Oliveira, R.C.; et al. Unraveling the accumulation and localization of selenium and barium in Brazil nuts using spectroanalytical techniques. J. Food Compos. Anal. 2022, 106, 10. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023, 21, 194. [Google Scholar] [CrossRef]
- da Silva, E.G.; Mataveli, L.R.V.; Arruda, M.A.Z. Speciation analysis of selenium in plankton, Brazil nut and human urine samples by HPLC-ICP-MS. Talanta 2013, 110, 53–57. [Google Scholar] [CrossRef]
- Kafaoglu, B.; Fisher, A.; Hill, S.; Kara, D. Chemometric evaluation of trace metal concentrations in some nuts and seeds. Food Addit. Contam. Part A-Chem. 2014, 31, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Lemire, M.; Fillion, M.; Barbosa, F.; Guimaraes, J.R.D.; Mergler, D. Elevated levels of selenium in the typical diet of Amazonian riverside populations. Sci. Total Environ. 2010, 408, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Stonehouse, G.C.; Walters, C.; El Mehdawi, A.F.; Fakra, S.C.; Pilon-Smits, E.A.H. Selenium Accumulation, Speciation and Localization in Brazil Nuts (Bertholletia excelsa H.B.K.). Plants-Basel 2019, 8, 17. [Google Scholar] [CrossRef]
- Martins, M.; Pacheco, A.M.; Lucas, A.C.S.; Andrello, A.C.; Appoloni, C.R.; Xavier, J.J.M. Brazil nuts: Determination of natural elements and aflatoxin. Acta Amaz. 2012, 42, 157–164. [Google Scholar] [CrossRef]
- Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Elemental composition and chemical characteristics of five edible nuts (almond, Brazil, pecan, macadamia and walnut) consumed in Southern Africa. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2007, 42, 585–591. [Google Scholar] [CrossRef]
- Moskwa, J.; Naliwajko, S.K.; Puscion-Jakubik, A.; Soroczynska, J.; Socha, K.; Koch, W.; Markiewicz-Zukowska, R. In Vitro Assessment of the Bioaccessibility of Zn, Ca, Mg, and Se from Various Types of Nuts. Foods 2023, 12, 12. [Google Scholar] [CrossRef]
- Ni, Z.L.; Yang, L.; Qu, M.H.; Li, Z.X.; Tang, F.B. Elemental Analysis of Tree Nuts and Their Pressed Oils by Triple Quadrupole Inductively Coupled Plasma-Mass Spectrometry (ICP-MS/MS). Anal. Lett. 2024, 58, 79–90. [Google Scholar] [CrossRef]
- Tosic, S.B.; Mitic, S.S.; Velimirovic, D.S.; Stojanovic, G.S.; Pavlovic, A.N.; Pecev-Marinkovic, E.T. Elemental composition of edible nuts: Fast optimization and validation procedure of an ICP-OES method. J. Sci. Food Agric. 2015, 95, 2271–2278. [Google Scholar] [CrossRef]
- Vonderheide, A.P.; Wrobel, K.; Kannamkumarath, S.S.; B’Hymer, C.; Montes-Bayon, M.; De Leon, C.P.; Caruso, J.A. Characterization of selenium species in brazil nuts by HPLC-ICP-MS and ES-MS. J. Agric. Food Chem. 2002, 50, 5722–5728. [Google Scholar] [CrossRef]
- Welna, M.; Szymczycha-Madeja, A. Improvement of a sample preparation procedure for multi-elemental determination in Brazil nuts by ICP-OES. Food Addit. Contam. Part A-Chem. 2014, 31, 658–665. [Google Scholar] [CrossRef]
- de Brito, R.C.M.; Pereira, J.B., Jr.; Dantas, K.D.F. Quantification of inorganic constituents in Brazil nuts and their products by inductively coupled plasma optical emission spectrometry. LWT-Food Sci. Technol. 2019, 116, 5. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Herbello-Hermelo, P.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Bioavailability assessment of essential and toxic metals in edible nuts and seeds. Food Chem. 2016, 205, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rodushkin, I.; Engström, E.; Sörlin, D.; Baxter, D. Levels of inorganic constituents in raw nuts and seeds on the Swedish market. Sci. Total Environ. 2008, 392, 290–304. [Google Scholar] [CrossRef]
- Lopes, G.S.; Silva, F.L.F.; Grinberg, P.; Sturgeon, R.E. An Evaluation of the Use of Formic Acid for Extraction of Trace Elements from Brazil Nut and Babassu Coconut and Its Suitability for Multi-Element Determination by ICP-MS. J. Braz. Chem. Soc. 2016, 27, 1229–1235. [Google Scholar] [CrossRef]
- Moreda-Pineiro, J.; Sanchez-Pinero, J.; Alonso-Rodriguez, E.; Turnes-Carou, I.; Lopez-Mahia, P.; Muniategui-Lorenzo, S. Major, minor and trace elements composition of Amazonian foodstuffs and its contribution to dietary intake. J. Food Meas. Charact. 2020, 14, 1314–1324. [Google Scholar] [CrossRef]
- Welna, M.; Klimpel, M.; Zyrnicki, W. Investigation of major and trace elements and their distributions between lipid and non-lipid fractions in Brazil nuts by inductively coupled plasma atomic optical spectrometry. Food Chem. 2008, 111, 1012–1015. [Google Scholar] [CrossRef]
- Koeder, C.; Keller, M. Radium levels in Brazil nuts: A review of the literature. Nutr. Bull. 2025, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Stoewsand, G.S.; Anderson, J.L.; Rutzke, M.; Lisk, D.J. Deposition of barium in the skeleton of rats fed brazil nuts. Nutr. Rep. Int. 1988, 38, 259–262. [Google Scholar]
- Brugge, D.; Buchner, V. Radium in the environment: Exposure pathways and health effects. Rev. Environ. Health 2012, 27, 1–17. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- IRIS (Integrated Risk Information System). Barium and Compounds; CASRN 7440-39-3; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- ATSDR. Toxicological Profile For Barium And Barium Compounds; Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services: Atlanta, Geogia, 2007.
- IRIS (Integrated Risk Information System). Strontium; CASRN 7440-24-6; U.S. Environmental Protection Agency: Washington, DC, USA, 1992.
- ATSDR. Toxicological Profile For Strontium; Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services: Atlanta, Geogia, 2004.
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103; Elsevier: Oxford, UK, 2007; Volume 37. [Google Scholar]
- Duarte, G.B.S.; Reis, B.Z.; Rogero, M.M.; Barbosa, F., Jr.; Cercato, C.; Cozzolino, S.M.F. Plasma Concentration of Essential and Toxic Trace Elements After Brazil Nut Intake: Results from a Randomized Controlled Trial. Biol. Trace Elem. Res. 2023, 201, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.M.; Fernandes, K.G.; Ramos, L.A.; Cavalheiro, E.T.G.; Nóbrega, J.A. Determination and Fractionation of Barium in Brazil Nuts. J. Braz. Chem. Soc. 2009, 20, 760–769. [Google Scholar] [CrossRef]
- Naozuka, J.; Vieira, E.C.; Nascimento, A.N.; Oliveira, P.V. Elemental analysis of nuts and seeds by axially viewed ICP OES. Food Chem. 2011, 124, 1667–1672. [Google Scholar] [CrossRef]
- Parekh, P.P.; Khan, A.R.; Torres, M.A.; Kitto, M.E. Concentrations of selenium, barium, and radium in Brazil nuts. J. Food Compos. Anal. 2008, 21, 332–335. [Google Scholar] [CrossRef]
- Smith, K.A. Comparative uptake and translocation by plants of calcium, strontium, barium and radium. 1. Bertholletia excelsa (Brazil nut tree). Plant Soil 1971, 34, 369–379. [Google Scholar] [CrossRef]
- Hiromoto, G.; Oliveira, J.; Carvalho, J.S.; Vicente, R.; Bellintani, S.A. Collective dose and risk assessment from Brazil nut consumption. Radiat. Prot. Dosim. 1996, 67, 229–230. [Google Scholar] [CrossRef]
- Ioannidis, I.; Paschalidou, P.; Sarrou, I.; Pashalidis, I. Radiometric analysis of potassium, radium and uranium levels in Brazil nuts. J. Radioanal. Nucl. Chem. 2023, 332, 1405–1408. [Google Scholar] [CrossRef]
- Kluczkovski, A.; Martins, M.; Lobo, E.; Junior, J.G.d.M.; Campelo, P.H.; Oliveira, T.; Martins, V.D.G.T. Trace Elements and Radionuclides in Brazil Nuts from the Brazilian Amazon. J. Agric. Stud. 2020, 8, 795–805. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Dai, Y.B.; Sun, S.; Li, Y.; Yang, J.J.; Zhang, C.B.; Cao, R.; Zhang, H.J.; Chen, J.P.; Geng, N.B. Residual levels and health risk assessment of rare earth elements in Chinese resident diet: A market-based investigation. Sci. Total Environ. 2022, 828, 9. [Google Scholar] [CrossRef]
- González, N.; Domingo, J.L. Levels of Rare Earth Elements in Food and Human Dietary Exposure: A Review. Biol. Trace Elem. Res. 2025, 203, 2240–2256. [Google Scholar] [CrossRef]
- Ferreira, M.D.; Fontes, M.P.F.; Lima, M.; Cordeiro, S.G.; Wyatt, N.L.P.; Lima, H.N.; Fendorf, S. Human health risk assessment and geochemical mobility of rare earth elements in Amazon soils. Sci. Total Environ. 2022, 806, 12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.; Fontes, M.P.F.; Bellato, C.R.; Neto, J.D.M.; Lima, H.N.; Fendorf, S. Geochemical signatures and natural background values of rare earth elements in soils of Brazilian Amazon. Environ. Pollut. 2021, 277, 14. [Google Scholar] [CrossRef] [PubMed]
- Klotzsche, M.; Drobot, B.; Schymura, S.; Vogel, M.; Raff, J.; Stumpf, T.; Steudtner, R. Follow me: Mechanistic insights into Eu(III) uptake, translocation and speciation in hydroponically grown Sand oat (Avena strigosa). Sci. Total Environ. 2025, 988, 179849. [Google Scholar] [CrossRef]
- Heller, A.; Barkleit, A.; Bok, F.; Wober, J. Effect of four lanthanides onto the viability of two mammalian kidney cell lines. Ecotoxicol. Environ. Saf. 2019, 173, 469–481. [Google Scholar] [CrossRef]
- Heller, A.; Pisarevskaja, A.; Bölicke, N.; Barkleit, A.; Bok, F.; Wober, J. The effect of four lanthanides onto a rat kidney cell line (NRK-52E) is dependent on the composition of the cell culture medium. Toxicology 2021, 456, 152771. [Google Scholar] [CrossRef] [PubMed]
- Senwitz, C.; Butscher, D.; Holtmann, L.; Vogel, M.; Steudtner, R.; Drobot, B.; Stumpf, T.; Barkleit, A.; Heller, A. Effect of Ba(II), Eu(III), and U(VI) on rat NRK-52E and human HEK-293 kidney cells in vitro. Sci. Total Environ. 2024, 923, 171374. [Google Scholar] [CrossRef]
- Senwitz, C.; Vogel, M.; Drobot, B.; Barkleit, A.; Stumpf, T.; Heller, A. Impact of DTPA and 3,4,3-(LI-1,2-HOPO) on Eu(III) interactions with renal cells in vitro. Sci. Total Environ. 2025, 966, 178736. [Google Scholar] [CrossRef]
- Sachs, S.; Heller, A.; Weiss, S.; Bok, F.; Bernhard, G. Interaction of Eu(III) with mammalian cells: Cytotoxicity, uptake, and speciation as a function of Eu(III) concentration and nutrient composition. Toxicol. Vitr. 2015, 29, 1555–1568. [Google Scholar] [CrossRef]
- Constantin, M.; Chioncel, M.F.; Petrescu, L.; Vrancianu, C.O.; Paun, M.; Cristian, R.E.; Sidoroff, M.; Dionisie, M.V.; Chifiriuc, M.C. From rock to living systems: Lanthanides toxicity and biological interactions. Ecotoxicol. Environ. Saf. 2025, 289, 20. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Juhasz, A.; Smith, E.; Naidu, R. Assessing the bioavailability and bioaccessibility of metals and metalloids. Environ. Sci. Pollut. Res. 2015, 22, 8802–8825. [Google Scholar] [CrossRef]
- Teixeira, J.L.D.; Rebellato, A.P.; Fioravanti, M.I.A.; Milani, R.F.; Morgano, M.A. Selenium in plant-based beverages: Total content, estimated bioaccessibility and contribution to daily intake. J. Trace Elem. Med. Biol. 2024, 81, 127329. [Google Scholar] [CrossRef]
- Wilke, C.; Barkleit, A.; Stumpf, T.; Ikeda-Ohno, A. Speciation of the trivalent f-elements Eu(III) and Cm(III) in digestive media. J. Inorg. Biochem. 2017, 175, 248–258. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.; Taylor, H.; Basta, N.; Brandon, E.; Casteel, S.; Gron, C.; Oomen, A.; van de Wiele, T. Inter-Laboratory Trial of a Unified Bioaccessibility Procedure; British Geological Survey Open Report OR/07/027; British Geological Survey: Nottingham, UK, 2009; p. 90. [Google Scholar]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an In Vitro Digestion Model for Estimating the Bioaccessibility of Soil Contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, R.; Dekimpe, J. Elemental speciation in biological-fluids. J. Anal. At. Spectrom. 1994, 9, 945–950. [Google Scholar] [CrossRef]
- Infante, H.G.; Hearn, R.; Catterick, T. Current mass spectrometry strategies for selenium speciation in dietary sources of high-selenium. Anal. Bioanal. Chem. 2005, 382, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Bodó, E.T.; Stefánka, Z.; Ipolyi, I.; Sörös, C.; Dernovics, M.; Fodor, P. Preparation, homogeneity and stability studies of a candidate LRM for Se speciation. Anal. Bioanal. Chem. 2003, 377, 32–38. [Google Scholar] [CrossRef]
- Ansoborlo, E.; Amekraz, B.; Moulin, C.; Moulin, V.; Taran, F.; Bailly, T.; Burgada, R.; Henge-Napoli, M.H.; Jeanson, A.; Den Auwer, C.; et al. Review of actinide decorporation with chelating agents. Comptes Rendus Chim. 2007, 10, 1010–1019. [Google Scholar] [CrossRef]
- Durbin, P.W.; Kullgren, B.; Xu, J.; Raymond, K.N. Development of decorporation agents for the actinides. Radiat. Prot. Dosim. 1998, 79, 433–443. [Google Scholar] [CrossRef]
- Gorden, A.E.; Xu, J.; Raymond, K.N.; Durbin, P. Rational design of sequestering agents for plutonium and other actinides. Chem. Rev. 2003, 103, 4207–4282. [Google Scholar] [CrossRef]
- Carbaugh, E.H.; Lynch, T.P.; Cannon, C.N.; Lewis, L.L. Case study: Three acute Am-241 inhalation exposures with DTPA therapy. Health Phys. 2010, 99, 539–546. [Google Scholar] [CrossRef]
- Abergel, R.J.; Durbin, P.W.; Kullgren, B.; Ebbe, S.N.; Xu, J.D.; Chang, P.Y.; Bunin, D.I.; Blakely, E.A.; Bjornstad, K.A.; Rosen, C.J.; et al. Biomimetic actinide chelators: An update on the preclinical development of the orally active hydroxypyridonate decorporation agents 3,4,3-LI(1,2-HOPO) and 5-LIO(Me-3,2-HOPO). Health Phys. 2010, 99, 401–407. [Google Scholar] [CrossRef]
- Choi, T.A.; Furimsky, A.M.; Swezey, R.; Bunin, D.I.; Byrge, P.; Iyer, L.V.; Chang, P.Y.; Abergel, R.J. In Vitro Metabolism and Stability of the Actinide Chelating Agent 3,4,3-LI(1,2-HOPO). J. Pharm. Sci. 2015, 104, 1832–1838. [Google Scholar] [CrossRef]
- HOPO Therapeutics. Harnessing Heavy Metals to Improve Human Health. Available online: https://hopotx.com/ (accessed on 28 July 2025).
- da Silva Junior, E.C.; Wadt, L.H.O.; da Silva, K.E.; de Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Junior, R.C.D.; dos Reis, A.R.; Lopes, G.; Broadley, M.R.; et al. Geochemistry of selenium, barium, and iodine in representative soils of the Brazilian Amazon rainforest. Sci. Total Environ. 2022, 828, 11. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Silveira, B.K.S.; de Freitas, B.V.M.; Hermsdorff, H.H.M.; Bressan, J. Effects of Regular Brazil Nut (Bertholletia excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods 2022, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.H.A.; Maia, J.G.S.; Streich, R.; Marx, F. Seed Composition of Amazonian Lecythidaceae Species: Part 3 in the Series “Studies of Edible Amazonian Plants”. J. Food Compos. Anal. 1999, 12, 37–51. [Google Scholar] [CrossRef]
- Bageri, B.S.; Mahmoud, M.A.; Shawabkeh, R.A.; Abdulraheem, A. Evaluation of Barium Sulfate (Barite) Solubility Using Different Chelating Agents at a High Temperature. J. Pet. Sci. Technol. 2017, 7, 42–56. [Google Scholar] [CrossRef]
- Cheryan, M. Phytic acid interactions in food systems. Crit. Rev. Food Sci. Nutr. 1980, 13, 297–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, D.X. Phytic acid and its interactions in food components, health benefits, and applications: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104201. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res. Int. 2021, 142, 110193. [Google Scholar] [CrossRef]
- Perera, I.; Seneweera, S.; Hirotsu, N. Manipulating the Phytic Acid Content of Rice Grain Toward Improving Micronutrient Bioavailability. Rice 2018, 11, 4. [Google Scholar] [CrossRef]
- Ogawa, K.; Higashi, T.; Mishiro, K.; Wakabayashi, H.; Shiba, K.; Odani, A.; Kinuya, S. Decreasing undesirable absorbed radiation to the intestine after administration of radium-223 dichloride for treatment of bone metastases. Sci. Rep. 2020, 10, 11917. [Google Scholar] [CrossRef]
- Harrison, G.E.; Carr, T.E.F.; Sutton, A. Distribution of radioactive calcium strontium barium and radium following intravenous injection into a healthy man. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1967, 13, 235–247. [Google Scholar] [CrossRef]
- Harrison, G.E.; Carr, T.E.F.; Sutton, A.; Rundo, J. Plasma concentration and excretion of calcium-47 strontium-85 barium-133 and radium-223 following successive intravenous doses to a healthy man. Nature 1966, 209, 526–527. [Google Scholar] [CrossRef]
- Griggs, J.L.; Thomas, D.J.; Fry, R.; Bradham, K.D. Improving the predictive value of bioaccessibility assays and their use to provide mechanistic insights into bioavailability for toxic metals/metalloids—A research prospectus. J. Toxicol. Environ. Health-Part B-Crit. Rev. 2021, 24, 307–324. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Buchko, G.W.; Cadet, J. Identification of 2-deoxy-d-ribono-1,4-lactone at the site of benzophenone photosensitized release of guanine in 2′-deoxyguanosine and thymidylyl-(3′-5′)-2′-deoxyguanosine. Can. J. Chem.-Rev. Can. Chim. 1992, 70, 1827–1832. [Google Scholar] [CrossRef]
- Colson, P.; King, R.R. C-13-NMR spectra of disaccharides of d-glucose, d-galactose, and l-rhamnose as models for immunological polysaccharides. Carbohydr. Res. 1976, 47, 1–13. [Google Scholar] [CrossRef]
- Debruyn, A.; Anteunis, M.; Degussem, R.; Dutton, G.G.S. H-1-NMR study of l-rhamnose, methyl alpha-l-rhamnopyranoside, and 4-o-beta-d-galactopyranosyl-l-rhamnose in deuterium-oxide. Carbohydr. Res. 1976, 47, 158–163. [Google Scholar] [CrossRef]
- Gheysen, K.; Mihai, C.; Conrath, K.; Martins, J.C. Rapid Identification of Common Hexapyranose Monosaccharide Units by a Simple TOCSY Matching Approach. Chem.-Eur. J. 2008, 14, 8869–8878. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Shen, F.; Smith, R.L.; Qi, X.H. Quantitative chemocatalytic production of lactic acid from glucose under anaerobic conditions at room temperature. Green Chem. 2017, 19, 76–81. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Nifantev, N.E.; Shashkov, A.S.; Kochetkov, N.K. NMR and conformational study of branched oligosaccharides containing 2,3-disubstituted residues of alpha-l-rhamnose. Can. J. Chem. 1990, 68, 1238–1250. [Google Scholar] [CrossRef]
- Ma, C.Y.; Cai, B.; Zhang, L.; Feng, J.F.; Pan, H. Acid-Catalyzed Conversion of Cellulose Into Levulinic Acid With Biphasic Solvent System. Front. Plant Sci. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Amador, R.M.; Salvador, M.D.; Fregapane, G.; Gómez-Alonso, S. Comprehensive Study of the Phenolic Compound Profile and Antioxidant Activity of Eight Pistachio Cultivars and Their Residual Cakes and Virgin Oils. J. Agric. Food Chem. 2019, 67, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Bigam, C.G.; Holm, A.; Hodges, R.S.; Sykes, B.D. H-1, C-13 and N-15 random coil nmr chemical-shifts of the common amino-acids. 1. Investigations of nearest-neighbor effects. J. Biomol. NMR 1995, 5, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Block, E.; Glass, R.S.; Jacobsen, N.E.; Johnson, S.; Kahakachchi, C.; KaminskI, R.; Skowronska, A.; Boakye, H.T.; Tyson, J.F.; Uden, P.C. Identification and synthesis of a novel selenium-sulfur amino acid found in selenized yeast: Rapid indirect detection NMR methods for characterizing low-level Organoselenium compounds in complex matrices. J. Agric. Food Chem. 2004, 52, 3761–3771. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Xu, S.P.; Lu, X.Y.; Boeri, M.V.; Pepelyayeva, Y.; Diaz, E.L.; Soni, S.D.; Allaire, M.; Forstner, M.B.; Bahnson, B.J.; et al. 77Se NMR Probes the Protein Environment of Selenomethionine. J. Phys. Chem. B 2020, 124, 601–616. [Google Scholar] [CrossRef]
- Ritchey, J.A.; Davis, B.M.; Pleban, P.A.; Bayse, C.A. Experimental and theoretical evidence for cyclic selenurane formation during selenomethionine oxidation. Org. Biomol. Chem. 2005, 3, 4337–4342. [Google Scholar] [CrossRef]
- Chen, A.; Zhu, L.Y.; Arai, Y. Solution NMR investigation of phytic acid adsorption mechanisms at the calcite-water interface. Sci. Total Environ. 2022, 840, 156700. [Google Scholar] [CrossRef]
- Qiu, D.; Guerry, P.; Knowles, J.C.; Smith, M.E.; Newport, R.J. Formation of functional phosphosilicate gels from phytic acid and tetraethyl orthosilicate. J. Sol-Gel Sci. Technol. 2008, 48, 378–383. [Google Scholar] [CrossRef]
- Mady, M.F.; Ortega, R. Fosfomycin and Its Derivatives: New Scale Inhibitors for Oilfield Applications. ACS Omega 2022, 7, 10701–10708. [Google Scholar] [CrossRef]
- Godinot, C.; Gaysinski, M.; Thomas, O.P.; Ferrier-Pagès, C.; Grover, R. On the use of 31P NMR for the quantification of hydrosoluble phosphorus-containing compounds in coral host tissues and cultured zooxanthellae. Sci. Rep. 2016, 6, 21760. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. Phytic acid | Properties and Determination. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4546–4555. [Google Scholar]
- Friedrich, S.; Barberon, A.; Shamoun, A.; Drobot, B.; Müller, K.; Stumpf, T.; Kretzschmar, J.; Barkleit, A. Efficacy of various complexing agents for displacing biologically important ligands from Eu(III) and Cm(III) complexes in artificial body fluids—An in vitro decorporation study. Int. J. Mol. Sci. 2025, 26, 7112. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Sieber, C.; Drobot, B.; Tsushima, S.; Barkleit, A.; Schmeide, K.; Stumpf, T.; Kretzschmar, J. Eu(III) and Cm(III) Complexation by the Aminocarboxylates NTA, EDTA, and EGTA Studied with NMR, TRLFS, and ITC-An Improved Approach to More Robust Thermodynamics. Molecules 2023, 28, 4881. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Nawrocki, P.R.; Kofod, N.; Sorensen, T.J. Seven Europium(III) Complexes in Solution–The Importance of Reporting Data When Investigating Luminescence Spectra and Electronic Structure. Eur. J. Inorg. Chem. 2022, 2022, e202200334. [Google Scholar] [CrossRef]
- Abergel, R.J.; D’Aleo, A.; Leung, C.N.P.; Shuh, D.K.; Raymond, K.N. Using the Antenna Effect as a Spectroscopic Tool: Photophysics and Solution Thermodynamics of the Model Luminescent Hydroxypyridonate Complex [Eu(III)(3,4,3-LI(1,2-HOPO))](-). Inorg. Chem. 2009, 48, 10868–10870. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Waurick, L.; Drobot, B.; Steudtner, R.; Müller, K.; Barkleit, A.; Stumpf, T.; Kretzschmar, J. Lanthanide Complexes of Aminopolycarboxylates Reveal Deuteration of Aminoacetate Carbons In Alkaline Aqueous Media. Chem. Commun. 2025, 61, 12598–12601. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 17294-2:2017-01; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. Beuth Verlag: Berlin, Germany, 2017.
- DIN EN ISO 20042 VDE 0493-2042: 2022-06; Measurement of Radioactivity—Gamma-Ray Emitting Radionuclides—Generic Test Method Using Gamma-Ray Spectrometry. VDE-Verlag GmbH: Berlin, Germany, 2022.
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water-molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Kimura, T.; Choppin, G.R.; Kato, Y.; Yoshida, Z. Determination of the hydration number of Cm(III) in various aqueous solutions. Radiochim. Acta 1996, 72, 61–64. [Google Scholar] [CrossRef]
- Denys, S.; Caboche, J.; Tack, K.; Rychen, G.; Wragg, J.; Cave, M.; Jondreville, C.; Feidt, C. In Vivo Validation of the Unified BARGE Method to Assess the Bioaccessibility of Arsenic, Antimony, Cadmium, and Lead in Soils. Environ. Sci. Technol. 2012, 46, 6252–6260. [Google Scholar] [CrossRef]
- Berenguel, O.; Pessôa, G.D.; Arruda, M.A.Z. Total content and in vitro bioaccessibility of tellurium in Brazil nuts. J. Trace Elem. Med. Biol. 2018, 48, 46–51. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Waterlot, C.; Guerin, A.; Proix, N.; Richard, A.; Douay, F. Use of an in vitro digestion method to estimate human bioaccessibility of Cd in vegetables grown in smelter-impacted soils: The influence of cooking. Environ. Geochem. Health 2015, 37, 767–778. [Google Scholar] [CrossRef]
- Herrera, M.A.; Rosende, M.; Arruda, M.A.Z.; Miró, M. On-line coupling of physiologically relevant bioaccessibility testing to inductively coupled plasma spectrometry: Proof of concept for fast assessment of gastrointestinal bioaccessibility of micronutrients from soybeans. Anal. Chim. Acta 2016, 939, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.F.; Zhang, Y.C.; Williams, P.N.; Lin, S.N.; Hou, Y.W.; Cai, C. In Vitro Model To Assess Arsenic Bioaccessibility and Speciation in Cooked Shrimp. J. Agric. Food Chem. 2018, 66, 4710–4715. [Google Scholar] [CrossRef] [PubMed]
- Intawongse, M.; Kongchouy, N.; Dean, J.R. Bioaccessibility of heavy metals in the seaweed Caulerpa racemosa var. corynephora: Human health risk from consumption. Instrum. Sci. Technol. 2018, 46, 628–644. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, S.; Su, F.; Li, F.; Zhou, X.F.; Mao, P.; Li, Y.W.; Li, Z.A.; Zhang, C.S. Dietary strategies to reduce the oral bioaccessibility of cadmium and arsenic in rice. Environ. Sci. Pollut. Res. 2018, 25, 33353–33360. [Google Scholar] [CrossRef]
- Wang, P.F.; Yin, N.Y.; Cai, X.L.; Du, H.L.; Li, Z.J.; Sun, G.X.; Cui, Y.S. Variability of chromium bioaccessibility and speciation in vegetables: The influence of in vitro methods, gut microbiota and vegetable species. Food Chem. 2019, 277, 347–352. [Google Scholar] [CrossRef]
- Ferreira, M.D.; Tarley, C.R.T. Bioaccessibility estimation of metallic macro and micronutrients Ca, Mg, Zn, Fe, Cu and Mn in flours of oat and passion fruit peel. LWT-Food Sci. Technol. 2021, 150, 111880. [Google Scholar] [CrossRef]
- Tokalioglu, S. Bioaccessibility of Cu, Mn, Fe, and Zn in Fruit and Vegetables by the In Vitro UBM and Statistical Evaluation of the Results. Biol. Trace Elem. Res. 2023, 201, 1538–1546. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Banata, B.B. Bioaccessibility of Fifteen Elements from Dried Fruits by the BARGE (Bioaccessibility Research Group of Europe) Unified Bioaccessibility Method (UBM) and Multivariate Statistical Analysis. Anal. Lett. 2024, 57, 1162–1181. [Google Scholar] [CrossRef]

| c in Defatted BNF (µg/g) | c in Whole Brazil Nut (µg/g) | |||
|---|---|---|---|---|
| Element | Present Work | Literature | Present Work | Literature |
| Se | 10.1 ± 0.8 | 2–84 [13,15,18,22,23] | 1.91 ± 0.25 | 1–55 [8,9,10,12,14,15,16,19,20,21,22,23,24,38] |
| Sr | 760 ± 40 | 188–426 [18,22,23] | 230 ± 13 | 115–198 [20,21,22,23,24,37] |
| Ba | 8000 ± 400 | 2200 –7097 [18,22,23] | 1720 ± 90 | 49–2476 [19,20,21,22,23,24,35,37] |
| La | 0.122 ± 0.008 | 0.0291 ± 0.002 | 0.0014 [21] | |
| Eu | 2.49 ± 0.17 | 0.65 ± 0.03 | <0.0004 [21] | |
| Radionuclide | a (mBq/g) | |||
| 226Ra | 74.0 ± 5.3 | 51.6 ± 3.7 | 17–205 [12,25,38,39,40,41,42] | |
| 228Ra | 63.0 ± 4.6 | 47.7 ± 3.0 | 18–100 [12,25,38,39,40,42] | |
| Element Concentrations c/RN Activities a/Bioaccessibility (%) | |||||||
|---|---|---|---|---|---|---|---|
| BNF a | Saliva | Stomach | GIT b | ||||
| c (µg/g) | c (µg/g) | Release (%) | c (µg/g) | Release (%) | c (µg/g) | Release (%) | |
| Se | 9.9 ± 0.9 | 3.5 ± 0.2 | 35 ± 2 | 5.7 ± 0.3 | 57 ± 3 | 8.4 ± 0.5 | 84 ± 5 (p.w.) 70 ± 23 [14] 74 [8] 19 ± 2 [20] |
| Sr | 640 ± 30 | 143 ± 22 | 23 ± 3 | 337 ±16 | 53 ± 3 | 332 ± 29 | 52 ± 5 (p.w.) 7.8 ± 0.8 [20] |
| Ba | 7100 ± 400 | 12 ± 2 | 0.18 ± 0.03 | 27 ± 6 | 0.38 ± 0.08 | 132 ± 5 | 1.9 ± 0.1 (p.w.) 2.2 ± 0.1 [20] |
| La | 0.031 ± 0.003 | 0.003 ± 0.002 | 8 ± 6 | 0.005 ± 0.004 | 17 ± 13 | 0.008 ± 0.004 | 27 ± 13 |
| Eu | 0.47 ± 0.05 | 0.028 ± 0.001 | 6.0 ± 0.2 | 0.029 ± 0.005 | 6.2 ± 1.1 | 0.13 ± 0.03 | 27 ± 6 |
| a (mBq/g) | a (mBq/g) | ||||||
| 226Ra | 74 ± 5 | 1.4 ± 0.3 | 1.9 ± 0.4 | ||||
| Sample (+10 µM Eu) | pH | Lifetime (µs) | n (H2O) ± 0.5 | 7F2/7F1 Intensity Ratio | Reference |
|---|---|---|---|---|---|
| GIT | 6.84 | 235 ± 26 | 4.0 | 3.4 | p.w. a |
| 6.8 | 261 ± 11 // 1300 ±32 | [58] | |||
| 6.5 | 315 ± 52 | [102] | |||
| GIT + BNF | 6.66 | 364 ± 16 | 2.3 | 3.8 | p.w. |
| GIT + 0.1 mM EDTA | 6.49 | 250 ± 23 | 3.7 | 3.4 | p.w. |
| GIT + 1 mM EDTA | 6.54 | 314 ± 12 | 2.8 | 3.0 | p.w. |
| GIT + BNF + 0.1 mM EDTA | 6.51 | 419 ± 30 | 1.9 | 3.2 | p.w. |
| GIT + BNF + 1 mM EDTA | 6.48 | 557 ± 27 | 1.3 | 3.3 | p.w. |
| 0.1 mM EDTA | 6.44 | 317 ± 3 | 2.8 | 2.4 | p.w. |
| 6.5 | 326 ± 8 | [102] | |||
| 3–9 | 299 ± 6 | [103] | |||
| 4–6 | 307 | [104] | |||
| GIT + 0.1 mM DTPA | 6.58 | 377 ± 31 | 2.2 | 2.7 | p.w. |
| GIT + 1 mM DTPA | 6.52 | 372 ± 36 | 2.3 | 2.1 | p.w. |
| GIT + BNF + 0.1 mM DTPA | 6.54 | 389 ± 22 | 2.1 | 2.9 | p.w. |
| GIT + BNF + 1 mM DTPA | 6.54 | 422 ± 31 | 1.9 | 2.1 | p.w. |
| DTPA | 6.53 | 628 ± 19 | 1.1 | 1.9 | p.w. |
| 7.4 | 618 ± 4 | 1.2 | 1.9 | [52] | |
| 6.5 | 545 ± 81 | [102] | |||
| 2–5 | 577 | [104] | |||
| GIT + 0.1 mM HOPO | 6.48 | 689 ± 24 | 1.0 | 10.2 | p.w. |
| GIT + BNF + 0.1 mM HOPO | 6.51 | 527 ± 16 | 1.4 | 10.5 | p.w. |
| HOPO | 6.47 | 661 ± 27 | 1.0 | 12.2 | p.w. |
| 7.4 | 829 ± 4 | 0.8 | 11.7 | [52] | |
| 6.5 | 713 ± 58 | [102] | |||
| 7.4 | 805 ± 81 | [105] |
| Components | Saliva | Gastric Juice | Pancreatic Juice | Bile Fluid | GIT |
|---|---|---|---|---|---|
| Inorganics (mmol/L) | |||||
| NaCl | 10.2 | 94.2 | 234 | 180 | 159 |
| KCl | 24.0 | 22.1 | 15.1 | 10.1 | 17.3 |
| NH4Cl | - | 11.4 | - | - | 2.63 |
| MgCl2 | - | - | 0.5 | - | 0.23 |
| CaCl2 | 1.0 | - | 1.4 | 1.5 | 2.28 |
| NaH2PO4 | 14.8 | 3.9 | - | - | 3.18 |
| KH2PO4 | - | - | 1.2 | - | 0.55 |
| NaHCO3 | - | - | 133.5 | 137.7 | 82.8 |
| KHCO3 | 15.0 | - | - | - | 2.31 |
| Na2SO4 | 8.0 | - | - | - | 1.23 |
| KSCN | 4.1 | - | - | - | 0.63 |
| Organics (mmol/L) | |||||
| urea | 6.7 | 2.8 | 3.3 | 8.3 | 4.58 |
| uric acid | 0.1 | - | - | - | 0.02 |
| glucose | - | 7.2 | - | - | 1.66 |
| glucosamine∙HCl | - | 3.1 | - | - | 0.72 |
| glucuronic acid | - | 0.2 | - | - | 0.05 |
| Enzymes (mg/mL) | |||||
| α-amylase | 1.0 | - | - | - | 0.15 |
| mucin | 0.5 | 3.0 | 3.0 | - | 2.15 |
| pepsin | - | 1.0 | - | - | 0.23 |
| pancreatin | - | - | 3.0 | - | 1.85 |
| trypsin | - | - | 1.0 | - | 0.46 |
| lipase | - | - | 0.5 | - | 0.23 |
| bile extract | - | - | - | 6.0 | 0.92 |
| pH | 6.5 ± 0.5 | 1.0 ± 0.2 | 7.4 ± 0.2 | 8.0 ± 0.2 | 6.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkleit, A.; Eum, J.; Walther, D.; Butscher, D.; Friedrich, S.; Müller, K.; Kretzschmar, J. In Vitro Bioaccessibility and Speciation of Toxic and Nutritional Trace Elements in Brazil Nuts. Int. J. Mol. Sci. 2025, 26, 8312. https://doi.org/10.3390/ijms26178312
Barkleit A, Eum J, Walther D, Butscher D, Friedrich S, Müller K, Kretzschmar J. In Vitro Bioaccessibility and Speciation of Toxic and Nutritional Trace Elements in Brazil Nuts. International Journal of Molecular Sciences. 2025; 26(17):8312. https://doi.org/10.3390/ijms26178312
Chicago/Turabian StyleBarkleit, Astrid, Jiyoung Eum, Diana Walther, Daniel Butscher, Sebastian Friedrich, Katharina Müller, and Jerome Kretzschmar. 2025. "In Vitro Bioaccessibility and Speciation of Toxic and Nutritional Trace Elements in Brazil Nuts" International Journal of Molecular Sciences 26, no. 17: 8312. https://doi.org/10.3390/ijms26178312
APA StyleBarkleit, A., Eum, J., Walther, D., Butscher, D., Friedrich, S., Müller, K., & Kretzschmar, J. (2025). In Vitro Bioaccessibility and Speciation of Toxic and Nutritional Trace Elements in Brazil Nuts. International Journal of Molecular Sciences, 26(17), 8312. https://doi.org/10.3390/ijms26178312








