Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity
Abstract
1. Introduction
2. Results
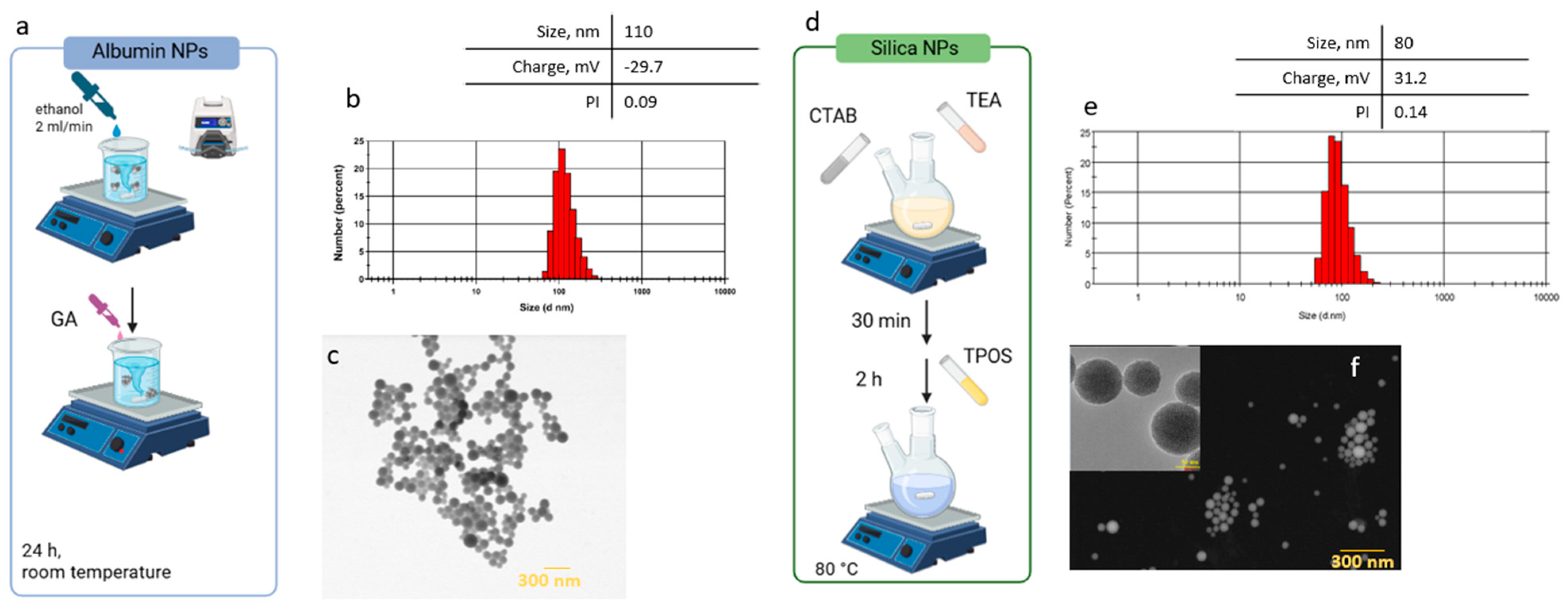
2.1. Synthesis and Characterization of Nanocarriers (Albumin and Silica NPs)
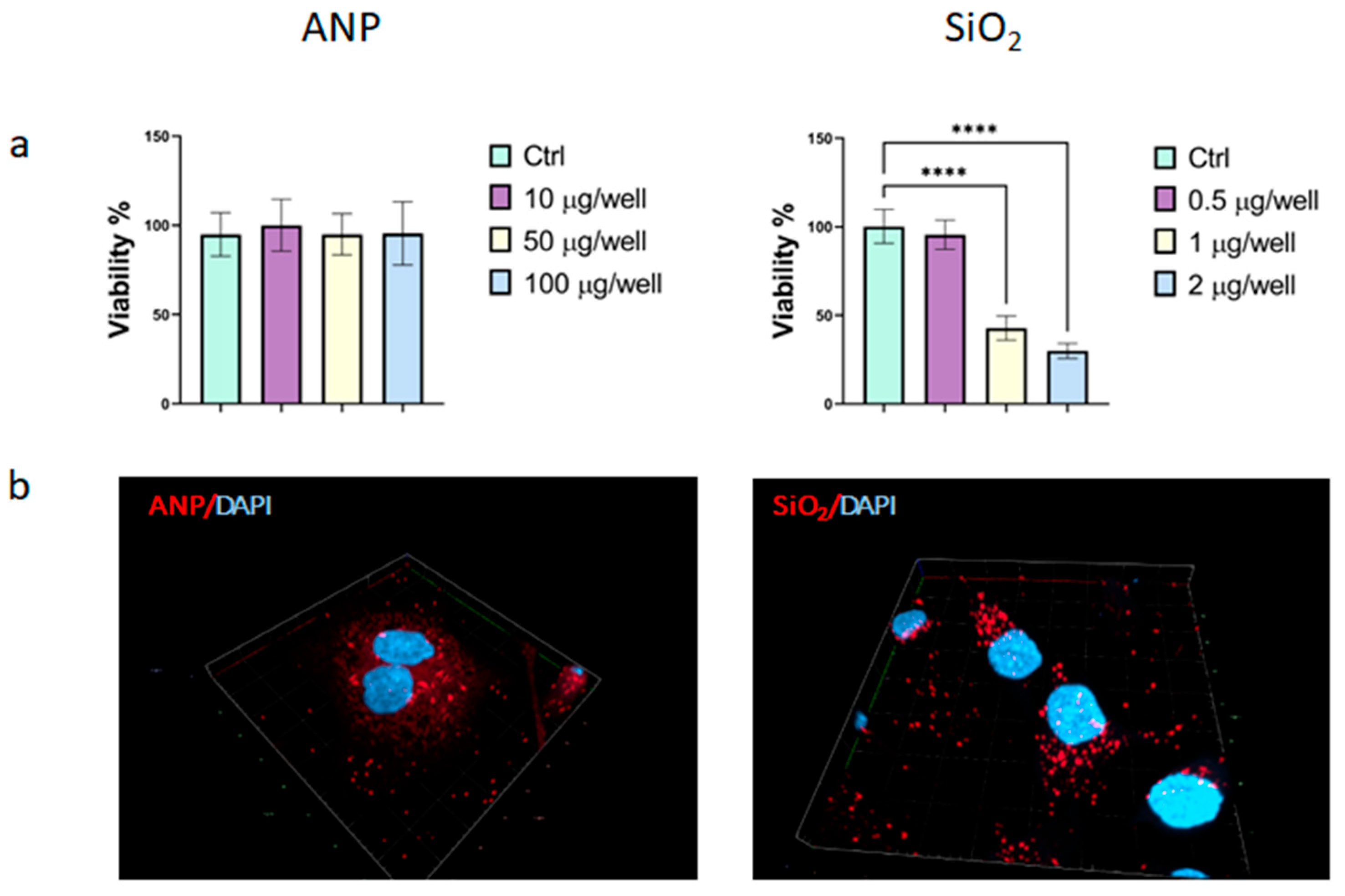
2.2. Efficiency of Cellular Internalization
2.3. Effect on Lysosomes
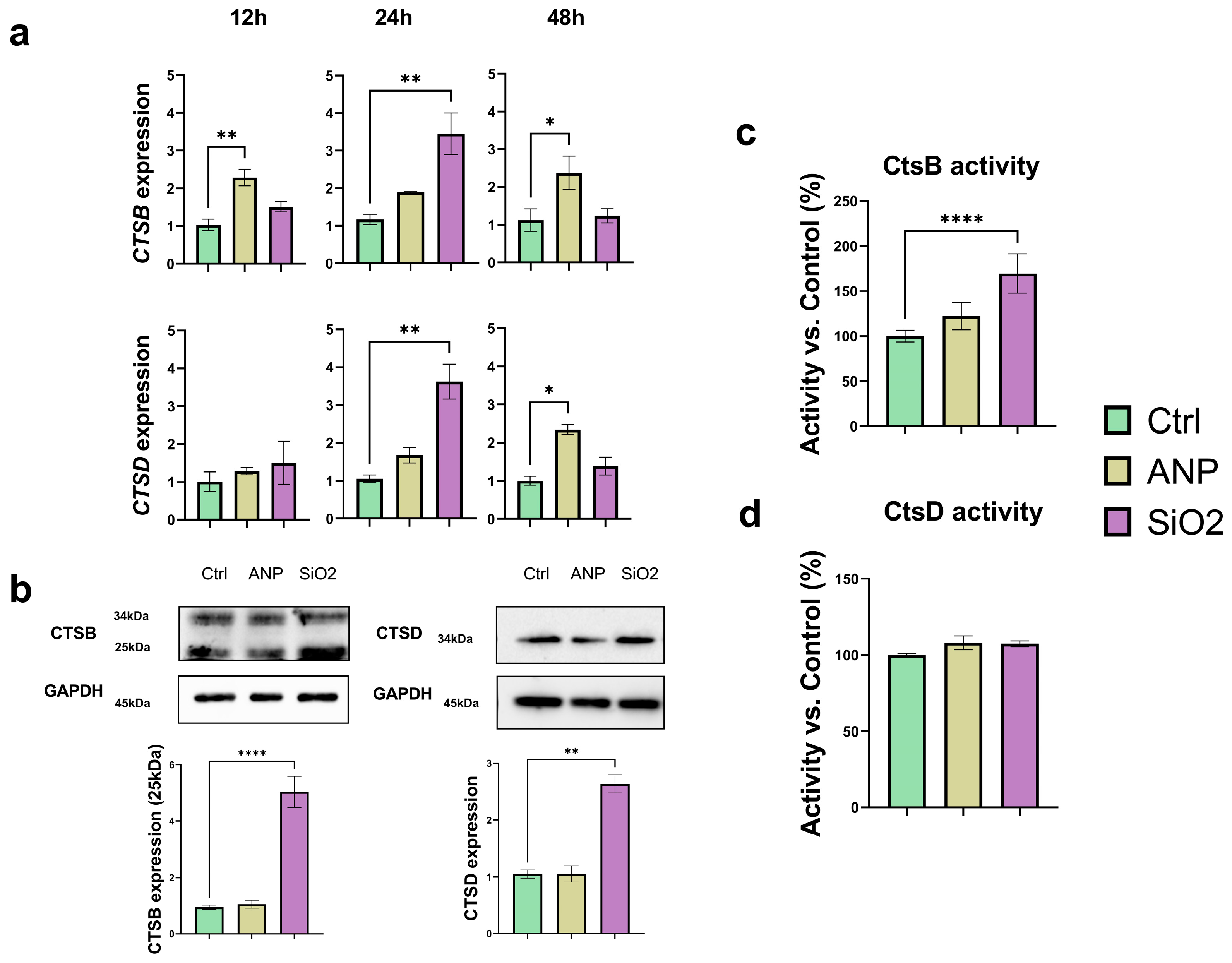
2.4. Effect on Catepsins (CtsB and CtsD) Expression and Activity
2.5. Effect on Cathepsins Localization
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Nanoparticles Synthesis
4.3. Nanoparticles Characterization
4.4. Nanoparticles Toxicity
4.5. Nanopartciles Localization and Internalization
4.6. Analysis of Catepsins’ Expression and Activity
4.6.1. RNA Isolation Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6.2. Western Blotting
4.6.3. Cathepsins Activity Assay
4.7. Immunostaining of Cathepsins
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahajan, K.; Bhattacharya, S. The advancement and obstacles in improving the stability of nanocarriers for precision drug delivery in the field of nanomedicine. Curr. Top. Med. Chem. 2024, 24, 686–721. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Ma, X. Transforming Healthcare with Nanomedicine: A SWOT Analysis of Drug Delivery Innovation. Drug Des. Dev. Ther. 2024, 18, 3499–3521. [Google Scholar] [CrossRef]
- Jadhav, V.; Roy, A.; Kaur, K.; Roy, A.; Sharma, K.; Verma, R.; Rustagi, S.; Malik, S. Current advancements in functional nanomaterials for drug delivery systems. Nano-Struct. Nano-Objects 2024, 38, 101177. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T. Nanomaterials in drug delivery: Strengths and opportunities in medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Kim, J.; Jo, C.; Lim, W.G.; Jung, S.; Lee, Y.M.; Lim, J.; Lee, H.; Lee, J.; Kim, W.J. Programmed nanoparticle-loaded nanoparticles for deep-penetrating 3D cancer therapy. Adv. Mater. 2018, 30, 1707557. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Lebelo, S.L.; Msagati, T.A. Nanocarrier design–function relationship: The prodigious role of properties in regulating biocompatibility for drug delivery applications. Chem. Biol. Interact. 2023, 377, 110466. [Google Scholar] [CrossRef]
- Jackson, M.P.; Hewitt, E.W. Cellular proteostasis: Degradation of misfolded proteins by lysosomes. Essays Biochem. 2016, 60, 173–180. [Google Scholar] [CrossRef]
- Lamming, D.W.; Bar-Peled, L. Lysosome: The metabolic signaling hub. Traffic 2019, 20, 27–38. [Google Scholar] [CrossRef]
- Voronina, M.V.; Frolova, A.S.; Kolesova, E.P.; Kuldyushev, N.A.; Parodi, A.; Zamyatnin, A.A., Jr. The Intricate Balance between Life and Death: ROS, cathepsins, and their interplay in cell death and autophagy. Int. J. Mol. Sci. 2024, 25, 4087. [Google Scholar] [CrossRef]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The ins and outs of cathepsins: Physiological function and role in disease management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Gill, M.R. Progress in mesoporous silica nanoparticles as drug delivery agents for cancer treatment. Pharmaceutics 2021, 13, 152. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Targeted delivery of albumin nanoparticles for breast cancer: A review. Colloids Surf. B Biointerfaces 2022, 213, 112422. [Google Scholar] [CrossRef]
- Salim, E.I.; Mosbah, A.M.; Elhussiny, F.; Hanafy, N.A.; Abdou, Y. Preparation and characterization of cetuximab-loaded egg serum albumin nanoparticles and their uses as a drug delivery system against Caco-2 colon cancer cells. Cancer Nanotechnol. 2023, 14, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chen, X.; Fang, W.; Yu, K.; Gu, W.; Wei, Y.; Zheng, H.; Piao, J.; Li, F. Strategies to regulate the degradation and clearance of mesoporous silica nanoparticles: A review. Int. J. Nanomed. 2024, 19, 5859–5878. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Fan, Z.; Fang, Y.; Zheng, Y.; Li, Y.; Yang, M.; Guo, C.; Li, Y.; Zhou, X. Silica nanoparticles trigger chaperone HSPB8-assisted selective autophagy via TFEB activation in hepatocytes. Small 2023, 19, 2204310. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Han, Z.; Yin, H.; Tian, J.; Zhang, J.; Li, N.; Ding, C.; Zhang, L. Characterization of cathepsin B in mediating silica nanoparticle-induced macrophage pyroptosis via an NLRP3-dependent manner. J. Inflamm. Res. 2022, 15, 4537–4545. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Li, F.; Yeh, S.; Shi, Q.; Wang, P.; Wu, H.; Xin, J. A novel thermal-driven self-assembly method to prepare albumin nanoparticles: Formation kinetics, degradation behavior and formation mechanism. AAPS PharmSciTech 2022, 23, 250. [Google Scholar] [CrossRef]
- Kolesova, E.P.; Egorova, V.S.; Syrocheva, A.O.; Frolova, A.S.; Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Trushina, D.B.; Fatkhutdinova, L.; Zyuzin, M. Proteolytic resistance determines albumin nanoparticle drug delivery properties and increases cathepsin B, D, and G expression. Int. J. Mol. Sci. 2023, 24, 10245. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hao, S.-L.; Yang, W.-X. Nanoparticles induce autophagy via mTOR pathway inhibition and reactive oxygen species generation. Nanomedicine 2020, 15, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Borm, P.J.; Müller-Schulte, D. Nanoparticles in drug delivery and environmental exposure: Same size, same risks? Nanomedicine 2006, 1, 235–249. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, L.; Guo, C.; Yan, B.; Su, G. Regulating protein corona formation and dynamic protein exchange by controlling nanoparticle hydrophobicity. Front. Bioeng. Biotechnol. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Yang, M.; Wu, E.; Tang, W.; Qian, J.; Zhan, C. Interplay between nanomedicine and protein corona. J. Mater. Chem. B 2021, 9, 6713–6727. [Google Scholar] [CrossRef]
- Breznica, P.; Koliqi, R.; Daka, A. A review of the current understanding of nanoparticles protein corona composition. Med. Pharm. Rep. 2020, 93, 342. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.-L.; Jiang, J.-G.; Calin, N.; Lam, K.-F.; Zhang, S.-J.; Wu, H.-H.; Wu, G.-D.; Albela, B.; Bonneviot, L. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef]
- Costanzo, F.D.; Gasperoni, S.; Rotella, V.; Costanzo, F.D. Targeted delivery of albumin bound paclitaxel in the treatment of advanced breast cancer. Onco. Targets Ther. 2009, 2, 179–188. [Google Scholar] [CrossRef][Green Version]
- Kokot, A.; Gadakh, S.; Saha, I.; Gajda, E.; Łaźniewski, M.; Rakshit, S.; Sengupta, K.; Mollah, A.F.; Denkiewicz, M.; Górczak, K. Unveiling the molecular mechanism of trastuzumab resistance in SKBR3 and BT474 cell lines for HER2 positive breast cancer. Curr. Issues Mol. Biol. 2024, 46, 2713–2740. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, Z.; Xie, J.; Yang, Q.; Mo, M.; Liu, J.; Chen, L. The Genetic and Epigenetic Toxicity of Silica Nanoparticles: An Updated Review. Int. J. Nanomed. 2024, 19, 13901–13923. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Jing, L.; Ma, R.; Liu, X.; Gao, L.; Cao, L.; Duan, J.; Zhou, X.; Li, Y. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ. Pollut. 2018, 236, 926–936. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Chen, C.; Liang, Z. Understanding of endo/lysosomal escape of nanomaterials in biomedical application. Smart Mol. 2025, e20240017. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar]
- Wang, J.; Liu, G. Imaging nano–bio interactions in the kidney: Toward a better understanding of nanoparticle clearance. Nanomater. Neoplasms 2021, 57, 579–586. [Google Scholar]
- Belyaev, I.B.; Mirkasymov, A.B.; Rodionov, V.I.; Babkova, J.S.; Nikitin, P.I.; Deyev, S.M.; Zelepukin, I.V. MPS blockade with liposomes controls pharmacokinetics of nanoparticles in a size-dependent manner. Biomed. Mater. 2024, 19, 065022. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, F.; Zhang, J.; Shi, Q.; Meng, Y.; Wang, C.; Pang, H.; Gu, L.; Xu, C.; Guo, Q. Advanced strategies for overcoming endosomal/lysosomal barrier in nanodrug delivery. Research 2023, 6, 0148. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Brule, S.V.D.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Burgers, T.C.; Vlijm, R.; Roos, W.H.; Åberg, C. Rapid internalization of nanoparticles by human cells at the single particle level. ACS Nano 2023, 17, 16517–16529. [Google Scholar] [CrossRef]
- Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Patil, S.; Gewirtz, D.A.; Bhutia, S.K. The lysosome as an imperative regulator of autophagy and cell death. Cell Mol. Life Sci. 2021, 78, 7435–7449. [Google Scholar] [CrossRef]
- Maysinger, D.; Gran, E.R.; Bertorelle, F.; Fakhouri, H.; Antoine, R.; Kaul, E.S.; Samhadaneh, D.M.; Stochaj, U. Gold nanoclusters elicit homeostatic perturbations in glioblastoma cells and adaptive changes of lysosomes. Theranostics 2020, 10, 1633–1648. [Google Scholar] [CrossRef]
- Parodi, A.; Evangelopoulos, M.; Arrighetti, N.; Cevenini, A.; Livingston, M.; Khaled, S.Z.; Brown, B.S.; Yazdi, I.K.; Paradiso, F.; Campa-Carranza, J.N. Endosomal escape of polymer-coated silica nanoparticles in endothelial cells. Small 2020, 16, 1907693. [Google Scholar] [CrossRef]
- Guo, C.; Xia, Y.; Niu, P.; Jiang, L.; Duan, J.; Yu, Y.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling. Int. J. Nanomed. 2015, 10, 1463–1477. [Google Scholar] [CrossRef]
- Schütz, I.; Lopez-Hernandez, T.; Gao, Q.; Puchkov, D.; Jabs, S.; Nordmeyer, D.; Schmudde, M.; Rühl, E.; Graf, C.M.; Haucke, V. Lysosomal dysfunction caused by cellular accumulation of silica nanoparticles. J. Biol. Chem. 2016, 291, 14170–14184. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.-J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, T.; Zhang, S.; Li, Y.; Chen, Y.; Miao, Z.; Wang, X.; Yang, G.; Li, Q.; Zhang, L. Cathepsin B: The dawn of tumor therapy. Eur. J. Med. Chem. 2024, 269, 116329. [Google Scholar] [CrossRef] [PubMed]
- Mijanovic, O.; Petushkova, A.I.; Brankovic, A.; Turk, B.; Solovieva, A.B.; Nikitkina, A.I.; Bolevich, S.; Timashev, P.S.; Parodi, A.; Zamyatnin, A.A., Jr. Cathepsin D—Managing the delicate balance. Pharmaceutics 2021, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Egorova, V.S.; Kolesova, E.P.; Lopus, M.; Yan, N.; Parodi, A.; Zamyatnin, A.A., Jr. Smart delivery systems responsive to cathepsin B activity for cancer treatment. Pharmaceutics 2023, 15, 1848. [Google Scholar] [CrossRef]
- Langer, K.; Anhorn, M.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef]
- Wang, D.-P.; Wang, Z.-J.; Zhao, R.; Lin, C.-X.; Sun, Q.-Y.; Yan, C.-P.; Zhou, X.; Cao, J.-M. Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part. Fibre Toxicol. 2020, 17, 12. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Lu, K.; Yang, M.; Li, Y.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int. J. Nanomed. 2017, 12, 809–825. [Google Scholar] [CrossRef]
- Woods, A.; Andrian, T.; Sharp, G.; Bicer, E.M.; Vandera, K.-K.A.; Patel, A.; Mudway, I.; Dailey, L.A.; Forbes, B. Development of new in vitro models of lung protease activity for investigating stability of inhaled biological therapies and drug delivery systems. Eur. J. Pharm. Biopharm. 2020, 146, 64–72. [Google Scholar] [CrossRef]
- Rhodes, J.; Andersen, Å.B. Role of cathepsin D in the degradation of human serum albumin by peritoneal macrophages and veiled cells in antigen presentation. Immunol. Lett. 1993, 37, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, M.; Zhang, Y.; Li, Z.; Li, X.; Liu, Z.; Liu, H.; Li, X. Relationship between lysosomal dyshomeostasis and progression of diabetic kidney disease. Cell Death Dis. 2021, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Bien, S.; Ritter, C.A.; Gratz, M.; Sperker, B.; Sonnemann, J.; Beck, J.F.; Kroemer, H.K. Nuclear factor-κB mediates up-regulation of cathepsin B by doxorubicin in tumor cells. Mol. Pharmacol. 2004, 65, 1092–1102. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J.H. Nanoparticle-mediated therapeutic application for modulation of lysosomal ion channels and functions. Pharmaceutics 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shi, A.; Yin, H.; Wang, Y.; Liu, Q.; Chen, W.; Wu, J. Nanomaterials respond to lysosomal function for tumor treatment. Cells 2022, 11, 3348. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrocheva, A.O.; Gorbacheva, V.I.; Egorova, V.S.; Zamyatnin, A.A., Jr.; Parodi, A.; Kolesova, E.P. Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity. Int. J. Mol. Sci. 2025, 26, 8291. https://doi.org/10.3390/ijms26178291
Syrocheva AO, Gorbacheva VI, Egorova VS, Zamyatnin AA Jr., Parodi A, Kolesova EP. Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity. International Journal of Molecular Sciences. 2025; 26(17):8291. https://doi.org/10.3390/ijms26178291
Chicago/Turabian StyleSyrocheva, Anastasiia O., Valentina I. Gorbacheva, Vera S. Egorova, Andrey A. Zamyatnin, Jr., Alessandro Parodi, and Ekaterina P. Kolesova. 2025. "Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity" International Journal of Molecular Sciences 26, no. 17: 8291. https://doi.org/10.3390/ijms26178291
APA StyleSyrocheva, A. O., Gorbacheva, V. I., Egorova, V. S., Zamyatnin, A. A., Jr., Parodi, A., & Kolesova, E. P. (2025). Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity. International Journal of Molecular Sciences, 26(17), 8291. https://doi.org/10.3390/ijms26178291







