Green Synthesized Titanium Oxide Nanoparticles Promote Salt Tolerance in Soybean
Abstract
1. Introduction
2. Results
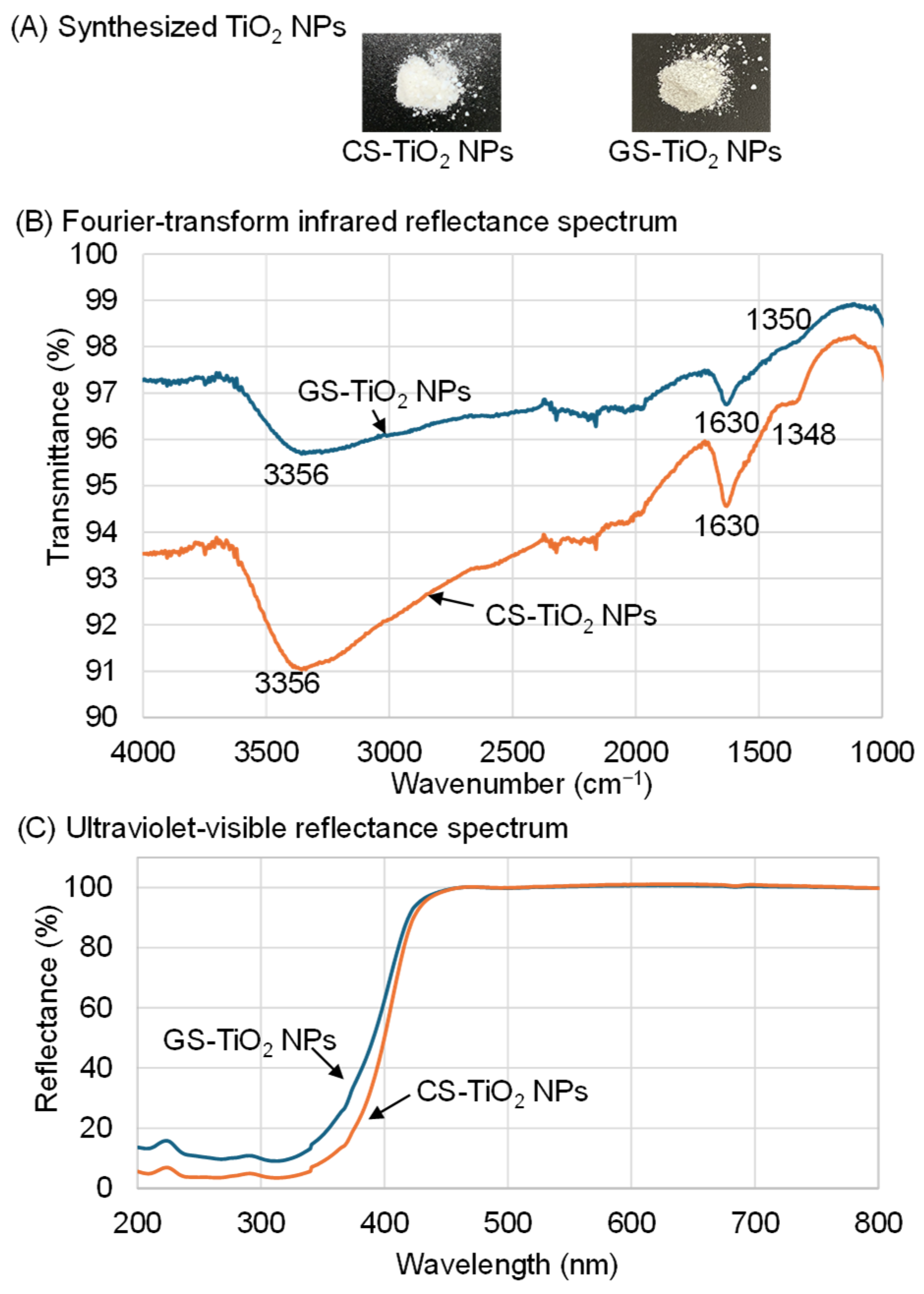
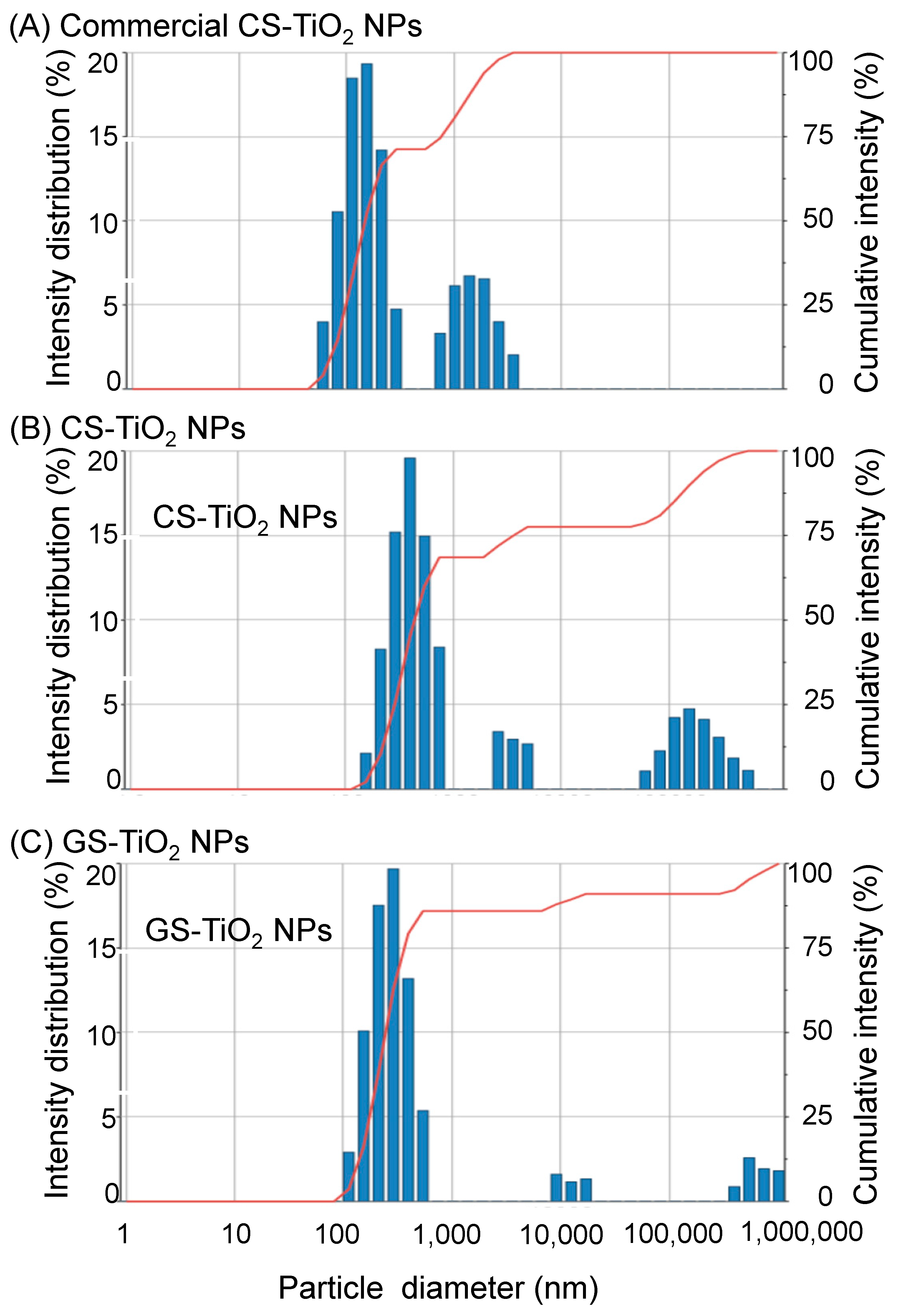
2.1. GS-TiO2 NPs Synthesis and Characterization with FTIR Spectroscopy, Ultraviolet-Visible Reflectance, and Multi-Angle Particle Size Measurement System
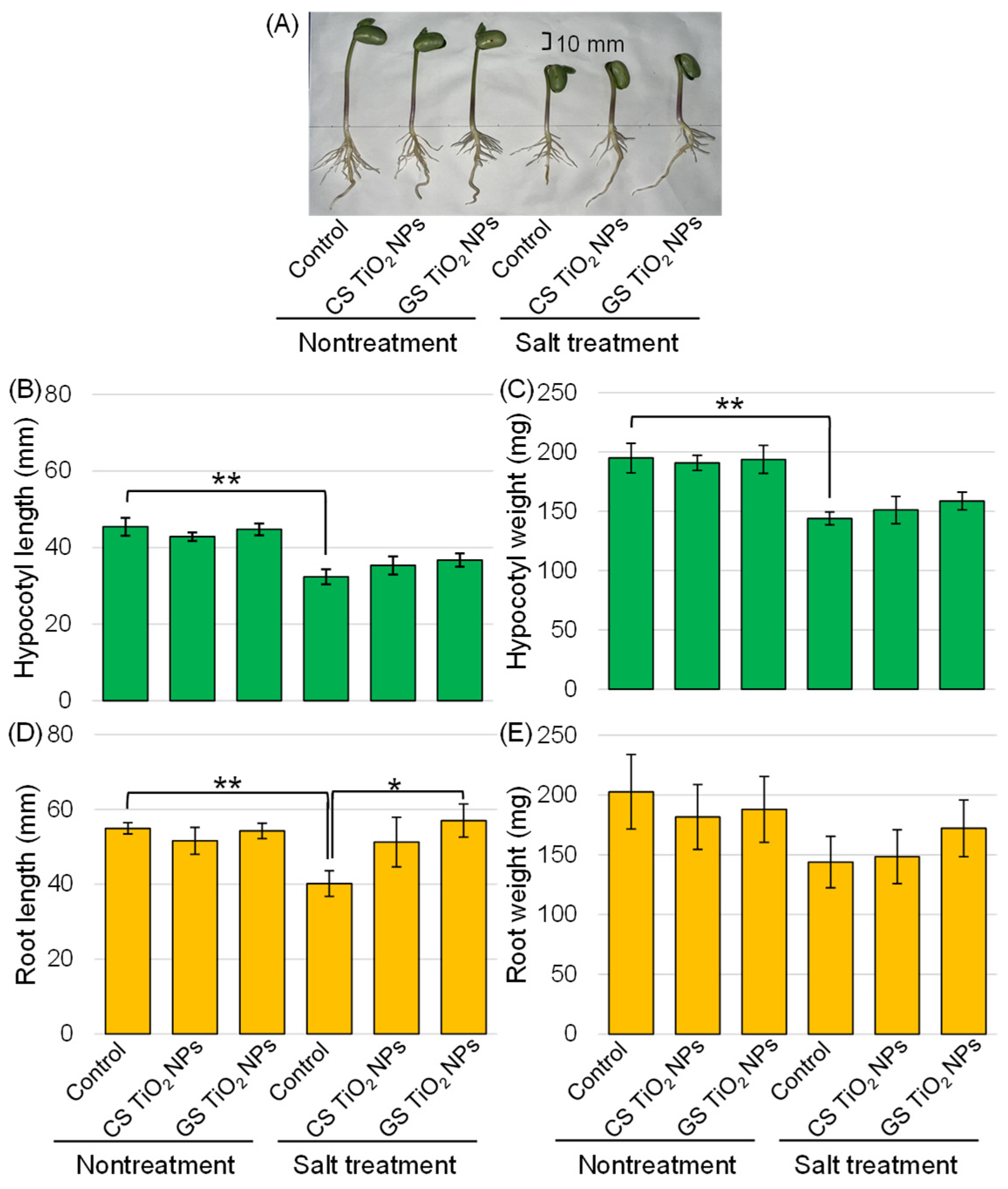
2.2. Morphological Analysis of Soybean Treated with TiO2 NPs Under Salt Stress
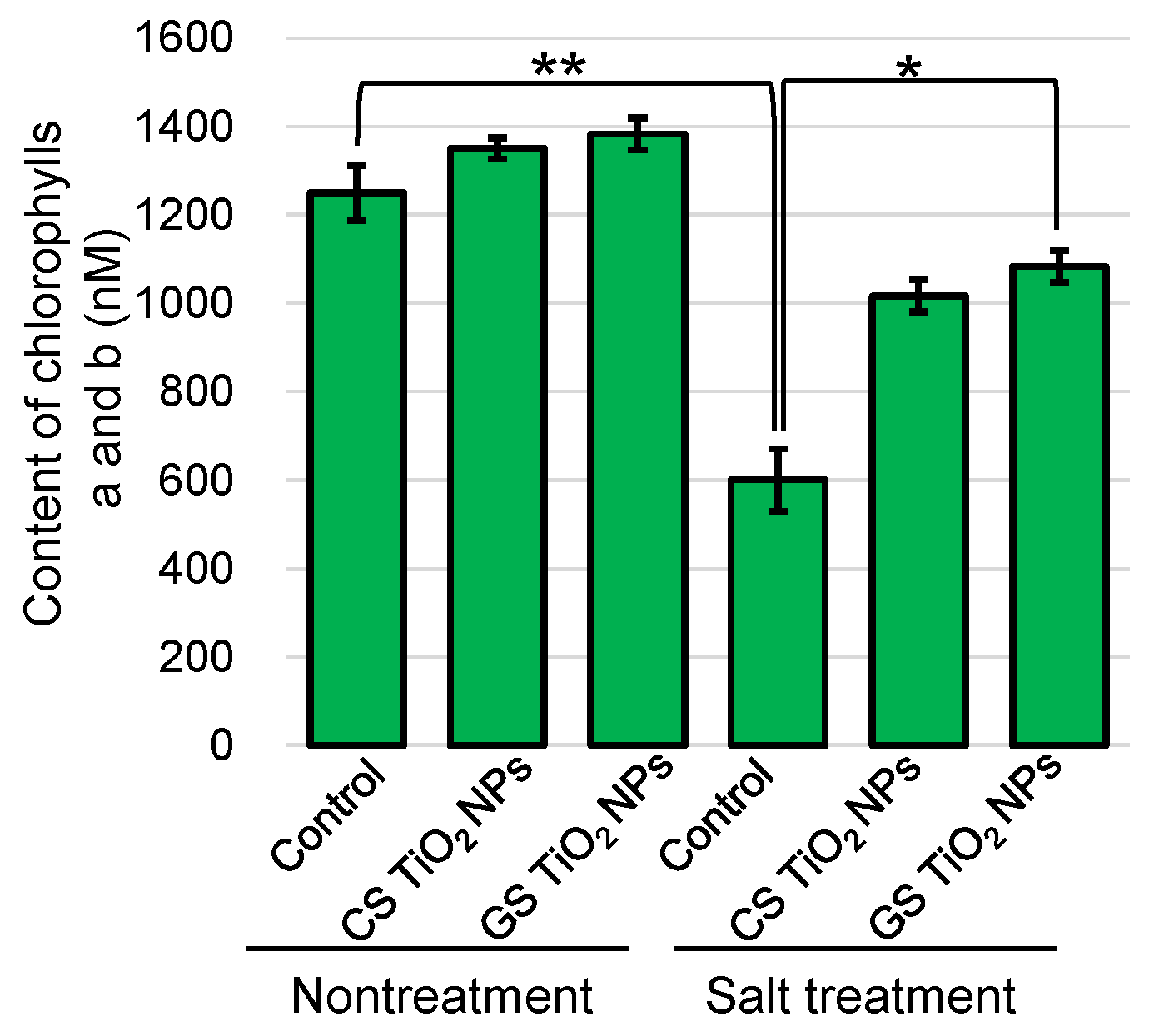
2.3. Analysis of Chlorophylls a and b Contents
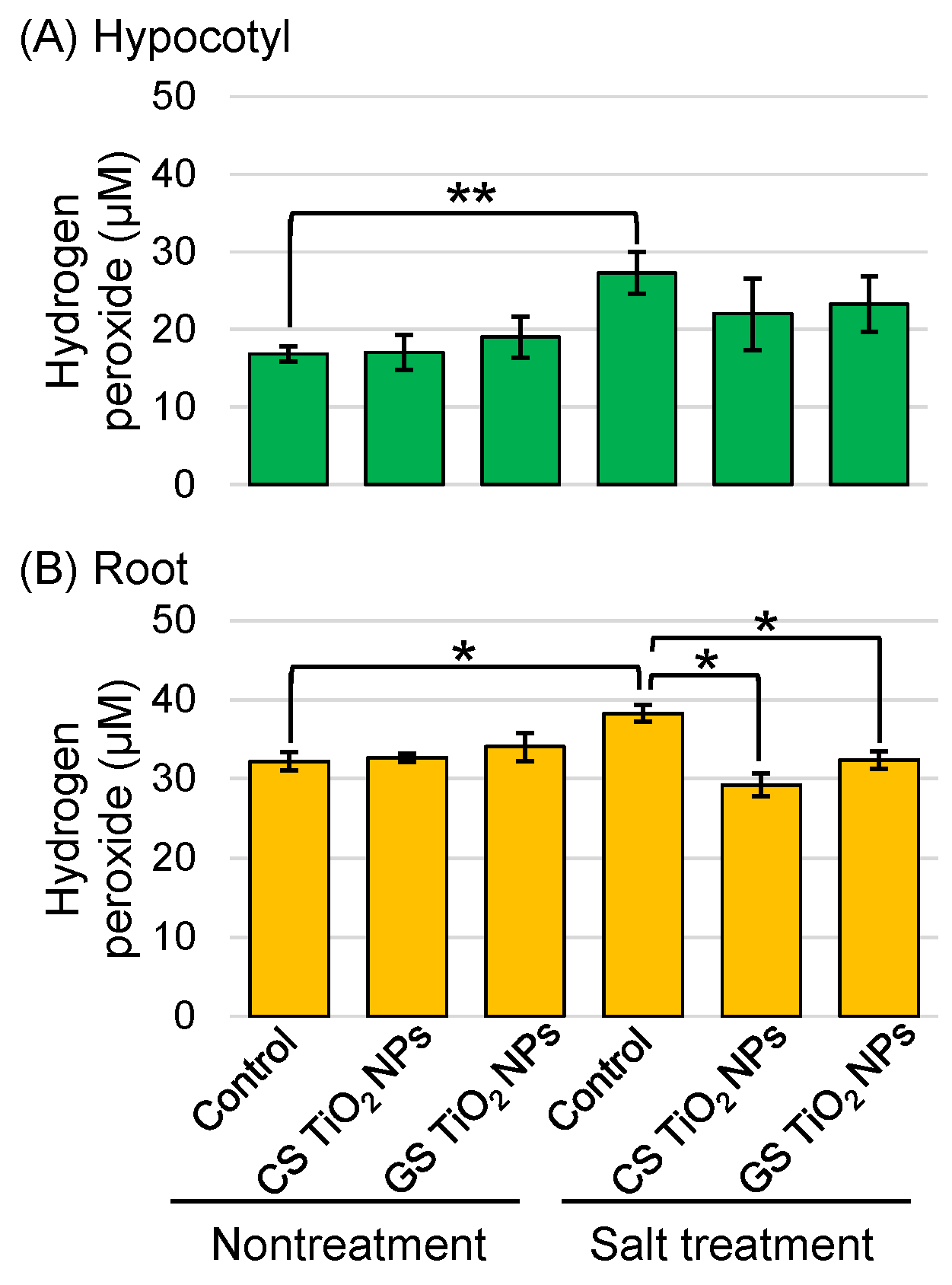
2.4. Analysis of Hydrogen Peroxide Content in Soybean Root Treated with TiO2 NPs Under Salt Stress
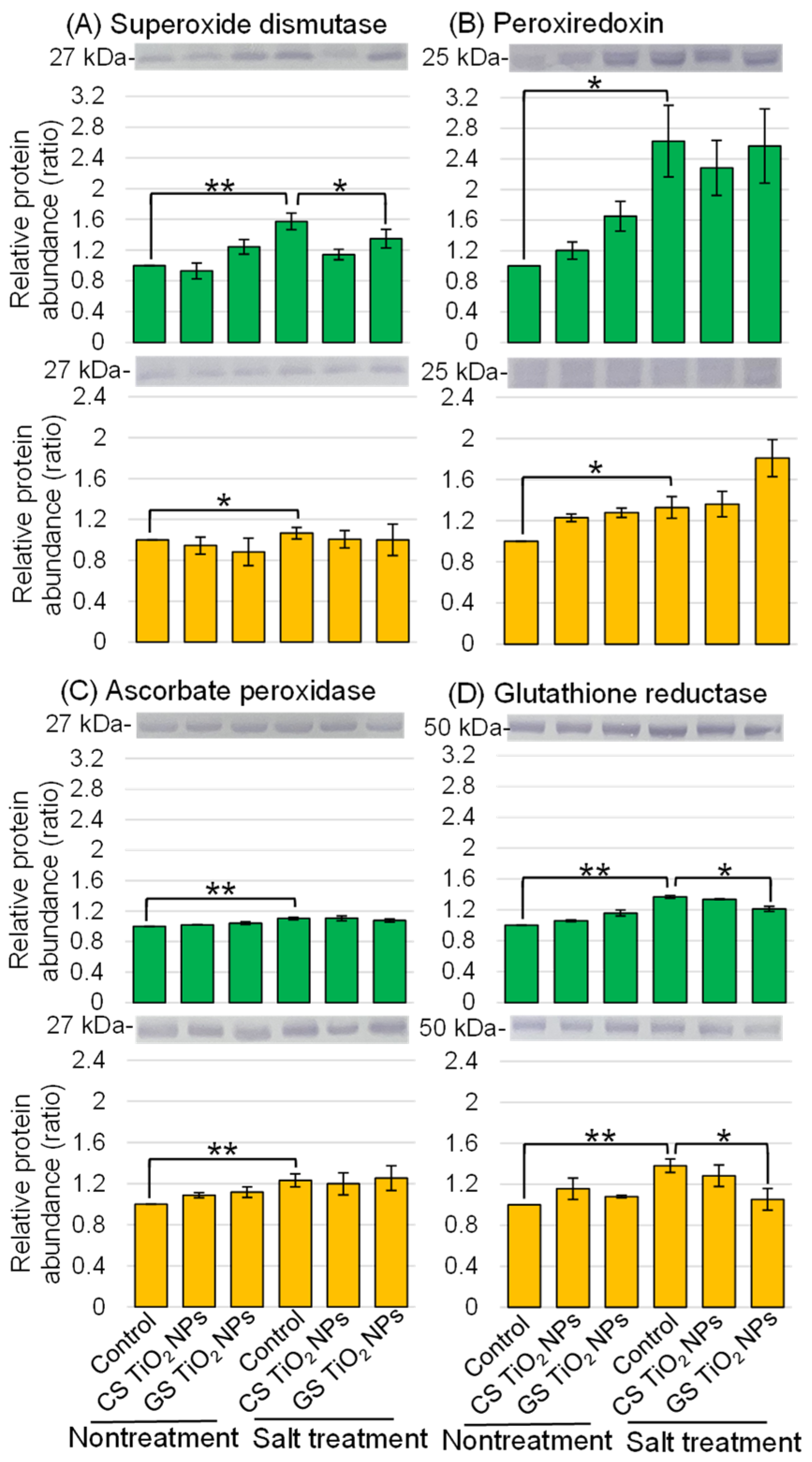
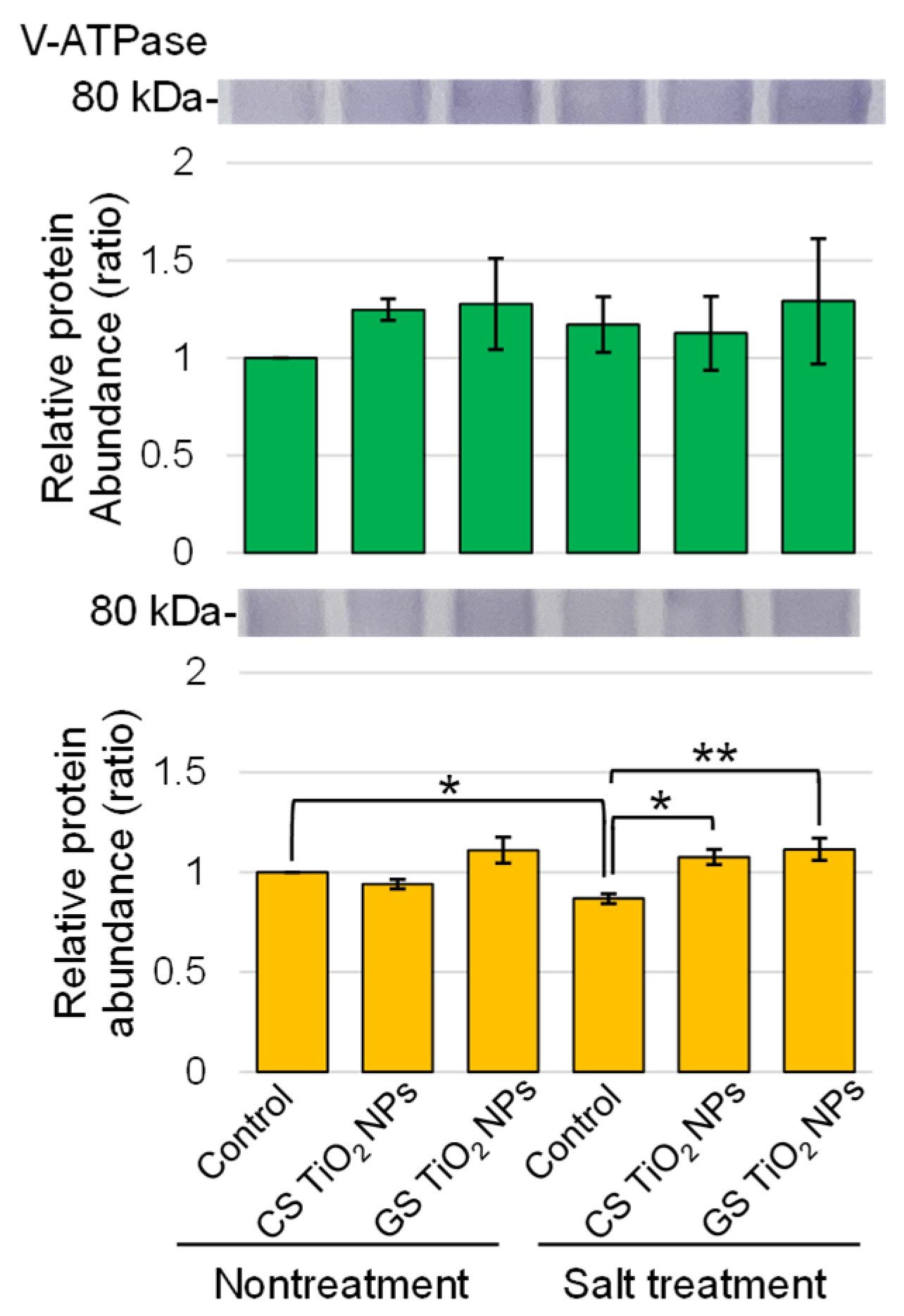
2.5. Immunoblot Analysis of Proteins in Soybean Treated with TiO2 NPs Under Salt Stress
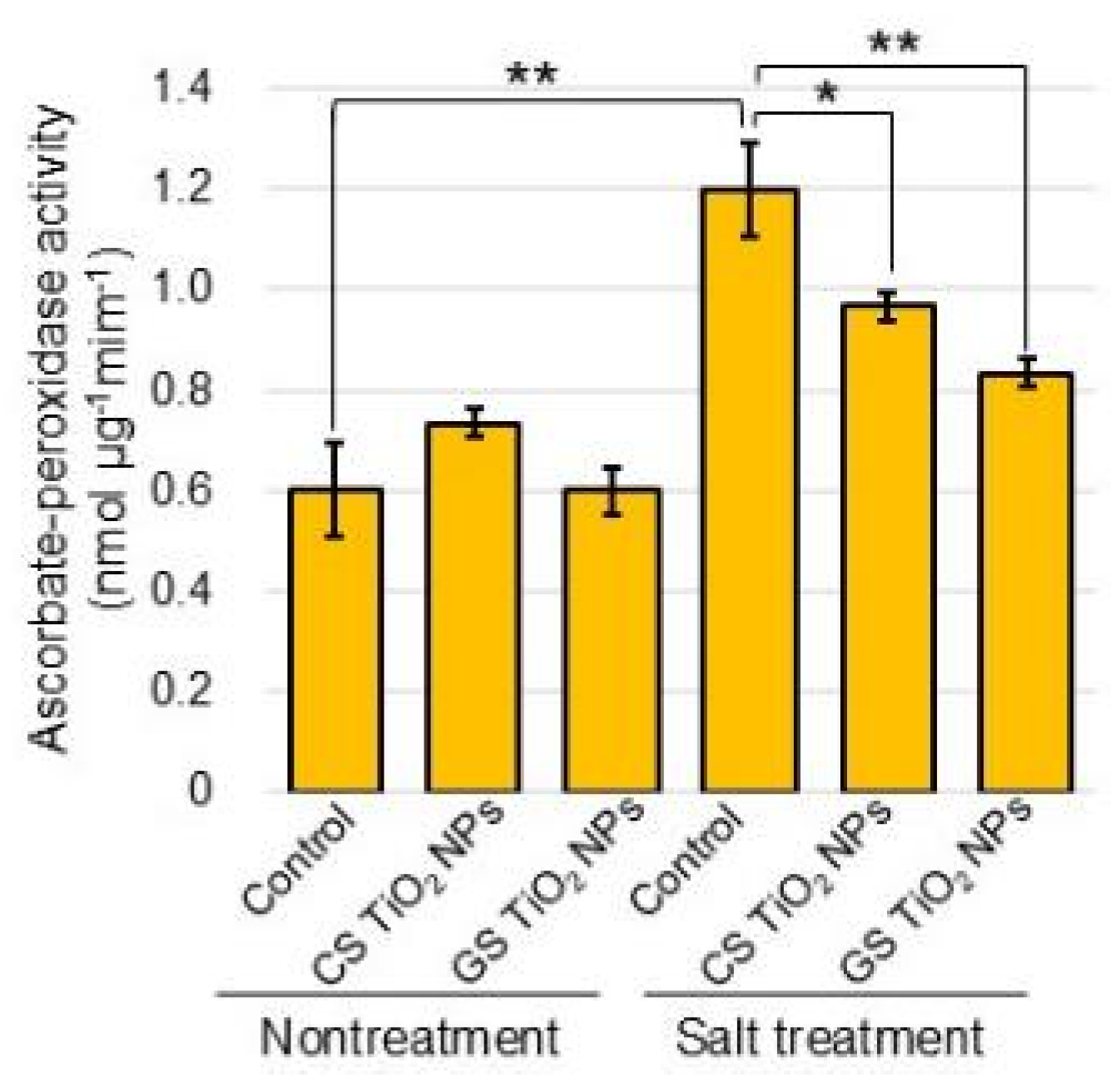
2.6. Analysis of Ascorbate Peroxidase Activity
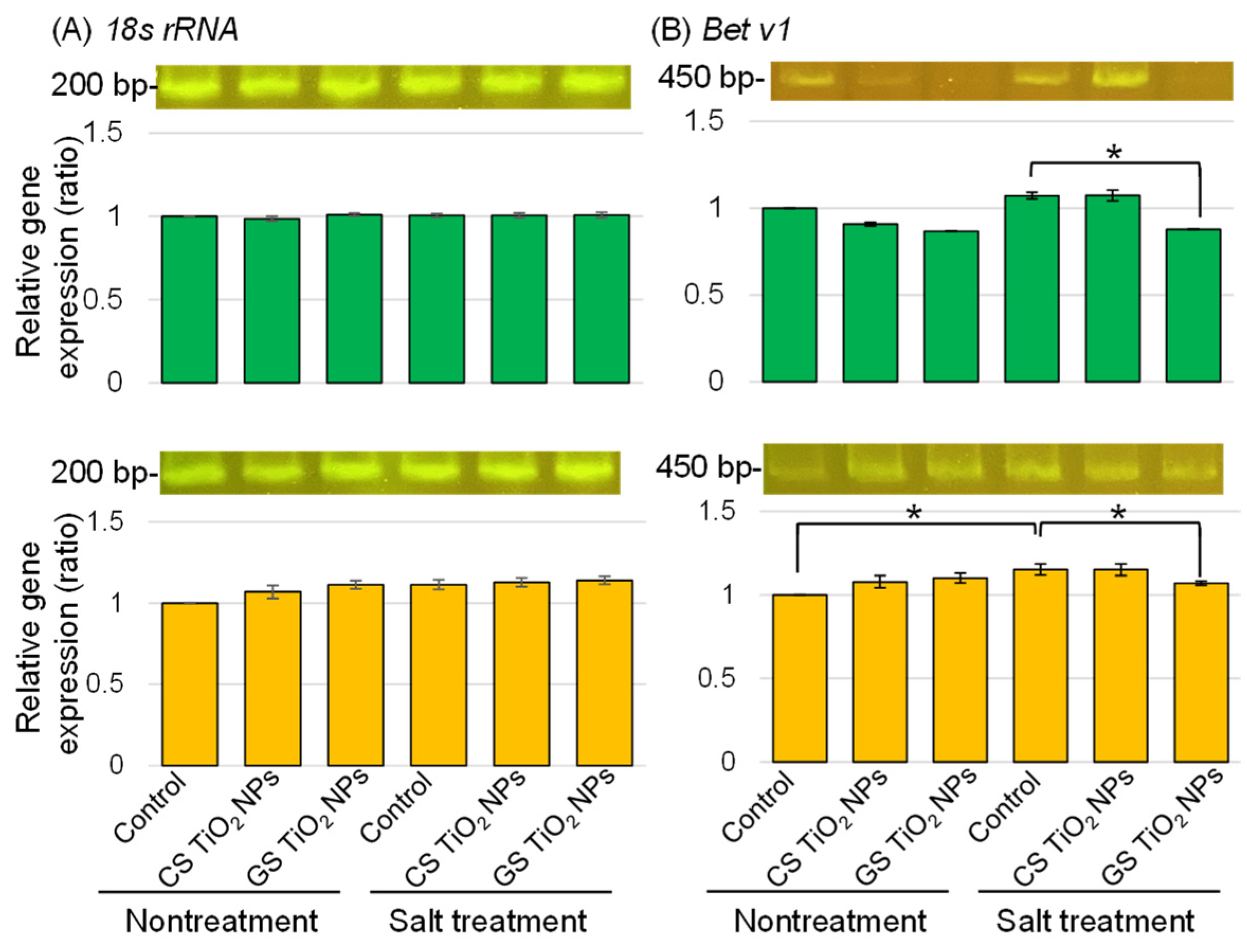
2.7. PCR Analysis of the Gene Encoding Bet v1 in Soybean with Application of TiO2 NPs Under Salt Stress
3. Discussion
3.1. GS-TiO2 NPs Promote Soybean Root Under Salt Stress
3.2. ROS-Scavenging System Relates to Salt-Tolerant Mechanism in Soybean with GS-TiO2 NPs
3.3. Soybean Tonoplast Functions with Application of GS-TiO2 NPs Under Salt Stress
3.4. Soybean Bet v1 Functions with Application of GS-TiO2 NPs Under Salt Stress
4. Materials and Methods
4.1. Preparation of Orange Peel Extract and Synthesis of TiO2 NPs
4.2. Analyses of TiO2 NPs with FTIR Spectroscopy, Ultraviolet-Visible Spectroscopy, and Multi-Angle Particle Size Measurement System
4.3. Plant Materials and Treatment
4.4. Measurement of Chlorophylls a and b Contents
4.5. Protein Extraction and Immunoblot Analysis
4.6. Assay of Ascorbate-Peroxidase Activity
4.7. RNA Extraction, cDNA Synthesis, and PCR Analysis
4.8. Hydrogen-Peroxide Content Measurement
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GS | green synthesized |
| CS | chemical synthesized |
| NPs | nanoparticles |
| FTIR | Fourier transform infrared |
| PCR | polymerase chain reaction |
| ROS | reactive oxygen species |
| PR10 | pathogenesis-related 10 |
| MLPs | major latex proteins |
References
- Demeke, E.D.; Benti, N.E.; Terefe, M.G.; Anbessa, T.T.; Mengistu, W.M.; Mekonnen, Y.S. A comprehensive review on nano-fertilizers: Preparation, development, utilization, and prospects for sustainable agriculture in Ethiopia. Nanoscale Adv. 2025, 7, 2131–2144. [Google Scholar] [CrossRef]
- Díaz-Parra, D.G.; García-Casillas, L.A.; Velasco-Ramírez, S.F.; Guevara-Martínez, S.J.; Zamudio-Ojeda, A.; Zuñiga-Mayo, V.M.; Rodríguez-Guzmán, E.; Melchor-González, A.; Lomelí-Rosales, D.A.; Velázquez-Juárez, G. Role of metal-based nanoparticles in Capsicum spp. plants. ACS Omega 2025, 10, 10756–10768. [Google Scholar] [CrossRef]
- Garg, D.; Sridhar, K.; Stephen Inbaraj, B.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-biofertilizer formulations for agriculture: A systematic review on recent advances and prospective applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef]
- Yaseen, R.; Ahmed, A.I.S.; Omer, A.M.; Agha, M.K.M.; Emam, T.M. Nano-fertilizers: Bio-fabrication, application and biosafety. Nov. Res. Microbiol. J. 2020, 4, 884–900. [Google Scholar]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.-H.; Chakrabartty, I. Nanofertilizers: A smart and sustainable attribute to modern agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; Omar, S.A.; John, S.V.; Zabochnicka-Swiątek, M.; Kalaji, H.M.; Rastogi, A. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S. Afr. J. Bot. 2020, 130, 90–102. [Google Scholar] [CrossRef]
- Marschner, H.; Romheld, V. Strategies of plants for acquisition of iron. Plant Soil 1994, 165, 261–274. [Google Scholar] [CrossRef]
- Ren, C.; Luo, G.; Li, X.; Yao, A.; Liu, W.; Zhang, L.; Wang, Y.; Li, W.; Han, D. MxFRO4 confers iron and salt tolerance through up-regulating antioxidant capacity associated with the ROS scavenging. J. Plant Physiol. 2023, 285, 154001. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Huang, P.; Shao, B.; Li, W.; Liu, W.; Wang, Y.; Xie, L.; Han, M.; Han, D. Overexpression of MxFRO6, a FRO gene from Malus xiaojinensis, increases iron and salt tolerance in Arabidopsis thaliana. In Vitr. Cell. Dev. Biol.-Plant 2022, 58, 189–199. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a Strawberry R2R3-MYB Transcription Factor, Improved Salt and Cold Stress Tolerance in Transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB transcription factor gene (FvMYB114) increases salt and cold tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, T.; Wang, Y.; Liang, X.; Han, J.; Han, D. MbMYBC1, a M. baccata MYB transcription factor, contribute to cold and drought stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1141446. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.M. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Cai, H.; Guo, M.; Chai, M.; She, Z.; Ye, L.; Cheng, Y.; Wang, B.; Qin, Y. The bZIP transcription factor GmbZIP15 negatively regulates salt- and drought-stress responses in soybean. Int. J. Mol. Sci. 2020, 21, 7778. [Google Scholar] [CrossRef]
- Li, M.; Chen, R.; Jiang, Q.; Sun, X.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.Q.; Vaciaxa, P.; Lo, T.T.M.; Nguyen, N.H.; Pham, N.T.T.; Nguyen, Q.H.; Do, P.T.; Nguyen, L.T.N.; Nguyen, Y.T.H.; Chu, M.H. GmDREB6, a soybean transcription factor, notably affects the transcription of the NtP5CS and NtCLC genes in transgenic tobacco under salt stress conditions. Saudi J. Biol. Sci. 2021, 28, 7175–7181. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Hu, X.; Xu, L.; An, X.; Jin, T.; Ma, R.; Li, Z.; Chen, S.; Du, S.; et al. Comparing the potential of silicon nanoparticles and conventional silicon for salinity stress alleviation in soybean (Glycine max L.): Growth and physiological traits and rhizosphere/endophytic bacterial communities. J. Agric. Food Chem. 2024, 72, 10781–10793. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive impacts of beneficial microbes and Si-Zn nanocomposite on growth and productivity of soybean subjected to water deficit under salt-affected soil conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Hegazy, H.S.; Abdalla, H.; Adarosy, M.H. Efficacy of green synthesized titanium dioxide nanoparticles in attenuation salt stress in Glycine max plants: Modulations in metabolic constituents and cell ultrastructure. BMC Plant Biol. 2025, 25, 221. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, H.; Adarosy, M.H.; Hegazy, H.S.; Abdelhameed, R.E. Potential of green synthesized titanium dioxide nanoparticles for enhancing seedling emergence, vigor and tolerance indices and DPPH free radical scavenging in two varieties of soybean under salinity stress. BMC Plant Biol. 2022, 22, 560. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, M.R.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of (Vicia narbonensis L.) and (Zea mays L.). Nano. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, A.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Srivastava, H.K.; El-Sadek, M.S.; Kordrostami, M.; Tran, L.-S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Mustafa, N.; Raja, N.I.; Ilyas, N.; Abasi, F.; Ahmad, M.S.; Ehsan, M.; Mehak, A.; Badshah, I.; Prócków, J. Exogenous application of green titanium dioxide nanoparticles (TiO2 NPs) to improve the germination, physiochemical, and yield parameters of wheat plants under salinity stress. Molecules 2022, 27, 4884. [Google Scholar] [CrossRef]
- Wai, P.P.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. Morphological and Proteomic Analyses to Reveal Salt-Tolerant Mechanisms in Soybean Seedlings Treated with Titanium-Oxide Nanoparticles. Oxygen 2025, 5, 4. [Google Scholar] [CrossRef]
- Helan, V.; Prince, J.J.; Al-Dhabi, N.A.; Arasu, M.V.; Ayeshamariam, A.; Madhumitha, G.; Roopan, S.M.; Jayachandran, M. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys. 2016, 6, 712–718. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Mobeen Amanulla, A.; Sundaram, R. Green synthesis of TiO2 nanoparticles using orange peel extract for antibacterial, cytotoxicity and humidity sensor applications. Mater. Today Proc. 2019, 8, 323–331. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Sony Michael Mary, M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-u.-R.; Ahmad, M.S. Titanium dioxide nanoparticles elicited agromorphological and physicochemical modifications in wheat plants to control (Bipolaris sorokiniana). PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar] [CrossRef]
- Fardood, S.T.; Ramazani, A.; Joo, S.W. Sol-gel synthesis and characterization of zinc oxide nanoparticles using black tea extract. J. Appl. Chem. Res. 2017, 11, 8–17. [Google Scholar]
- Fardood, S.T.; Forootan, R.; Moradnia, F.; Afshari, Z.; Ramazani, A. Green synthesis, characterization, and photocatalytic activity of cobalt chromite spinel nanoparticles. Mater. Res. Express 2020, 7, 015086. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Altynnikov, A.A.; Savinov, E.N.; Kurkin, E.N. Correlation of TiO2 photocatalytic activity and diffuse reflectance spectra. J. Photochem. Photobiol. A 2001, 144, 193–196. [Google Scholar] [CrossRef]
- Ramasamy, D.; Mukundan, G.; Ravipati, M.; Badhulika, S. ZrS2 Nanoparticles embedded in chitosan-based hydrogel for the electrochemical detection of antimalarial drug amodiaquine in serum samples. ACS Appl. Bio Mater. 2025, 8, 4707–4718. [Google Scholar] [CrossRef] [PubMed]
- Kweon, D.H.; Baek, J.H.; Park, S.O.; Noh, H.J.; Jeon, J.P.; Lee, J.H.; Shin, T.J.; Kwak, S.K.; Jeon, I.Y.; Baek, J.B. Platinum nanoparticles on metalloid antimony functionalized graphitic nanoplatelets for enhanced water electrolysis. Small 2025, 21, e2501408. [Google Scholar] [CrossRef] [PubMed]
- Kausar, R.; Komatsu, S. Proteomic approaches to uncover salt stress response mechanisms in crops. Int. J. Mol. Sci. 2022, 24, 518. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000, 30, 151–155. [Google Scholar] [CrossRef]
- Mohammadi, R.; Reza, M.; Alireza, A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013, 152, 403–410. [Google Scholar] [CrossRef]
- Laware, S.L.; Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 749–760. [Google Scholar]
- Kriegel, A.; Andrés, Z.; Medzihradszky, A.; Krüger, F.; Scholl, S.; Delang, S.; Patir-Nebioglu, M.G.; Gute, G.; Yang, H.; Murphy, A.S.; et al. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 2015, 27, 3383–3396. [Google Scholar] [CrossRef]
- Wang, C.; Xiang, Y.; Qian, D. Current progress in plant V-ATPase: From biochemical properties to physiological functions. J. Plant Physiol. 2021, 266, 153525. [Google Scholar] [CrossRef]
- Apse, M.P.; Sottosanto, J.B.; Blumwald, E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 2003, 36, 229–239. [Google Scholar] [CrossRef]
- Wolf, S.; Hematy, K.; Hofte, H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Luo, L.; Gu, L.; Li, H.; Zhang, Q.; Ye, Y.; Li, L. Vacuolar H+ -ATPase subunit VAB3 regulates cell growth and ion homeostasis in Arabidopsis. Plant J. 2022, 112, 664–676. [Google Scholar] [CrossRef]
- Qiao, Q.; Huang, Y.; Dong, H.; Xing, C.; Han, C.; Lin, L.; Wang, X.; Su, Z.; Qi, K.; Xie, Z.; et al. The PbbHLH62/PbVHA-B1 module confers salt tolerance through modulating intracellular Na+/K+ homeostasis and reactive oxygen species removal in pear. Plant Physiol. Biochem. 2024, 210, 108663. [Google Scholar] [CrossRef]
- Noori, A.; Bharath, L.P.; White, J.C. Type-specific impacts of silver on the protein profile of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediation 2022, 24, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Lackner, P.; Breiteneder, H. The Bet v 1 fold: An ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Biol. 2008, 8, 286. [Google Scholar] [CrossRef]
- Yerlikaya, B.A.; Yerlikaya, S.; Aydin, A.; Yilmaz, N.N.; Bahadır, S.; Abdulla, M.F.; Mostafa, K.; Kavas, M. Enhanced drought and salt stress tolerance in Arabidopsis via ectopic expression of the PvMLP19 gene. Plant Cell Rep. 2025, 44, 130. [Google Scholar] [CrossRef]
- Wang, T.; Xie, M.; Hou, S.; Ma, J.; Lin, Y.; Chen, S.; Li, D.; Yang, G. Walnut pr10/bet v1-like proteins interact with chitinase in response to anthracnose stress. J. Evol. Biol. 2025, 38, 391–403. [Google Scholar] [CrossRef]
- Fujita, K.; Inui, H. Review: Biological functions of major latex-like proteins in plants. Plant Sci. 2021, 306, 110856. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Dai, X.-F. Cloning and characterization of the Gossypium hirsutum major latex protein gene and functional analysis in Arabidopsis thaliana. Planta 2010, 231, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Liang, S.; Wang, H.Y.; Han, L.B.; Wang, F.X.; Cheng, H.Q.; Wu, X.M.; Qu, Z.L.; Wu, J.H.; Xia, G.X. Cotton major latex protein 28 functions as a positive regulator of the ethylene responsive factor 6 in defense against Verticillium dahliae. Mol. Plant 2015, 8, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Mao, X.; Li, A.; Jing, R. Wheat transcription factor TaAREB3 participates in drought and freezing tolerances in Arabidopsis. Int. J. Biol. Sci. 2016, 12, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Chen, X.; Ye, T.; Zhong, B.; Liu, R.; Wu, Y.; Chan, Z. Major latex protein-like protein 43 (MLP43) functions as a positive regulator during abscisic acid responses and confers drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 421–434. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamamoto, A.; Nakamura, T.; Nouri, M.Z.; Nanjo, Y.; Nishizawa, K.; Furukawa, K. Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under fooding stress using proteomics and metabolomics techniques. J. Proteome Res. 2011, 10, 3993–4004. [Google Scholar] [CrossRef]
- Yin, X.; Komatsu, S. Nuclear proteomics reveals the role of protein synthesis and chromatin structure in root tip of soybean during the initial stage of fooding stress. J. Proteome Res. 2016, 15, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, S.; Wai, P.P.; Takeshita, T.; Shiraishi, Y. Green Synthesized Titanium Oxide Nanoparticles Promote Salt Tolerance in Soybean. Int. J. Mol. Sci. 2025, 26, 8309. https://doi.org/10.3390/ijms26178309
Komatsu S, Wai PP, Takeshita T, Shiraishi Y. Green Synthesized Titanium Oxide Nanoparticles Promote Salt Tolerance in Soybean. International Journal of Molecular Sciences. 2025; 26(17):8309. https://doi.org/10.3390/ijms26178309
Chicago/Turabian StyleKomatsu, Setsuko, Pwint Phoo Wai, Tatsuya Takeshita, and Yuta Shiraishi. 2025. "Green Synthesized Titanium Oxide Nanoparticles Promote Salt Tolerance in Soybean" International Journal of Molecular Sciences 26, no. 17: 8309. https://doi.org/10.3390/ijms26178309
APA StyleKomatsu, S., Wai, P. P., Takeshita, T., & Shiraishi, Y. (2025). Green Synthesized Titanium Oxide Nanoparticles Promote Salt Tolerance in Soybean. International Journal of Molecular Sciences, 26(17), 8309. https://doi.org/10.3390/ijms26178309





