A Review of FDG-PET in Progressive Supranuclear Palsy and Corticobasal Syndrome
Abstract
1. Introduction
2. Results
2.1. FDG-PET in Progressive Supranuclear Palsy
2.2. FDG-PET in Corticobasal Syndrome
2.3. FDG-PET in Atypical Parkinsonian Disorders
2.4. FDG-PET in Progressive Supranuclear Palsy and/or Corticobasal Syndrome Compared to Frontotemporal Dementia, Alzheimer’s Disease, and/or Neurodegenerative Diseases
3. Discussion
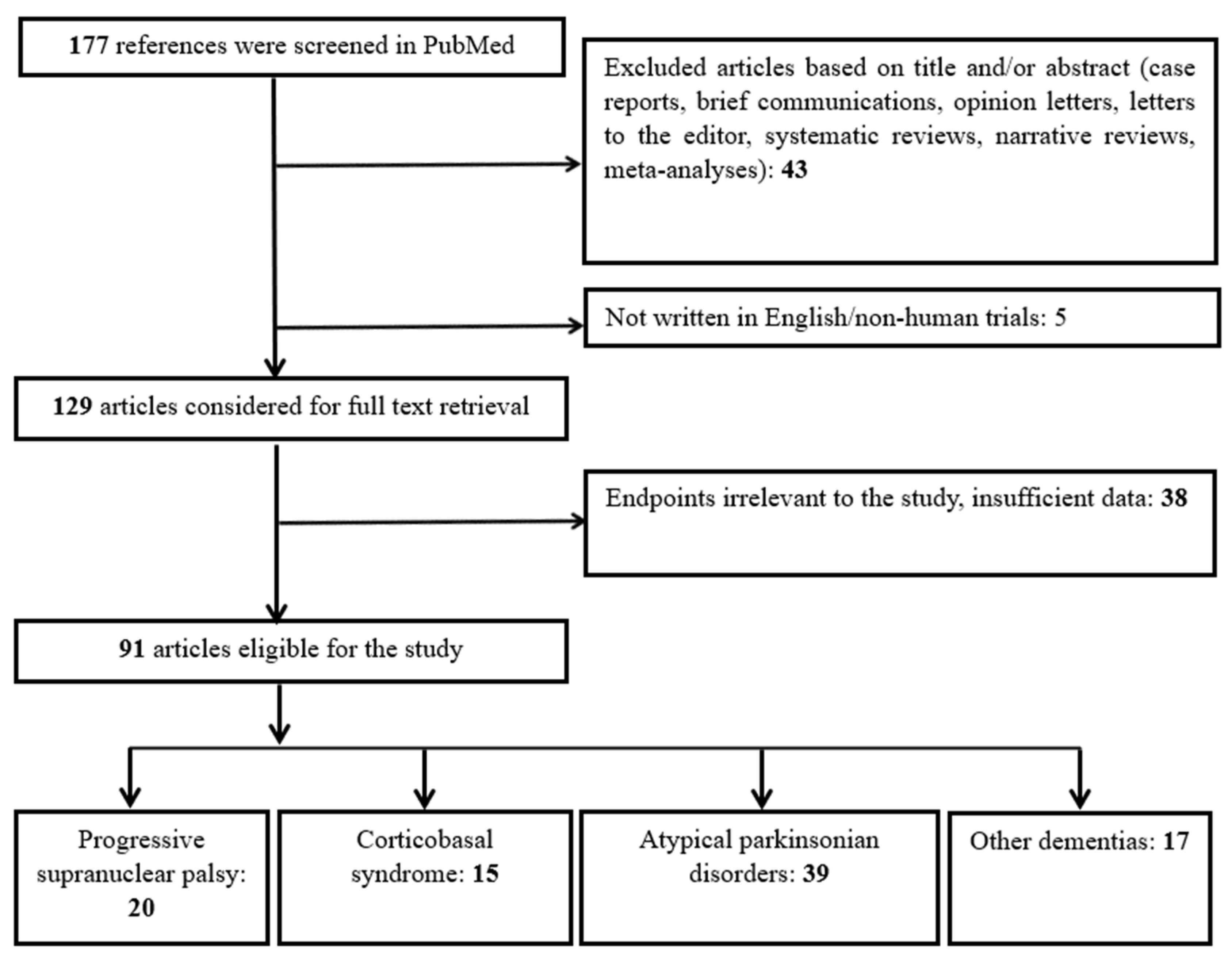
4. Materials and Methods
4.1. Search Strategy
4.2. Study Selection and Categorization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4R | four-repeat |
| AD | Alzheimer’s disease |
| APD | atypical Parkinsonian disorder |
| AUC | area under the curve |
| bvFTD | behavioral variant frontotemporal dementia |
| CBD | corticobasal degeneration |
| CBS | corticobasal syndrome |
| CSF | cerebrospinal fluid |
| DaT-SPECT | dopamine transporter imaging with single-photon emission computed tomography |
| DLB | dementia with Lewy bodies |
| FAB | Frontal Assessment Battery |
| FDG-PET | fludeoxyglucose-18–positron emission tomography |
| FTD | frontotemporal dementia |
| GGT | globular glial tauopathy |
| IBZM | 123I-iodobenzamide |
| lvPPA | logopenic variant PPA |
| MCI | mild cognitive impairment |
| MDS | Movement Disorders Society |
| MDS-UPDRS | MDS-Unified Parkinson’s Disease Rating Scale |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MRI | magnetic resonance imaging |
| MSA | multiple system atrophy |
| MSA-P | multiple system atrophy-parkinsonian type |
| nfvPPA | nonfluent variant PPA |
| PAGF | pure akinesia with gait freezing |
| PCA | posterior cortical atrophy |
| PD | Parkinson’s disease |
| PDD | Parkinson’s disease dementia |
| PET | Positron emission tomography |
| PPA | primary progressive aphasia |
| PPAOS | primary progressive apraxia of speech |
| PSP | progressive supranuclear palsy |
| PSP-CBS | PSP with predominant corticobasal syndrome |
| PSP-F | PSP with predominant frontal presentation |
| PSP-OM | PSP with predominant ocular motor dysfunction |
| PSP-P | PSP with predominant Parkinsonism |
| PSP-PGF | PSP with progressive gait freezing |
| PSP-PI | PSP with postural instability |
| PSP-PLS | PSP–primary lateral sclerosis |
| PSP-SL | PSP with predominant speech/language impairment |
| PSP-RS | PSP–Richardson syndrome |
| ROI | region of interest |
| SIS | Saccadic Impairment Scale |
| SPECT | single-photon emission computed tomography |
| svPPA | semantic variant PPA |
References
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the Diagnosis of Corticobasal Degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, A.; Sioka, C.; Kloufetou, E.; Konitsiotis, S. Cognitive Impairment in Parkinson’s Disease and Other Parkinsonian Syndromes. J. Neural Transm. 2025, 132, 341–355. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S. A New Paradigm for Neurodegenerative Diseases Classification: A Clinical Perspective. J. Clin. Neurosci. 2025, 134, 111099. [Google Scholar] [CrossRef]
- Shir, D.; Pham, N.T.T.; Botha, H.; Koga, S.; Kouri, N.; Ali, F.; Knopman, D.S.; Petersen, R.C.; Boeve, B.F.; Kremers, W.K.; et al. Clinicoradiologic and Neuropathologic Evaluation of Corticobasal Syndrome. Neurology 2023, 101, E289–E299. [Google Scholar] [CrossRef] [PubMed]
- Crutch, S.J.; Schott, J.M.; Rabinovici, G.D.; Murray, M.; Snowden, J.S.; van der Flier, W.M.; Dickerson, B.C.; Vandenberghe, R.; Ahmed, S.; Bak, T.H.; et al. Consensus Classification of Posterior Cortical Atrophy. Alzheimer’s Dement. 2017, 13, 870–884. [Google Scholar] [CrossRef]
- Heikkinen, S.; Katisko, K.; Haapasalo, A.; Portaankorva, A.; Hartikainen, P.; Solje, E. Overlap in the Diagnostic Criteria of Frontotemporal Dementia Syndromes with Parkinsonism. J. Alzheimer’s Dis. 2025, 104, 374–381. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Sioka, C. Differentiating Progressive Supranuclear Palsy and Corticobasal Syndrome: Insights from Cerebrospinal Fluid Biomarkers—A Narrative Review. Medicina. 2025, 61, 701. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Sioka, C. A Narrative Review of Serum Biomarkers in Progressive Supranuclear Palsy, Corticobasal Syndrome, and Related Disorders. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Li, G.; Wu, R.; Tong, R.; Bo, B.; Zhao, Y.; Gillen, K.M.; Spincemaille, P.; Ku, Y.; Du, Y.; Wang, Y.; et al. Quantitative Measurement of Metal Accumulation in Brain of Patients With Wilson’s Disease. Mov. Disord. 2020, 35, 1787–1795. [Google Scholar] [CrossRef]
- Bellini, G.; Di Rauso, G.; Fontanelli, L.; Benevento, E.; Becattini, L.; Frosini, D.; Ceravolo, R.; Del Prete, E. Deep Brain Stimulation in Progressive Supranuclear Palsy: A Dead-End Story? A Narrative Review. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M. European Association of Nuclear Medicine and European Academy of Neurology Recommendations for the Use of Brain 18F-fluorodeoxyglucose Positron Emission Tomography in Neurodegenerative Cognitive Impairment and Dementia: Delphi Consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Festari, C.; Massa, F.; Cotta Ramusino, M.; Orini, S.; Aarsland, D.; Agosta, F.; Babiloni, C.; Borroni, B.; Cappa, S.F.; et al. European Intersocietal Recommendations for the Biomarker-Based Diagnosis of Neurocognitive Disorders. Lancet Neurol. 2024, 23, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, S.P.; Ballarini, T.; Sala, A.; Cerami, C.; Presotto, L.; Santangelo, R.; Fallanca, F.; Vanoli, E.G.; Gianolli, L.; Iannaccone, S.; et al. FDG-PET and CSF Biomarker Accuracy in Prediction of Conversion to Different Dementias in a Large Multicentre MCI Cohort. Neuroimage Clin. 2018, 18, 167–177. [Google Scholar] [CrossRef]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef]
- Foster, N.L.; Gilman, S.; Berent, S.; Morin, E.M.; Brown, M.B.; Koeppe, R.A. Cerebral Hypometabolism in Progressive Supranuclear Palsy Studied with Positron Emission Tomography. Ann. Neurol. 1988, 24, 399–406. [Google Scholar] [CrossRef]
- Goffinet, A.M.; De Voider, A.G.; Gillain, C.; Rectem, D.; Bol, A.; Michel, C.; Cogneau, M.; Labar, D.; Laterre, C. Positron Tomography Demonstrates Frontal Lobe Hypometabolism in Progressive Supranuclear Palsy. Ann. Neurol. 1989, 25, 131–139. [Google Scholar] [CrossRef]
- Karbe, H.; Grond, M.; Huber, M.; Herholz, K.; Kessler, J.; Heiss, W.-D. Subcortical Damage and Cortical Dysfunction in Progressive Supranuclear Palsy Demonstrated by Positron Emission Tomography. J. Neurol. 1992, 239, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, B.; Gao, S.; Li, X. Clinical, MRI and 18F-FDG-PET/CT Analysis of Progressive Supranuclear Palsy. J. Clin. Neurosci. 2020, 80, 318–323. [Google Scholar] [CrossRef]
- Yamauchi, H.; Fukuyama, H.; Nagahama, Y.; Katsumi, Y.; Dong, Y.; Konishi, J.; Kimura, J. Atrophy of the Corpus Callosum, Cognitive Impairment, and Cortical Hypometabolism in Progressive Supranuclear Palsy. Ann. Neurol. 1997, 41, 606–614. [Google Scholar] [CrossRef]
- Mishina, M.; Ishii, K.; Mitani, K.; Ohyama, M.; Yamazaki, M.; Ishiwata, K.; Senda, M.; Kobayashi, S.; Kitamura, S.; Katayama, Y. Midbrain Hypometabolism as Early Diagnostic Sign for Progressive Supranuclear Palsy. Acta Neurol. Scand. 2004, 110, 128–135. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishii, K.; Kakigi, T.; Yokoyama, K.; Mori, E.; Murakami, T. Brain Alterations and Mini-Mental State Examination in Patients with Progressive Supranuclear Palsy: Voxel-Based Investigations Using 18F-Fluorodeoxyglucose Positron Emission Tomography and Magnetic Resonance Imaging. Dement. Geriatr. Cogn. Dis. Extra 2011, 1, 381–392. [Google Scholar] [CrossRef]
- Zwergal, A.; La Fougère, C.; Lorenzl, S.; Rominger, A.; Xiong, G.; Deutschenbaur, L.; Linn, J.; Krafczyk, S.; Dieterich, M.; Brandt, T.; et al. Postural Imbalance and Falls in PSP Correlate with Functional Pathology of the Thalamus. Neurology 2011, 77, 101–109. [Google Scholar] [CrossRef]
- Zwergal, A.; Fougère, C.L.; Lorenzl, S.; Rominger, A.; Xiong, G.; Deutschenbaur, L.; Schöberl, F.; Linn, J.; Dieterich, M.; Brandt, T.; et al. Functional Disturbance of the Locomotor Network in Progressive Supranuclear Palsy. Neurology 2013, 80, 634–641. [Google Scholar] [CrossRef]
- Amtage, F.; Maurer, C.; Hellwig, S.; Tüscher, O.; Kreft, A.; Weiller, C.; Rijntjes, M.; Winkler, C.; Meyer, P.T. Functional Correlates of Vertical Gaze Palsy and Other Ocular Motor Deficits in PSP: An FDG-PET Study. Park. Relat. Disord. 2014, 20, 898–906. [Google Scholar] [CrossRef]
- Isella, V.; Licciardo, D.; Ferri, F.; Crivellaro, C.; Morzenti, S.; Appollonio, I.; Ferrarese, C. Reduced Phonemic Fluency in Progressive Supranuclear Palsy Is Due to Dysfunction of Dominant BA6. Front. Aging Neurosci. 2022, 14, 969875. [Google Scholar] [CrossRef]
- Doll-Lee, J.; Klietz, M.; Greten, S.; Kopp, B.; Berding, G.; Brendel, M.; Wilkens, I.; Katzdobler, S.; Levin, J.; Danek, A.; et al. Associations between Neuropsychological Profile and Regional Brain FDG Uptake in Progressive Supranuclear Palsy. J. Park. Dis. 2025, 15, 904–912. [Google Scholar] [CrossRef]
- Buchert, R.; Wegner, F.; Huppertz, H.J.; Berding, G.; Brendel, M.; Apostolova, I.; Buhmann, C.; Dierks, A.; Katzdobler, S.; Klietz, M.; et al. Automatic Covariance Pattern Analysis Outperforms Visual Reading of 18F-Fluorodeoxyglucose-Positron Emission Tomography (FDG-PET) in Variant Progressive Supranuclear Palsy. Mov. Disord. 2023, 38, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Garraux, G.; Salmon, E.; Peigneux, P.; Kreisler, A.; Degueldre, C.; Lemaire, C.; Destée, A.; Franck, G. Voxel-Based Distribution of Metabolic Impairment in Corticobasal Degeneration. Mov. Disord. 2000, 15, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Pham, N.T.T.; Ali, F.; Machulda, M.M.; Lowe, V.J.; Josephs, K.A.; Whitwell, J.L. Frontal Hypometabolism in the Diagnosis of Progressive Supranuclear Palsy Clinical Variants. J. Neurol. 2024, 271, 4267–4280. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Ishii, K.; Sakamoto, S.; Mori, T.; Sasaki, M.; Hirono, N.; Mori, E. Voxel-Based Comparison of Regional Cerebral Glucose Metabolism between PSP and Corticobasal Degeneration. J. Neurol. Sci. 2002, 199, 67–71. [Google Scholar] [CrossRef]
- Juh, R.; Pae, C.U.; Kim, T.S.; Lee, C.U.; Choe, B.; Suh, T. Cerebral Glucose Metabolism in Corticobasal Degeneration Comparison with Progressive Supranuclear Palsy Using Statistical Mapping Analysis. Neurosci. Lett. 2005, 383, 22–27. [Google Scholar] [CrossRef]
- Amtage, F.; Hellwig, S.; Kreft, A.; Spehl, T.; Glauche, V.; Winkler, C.; Rijntjes, M.; Hellwig, B.; Weiller, C.; Weber, W.A.; et al. Neuronal Correlates of Clinical Asymmetry in Progressive Supranuclear Palsy. Clin. Nucl. Med. 2014, 39, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, N.; Botha, H.; Whitwell, J.L.; Lowe, V.; Dickson, D.W.; Josephs, K.A. FDG-PET in Pathologically Confirmed Spontaneous 4R-Tauopathy Variants. J. Neurol. 2014, 261, 710–716. [Google Scholar] [CrossRef]
- Buchert, R.; Huppertz, H.J.; Wegner, F.; Berding, G.; Brendel, M.; Apostolova, I.; Buhmann, C.; Poetter-Nerger, M.; Dierks, A.; Katzdobler, S.; et al. Added Value of FDG-PET for Detection of Progressive Supranuclear Palsy. J. Neurol. Neurosurg. Psychiatry 2024, 96, 287–295. [Google Scholar] [CrossRef]
- Blin, J.; Vidailhet, M.-J.; Pillon, B.; Dubois, B.; Feve, J.-R.; Agid, Y. Corticobasal Degeneration: Decreased and Asymmetrical Glucose Consumption as Studied with PET. Mov. Disord. 1992, 7, 348–354. [Google Scholar] [CrossRef]
- Klaffke, S.; Kuhn, A.A.; Plotkin, M.; Amthauer, H.; Harnack, D.; Felix, R.; Kupsch, A. Dopamine Transporters, D2 Receptors, and Glucose Metabolism in Corticobasal Degeneration. Mov. Disord. 2006, 21, 1724–1727. [Google Scholar] [CrossRef]
- Turaga, S.P.; Mridula, R.; Borgohain, R. Cerebral Glucose Metabolism, Clinical, Neuropsychological, and Radiological Profile in Patients with Corticobasal Syndrome. Neurol. India 2013, 61, 7–11. [Google Scholar] [CrossRef]
- Franceschi, A.; Clifton, M.; Naser-Tavakolian, K.; Ahmed, O.; Cruciata, G.; Bangiyev, L.; Clouston, S.; Franceschi, D. (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Assessment of Hypometabolism Patterns in Clinical Phenotypes of Suspected Corticobasal Degeneration. World J. Nucl. Med. 2021, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.J.; Ghosh, P.M.; Lee, S.E.; Corbetta-Rastelli, C.; Jagust, W.J.; Kornak, J.; Rankin, K.P.; Grinberg, L.T.; Vinter, H.V.; Mendez, M.F.; et al. Predicting Amyloid Status in Corticobasal Syndrome Using Modified Clinical Criteria, Magnetic Resonance Imaging and Fluorodeoxyglucose Positron Emission Tomography. Alzheimers Res. Ther. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Boman, A.; Svensson, S.; Boxer, A.; Rojas, J.C.; Seeley, W.W.; Karydas, A.; Miller, B.; Kagedal, K.; Svenningsson, P. Distinct Lysosomal Network Protein Profiles in Parkinsonian Syndrome Cerebrospinal Fluid. J. Park. Dis. 2016, 6, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Grisanti, S.G.; Ferri, F.; Morzenti, S.; Crivellaro, C.; Musarra, M.; Ferrarese, C. Cognitive Reserve Maps the Core Loci of Neurodegeneration in Corticobasal Degeneration. Eur. J. Neurol. 2018, 25, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Licciardo, D.; Ferri, F.; Crivellaro, C.; Morzenti, S.; Appollonio, I.M.; Ferrarese, C. Left and Right Corticobasal Syndrome: Comparison of Cognitive Profiles between Metabolic Imaging—Matched Groups. Neurol. Sci. 2024, 45, 1499–1506. [Google Scholar] [CrossRef]
- Pardini, M.; Huey, E.D.; Spina, S.; Kreisl, W.C.; Morbelli, S.; Wassermann, E.M.; Nobili, F.; Ghetti, B.; Grafman, J. FDG-PET Patterns Associated with Underlying Pathology in Corticobasal Syndrome. Neurology 2019, 92, E1121–E1135. [Google Scholar] [CrossRef]
- Jo, S.; Oh, J.S.; Cheong, E.N.; Kim, H.J.; Lee, S.; Oh, M.; Kim, J.S.; Chung, S.J.; Lee, C.S.; Kwon, M.; et al. FDG-PET Patterns Associated with Ideomotor Apraxia and Imitation Apraxia in Patients with Corticobasal Syndrome. Park. Relat. Disord. 2021, 88, 96–101. [Google Scholar] [CrossRef]
- Parmera, J.B.; de Almeida, I.J.; de Oliveira, M.C.B.; Silagi, M.L.; de Godoi Carneiro, C.; Studart-Neto, A.; Ono, C.R.; Reis Barbosa, E.; Nitrini, R.; Buchpiguel, C.A.; et al. Metabolic and Structural Signatures of Speech and Language Impairment in Corticobasal Syndrome: A Multimodal PET/MRI Study. Front. Neurol. 2021, 12, 702052. [Google Scholar] [CrossRef]
- Parmera, J.B.; de Godoi Carneiro, C.; de Almeida, I.J.; de Oliveira, M.C.B.; Barbosa, P.M.; Studart-Neto, A.; Ono, C.R.; Nitrini, R.; Buchpiguel, C.A.; Barbosa, E.R.; et al. Probable 4-Repeat Tauopathy Criteria Predict Brain Amyloid Negativity, Distinct Clinical Features, and FDG-PET/MRI Neurodegeneneration Patterns in Corticobasal Syndrome. Mov. Disord. Clin. Pract. 2024, 11, 238–247. [Google Scholar] [CrossRef]
- Parmera, J.B.; Coutinho, A.M.; Aranha, M.R.; Studart-Neto, A.; de Godoi Carneiro, C.; de Almeida, I.J.; Fontoura Solla, D.J.; Ono, C.R.; Barbosa, E.R.; Nitrini, R.; et al. FDG-PET Patterns Predict Amyloid Deposition and Clinical Profile in Corticobasal Syndrome. Mov. Disord. 2021, 36, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Shimada, H.; Shinotoh, H.; Hirano, S.; Tagai, K.; Sano, Y.; Yamamoto, Y.; Endo, H.; Matsuoka, K.; Takahata, K.; et al. PET-Based Classification of Corticobasal Syndrome. Park. Relat. Disord. 2022, 98, 92–98. [Google Scholar] [CrossRef]
- Ghirelli, A.; Goodrich, A.W.; Stephens, Y.C.; Graff-Radford, J.; Ali, F.; Machulda, M.M.; Schwarz, C.G.; Senjem, M.L.; Agosta, F.; Filippi, M.; et al. Relationships between Hypometabolism and Both β-Amyloid and Tau PET in Corticobasal Syndrome. Alzheimer’s Dement. 2025, 21, e70018. [Google Scholar] [CrossRef]
- Mille, E.; Levin, J.; Brendel, M.; Zach, C.; Barthel, H.; Sabri, O.; Bötzel, K.; Bartenstein, P.; Danek, A.; Rominger, A. Cerebral Glucose Metabolism and Dopaminergic Function in Patients with Corticobasal Syndrome. J. Neuroimaging 2017, 27, 255–261. [Google Scholar] [CrossRef]
- Eckert, T.; Tang, C.; Ma, Y.; Brown, N.; Lin, T.; Frucht, S.; Feigin, A.; Eidelberg, D. Abnormal Metabolic Networks in Atypical Parkinsonism. Mov. Disord. 2008, 23, 727–733. [Google Scholar] [CrossRef]
- Mudali, D.; Teune, L.K.; Renken, R.J.; Leenders, K.L.; Roerdink, J.B.T.M. Classification of Parkinsonian Syndromes from FDG-PET Brain Data Using Decision Trees with SSM/PCA Features. Comput. Math. Methods Med. 2015, 2015, 136921. [Google Scholar] [CrossRef]
- Srulijes, K.; Reimold, M.; Liscic, R.M.; Bauer, S.; Dietzel, E.; Liepelt-Scarfone, I.; Berg, D.; Maetzler, W. Fluorodeoxyglucose Positron Emission Tomography in Richardson’s Syndrome and Progressive Supranuclear Palsy-parkinsonism. Mov. Disord. 2012, 27, 151–155. [Google Scholar] [CrossRef]
- Eckert, T.; Barnes, A.; Dhawan, V.; Frucht, S.; Gordon, M.F.; Feigin, A.S.; Eidelberg, D. FDG PET in the Differential Diagnosis of Parkinsonian Disorders. Neuroimage 2005, 26, 912–921. [Google Scholar] [CrossRef]
- Niethammer, M.; Tang, C.C.; Feigin, A.; Allen, P.J.; Heinen, L.; Hellwig, S.; Amtage, F.; Hanspal, E.; Vonsattel, J.P.; Poston, K.L.; et al. A Disease-Specific Metabolic Brain Network Associated with Corticobasal Degeneration. Brain 2014, 137, 3036–3046. [Google Scholar] [CrossRef]
- Hellwig, S.; Reinhard, M.; Amtage, F.; Guschlbauer, B.; Buchert, R.; Tüscher, O.; Weiller, C.; Niesen, W.D.; Meyer, P.T. Transcranial Sonography and [18F]Fluorodeoxyglucose Positron Emission Tomography for the Differential Diagnosis of Parkinsonism: A Head-to-head Comparison. Eur. J. Neurol. 2014, 21, 860–866. [Google Scholar] [CrossRef]
- Tripathi, M.; Dhawan, V.; Peng, S.; Kushwaha, S.; Batla, A.; Jaimini, A.; D’Souza, M.M.; Sharma, R.; Saw, S.; Mondal, A. Differential Diagnosis of Parkinsonian Syndromes Using F-18 Fluorodeoxyglucose Positron Emission Tomography. Neuroradiology 2013, 55, 483–492. [Google Scholar] [CrossRef]
- Hellwig, S.; Frings, L.; Amtage, F.; Buchert, R.; Spehl, T.S.; Rijntjes, M.; Tüscher, O.; Weiller, C.; Weber, W.A.; Vach, W.; et al. 18F-FDG PET Is an Early Predictor of Overall Survival in Suspected Atypical Parkinsonism. J. Nucl. Med. 2015, 56, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Poston, K.L.; Eckert, T.; Feigin, A.; Frucht, S.; Gudesblatt, M.; Dhawan, V.; Lesser, M.; Vonsattel, J.-P.; Fahn, S.; et al. Differential Diagnosis of Parkinsonism: A Metabolic Imaging Study Using Pattern Analysis. Lancet Neurol. 2010, 9, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Juh, R.; Kim, J.; Moon, D.; Choe, B.; Suh, T. Different Metabolic Patterns Analysis of Parkinsonism on the 18F-FDG PET. Eur. J. Radiol. 2004, 51, 223–233. [Google Scholar] [CrossRef]
- Garraux, G.; Phillips, C.; Schrouff, J.; Kreisler, A.; Lemaire, C.; Degueldre, C.; Delcour, C.; Hustinx, R.; Luxen, A.; Destée, A.; et al. Multiclass Classification of FDG PET Scans for the Distinction between Parkinson’s Disease and Atypical Parkinsonian Syndromes. Neuroimage Clin. 2013, 2, 883–893. [Google Scholar] [CrossRef]
- Tripathi, M.; Tang, C.C.; Feigin, A.; De Lucia, I.; Nazem, A.; Dhawan, V.; Eidelberg, D. Automated Differential Diagnosis of Early Parkinsonism Using Metabolic Brain Networks: A Validation Study. J. Nucl. Med. 2016, 57, 60–66. [Google Scholar] [CrossRef]
- Brajkovic, L.; Kostic, V.; Sobic-Saranovic, D.; Stefanova, E.; Jecmenica-Lukic, M.; Jesic, A.; Stojiljkovic, M.; Odalovic, S.; Gallivanone, F.; Castiglioni, I.; et al. The Utility of FDG-PET in the Differential Diagnosis of Parkinsonism. Neurol. Res. 2017, 39, 675–684. [Google Scholar] [CrossRef]
- Martí-Andrés, G.; van Bommel, L.; Meles, S.K.; Riverol, M.; Valentí, R.; Kogan, R.V.; Renken, R.J.; Gurvits, V.; van Laar, T.; Pagani, M.; et al. Multicenter Validation of Metabolic Abnormalities Related to PSP According to the MDS-PSP Criteria. Mov. Disord. 2020, 35, 2009–2018. [Google Scholar] [CrossRef]
- Shen, B.; Wei, S.; Ge, J.; Peng, S.; Liu, F.; Li, L.; Guo, S.; Wu, P.; Zuo, C.; Eidelberg, D.; et al. Reproducible Metabolic Topographies Associated with Multiple System Atrophy: Network and Regional Analyses in Chinese and American Patient Cohorts. Neuroimage Clin. 2020, 28, 102416. [Google Scholar] [CrossRef]
- Amod, F.H.; Bhigjee, A.I.; Nyakale, N. Utility of 18FFDG-PET in Parkinsonism in an African Population. eNeurologicalSci 2022, 27, 100399. [Google Scholar] [CrossRef]
- Tomše, P.; Rebec, E.; Studen, A.; Perovnik, M.; Rus, T.; Ležaić, L.; Tang, C.C.; Eidelberg, D.; Trošt, M. Abnormal Metabolic Covariance Patterns Associated with Multiple System Atrophy and Progressive Supranuclear Palsy. Phys. Medica 2022, 98, 131–138. [Google Scholar] [CrossRef]
- Eidelberg, D.; Dhawan, V.; Moeller, J.R.; Sidtis, J.J.; Ginos, J.Z.; Strother, S.C.; Cederbaum, J.; Greene, P.; Fahn, S.; Powers, J.M.; et al. The Metabolic Landscape of Cortico-Basal Ganglionic Degeneration: Regional Asymmetries Studied with Positron Emission Tomography. J. Neurol. Neurosurg. Psychiatry 1991, 54, 856–862. [Google Scholar] [CrossRef][Green Version]
- Karbe, H.; Holthoff, V.; Huber, M.; Herholz, K.; Wienhard, K.; Wagner, R.; Heiss, W.-D. Positron Emission Tomography in Degenerative Disorders of the Dopaminergic System. J. Neural Transm. Park. Dis. Dement. Sect. 1992, 4, 121–130. [Google Scholar] [CrossRef]
- Klein, R.C.; de Jong, B.M.; de Vries, J.J.; Leenders, K.L. Direct Comparison between Regional Cerebral Metabolism in Progressive Supranuclear Palsy and Parkinson’s Disease. Mov. Disord. 2005, 20, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Herting, B.; Beuthien-Baumann, B.; Pöttrich, K.; Donix, M.; Triemer, A.; Lampe, J.B.; Von Kummer, R.; Herholz, K.; Reichmann, H.; Holthoff, V.A. Prefrontal Cortex Dysfunction and Depression in Atypical Parkinsonian Syndromes. Mov. Disord. 2007, 22, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kim, J.S.; Im, K.C.; Oh, S.J.; Kim, M.J.; Lee, J.H.; Chung, S.J.; Lee, M.C. Functional Brain Imaging in Pure Akinesia with Gait Freezing: [18F] FDG PET and [18F] FP-CIT PET Analyses. Mov. Disord. 2009, 24, 237–245. [Google Scholar] [CrossRef]
- Hellwig, S.; Amtage, F.; Kreft, A.; Buchert, R.; Winz, O.H.; Vach, W.; Spehl, T.S.; Rijntjes, M.; Hellwig, B.; Weiller, C.; et al. [18F]FDG-PET Is Superior to [123I]IBZM-SPECT for the Differential Diagnosis of Parkinsonism. Neurology 2012, 79, 1314–1322. [Google Scholar] [CrossRef]
- Teune, L.K.; Renken, R.J.; Mudali, D.; De Jong, B.M.; Dierckx, R.A.; Roerdink, J.B.T.M.; Leenders, K.L. Validation of Parkinsonian Disease-Related Metabolic Brain Patterns. Mov. Disord. 2013, 28, 547–551. [Google Scholar] [CrossRef]
- Akdemir, Ü.Ö.; Tokçaer, A.B.; Karakuş, A.; Kapucu, L.Ö. Brain 18F-FDG PET Imaging in the Differential Diagnosis of Parkinsonism. Clin. Nucl. Med. 2014, 39, e220–e226. [Google Scholar] [CrossRef]
- Baudrexel, S.; Seifried, C.; Penndorf, B.; Klein, J.C.; Middendorp, M.; Steinmetz, H.; Grünwald, F.; Hilker, R. The Value of Putaminal Diffusion Imaging versus 18-fluorodeoxyglucose Positron Emission Tomography for the Differential Diagnosis of the Parkinson Variant of Multiple System Atrophy. Mov. Disord. 2014, 29, 380–387. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Senjem, M.L.; Gunter, J.L.; Schwarz, C.G.; Reid, R.I.; Spychalla, A.J.; Lowe, V.J.; et al. The Evolution of Primary Progressive Apraxia of Speech. Brain 2014, 137, 2783–2795. [Google Scholar] [CrossRef]
- Ko, J.H.; Lee, C.S.; Eidelberg, D. Metabolic Network Expression in Parkinsonism: Clinical and Dopaminergic Correlations. J. Cereb. Blood Flow. Metab. 2017, 37, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, J.; Peng, S.; Wu, P.; Wang, J.; Zhang, H.; Guan, Y.; Eidelberg, D.; Zuo, C.; Ma, Y. Reproducible Network and Regional Topographies of Abnormal Glucose Metabolism Associated with Progressive Supranuclear Palsy: Multivariate and Univariate Analyses in American and Chinese Patient Cohorts. Hum. Brain Mapp. 2018, 39, 2842–2858. [Google Scholar] [CrossRef]
- Arnone, A.; Allocca, M.; Di Dato, R.; Puccini, G.; Laghai, I.; Rubino, F.; Nerattini, M.; Ramat, S.; Lombardi, G.; Ferrari, C.; et al. FDG PET in the Differential Diagnosis of Degenerative Parkinsonian Disorders: Usefulness of Voxel-Based Analysis in Clinical Practice. Neurol. Sci. 2022, 43, 5333–5341. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Wu, P.; Yakushev, I.; Zhang, H.; Ziegler, S.; Jiang, J.; Förster, S.; Wang, J.; Schwaiger, M.; et al. Adjustment for the Age- and Gender-Related Metabolic Changes Improves the Differential Diagnosis of Parkinsonism. Phenomics 2023, 3, 50–63. [Google Scholar] [CrossRef]
- El Ouartassi, A.; Giordana, C.; Schiazza, A.; Chardin, D.; Darcourt, J. [18F]-FDopa Positron Emission Tomography Imaging in Corticobasal Syndrome. Brain Imaging Behav. 2023, 17, 619–627. [Google Scholar] [CrossRef]
- Ali, F.; Clark, H.; Machulda, M.; Senjem, M.L.; Lowe, V.J.; Jack, C.R.; Josephs, K.A.; Whitwell, J.; Botha, H. Patterns of Brain Volume and Metabolism Predict Clinical Features in the Progressive Supranuclear Palsy Spectrum. Brain Commun. 2024, 6, fcae233. [Google Scholar] [CrossRef]
- Du, X.; Zhao, H.; Li, Y.; Dai, Y.; Gao, L.; Li, Y.; Fan, K.; Sun, Z.; Zhang, Y. The Value of PET/CT in the Diagnosis and Differential Diagnosis of Parkinson’s Disease: A Dual-Tracer Study. NPJ Park. Dis. 2024, 10, 171. [Google Scholar] [CrossRef]
- Ling, R.; Wang, M.; Lu, J.; Wu, S.; Wu, P.; Ge, J.; Wang, L.; Liu, Y.; Jiang, J.; Shi, K.; et al. Radiomics-Guided Deep Learning Networks Classify Differential Diagnosis of Parkinsonism. Brain Sci. 2024, 14, 680. [Google Scholar] [CrossRef]
- Ling, R.; Cen, X.; Wu, S.; Wang, M.; Zhang, Y.; Jiang, J.; Lu, J.; Liu, Y.; Zuo, C.; Jiang, J.; et al. Explainable Graph Neural Network Based on Metabolic Brain Imaging for Differential Diagnosis of Parkinsonism. Front. Aging Neurosci. 2025, 17, 1580910. [Google Scholar] [CrossRef]
- Pillai, K.S.; Rajeswari, P.; Kamble, R.B.; Gopalan Nair Santhamma, S.; Chacko, M.; Jayakrishnan, V.; Ramachandran, R.; Avarachan, A.; Kishore, A. Multimodality Brain Imaging Markers in Progressive Supranuclear Palsy Subtypes and Parkinson’s Disease. Mov. Disord. Clin. Pract. 2025, 12, 664–669. [Google Scholar] [CrossRef]
- Štokelj, E.; Rus, T.; Jamšek, J.; Trošt, M.; Simončič, U. Multinomial Logistic Regression Algorithm for the Classification of Patients with Parkinsonisms. EJNMMI Res. 2025, 15, 24. [Google Scholar] [CrossRef]
- Botha, H.; Whitwell, J.L.; Madhaven, A.; Senjem, M.L.; Lowe, V.; Josephs, K.A. The Pimple Sign of Progressive Supranuclear Palsy Syndrome. Park. Relat. Disord. 2014, 20, 180–185. [Google Scholar] [CrossRef]
- Cerami, C.; Dodich, A.; Lettieri, G.; Iannaccone, S.; Magnani, G.; Marcone, A.; Gianolli, L.; Cappa, S.F.; Perani, D. Different FDG-PET Metabolic Patterns at Single-Subject Level in the Behavioral Variant of Fronto-Temporal Dementia. Cortex 2016, 83, 101–112. [Google Scholar] [CrossRef]
- Cerami, C.; Dodich, A.; Iannaccone, S.; Magnani, G.; Marcone, A.; Guglielmo, P.; Vanoli, G.; Cappa, S.F.; Perani, D. Individual Brain Metabolic Signatures in Corticobasal Syndrome. J. Alzheimer’s Dis. 2020, 76, 517–528. [Google Scholar] [CrossRef]
- Bergeron, D.; Beauregard, J.-M.; Jean-Guimond; Soucy, J.-P.; Verret, L.; Poulin, S.; Matias-Guiu, J.A.; Cabrera-Martín, M.N.; Bouchard, R.W.; Laforce, R. Posterior Cingulate Cortex Hypometabolism in Non-Amnestic Variants of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, S.; Cajanus, A.; Katisko, K.; Hartikainen, P.; Vanninen, R.; Haapasalo, A.; Krüger, J.; Remes, A.M.; Solje, E. Brainstem Atrophy Is Linked to Extrapyramidal Symptoms in Frontotemporal Dementia. J. Neurol. 2022, 269, 4488–4497. [Google Scholar] [CrossRef]
- Isella, V.; Crivellaro, C.; Formenti, A.; Musarra, M.; Pacella, S.; Morzenti, S.; Ferri, F.; Mapelli, C.; Gallivanone, F.; Guerra, L.; et al. Validity of Cingulate–Precuneus–Temporo-Parietal Hypometabolism for Single-Subject Diagnosis of Biomarker-Proven Atypical Variants of Alzheimer’s Disease. J. Neurol. 2022, 269, 4440–4451. [Google Scholar] [CrossRef]
- Teune, L.K.; Bartels, A.L.; de Jong, B.M.; Willemsen, A.T.M.; Eshuis, S.A.; de Vries, J.J.; van Oostrom, J.C.H.; Leenders, K.L. Typical Cerebral Metabolic Patterns in Neurodegenerative Brain Diseases. Mov. Disord. 2010, 25, 2395–2404. [Google Scholar] [CrossRef]
- Wenzel, F.; Young, S.; Wilke, F.; Apostolova, I.; Arlt, S.; Jahn, H.; Thiele, F.; Buchert, R. B-Spline-Based Stereotactical Normalization of Brain FDG PET Scans in Suspected Neurodegenerative Disease: Impact on Voxel-Based Statistical Single-Subject Analysis. Neuroimage 2010, 50, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, V.; Montandon, M.L.; Viaud, C.T.; Allaoua, M.; Assal, F.; Burkhard, P.R.; Ratib, O.; Zaidi, H. Regions of Interest–Based Discriminant Analysis of DaTSCAN SPECT and FDG-PET for the Classification of Dementia. Clin. Nucl. Med. 2013, 38, e112–e117. [Google Scholar] [CrossRef]
- Taswell, C.; Villemagne, V.L.; Yates, P.; Shimada, H.; Leyton, C.E.; Ballard, K.J.; Piguet, O.; Burrell, J.R.; Hodges, J.R.; Rowe, C.C. 18F-FDG PET Improves Diagnosis in Patients with Focal-Onset Dementias. J. Nucl. Med. 2015, 56, 1547–1553. [Google Scholar] [CrossRef]
- Franceschi, A.; Naser-Tavakolian, K.; Clifton, M.; Ahmed, O.; Stoffers, K.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Hybrid Imaging in Dementia: A Semi-Quantitative (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Approach in Clinical Practice. World J. Nucl. Med. 2021, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Clifton, M.A.; Naser-Tavakolian, K.; Ahmed, O.; Bangiyev, L.; Clouston, S.; Franceschi, D. FDG PET/MRI for Visual Detection of Crossed Cerebellar Diaschisis in Patients with Dementia. Am. J. Roentgenol. 2021, 216, 165–171. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Naser-Tavakolian, K.; Clifton, M.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Metabolic Positron-Emission Tomography/Magnetic Resonance Imaging in Primary Progressive Aphasia and Frontotemporal Lobar Degeneration Subtypes: Reassessment of Expected [18F]-Fluorodeoxyglucose Uptake Patterns. World J. Nucl. Med. 2021, 20, 294–304. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Strand, E.A.; Machulda, M.M.; Botha, H.; Martin, P.R.; Pham, N.T.T.; Stierwalt, J.; et al. A Molecular Pathology, Neurobiology, Biochemical, Genetic and Neuroimaging Study of Progressive Apraxia of Speech. Nat. Commun. 2021, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Seckin, Z.I.; Duffy, J.R.; Strand, E.A.; Clark, H.M.; Utianski, R.L.; Machulda, M.M.; Botha, H.; Ali, F.; Thu Pham, N.T.; Lowe, V.J.; et al. The Evolution of Parkinsonism in Primary Progressive Apraxia of Speech: A 6-Year Longitudinal Study. Park. Relat. Disord. 2020, 81, 34–40. [Google Scholar] [CrossRef]
- Gan, J.; Shi, Z.; Zuo, C.; Zhao, X.; Liu, S.; Chen, Y.; Zhang, N.; Cai, L.; Cui, R.; Ai, L.; et al. Analysis of Positron Emission Tomography Hypometabolic Patterns and Neuropsychiatric Symptoms in Patients with Dementia Syndromes. CNS Neurosci. Ther. 2023, 29, 2193–2205. [Google Scholar] [CrossRef]
- Braun, A.S.; Satoh, R.; Pham, N.T.T.; Singh-Reilly, N.; Ali, F.; Dickson, D.W.; Lowe, V.J.; Whitwell, J.L.; Josephs, K.A. Machine Learning Models of Voxel-Level [18F] Fluorodeoxyglucose Positron Emission Tomography Data Excel at Predicting Progressive Supranuclear Palsy Pathology. Ann. Neurol. 2025, 98, 410–420. [Google Scholar] [CrossRef]
- Armstrong, M.J. Lewy Body Dementias. Contin. Lifelong Learn. Neurol. 2019, 25, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-C.; Ye, Q.; Yuan, C.-X. Metabolic Pattern Analysis of 18F-FDG PET as a Marker for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2019, 30, 743–756. [Google Scholar] [CrossRef]
- Walker, Z.; Gandolfo, F.; Orini, S.; Garibotto, V.; Agosta, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Nestor, P.; Boccardi, M.; et al. Clinical Utility of FDG PET in Parkinson’s Disease and Atypical Parkinsonism Associated with Dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko, N.K.; Koziorowski, D.M.; Królicki, L.; Budrewicz, S.; Friedman, A. Accumulation of Tau Protein, Metabolism and Perfusion—Application and Efficacy of Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) Imaging in the Examination of Progressive Supranuclear Palsy (PSP) and Corticobasal Syndrome (CBS). Front. Neurol. 2019, 10, 101. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies—Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Höglinger, G.U.; Antonini, A.; Bordelon, Y.; Boxer, A.L.; Colosimo, C.; van Eimeren, T.; Golbe, L.I.; Kassubek, J.; Kurz, C.; et al. Radiological Biomarkers for Diagnosis in PSP: Where Are We and Where Do We Need to Be? Mov. Disord. 2017, 32, 955–971. [Google Scholar] [CrossRef]
- Vasilevskaya, A.; Taghdiri, F.; Multani, N.; Anor, C.; Misquitta, K.; Houle, S.; Burke, C.; Tang-Wai, D.; Lang, A.E.; Fox, S.; et al. PET Tau Imaging and Motor Impairments Differ Between Corticobasal Syndrome and Progressive Supranuclear Palsy With and Without Alzheimer’s Disease Biomarkers. Front. Neurol. 2020, 11, 574. [Google Scholar] [CrossRef]
- Liang, M.; Jia, C.; Yen, T.-C.; Liu, L.; Li, M.; Cui, R. Complementary Value of Metabolic and Tau PET Imaging in the Diagnosis of Corticobasal Degeneration. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4286–4288. [Google Scholar] [CrossRef]
- Santibanez, R.A.; Mendez-Orellana, C.; Juri, C.; Escobar, S.; Bustamante, G.; Quiroga, C.; Contreras, N.; Haeger, A.; Amaral, H.; Kramer, V. Tau PET Imaging with 18F-PI-2620 in Patients with Corticobasal Syndrome: A Case Series. Alzheimer’s Dement. 2022, 18, e067105. [Google Scholar] [CrossRef]
- Giannakis, A.; Konitsiotis, S.; Xiromerisiou, G. Diffusion Tensor Imaging in Progressive Supranuclear Palsy Versus Other Neurodegenerative Diseases: A Review. J. Neuroimaging 2025, 35, e70063. [Google Scholar] [CrossRef]
- Klietz, M.; Mahmoudi, N.; Maudsley, A.A.; Sheriff, S.; Bronzlik, P.; Almohammad, M.; Nösel, P.; Wegner, F.; Höglinger, G.U.; Lanfermann, H.; et al. Whole-Brain Magnetic Resonance Spectroscopy Reveals Distinct Alterations in Neurometabolic Profile in Progressive Supranuclear Palsy. Mov. Disord. 2023, 38, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Gao, Y.; Li, F.; Wei, L.; Sun, Y.; Liu, F.; Zhu, Y.; Qiu, J.; Wang, Z.; Zhang, Y. Iron Deposition Is Associated with Motor and Non-Motor Network Breakdown in Parkinsonism. Front. Aging Neurosci. 2025, 16, 1518155. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Banno, F.; Mizutani, Y.; Maeda, T.; Nagao, R.; Shima, S.; Murayama, K.; Ohno, Y.; Maeda, T.; Sasaki, M.; et al. Flattened Red Nucleus in Progressive Supranuclear Palsy Detected by Quantitative Susceptibility Mapping. Park. Relat. Disord. 2025, 131, 107251. [Google Scholar] [CrossRef]
- Miyata, M.; Kakeda, S.; Yoneda, T.; Ide, S.; Okada, K.; Adachi, H.; Korogi, Y. Signal Intensity of Cerebral Gyri in Corticobasal Syndrome on Phase Difference Enhanced Magnetic Resonance Images: Comparison of Progressive Supranuclear Palsy and Parkinson’s Disease. J. Neurol. Sci. 2020, 419, 117210. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Weigand, S.D.; Pham, N.T.T.; Ali, F.; Arani, A.; Senjem, M.L.; Jack, C.R.; Whitwell, J.L.; Josephs, K.A. Magnetic Susceptibility in Progressive Supranuclear Palsy Variants, Parkinson’s Disease, and Corticobasal Syndrome. Mov. Disord. 2023, 38, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
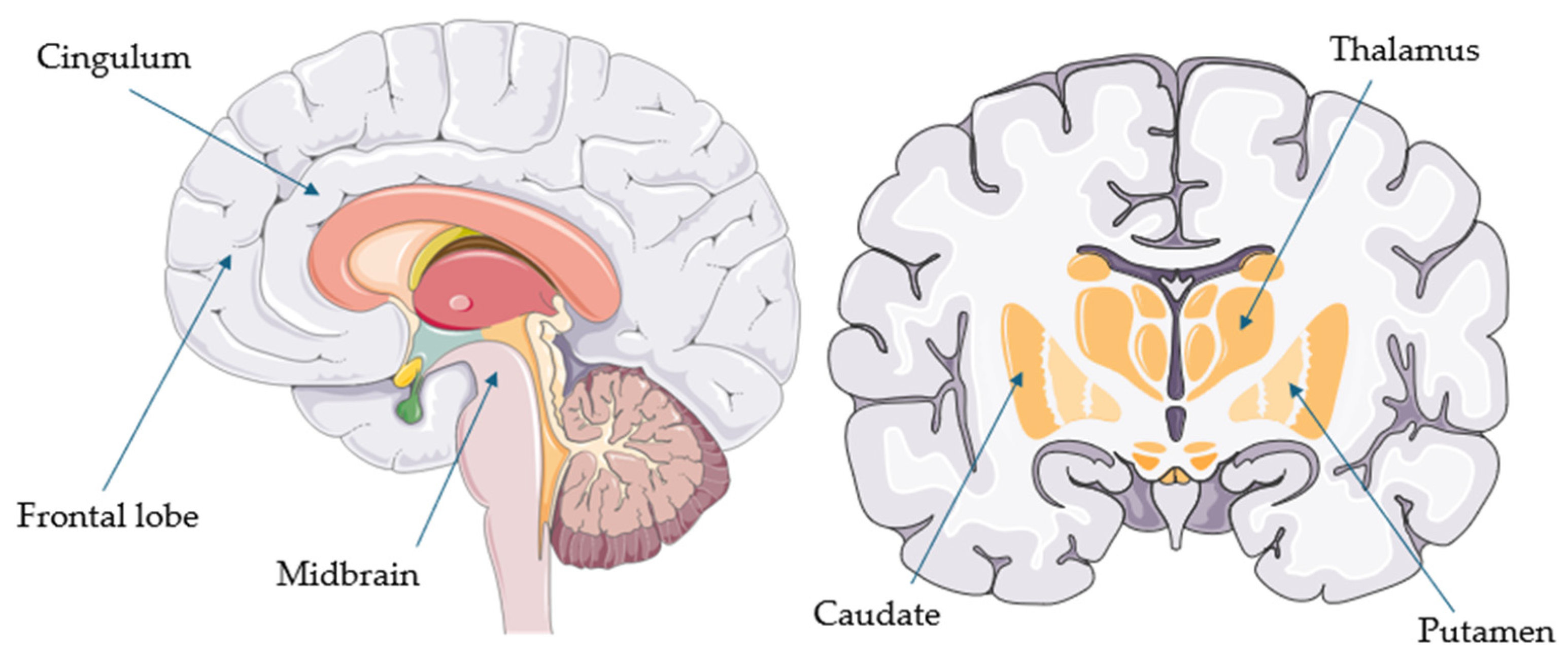
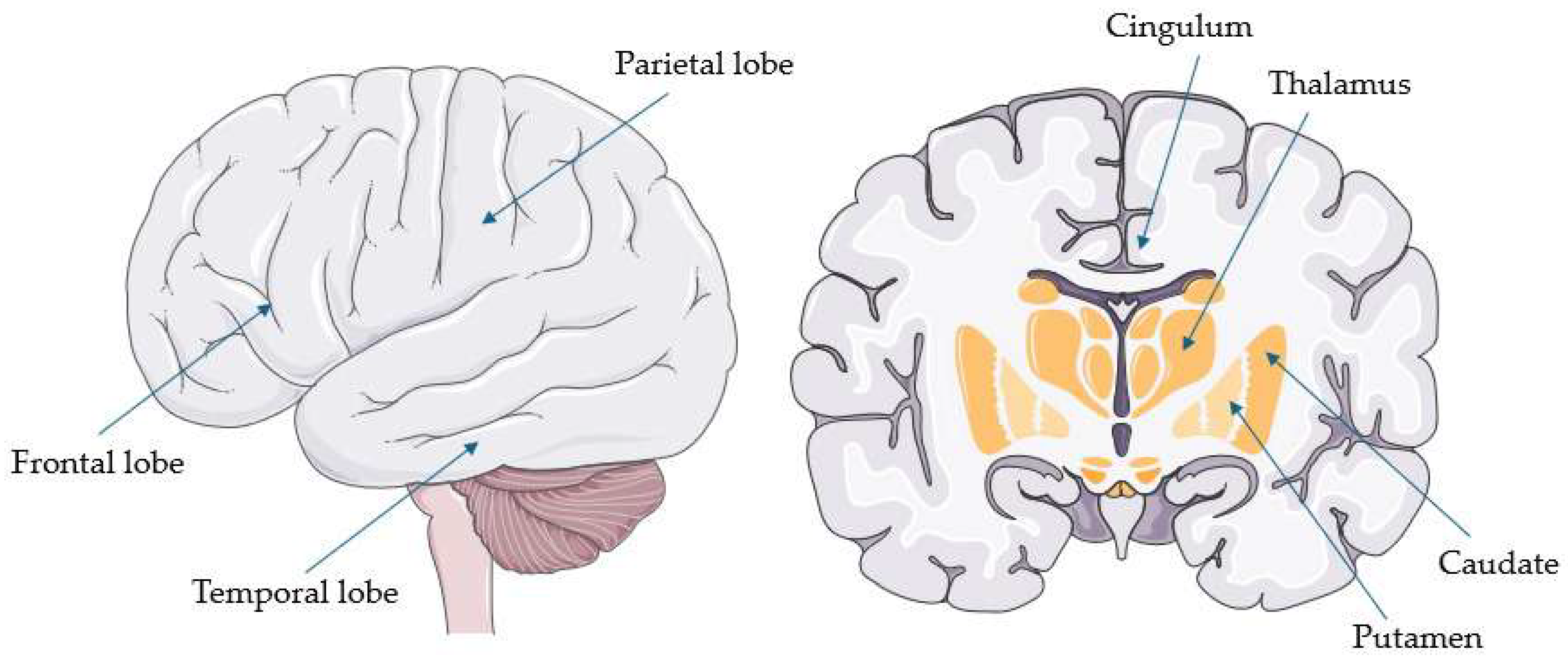
| Study | 4Rtauopathy 3 and Number of Patients (n) | Comparison Groups | Autopsy—Number of Confirmed Cases (n) | Main Findings |
|---|---|---|---|---|
| Foster et al., 1988 [16] | PSP (14) | NC 4 | 1 (confirmed multiple system atrophy) |
|
| Goffinet et al., 1989 [17] | PSP (9) | - | 0 |
|
| Karbe et al., 1992 [18] | PSP (9) | NC | 0 |
|
| Zhao et al., 2020 [19] | PSP (19) | - | 0 |
|
| Yamauchi et al., 1997 [20] | PSP (10) | NC | 0 |
|
| Mishina et al., 2004 [21] | PSP (16) | NC | 2 |
|
| Takahashi et al., 2011 [22] | PSP (16) | NC | 0 |
|
| Zwergal et al., 2011 [23] | PSP (16) | NC | 0 |
|
| Zwergal et al., 2013 [24] | PSP (12) | NC | 0 |
|
| Amtage et al., 2014 [25] | PSP (26) | - | 0 |
|
| Isella et al., 2022 [26] | PSP (31) | - | 0 |
|
| Doll-Lee et, 2025 [27] | PSP (22) | NC | 0 |
|
| Buchert et al., 2023 [28] | PSP-RS 7 (21), non-PSP-RS (20) | NC | 0 |
|
| Garraux et al., 2001 [29] | PSP-RS (21), non-PSP-RS (20) | NC | 0 |
|
| Black et al., 2024 [30] | PSP-RS (59), PSP-P (22) 9, PSP-CBS (6) 10, PSP-SL (28) 11, PSP-F (8) 12, PSP-PGF 13 (8), PSP-OM 14 (2), PSP-PLS (5) 15, PSP-PI (5) 16 | - | 43 (28 diagnosed with PSP, 15 with other neurodegenerative diseases) |
|
| Hosaka et al., 2002 [31] | PSP (21), CBD 18 (22) | NC | 0 |
|
| Juh et al., 2005 [32] | PSP (8), CBD (8) | NC | 0 |
|
| Amtage et al., 2014 [33] | PSP (12), CBS 19 (12) | NC | 0 |
|
| Zalewski et al., 2014 [34] | PSP (7), CBD (1) | - | 8 |
|
| Study | Caudate Nucleus | Putamen | Globus Pallidus | Thalamus | Pons | Midbrain | Superior Cerebellar Peduncle | Cerebellum | Frontal Cortex | Parietal Cortex | Temporal Cortex | Occipital Cortex | Cingulate | Insular Cortex | Hippocampus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foster et al., 1988 [16] | + | + | - | + | + | - | - | - | - | - | - | - | - | - | - |
| Goffinet et al., 1989 [17] | + | + | + | + | - | - | - | + | + (motor/premotor) | - | - | - | - | - | - |
| Karbe et al., 1992 [18] | + | + | - | - | - | + | - | - | + (sensorimotor) | + (sensorimotor) | - | - | - | - | - |
| Zhao et al., 2020 [19] | + | + | - | - | - | + | - | - | + (medial) | - | - | - | - | - | - |
| Yamauchi et al., 1997 [20] | - | - | - | - | - | - | - | - | + (dorsolateral/medial) | - | - | - | - | - | - |
| Mishina et al., 2004 [21] | + (head) | + (anterior) | + (anterior) | - | - | + | - | - | + (superior/inferior/medial) | - | - | - | - | + | - |
| Takahashi et al., 2011 [22] | - | - | - | - | - | + (tegmentum) | - | - | + | - | - | - | - | - | - |
| Zwergal et al., 2011 [23] | - | - | - | + | - | + (tegmentum) | - | - | + (middle) | - | - | - | + (anterior) | - | - |
| Zwergal et al., 2013 [24] | - | - | - | + | - | + | - | + (vermis) | + (middle/inferior) | - | - | - | + (anterior) | - | - |
| Amtage et al., 2014 [25] | - | - | - | - | - | - | - | + (vermis) | + (frontal eye filed) | + (lingual/inferior) | + (anterior) | ||||
| Isella et al., 2022 [26] | + | + | + | - | - | + | - | - | + (dorsolateral/mesial prefrontal/inferior frontal) | - | - | - | - | - | - |
| Doll-Lee et, 2025 [27] | - | - | - | - | - | - | - | - | + (inferior) | + (angular) | - | - | - | - | - |
| Garraux et al., 2001 [29] | - | - | - | - | + | + | - | + (orbitofrontal) | - | - | - | + (anterior) | - | ||
| Black et al., 2024 [30] | - | - | - | - | - | - | - | - | + (premotor/prefrontal/sensorimotor) | + (sensorimotor) | - | - | - | - | - |
| Hosaka et al., 2002 [31] | + | - | - | - | - | + | - | - | + (inferior) | - | - | - | + (anterior) | - | - |
| Juh et al., 2005 [32] | - | - | - | + | - | + | - | - | + (orbitofrontal/middle) | - | - | - | + | - | - |
| Amtage et al., 2014 [33] | - | - | - | + | - | - | - | - | + (medial/sensorimotor) | + (sensorimotor) | - | - | + (middle) | - | - |
| Zalewski et al., 2014 [34] | + | - | - | + | - | + | - | - | + (premotor/supplementary motor/precentral) | - | - | - | - | - | - |
| Study | 4Rtauopathy 3 and Patient Numbers (n) | Comparison Groups | Autopsy—Number of Confirmed Cases (n) | Main Findings |
|---|---|---|---|---|
| Blin et al., 1992 [36] | CBD (5) 4 | NC 5 | 0 |
|
| Klaffke et al., 2006 [37] | CBD (8) | - | 0 |
|
| Turaga et al., 2012 [38] | CBS (17) | - | 0 |
|
| Franceschi et al., 2020 [39] | CBD (12) | - | 0 |
|
| Sha et al., 2015 [40] | CBS (25) | - | 8 |
|
| Mille et al., 2016 [41] | CBS (34) | - | 0 |
|
| Isella et al., 2018 [42] | CBD (35) | - | 0 |
|
| Isella et al., 2024 [43] | CBS (49) | - | 0 |
|
| Pardini et al., 2019 [44] | CBS (29) | NC | 29 |
|
| Jo et al., 2021 [45] | CBD (52) | NC | 0 |
|
| Parmera et al., 2021 [46] | CBS (31) | - | 0 |
|
| Parmera et al., 2024 [47] | CBS (32) | - | 0 |
|
| Parmera et al., 2020 [48] | CBS (45) | - | 0 |
|
| Nakano et al., 2022 [49] | CBS (16) | NC | 1 (biopsy-confirmed) |
|
| Ghirelli et al., 2025 [50] | CBS (33) | - | 0 |
|
| Study | Caudate Nucleus | Putamen | Thalamus | Midbrain | Cerebellum | Frontal Cortex | Parietal Cortex | Temporal Cortex | Occipital Cortex | Cingular Cortex | Insular Cortex | Hippocampus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blin et al., 1992 [36] | + C 3 | + C | + C | - | - | + C (sensorimotor) | + C (associative) | + C | - | - | - | - |
| Klaffke et al., 2006 [37] | - | - | + C | - | - | + C | + C | - | - | - | - | - |
| Turaga et al., 2012 [38] | + C | + C | + C | - | - | + C (dorsolateral/prefrontal) | + C (inferior) | + L 4 | - | - | - | - |
| Franceschi et al., 2020 [39] | + C | + C | + C | - | - | + (sensorimotor) | + C (postcentral/precuneus) | - | + C | - | - | - |
| Sha et al., 2015 [40] | + B 5 | - | - | - | - | + B (sensorimotor) | + B | + B | - | - | - | - |
| Mille et al., 2016 [41] | + C | + L | + B | - | - | + C (precentral/middle) | + C (postcentral/paracentral lobule) | - | - | + C | - | - |
| Isella et al., 2018 [42] | - | - | - | - | - | + L (precentral/pars triangularis) | + L (postcentral/supramarginal) | + L (postcentral/superior) | - | - | + L (posterior) | - |
| Pardini et al., 2019 [44] | + C | - | + C | - | - | + C (precentral/superior/middle/inferior) | + C (postcentral/superior parietal/supramarginal) | - | - | - | - | - |
| Jo et al., 2021 [45] | + C | - | - | - | - | + C (superior/middle/precentral) | + C (postcentral/angular/precuneus) | - | - | + (anterior/posterior) | + | - |
| Parmera et al., 2021 [46] | + B | + B | + B | - | - | + L (premotor/inferior) | + L (inferior) + B (prefrontal) | + B (posterior) | + (posterior) | + B (posterior) | - | - |
| Parmera et al., 2024 [47] | + C | + C | + C | - | - | + C (supplementary motor/middle/inferior/prefrontal) | + C (superior) | + C (posterior) | - | + C (anterior/middle/posterior) | - | - |
| Parmera et al., 2020 [48] | - | - | + C | + B | - | + C (supplementary motor) | + C (angular/superius parietal/paracentral lobule) | + B (posterior superior/middle/inferior/fusiform) | - | - | - | - |
| Nakano et al., 2022 [49] | - | - | + C | - | - | + (precentral) | - | - | - | - | - | - |
| Ghirelli et al., 2025 [50] | - | - | - | - | - | + C (middle) | + B (postcentral/superior) + C (inferior/medial) | + C (lateral) | + C (lateral) | - | - | - |
| Study | 4Rtauopathy 4 and Number of Patients (n) | Comparison Groups | Autopsy—Number of Confirmed Cases (n) | Main Findings |
|---|---|---|---|---|
| Eckert et al., 2008 [52] | PSP (21) | MSA 5, NC 6 | 0 |
|
| Mudali et al., 2015 [53] | PSP (17) | PD 7, MSA, NC | 0 |
|
| Srulijes et al., 2012 [54] | PSP-RS (11) 9, PSP-P (8) 10 | PD, NC | 0 |
|
| Eckert et al., 2005 [55] | PSP (20), CBD (11) 11 | PD, MSA | 2 (MSA) |
|
| Botha et al., 2014 [34] | PSP (28), CBS (26) | MSA | 3 (PSP) |
|
| Niethammer et al., 2014 [56] | PSP (51), CBD (27) | MSA, NC | 3 (CBD) |
|
| Hellwig et al., 2014 [57] | PSP (7), CBD (5) | PD, MSA | 0 |
|
| Tripathi et al., 2013 [58] | PSP (30), CBS (2) | PD, MSA | 0 |
|
| Hellwig et al., 2015 [59] | PSP (22), CBD (8) | PD, DLB 12, MSA | 0 |
|
| Tang et al., 2010 [60] | PSP (37) | PD, MSA | 2 PSP, 1 MSA (CBD at autopsy) |
|
| Juh et al., 2004 [61] | PSP (7) | PD, MSA, NC | 0 |
|
| Garraux et al., 2013 [62] | PSP (26), CBS (21) | PD, MSA | 0 |
|
| Tripathi et al., 2016 [63] | PSP (30) | PD, MSA | 26 |
|
| Brajkovic et al., 2017 [64] | PSP, CBS (21) | PD, MSA | 0 |
|
| Marti-Andres et al., 2020 [65] | PSP-RS (47), PSP-P (18), PSP-PGF (8) 13 | PD, NC | 0 |
|
| Shen et al., 2020 [66] | PSP (34) | PD, MSA, NC | 0 |
|
| Amod et al., 2022 [67] | PSP (11), CBS (2) | PD, DLB, MSA, NC | 0 |
|
| Tomse et al., 2022 [68] | PSP (11) | PD, MSA-P 14, NC | 0 |
|
| Eidelberg et al., 1991 [69] | CBD (5) | PD, NC | 1 |
|
| Karbe et al., 1992 [70] | PSP (9) | PD, PD plus syndrome | 0 |
|
| Klein et al., 2005 [71] | PSP (10) | PD | 1 |
|
| Herting et al., 2007 [72] | PSP (9) | MSA, NC | 0 |
|
| Park et al., 2009 [73] | PSP (14) | PD, PAGF 15, NC | 0 |
|
| Hellwig et al., 2012 [74] | PSP (20), CBD (9) | PD, PDD 16, DLB, MSA | 0 |
|
| Teune et al., 2013 [75] | PSP (17) | PD, MSA, NC | 0 |
|
| Akdemir et al., 2014 [76] | PSP (4), CBD (2) | PD, DLB, MSA | 0 |
|
| Baudrexel et al., 2014 [77] | PSP (8) | PD, MSA-P, NC | 0 |
|
| Josephs et al., 2014 [78] | PSP (5) | PPAOS 17 | 0 |
|
| Ko et al., 2017 [79] | PSP (38), CBS (42) | PD, DLB, MSA | 0 |
|
| Ge et al., 2018 [80] | PSP (20) | PD, MSA, NC | 0 |
|
| Arnone et al., 2022 [81] | PSP (10), CBS (8) | PD, MSA | 0 |
|
| Lu et al., 2023 [82] | PSP (155) | PD, MSA | 0 |
|
| Ouartassi et al., 2023 [83] | PSP (12), CBS (27) | PD, NC | 0 |
|
| Ali et al., 2024 [84] | PSP-RS (60), other PSP subtypes (51) | - | 0 |
|
| Du et al., 2024 [85] | PSP (15) | PD, MSA-P, VP 18 | 0 |
|
| Ling et al., 2024 [86] | PSP (234) | PD, MSA, NC | 0 |
|
| Ling et al., 2025 [87] | PSP (234) | PD, MSA, NC | 0 |
|
| Pillai et al., 2025 [88] | PSP-RS (32), PSP-P (35), PSP-PI 19 (19), PSP-CBS 20 (4), PSP-SL 21 (3), PSP-OM 22 (1) | PD | 0 |
|
| Stokelj et al., 2025 [89] | PSP (37) | PD, MSA | 0 |
|
| Study | 4Rtauopathy 4 and Number of Patients (n) | Comparison Groups | Autopsy—Number of Confirmed Cases(n) | Main Findings |
|---|---|---|---|---|
| Cerami et al., 2016 [91] | PSP, CBD 5 | nfvPPA 6, svPPA 7, lvPPA 8 | 0 |
|
| Cerami et al., 2020 [92] | CBS | AD 9 | 0 |
|
| Caminiti et al., 2018 [14] | PSP, CBD | MCI 11, AD, FTD 12, DLB 13, bvFTD 14, PCA 15 | 0 |
|
| Bergeron et al., 2020 [93] | CBS | AD, bvFTD, nfvPPA, svPPA, lvPPA, PCA | 0 |
|
| Heikkinen et al., 2022 [94] | PSP, CBD | bvFTD | 0 |
|
| Isella et al., 2022 [95] | PSP, CBS | AD, PPA 16, PCA, DLB, bvFTD | 0 |
|
| Teune et al., 2010 [96] | PSP (17), CBD (10) | PD 17, DLB 18, MSA 19, AD, FTD | 0 |
|
| Wenzel et al., 2010 [97] | CBD (4) | AD, DLB, FTD, NC 20 | 0 |
|
| Garibotto et al., 2013 [98] | CBD (6) | AD, DLB, FTD, PDD 21 | 0 |
|
| Taswell et al., 2015 [99] | CBS (14) | AD, nfvPPA, svPPA, lvPPA | 0 |
|
| Franceschi et al., 2020 [100] | CBD (12) | AD, DLB, FTD | 0 |
|
| Franceschi et al., 2020 [101] | CBD (3) | AD, lvPPA, svPPA, bvFTD | 0 |
|
| Franceschi et al., 2021 [102] | PSP (5), CBD (12) | bvFTD, lvPPA, svPPA, nfvPPA | 0 |
|
| Joesphs et al, 2021 [103] | PSP (10), CBD (17) | TDP 23, AGD 24, Pick’s disease | 32 |
|
| Seckin et al., 2020 [104] | PSP (6), CBS (3) | - | 0 |
|
| Gan et al., 2023 [105] | PSP (37), CBD (16) | AD, DLB, bvFTD, nfvPPA, FTD-ALS 25, VaD 26, MCI 27 | 0 |
|
| Braun et al., 2025 [106] | PSP-RS 28 (18), PSP-P (7) 29, PSP-CBS 30 (3), PSP-F 31 (1), PSP-PGF 32 (2), PSP-PLS 33 (1), PSP-PI 34 (2), CBD (26) | AD, PD, DLB, MSA, TDP, PPAOS, PCA, AGD, Pick’s disease | 137 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakis, A.; Kloufetou, E.; Pechlivani, L.; Sioka, C.; Alexiou, G.; Konitsiotis, S.; Kyritsis, A.P. A Review of FDG-PET in Progressive Supranuclear Palsy and Corticobasal Syndrome. Int. J. Mol. Sci. 2025, 26, 8278. https://doi.org/10.3390/ijms26178278
Giannakis A, Kloufetou E, Pechlivani L, Sioka C, Alexiou G, Konitsiotis S, Kyritsis AP. A Review of FDG-PET in Progressive Supranuclear Palsy and Corticobasal Syndrome. International Journal of Molecular Sciences. 2025; 26(17):8278. https://doi.org/10.3390/ijms26178278
Chicago/Turabian StyleGiannakis, Alexandros, Eugenia Kloufetou, Louisa Pechlivani, Chrissa Sioka, George Alexiou, Spiridon Konitsiotis, and Athanassios P. Kyritsis. 2025. "A Review of FDG-PET in Progressive Supranuclear Palsy and Corticobasal Syndrome" International Journal of Molecular Sciences 26, no. 17: 8278. https://doi.org/10.3390/ijms26178278
APA StyleGiannakis, A., Kloufetou, E., Pechlivani, L., Sioka, C., Alexiou, G., Konitsiotis, S., & Kyritsis, A. P. (2025). A Review of FDG-PET in Progressive Supranuclear Palsy and Corticobasal Syndrome. International Journal of Molecular Sciences, 26(17), 8278. https://doi.org/10.3390/ijms26178278








