Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease
Abstract
1. Introduction
2. Biological Basis and Clinical Potential of EVs
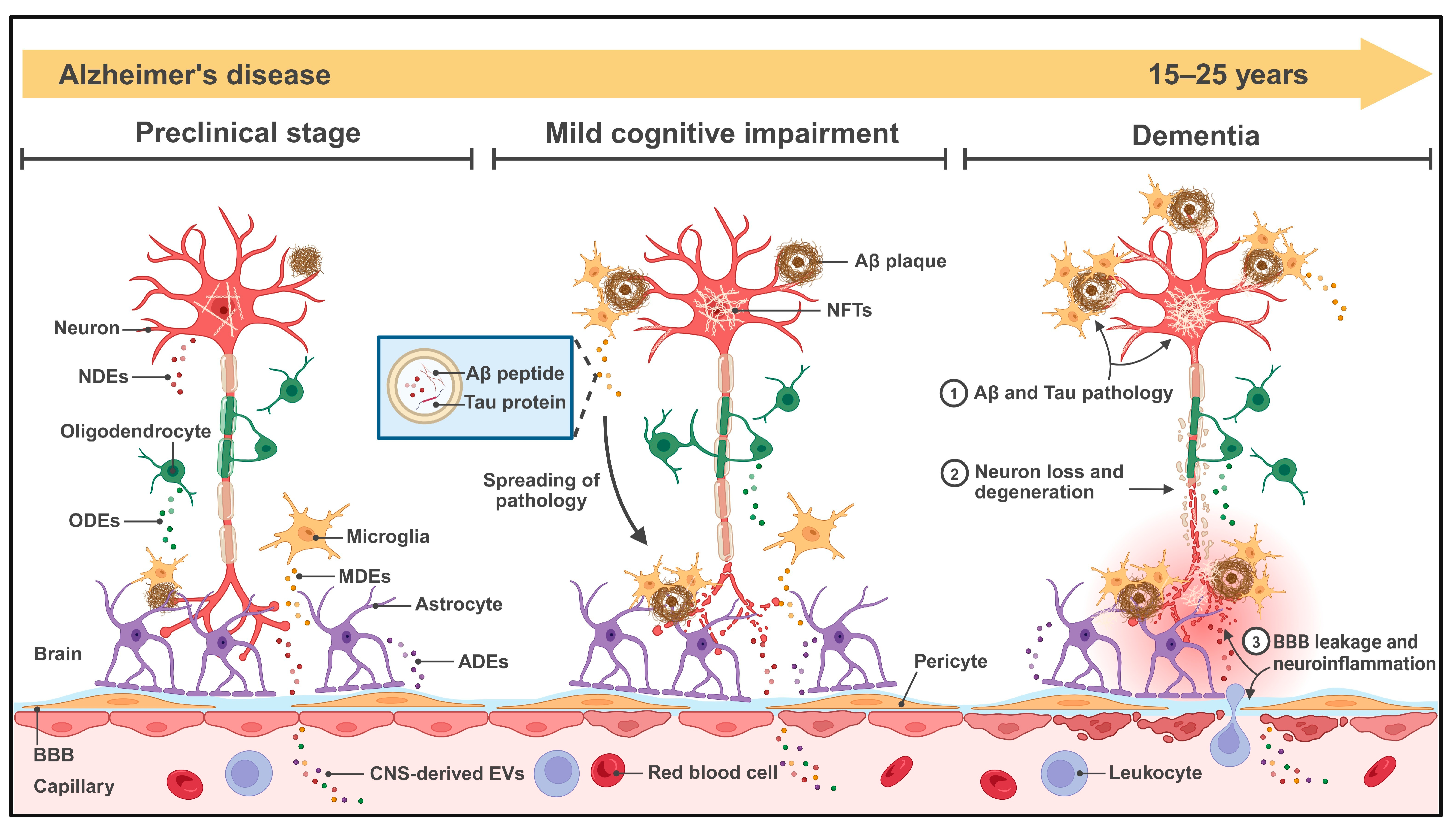
3. The Role of CNS-Derived EVs in AD Pathogenesis
3.1. CNS-Derived EVs and Aβ Pathology
3.2. CNS-Derived EVs and Tau Pathology
3.3. CNS-Derived EVs and Other Mechanisms in AD Progression
4. Promising CNS-Derived EV Biomarkers in AD Progression
4.1. NDE Biomarkers
4.2. Glia-Derived EV Biomarkers
5. Isolation and Enrichment Technologies for CNS-Derived EVs in AD Diagnostics
5.1. Density-Based Separation
5.2. Size-Based Separation
5.3. Precipitation Methods
5.4. Charge-/Dielectric-Based Separation
5.5. Immunoaffinity-Based Separation
5.6. Acoustic Separation
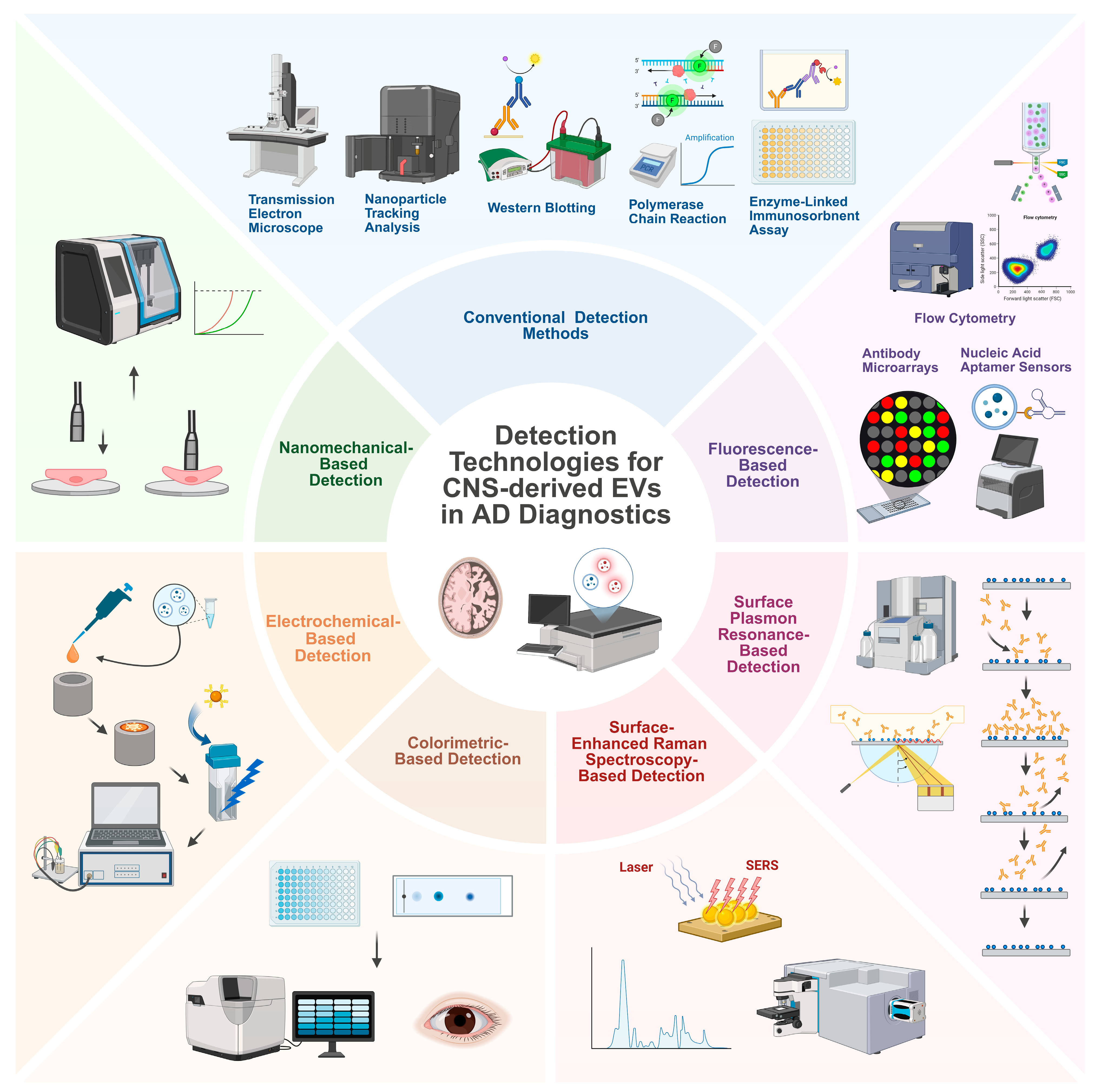
6. New Detection Technologies for CNS-Derived EVs in AD Diagnostics
6.1. Fluorescence-Based Detection
6.1.1. FCM
6.1.2. Antibody Microarrays
6.1.3. Nucleic Acid Aptamer Sensors
6.2. SPR-Based Detection
6.3. SERS-Based Detection
6.4. Colorimetric-Based Detection
6.5. Electrochemical-Based Detection
6.6. Nanomechanical-Based Detection
7. Discussion
7.1. Clinical Utility and Applications
7.2. Current Limitations and Challenges
7.3. Emerging Directions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Ogbodo, J.O.; Agbo, C.P.; Njoku, U.O.; Ogugofor, M.O.; Egba, S.I.; Ihim, S.A.; Echezona, A.C.; Brendan, K.C.; Upaganlawar, A.B.; Upasani, C.D. Alzheimer’s Disease: Pathogenesis and Therapeutic Interventions. Curr. Aging Sci. 2022, 15, 2–25. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Jia, J.; Ning, Y.; Chen, M.; Wang, S.; Yang, H.; Li, F.; Ding, J.; Li, Y.; Zhao, B.; Lyu, J.; et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 712–722. [Google Scholar] [CrossRef]
- Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [CrossRef]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Kumar, A.; Nader, M.A.; Deep, G. Emergence of Extracellular Vesicles as “Liquid Biopsy” for Neurological Disorders: Boom or Bust. Pharmacol. Rev. 2024, 76, 199–227. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Watson, L.S.; Hamlett, E.D.; Stone, T.D.; Sims-Robinson, C. Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 22. [Google Scholar] [CrossRef]
- Park, C.; Weerakkody, J.S.; Schneider, R.; Miao, S.; Pitt, D. CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases. Front. Neurosci. 2024, 18, 1426700. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Bravo-Miana, R.D.C.; Arizaga-Echebarria, J.K.; Otaegui, D. Central nervous system-derived extracellular vesicles: The next generation of neural circulating biomarkers? Transl. Neurodegener. 2024, 13, 32. [Google Scholar] [CrossRef]
- Xu, X.; Iqbal, Z.; Xu, L.; Wen, C.; Duan, L.; Xia, J.; Yang, N.; Zhang, Y.; Liang, Y. Brain-derived extracellular vesicles: Potential diagnostic biomarkers for central nervous system diseases. Psychiatry Clin. Neurosci. 2024, 78, 83–96. [Google Scholar] [CrossRef]
- Fan, B.; Gu, J.; Wu, J.; Sun, Y.; Huang, R.; Shen, H.; Zhang, X.; Li, Z. Circulating Abnormal Extracellular Vesicles: Their Mechanism for Crossing Blood-Brain Barrier, Effects on Central Nervous System and Detection Methods. J. Biomed. Nanotechnol. 2022, 18, 640–659. [Google Scholar] [CrossRef]
- Visan, K.S.; Wu, L.Y.; Voss, S.; Wuethrich, A.; Möller, A. Status quo of Extracellular Vesicle isolation and detection methods for clinical utility. Semin. Cancer Biol. 2023, 88, 157–171. [Google Scholar] [CrossRef]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Matos, M.; Gutiérrez, G.; Moyano, A.; Salvador, M.; Rivas, M.; Blanco-López, M.C. Extracellular Vesicles: Current Analytical Techniques for Detection and Quantification. Biomolecules 2020, 10, 824. [Google Scholar] [CrossRef]
- Adnan, S.-s.; Benjamin, G.T.; David, R.W. Advances in extracellular vesicle isolation methods: A path towards cell-type specific EV isolation. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 447–460. [Google Scholar] [CrossRef]
- Tran, H.L.; Zheng, W.; Issadore, D.A.; Im, H.; Cho, Y.-K.; Zhang, Y.; Liu, D.; Liu, Y.; Li, B.; Liu, F.; et al. Extracellular Vesicles for Clinical Diagnostics: From Bulk Measurements to Single-Vesicle Analysis. ACS Nano 2025, 19, 28021–28109. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Fourcade, O.; Simon, M.F.; Viodé, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournié, B.; Sarda, L.; Chap, H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Cabrera-Pastor, A. Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies. Int. J. Mol. Sci. 2024, 25, 10068. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Delport, A.; Hewer, R. The amyloid precursor protein: A converging point in Alzheimer’s disease. Mol. Neurobiol. 2022, 59, 4501–4516. [Google Scholar] [CrossRef]
- Yuyama, K.; Yamamoto, N.; Yanagisawa, K. Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality: Another putative pathway for GM1-induced amyloid fibril formation. J. Neurochem. 2008, 105, 217–224. [Google Scholar] [CrossRef]
- Khursheed, A.; Viles, J.H. Impact of Membrane Phospholipids and Exosomes on the Kinetics of Amyloid-β Fibril Assembly. J. Mol. Biol. 2024, 436, 168464. [Google Scholar] [CrossRef]
- Falker, C.; Hartmann, A.; Guett, I.; Dohler, F.; Altmeppen, H.; Betzel, C.; Schubert, R.; Thurm, D.; Wegwitz, F.; Joshi, P.; et al. Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J. Neurochem. 2016, 137, 88–100. [Google Scholar] [CrossRef]
- Sharples, R.A.; Vella, L.J.; Nisbet, R.M.; Naylor, R.; Perez, K.; Barnham, K.J.; Masters, C.L.; Hill, A.F. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008, 22, 1469–1478. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Eitan, E.; Hutchison, E.R.; Marosi, K.; Comotto, J.; Mustapic, M.; Nigam, S.M.; Suire, C.; Maharana, C.; Jicha, G.A.; Liu, D.; et al. Extracellular Vesicle-Associated Aβ Mediates Trans-Neuronal Bioenergetic and Ca(2+)-Handling Deficits in Alzheimer’s Disease Models. NPJ Aging Mech. Dis. 2016, 2, 16019. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1792–1800. [Google Scholar] [CrossRef]
- Huang, S.; Liao, X.; Wu, J.; Zhang, X.; Li, Y.; Xiang, D.; Luo, S. The Microglial membrane receptor TREM2 mediates exosome secretion to promote phagocytosis of amyloid-β by microglia. FEBS Lett. 2022, 596, 1059–1071. [Google Scholar] [CrossRef]
- Tamboli, I.Y.; Barth, E.; Christian, L.; Siepmann, M.; Kumar, S.; Singh, S.; Tolksdorf, K.; Heneka, M.T.; Lütjohann, D.; Wunderlich, P.; et al. Statins promote the degradation of extracellular amyloid {beta}-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 2010, 285, 37405–37414. [Google Scholar] [CrossRef]
- Fowler, S.L.; Behr, T.S.; Turkes, E.; O’Brien, D.P.; Cauhy, P.M.; Rawlinson, I.; Edmonds, M.; Foiani, M.S.; Schaler, A.; Crowley, G.; et al. Tau filaments are tethered within brain extracellular vesicles in Alzheimer’s disease. Nat. Neurosci. 2025, 28, 40–48. [Google Scholar] [CrossRef]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.; et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Ruan, Z.; Delpech, J.C.; Venkatesan Kalavai, S.; Van Enoo, A.A.; Hu, J.; Ikezu, S.; Ikezu, T. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol. Neurodegener. 2020, 15, 47. [Google Scholar] [CrossRef]
- Ruan, Z.; Pathak, D.; Venkatesan Kalavai, S.; Yoshii-Kitahara, A.; Muraoka, S.; Bhatt, N.; Takamatsu-Yukawa, K.; Hu, J.; Wang, Y.; Hersh, S.; et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 2021, 144, 288–309, Erratum in Brain 2021, 144, e42. [Google Scholar] [CrossRef]
- Weng, S.; Lai, Q.L.; Wang, J.; Zhuang, L.; Cheng, L.; Mo, Y.; Liu, L.; Zhao, Z.; Zhang, Y.; Qiao, S. The Role of Exosomes as Mediators of Neuroinflammation in the Pathogenesis and Treatment of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 899944. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun. 2020, 83, 270–282, Erratum in Brain Behav. Immun. 2020, 88, 959. [Google Scholar] [CrossRef]
- Gabrielli, M.; Tozzi, F.; Verderio, C.; Origlia, N. Emerging Roles of Extracellular Vesicles in Alzheimer’s Disease: Focus on Synaptic Dysfunction and Vesicle-Neuron Interaction. Cells 2022, 12, 63. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Y.; Pang, A.; Li, P.; Mei, S. M1-type microglia-derived exosomes contribute to blood-brain barrier damage. Brain Res. 2024, 1835, 148919. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Akers, J.C.; Carter, B.S.; Rockenstein, E.M.; Galasko, D.; Masliah, E.; Rissman, R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- Li, T.R.; Yao, Y.X.; Jiang, X.Y.; Dong, Q.Y.; Yu, X.F.; Wang, T.; Cai, Y.N.; Han, Y. β-Amyloid in blood neuronal-derived extracellular vesicles is elevated in cognitively normal adults at risk of Alzheimer’s disease and predicts cerebral amyloidosis. Alzheimers Res. Ther. 2022, 14, 66. [Google Scholar] [CrossRef]
- Gu, D.; Liu, F.; Meng, M.; Zhang, L.; Gordon, M.L.; Wang, Y.; Cai, L.; Zhang, N. Elevated matrix metalloproteinase-9 levels in neuronal extracellular vesicles in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 1681–1691. [Google Scholar] [CrossRef]
- Eitan, E.; Thornton-Wells, T.; Elgart, K.; Erden, E.; Gershun, E.; Levine, A.; Volpert, O.; Azadeh, M.; Smith, D.G.; Kapogiannis, D. Synaptic proteins in neuron-derived extracellular vesicles as biomarkers for Alzheimer’s disease: Novel methodology and clinical proof of concept. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 133–150. [Google Scholar] [CrossRef]
- Li, Y.; Meng, S.; Di, W.; Xia, M.; Dong, L.; Zhao, Y.; Ling, S.; He, J.; Xue, X.; Chen, X.; et al. Amyloid-β protein and MicroRNA-384 in NCAM-Labeled exosomes from peripheral blood are potential diagnostic markers for Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 1093–1107. [Google Scholar] [CrossRef]
- Li, Y.; Xia, M.; Meng, S.; Wu, D.; Ling, S.; Chen, X.; Liu, C. MicroRNA-29c-3p in dual-labeled exosome is a potential diagnostic marker of subjective cognitive decline. Neurobiol. Dis. 2022, 171, 105800. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mustapic, M.; Shardell, M.D.; Berkowitz, S.T.; Diehl, T.C.; Spangler, R.D.; Tran, J.; Lazaropoulos, M.P.; Chawla, S.; Gulyani, S.; et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2019, 76, 1340–1351. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Winston, C.N.; Barlow, J.W.; Sarsoza, F.M.; Alvarez, I.; Aleixandre, M.; Linares, C.; García-Fantini, M.; Kastberger, B.; Winter, S.; et al. Modulation of Amyloid-β and Tau in Alzheimer’s Disease Plasma Neuronal-Derived Extracellular Vesicles by Cerebrolysin® and Donepezil. J. Alzheimers Dis. 2022, 90, 705–717. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Kapogiannis, D.; Schwartz, J.B.; Lobach, I.V.; Goetzl, L.; Abner, E.L.; Jicha, G.A.; Karydas, A.M.; Boxer, A.; Miller, B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016, 30, 4141–4148. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimers Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D.; Schwartz, J.B. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018, 32, 888–893. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bianchi, A.; Nemni, R.; Clerici, M. SNAP-25 in Serum Is Carried by Exosomes of Neuronal Origin and Is a Potential Biomarker of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 5792–5798. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Wang, T.; Yao, Y.; Han, C.; Li, T.; Du, W.; Xue, J.; Han, Y.; Cai, Y. MCP-1 levels in astrocyte-derived exosomes are changed in preclinical stage of Alzheimer’s disease. Front. Neurol. 2023, 14, 1119298. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J. 2019, 33, 231–238. [Google Scholar] [CrossRef]
- Zhong, J.; Ren, X.; Liu, W.; Wang, S.; Lv, Y.; Nie, L.; Lin, R.; Tian, X.; Yang, X.; Zhu, F.; et al. Discovery of Novel Markers for Identifying Cognitive Decline Using Neuron-Derived Exosomes. Front. Aging Neurosci. 2021, 13, 696944. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tufekci, K.U.; Olcum, M.; Durur, D.Y.; Akarlar, B.A.; Ozlu, N.; Bagriyanik, H.A.; Keskinoglu, P.; Yener, G.; Genc, S. Proteome profiling of neuron-derived exosomes in Alzheimer’s disease reveals hemoglobin as a potential biomarker. Neurosci. Lett. 2021, 755, 135914. [Google Scholar] [CrossRef]
- Yao, P.J.; Eren, E.; Goetzl, E.J.; Kapogiannis, D. Mitochondrial Electron Transport Chain Protein Abnormalities Detected in Plasma Extracellular Vesicles in Alzheimer’s Disease. Biomedicines 2021, 9, 1587. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Kapogiannis, D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015, 85, 40–47. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Biragyn, A.; Masharani, U.; Frassetto, L.; Petersen, R.C.; Miller, B.L.; Goetzl, E.J. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015, 29, 589–596. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef]
- Durur, D.Y.; Tastan, B.; Ugur Tufekci, K.; Olcum, M.; Uzuner, H.; Karakülah, G.; Yener, G.; Genc, S. Alteration of miRNAs in Small Neuron-Derived Extracellular Vesicles of Alzheimer’s Disease Patients and the Effect of Extracellular Vesicles on Microglial Immune Responses. J. Mol. Neurosci. 2022, 72, 1182–1194. [Google Scholar] [CrossRef]
- Pounders, J.; Hill, E.J.; Hooper, D.; Zhang, X.; Biesiada, J.; Kuhnell, D.; Greenland, H.L.; Esfandiari, L.; Timmerman, E.; Foster, F.; et al. MicroRNA expression within neuronal-derived small extracellular vesicles in frontotemporal degeneration. Medicine 2022, 101, e30854. [Google Scholar] [CrossRef]
- Kumar, A.; Su, Y.; Sharma, M.; Singh, S.; Kim, S.; Peavey, J.J.; Suerken, C.K.; Lockhart, S.N.; Whitlow, C.T.; Craft, S.; et al. MicroRNA expression in extracellular vesicles as a novel blood-based biomarker for Alzheimer’s disease. Alzheimers Dement. 2023, 19, 4952–4966. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef]
- Serpente, M.; Fenoglio, C.; D’Anca, M.; Arcaro, M.; Sorrentino, F.; Visconte, C.; Arighi, A.; Fumagalli, G.G.; Porretti, L.; Cattaneo, A.; et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells 2020, 9, 1443. [Google Scholar] [CrossRef]
- Bigbee, J.W. Cells of the Central Nervous System: An Overview of Their Structure and Function. Adv. Neurobiol. 2023, 29, 41–64. [Google Scholar]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Krämer-Albers, E.M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom. Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef]
- Momen-Heravi, F. Isolation of Extracellular Vesicles by Ultracentrifugation. Methods Mol. Biol. 2017, 1660, 25–32. [Google Scholar]
- André-Grégoire, G.; Roux, Q.; Gavard, J. Isolating plasma extracellular vesicles from mouse blood using size-exclusion chromatography, density gradient, and ultracentrifugation. STAR Protoc. 2023, 4, 102740. [Google Scholar] [CrossRef]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef]
- Allelein, S.; Medina-Perez, P.; Lopes, A.L.H.; Rau, S.; Hause, G.; Kölsch, A.; Kuhlmeier, D. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci. Rep. 2021, 11, 11585. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, J.; Ning, H.; Liu, W.; Han, X. Exosome isolation based on polyethylene glycol (PEG): A review. Mol. Cell. Biochem. 2025, 480, 2847–2861. [Google Scholar] [CrossRef]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; da Cruz e Silva, O.A.B.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef]
- Back, W.; Bang, M.; Jung, J.H.; Kang, K.W.; Choi, B.H.; Choi, Y.; Hong, S.; Kim, H.K.; Park, Y.; Park, J.H. Charge-Based Isolation of Extracellular Vesicles from Human Plasma. ACS Omega 2024, 9, 17832–17838. [Google Scholar] [CrossRef]
- Preußer, C.; Stelter, K.; Tertel, T.; Linder, M.; Helmprobst, F.; Szymanski, W.; Graumann, J.; Giebel, B.; Reinartz, S.; Müller, R.; et al. Isolation of native EVs from primary biofluids-Free-flow electrophoresis as a novel approach to purify ascites-derived EVs. J. Extracell. Biol. 2022, 1, e71, Erratum in J. Extracell. Biol. 2023, 2, e80 and J. Extracell. Biol. 2023, 2, e81. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Rafatmah, E.; Shojaeifard, Z. Microfluidic paper and thread-based separations: Chromatography and electrophoresis. J. Chromatogr. A 2023, 1704, 464117. [Google Scholar] [CrossRef]
- Mondal, S.K.; Whiteside, T.L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol. Biol. 2021, 2265, 305–321. [Google Scholar]
- Xu, W.M.; Li, A.; Chen, J.J.; Sun, E.J. Research Development on Exosome Separation Technology. J. Membr. Biol. 2023, 256, 25–34. [Google Scholar] [CrossRef]
- Hou, G.S.; Yuan, H.M.; Liang, Z.; Zhang, L.H.; Zhang, Y.K. Exosome separation and enrichment technologies and their applications in disease diagnosis and treatment. Se Pu 2025, 43, 434–445. [Google Scholar] [CrossRef]
- Ku, A.; Lim, H.C.; Evander, M.; Lilja, H.; Laurell, T.; Scheding, S.; Ceder, Y. Acoustic Enrichment of Extracellular Vesicles from Biological Fluids. Anal. Chem. 2018, 90, 8011–8019. [Google Scholar] [CrossRef]
- Havers, M.; Broman, A.; Lenshof, A.; Laurell, T. Advancement and obstacles in microfluidics-based isolation of extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1265–1285. [Google Scholar] [CrossRef]
- Liu, J.; Qu, Y.; Wang, H. Immuno-Acoustic Sorting of Disease-Specific Extracellular Vesicles by Acoustophoretic Force. Micromachines 2021, 12, 1534. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef]
- Chatterjee, M.; Özdemir, S.; Kunadt, M.; Koel-Simmelink, M.; Boiten, W.; Piepkorn, L.; Pham, T.V.; Chiasserini, D.; Piersma, S.R.; Knol, J.C.; et al. C1q is increased in cerebrospinal fluid-derived extracellular vesicles in Alzheimer’s disease: A multi-cohort proteomics and immuno-assay validation study. Alzheimers Dement. 2023, 19, 4828–4840. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Kangas, P.; Nyman, T.A.; Metsähonkala, L.; Burns, C.; Tempest, R.; Williams, T.; Karttunen, J.; Jokinen, T.S. Towards optimised extracellular vesicle proteomics from cerebrospinal fluid. Sci. Rep. 2023, 13, 9564. [Google Scholar] [CrossRef]
- Tzaridis, T.; Bachurski, D.; Liu, S.; Surmann, K.; Babatz, F.; Gesell Salazar, M.; Völker, U.; Hallek, M.; Herrlinger, U.; Vorberg, I.; et al. Extracellular Vesicle Separation Techniques Impact Results from Human Blood Samples: Considerations for Diagnostic Applications. Int. J. Mol. Sci. 2021, 22, 9211. [Google Scholar] [CrossRef]
- Lee, E.E.; Winston-Gray, C.; Barlow, J.W.; Rissman, R.A.; Jeste, D.V. Plasma Levels of Neuron- and Astrocyte-Derived Exosomal Amyloid Beta1-42, Amyloid Beta1-40, and Phosphorylated Tau Levels in Schizophrenia Patients and Non-psychiatric Comparison Subjects: Relationships With Cognitive Functioning and Psychopathology. Front. Psychiatry 2020, 11, 532624. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Serra, A.; Sze, S.K. Enrichment of extracellular vesicles from tissues of the central nervous system by PROSPR. Mol. Neurodegener. 2016, 11, 41. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef]
- Lee, S.; Roh, S.M.; Lee, E.; Park, Y.; Lee, B.C.; Kwon, Y.; Kim, H.J.; Kim, J. Applications of Converged Various Forces for Detection of Biomolecules and Novelty of Dielectrophoretic Force in the Applications. Sensors 2020, 20, 3242. [Google Scholar] [CrossRef]
- Chen, W.; Xie, Y.; Chang, Y.; Xu, Y.; Zhao, M.; Deng, P.; Qin, J.; Li, H. A Portable Device for Simple Exosome Separation from Biological Samples. Micromachines 2021, 12, 1182. [Google Scholar] [CrossRef]
- Dilsiz, N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef]
- Solovicová, V.; Ďatková, A.; Bertók, T.; Kasák, P.; Vikartovská, A.; Lorencová, L.; Tkac, J. Advances in magnetic affinity-based isolation/detection of exosomes for robust diagnostics. Mikrochim. Acta 2025, 192, 206. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Nogueras-Ortiz, C.J.; Eren, E.; Yao, P.; Calzada, E.; Dunn, C.; Volpert, O.; Delgado-Peraza, F.; Mustapic, M.; Lyashkov, A.; Rubio, F.J.; et al. Single-extracellular vesicle (EV) analyses validate the use of L1 Cell Adhesion Molecule (L1CAM) as a reliable biomarker of neuron-derived EVs. J. Extracell. Vesicles 2024, 13, e12459. [Google Scholar] [CrossRef]
- Collier, M.E.W.; Allcock, N.; Sylvius, N.; Cassidy, J.; Giorgini, F. Examination of the enrichment of neuronal extracellular vesicles from cell conditioned media and human plasma using an anti-NCAM immunocapture bead approach. BMC Methods 2025, 2, 12. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Ciappelloni, S.; Bouchet, D.; Dubourdieu, N.; Boué-Grabot, E.; Kellermayer, B.; Manso, C.; Marignier, R.; Oliet, S.H.R.; Tourdias, T.; Groc, L. Aquaporin-4 Surface Trafficking Regulates Astrocytic Process Motility and Synaptic Activity in Health and Autoimmune Disease. Cell Rep. 2019, 27, 3860–3872.e3864. [Google Scholar] [CrossRef]
- Willis, C.M.; Ménoret, A.; Jellison, E.R.; Nicaise, A.M.; Vella, A.T.; Crocker, S.J. A Refined Bead-Free Method to Identify Astrocytic Exosomes in Primary Glial Cultures and Blood Plasma. Front. Neurosci. 2017, 11, 335. [Google Scholar] [CrossRef]
- Valle-Tamayo, N.; Pérez-González, R.; Chiva-Blanch, G.; Belbin, O.; Serrano-Requena, S.; Sirisi, S.; Cervantes González, A.; Giró, O.; Sánchez-Aced, É.; Dols-Icardo, O.; et al. Enrichment of Astrocyte-Derived Extracellular Vesicles from Human Plasma. J. Vis. Exp. 2022, 186, e64107. [Google Scholar]
- Visconte, C.; Golia, M.T.; Fenoglio, C.; Serpente, M.; Gabrielli, M.; Arcaro, M.; Sorrentino, F.; Busnelli, M.; Arighi, A.; Fumagalli, G.; et al. Plasma microglial-derived extracellular vesicles are increased in frail patients with Mild Cognitive Impairment and exert a neurotoxic effect. Geroscience 2023, 45, 1557–1571. [Google Scholar] [CrossRef]
- Arvanitaki, E.S.; Goulielmaki, E.; Gkirtzimanaki, K.; Niotis, G.; Tsakani, E.; Nenedaki, E.; Rouska, I.; Kefalogianni, M.; Xydias, D.; Kalafatakis, I.; et al. Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. USA 2024, 121, e2317402121. [Google Scholar] [CrossRef]
- You, Y.; Muraoka, S.; Jedrychowski, M.P.; Hu, J.; McQuade, A.K.; Young-Pearse, T.; Aslebagh, R.; Shaffer, S.A.; Gygi, S.P.; Blurton-Jones, M.; et al. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. J. Extracell. Vesicles 2022, 11, e12183. [Google Scholar] [CrossRef]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef]
- Chen, M.; Pei, Z.; Wang, Y.; Song, F.; Zhong, J.; Wang, C.; Ma, Y. Small extracellular vesicles’ enrichment from biological fluids using an acoustic trap. Analyst 2024, 149, 3169–3177. [Google Scholar] [CrossRef]
- Sattarov, R.; Havers, M.; Orbjörn, C.; Stomrud, E.; Janelidze, S.; Laurell, T.; Mattsson-Carlgren, N. Phosphorylated tau in cerebrospinal fluid-derived extracellular vesicles in Alzheimer’s disease: A pilot study. Sci. Rep. 2024, 14, 25419. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Zhang, W.; Zhou, W.; Liu, X.; Quan, H.; Li, J.; Li, P.; Li, Y.; Hu, C.; et al. Oscillating microbubble array-based metamaterials (OMAMs) for rapid isolation of high-purity exosomes. Sci. Adv. 2025, 11, eadu8915. [Google Scholar] [CrossRef]
- Das, S.; Lyon, C.J.; Hu, T. A Panorama of Extracellular Vesicle Applications: From Biomarker Detection to Therapeutics. ACS Nano 2024, 18, 9784–9797. [Google Scholar] [CrossRef]
- Khaksari, S.; Abnous, K.; Hadizadeh, F.; Ramezani, M.; Taghdisi, S.M.; Mousavi Shaegh, S.A. Signal amplification strategies in biosensing of extracellular vesicles (EVs). Talanta 2023, 256, 124244. [Google Scholar] [CrossRef]
- Manohar, S.M.; Shah, P.; Nair, A. Flow cytometry: Principles, applications and recent advances. Bioanalysis 2021, 13, 181–198. [Google Scholar] [CrossRef]
- Tian, C.; Stewart, T.; Hong, Z.; Guo, Z.; Aro, P.; Soltys, D.; Pan, C.; Peskind, E.R.; Zabetian, C.P.; Shaw, L.M.; et al. Blood extracellular vesicles carrying synaptic function- and brain-related proteins as potential biomarkers for Alzheimer’s disease. Alzheimers Dement. 2023, 19, 909–923. [Google Scholar] [CrossRef]
- Dayarathna, T.; Roseborough, A.D.; Gomes, J.; Khazaee, R.; Silveira, C.R.A.; Borron, K.; Yu, S.; Coleman, K.; Jesso, S.; Finger, E.; et al. Nanoscale flow cytometry-based quantification of blood-based extracellular vesicle biomarkers distinguishes MCI and Alzheimer’s disease. Alzheimers Dement. 2024, 20, 6094–6106. [Google Scholar] [CrossRef]
- Brahmer, A.; Geiß, C.; Lygeraki, A.; Neuberger, E.; Tzaridis, T.; Nguyen, T.T.; Luessi, F.; Régnier-Vigouroux, A.; Hartmann, G.; Simon, P.; et al. Assessment of technical and clinical utility of a bead-based flow cytometry platform for multiparametric phenotyping of CNS-derived extracellular vesicles. Cell Commun. Signal. 2023, 21, 276. [Google Scholar] [CrossRef]
- Bettin, B.A.; Varga, Z.; Nieuwland, R.; van der Pol, E. Standardization of extracellular vesicle concentration measurements by flow cytometry: The past, present, and future. J. Thromb. Haemost. 2023, 21, 2032–2044. [Google Scholar] [CrossRef]
- Gul, B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of extracellular vesicles by flow cytometry: Challenges and promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef]
- Aparna, G.M.; Tetala, K.K.R. Recent Progress in Development and Application of DNA, Protein, Peptide, Glycan, Antibody, and Aptamer Microarrays. Biomolecules 2023, 13, 602. [Google Scholar] [CrossRef]
- Martel, R.; Shen, M.L.; DeCorwin-Martin, P.; de Araujo, L.O.F.; Juncker, D. Extracellular Vesicle Antibody Microarray for Multiplexed Inner and Outer Protein Analysis. ACS Sens. 2022, 7, 3817–3828. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Igarashi, Y.; Monde, K.; Hirase, T.; Nakayama, M.; Makino, Y. Immuno-digital invasive cleavage assay for analyzing Alzheimer’s amyloid ß-bound extracellular vesicles. Alzheimers Res. Ther. 2022, 14, 140. [Google Scholar] [CrossRef]
- Chen, Z.; Dodig-Crnković, T.; Schwenk, J.M.; Tao, S.C. Current applications of antibody microarrays. Clin. Proteom. 2018, 15, 7. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef]
- Hu, J.; Gao, D. Recent Advances in Aptamer-Based Microfluidic Biosensors for the Isolation, Signal Amplification and Detection of Exosomes. Sensors 2025, 25, 848. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, L.; Ye, W.; Wang, Q.; Geng, S.; Sun, C. A sensitive detection assay based on signal amplification technology for Alzheimer’s disease’s early biomarker in exosome. Anal. Chim. Acta 2018, 1022, 124–130. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Zhang, J.; Luo, F.; Ma, H.; Guan, M.; Feng, J.; Dong, X. Dumbbell Aptamer Sensor Based on Dual Biomarkers for Early Detection of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2023, 15, 16394–16407. [Google Scholar] [CrossRef]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef]
- Zhao, L.; Kuang, J.; Xiang, K.; Gan, J.; Zeng, Y.; Zhang, X.; Yan, Y.; Zhang, M.; Zhang, H.; Hu, P. Dual-mode exosome detection leveraging a nanozyme-active artificial receptor: PDA@Fe@Zn-based nucleic acid aptamer sensor. Talanta 2025, 285, 127380. [Google Scholar] [CrossRef]
- Chiu, N.F. The Current Status and Future Promise of SPR Biosensors. Biosensors 2022, 12, 933. [Google Scholar] [CrossRef]
- Chin, L.K.; Son, T.; Hong, J.S.; Liu, A.Q.; Skog, J.; Castro, C.M.; Weissleder, R.; Lee, H.; Im, H. Plasmonic Sensors for Extracellular Vesicle Analysis: From Scientific Development to Translational Research. ACS Nano 2020, 14, 14528–14548. [Google Scholar] [CrossRef]
- Song, S.; Lee, J.U.; Jeon, M.J.; Kim, S.; Sim, S.J. Detection of multiplex exosomal miRNAs for clinically accurate diagnosis of Alzheimer’s disease using label-free plasmonic biosensor based on DNA-Assembled advanced plasmonic architecture. Biosens. Bioelectron. 2022, 199, 113864. [Google Scholar] [CrossRef]
- Song, S.; Lee, J.U.; Jeon, M.J.; Kim, S.; Lee, C.N.; Sim, S.J. Precise profiling of exosomal biomarkers via programmable curved plasmonic nanoarchitecture-based biosensor for clinical diagnosis of Alzheimer’s disease. Biosens. Bioelectron. 2023, 230, 115269. [Google Scholar] [CrossRef]
- Qiu, G.; Thakur, A.; Xu, C.; Ng, S.-P.; Lee, Y.; Wu, C.-M.L. Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 2019, 29, 1806761. [Google Scholar] [CrossRef]
- Špringer, T.; Bocková, M.; Slabý, J.; Sohrabi, F.; Čapková, M.; Homola, J. Surface plasmon resonance biosensors and their medical applications. Biosens. Bioelectron. 2025, 278, 117308. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Shin, H.; Seo, D.; Choi, Y. Extracellular Vesicle Identification Using Label-Free Surface-Enhanced Raman Spectroscopy: Detection and Signal Analysis Strategies. Molecules 2020, 25, 5209. [Google Scholar] [CrossRef]
- Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. [Google Scholar] [CrossRef]
- Yan, Z.; Dutta, S.; Liu, Z.; Yu, X.; Mesgarzadeh, N.; Ji, F.; Bitan, G.; Xie, Y.H. A Label-Free Platform for Identification of Exosomes from Different Sources. ACS Sens. 2019, 4, 488–497. [Google Scholar] [CrossRef]
- Stremersch, S.; Marro, M.; Pinchasik, B.E.; Baatsen, P.; Hendrix, A.; De Smedt, S.C.; Loza-Alvarez, P.; Skirtach, A.G.; Raemdonck, K.; Braeckmans, K. Identification of Individual Exosome-Like Vesicles by Surface Enhanced Raman Spectroscopy. Small 2016, 12, 3292–3301. [Google Scholar] [CrossRef]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced Raman spectroscopy–based biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Song, X.; Wang, H.; Wang, J.; Wang, Y.; Huang, J.; Yu, J. Robust and Universal SERS Sensing Platform for Multiplexed Detection of Alzheimer’s Disease Core Biomarkers Using PAapt-AuNPs Conjugates. ACS Sens. 2019, 4, 2140–2149. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, X. Surface-Enhanced Raman Scattering (SERS) for exosome detection. Clin. Chim. Acta 2025, 568, 120148. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Liu, H.; Kang, C.; Wang, C. Cancer diagnosis using label-free SERS-based exosome analysis. Theranostics 2024, 14, 1966–1981. [Google Scholar] [CrossRef]
- Huang, C.C.; Isidoro, C. Raman Spectrometric Detection Methods for Early and Non-Invasive Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1145–1156. [Google Scholar] [CrossRef]
- Bari, S.M.I.; Hossain, F.B.; Nestorova, G.G. Advances in Biosensors Technology for Detection and Characterization of Extracellular Vesicles. Sensors 2021, 21, 7645. [Google Scholar] [CrossRef]
- Duy Mac, K.; Su, J. Optical biosensors for diagnosing neurodegenerative diseases. NPJ Biosens 2025, 2, 20. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, M.; Liu, Y.; Wan, S.; Cui, C.; Zhang, L.; Tan, W. Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angew. Chem. Int. Ed. Engl. 2017, 56, 11916–11920. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Li, X.; Gu, M.; Xu, L.; Zhao, J.; Zhang, H. Rapid and simple separation and detection of Aβ42 in blood neuronal-derived extracellular vesicles using nanozyme-catalyzed colorimetric sensor. Talanta 2025, 296, 128439. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Hu, H.; Lv, M.; Zhang, H. A colorimetric nano-enzyme assay with Ni@Pt nanoparticles as signal labels for rapid and sensitive detection of exosomal Aβ42 in plasma. Mikrochim. Acta 2025, 192, 53. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Xu, W.; Zeng, R.; Weng, J.; Sun, L. A label-free colorimetric biosensor utilizing natural material for highly sensitive exosome detection. Talanta 2024, 275, 126182. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Tu, H.; Pan, C.; Chai, Y.; Chen, W. Advances in colorimetric biosensors of exosomes: Novel approaches based on natural enzymes and nanozymes. Nanoscale 2024, 16, 1005–1024. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xie, J.; Han, Y.; Huang, Y.; Zhang, H. Exosomal analysis: Advances in biosensor technology. Clin. Chim. Acta 2021, 518, 142–150. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Du, W.; Zhou, M.; Wang, G.; Lu, M.; Wang, X.; Guo, Z.; Ma, M.; Zhang, Y. A HPRR-based diatomic catalyst electrochemical biosensor for detecting cancer-related extracellular vesicles. Anal. Methods 2024, 16, 7381–7389. [Google Scholar] [CrossRef]
- Li, D.; Zou, S.; Huang, Z.; Sun, C.; Liu, G. Isolation and quantification of L1CAM-positive extracellular vesicles on a chip as a potential biomarker for Parkinson’s Disease. J. Extracell. Vesicles 2024, 13, e12467. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, X.; Bai, S.; Lai, M.; Yu, J.; Chen, M.; Zhou, R.; Jia, Y.; Yan, H.; Liang, Z.; et al. Clinically Accurate Diagnosis of Alzheimer’s Disease via Single-Molecule Bioelectronic Label-Free Profiling of Multiple Blood Extracellular Vesicle Biomarkers. Adv. Mater. 2025, e2505262. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yang, Y.; Cai, H.; Ao, Z.; Xing, Y.; Li, K.; Yang, K.; Guan, W.; Friend, J.; et al. Extracellular vesicle-based point-of-care testing for diagnosis and monitoring of Alzheimer’s disease. Microsyst. Nanoeng. 2025, 11, 65. [Google Scholar] [CrossRef]
- Babamiri, B.; Bahari, D.; Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: Recent advances and future directions. Biosens. Bioelectron. 2019, 142, 111530. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, R.; Liu, S.; Zheng, J.; Yan, H.; Su, S.; Chai, N.; Segal, E.; Jiang, C.; Guo, K.; et al. Electrochemical Biosensors for the Detection of Exosomal microRNA Biomarkers for Early Diagnosis of Neurodegenerative Diseases. Anal. Chem. 2025, 97, 5355–5371. [Google Scholar] [CrossRef]
- Matlyuba Jakhonkulovna, S.; Bahodirova Kamolovna, G.; Zokirov, M.; Umida Tajimuratovna, B.; Yumashev, A.; Shichiyakh, R.; Safarova, N.I.; Nargiza Nusratovna, A.; Esanmuradova, N.; Muyassar Karimbaevna, T.; et al. Electrochemical biosensors for early detection of Alzheimer’s disease. Clin. Chim. Acta 2025, 572, 120278. [Google Scholar] [CrossRef]
- Bae, M.; Kim, N.; Cho, E.; Lee, T.; Lee, J.H. Recent Advances in Electrochemical Biosensors for Neurodegenerative Disease Biomarkers. Biosensors 2025, 15, 151. [Google Scholar] [CrossRef]
- Ruz, J.J.; Malvar, O.; Gil-Santos, E.; Ramos, D.; Calleja, M.; Tamayo, J. A Review on Theory and Modelling of Nanomechanical Sensors for Biological Applications. Processes 2021, 9, 164. [Google Scholar] [CrossRef]
- Rao, D.; Mei, K.; Yan, T.; Wang, Y.; Wu, W.; Chen, Y.; Wang, J.; Zhang, Q.; Wu, S. Nanomechanical sensor for rapid and ultrasensitive detection of tumor markers in serum using nanobody. Nano Res. 2022, 15, 1003–1012. [Google Scholar] [CrossRef]
- Cafolla, C.; Philpott-Robson, J.; Elbourne, A.; Voïtchovsky, K. Quantitative Detection of Biological Nanovesicles in Drops of Saliva Using Microcantilevers. ACS Appl. Mater. Interfaces 2024, 16, 44–53. [Google Scholar] [CrossRef]
- Mei, K.; Yan, T.; Wang, Y.; Rao, D.; Peng, Y.; Wu, W.; Chen, Y.; Ren, M.; Yang, J.; Wu, S.; et al. Magneto-Nanomechanical Array Biosensor for Ultrasensitive Detection of Oncogenic Exosomes for Early Diagnosis of Cancers. Small 2023, 19, e2205445. [Google Scholar] [CrossRef]
- Suthar, J.; Parsons, E.S.; Hoogenboom, B.W.; Williams, G.R.; Guldin, S. Acoustic Immunosensing of Exosomes Using a Quartz Crystal Microbalance with Dissipation Monitoring. Anal. Chem. 2020, 92, 4082–4093. [Google Scholar] [CrossRef]
- Suthar, J.; Prieto-Simon, B.; Williams, G.R.; Guldin, S. Dual-Mode and Label-Free Detection of Exosomes from Plasma Using an Electrochemical Quartz Crystal Microbalance with Dissipation Monitoring. Anal. Chem. 2022, 94, 2465–2475, Erratum in Anal. Chem. 2022, 94, 11457. [Google Scholar] [CrossRef]
- Etayash, H.; McGee, A.R.; Kaur, K.; Thundat, T. Nanomechanical sandwich assay for multiple cancer biomarkers in breast cancer cell-derived exosomes. Nanoscale 2016, 8, 15137–15141. [Google Scholar] [CrossRef]
- Calleja, M.; Kosaka, P.M.; San Paulo, Á.; Tamayo, J. Challenges for nanomechanical sensors in biological detection. Nanoscale 2012, 4, 4925–4938. [Google Scholar] [CrossRef]
- Gomes, P.; Tzouanou, F.; Skolariki, K.; Vamvaka-Iakovou, A.; Noguera-Ortiz, C.; Tsirtsaki, K.; Waites, C.L.; Vlamos, P.; Sousa, N.; Costa-Silva, B.; et al. Extracellular vesicles and Alzheimer’s disease in the novel era of Precision Medicine: Implications for disease progression, diagnosis and treatment. Exp. Neurol. 2022, 358, 114183. [Google Scholar] [CrossRef]
- Cheng, C.A. Before Translating Extracellular Vesicles into Personalized Diagnostics and Therapeutics: What We Could Do. Mol. Pharm. 2024, 21, 2625–2636. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, X.; Zhu, L.; Luo, L.; Sun, N.; Pei, R. Current Advances in Technologies for Single Extracellular Vesicle Analysis and Its Clinical Applications in Cancer Diagnosis. Biosensors 2023, 13, 129. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, K.; Yan, A.; Wang, J.G.; Zhang, Z.; Li, D. Intelligent Probabilistic System for Digital Tracing Cellular Origin of Individual Clinical Extracellular Vesicles. Anal. Chem. 2021, 93, 10343–10350. [Google Scholar] [CrossRef]
- Woud, W.W.; van der Pol, E.; Mul, E.; Hoogduijn, M.J.; Baan, C.C.; Boer, K.; Merino, A. An imaging flow cytometry-based methodology for the analysis of single extracellular vesicles in unprocessed human plasma. Commun. Biol. 2022, 5, 633. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; McCafferty, E.; Soper, S.A.; Witek, M.A.; Hu, M.; Ford, J.M.; Song, S.; Kapogiannis, D.; Glesby, M.J.; et al. Microfluidic Isolation of Neuronal-Enriched Extracellular Vesicles Shows Distinct and Common Neurological Proteins in Long COVID, HIV Infection and Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 3830. [Google Scholar] [CrossRef]
- Zare, H.; Kasdorf, M.M.; Bakhshian Nik, A. Microfluidics in neural extracellular vesicles characterization for early Alzheimer’s disease diagnosis. Mol. Cell. Neurosci. 2025, 132, 103982. [Google Scholar] [CrossRef]
| Biomarkers | Source | EV Subpopulation | Surface Marker | Findings in AD vs. Controls | References |
|---|---|---|---|---|---|
| Amyloid-related biomarkers | |||||
| Aβ42, Aβ40, Aβ1-42 | Plasma | NDEs | L1CAM, GAP43, NLGN3, NCAM | Aβ42, Aβ1-42: MCI, AD (↑) Aβ42/40: AD (↑) | [56,57,58,59,60,61,62] |
| Tau pathology biomarkers | |||||
| P-T181-tau, P-S396-tau, P-T231-tau, T-tau | Plasma | NDEs | L1CAM, NCAM, GAP43, NLGN3, amphiphysin 1 | P-T181-tau, P-S396-tau, T-tau: MCI, AD (↑) P-T231-tau: AD (↑) | [56,57,59,60,61,62,63,64] |
| Neurodegeneration/neuronal injury biomarkers | |||||
| synaptophysin, synaptopodin, synaptotagmin-1, synaptotagmin-2, SNAP-25, syntaxin-1, NRGN, GAP43, PSD95, GluR2, AMPA4, NPTX2, NLGN1, NRXN2α, REST, proBDNF | Plasma Serum | NDEs | L1CAM, NCAM, GAP43, NLGN3 | synaptotagmin-1, SNAP-25, NRGN, GAP43, REST: MCI, AD (↓) synaptophysin, synaptopodin, synaptotagmin-2, syntaxin-1, PSD95, GluR2, AMPA4, NPTX2, NLGN1, NRXN2α, proBDNF: AD (↓) | [56,60,64,65,66,67,68] |
| Inflammatory/immune mechanism-related biomarkers | |||||
| C1q, C4b, C3b, C3d, factor B, factor D, fragment Bb, C5b-C9 TCC, IL-6, TNF-α, IL-1β, CD59, CD46, DAF, CR1, MCP-1, HGF, FGF-2, FGF-13, IGF-1 | Plasma | ADEs | GLAST | C1q, C4b, C3b, C3d, factor B, factor D, fragment Bb, C5b-C9 TCC, IL-6, TNF-α, IL-1β: AD (↑) CD59, CD46, DAF, CR1, HGF, FGF-2, FGF-13, IGF-1: AD (↓) MCP-1: SCD (↓) | [69,70,71] |
| C7, HGF, FGF-13, IGF-1, MMP-9 | Plasma Serum | NDEs | L1CAM | C7, MMP-9: AD (↑) HGF, FGF-13, IGF-1: AD (↓) | [59,71,72] |
| Vascular brain injury-related biomarkers | |||||
| Hemoglobin, Hemoglobin subunit α, β, and δ | Plasma | NDEs | L1CAM | Hemoglobin, Hemoglobin subunit α, β, and δ: AD (↑) | [73] |
| Other biomarkers—proteins | |||||
| ZYX, pY-IRS-1, p-S312-IRS-1, p-panY-IRS-1, SOD1, mitochondrial electron transport chain complexes I, III, IV, ATP synthase, cathepsin D, LAMP-1, ubiquitin, HSP70 | Plasma Serum | NDEs | L1CAM | pY-IRS-1, p-S312-IRS-1, cathepsin D, LAMP-1, ubiquitin: AD (↑) ZYX, p-panY-IRS-1, SOD1, mitochondrial electron transport chain complexes I, III, IV, ATP synthase, HSP70: AD (↓) | [63,72,74,75,76] |
| Other biomarkers—proteins | |||||
| BACE1, sAPPβ, GDNF | Plasma | ADEs | GLAST | BACE1, sAPPβ: AD (↑) GDNF: AD (↓) | [77] |
| Other biomarkers—microRNAs | |||||
| miR-384, miR-29c-3p, miR-let-7e-5p, miR-122, miR-3591, miR-9-5p, miR-106b-5p, miR-125b-5p, miR-132-5p, miR-29a-5p, miR-210-3p, miR-212-3p, miR-132-3, miR-23a-3p, miR-223-3p, miR-190-5p, miR-100-3p | Plasma | NDEs | L1CAM, NCAM, amphiphysin 1 | miR-384, miR-29c-3p, miR-let-7e-5p, miR-9-5p, miR-106b-5p, miR-125b-5p, miR-132-5p, miR-23a-3p, miR-223-3p, miR-190-5p: AD (↑) miR-122, miR-3591, miR-29a-5p, miR-212-3p, miR-132-3, miR-100-3p: AD (↓) miR-210-3p: MCI (↑) | [61,62,78,79,80,81,82] |
| miR-29a-5p, miR-107, miR-125b-5p, miR-132-5p, miR-210-3p | Plasma | ADEs | GLAST | miR-29a-5p, miR-107, miR-125b-5p, miR-132-5p: AD (↑) miR-210-3p: MCI (↑) | [80] |
| miR-29a-5p, miR-125b-5p, miR-132-5p, miR-210-3p, miR-106b-5p | Plasma | MDEs | TMEM119 | miR-29a-5p: AD (↓) miR-125b-5p: MCI (↓) miR-132-5p, miR-106b-5p: AD (↑) miR-210-3p: MCI-AD (↑) | [80] |
| Isolation Techniques | Principle | Yield | Purity | Scalability | Throughput | Time | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|---|---|
| Ultracentrifugation-Based Separation | Separates EVs based on their buoyant density using ultracentrifugation or density gradients | Low to moderate | High | Low | Low | Long | Well-established; suitable for bulk EV isolation; high yield | Time-consuming; labor-intensive; large sample volume | [89,90,91,92] |
| Size-Based Separation | Filters or retains EVs based on size using ultrafiltration membranes or SEC columns | Moderate to high | Moderate to high | High | Moderate | Short | Preserves EV integrity; mild conditions | Risk of filter clogging; co-isolation of similar-sized contaminants | [93,94,95] |
| Precipitation Methods | Uses polymers to precipitate EVs by reducing their solubility | High | Low | High | High | Moderate | Simple; low-cost; compatible with clinical workflows | Low specificity; potential contamination by non-EV proteins or particles | [96,97,98] |
| Charge-/Dielectric-Based Separation | Uses charge or dielectric differences to separate EVs through electrophoresis or dielectrophoresis | Moderate | High | Moderate to high | Low | Moderate | High purity; potential for fast sorting | Low throughput; less established for clinical use | [99,100,101] |
| Immunoaffinity-Based Separation | Employs antibodies specific to capture target EVs via magnetic beads or affinity columns | Low to moderate | High | Low to moderate | Low | Moderate | High specificity; enriches cell-type or disease-specific EVs | High-cost; limited to known markers | [102,103,104] |
| Acoustic Separation | Applies acoustic forces to separate EVs based on size and compressibility | Moderate to high | High | Moderate | Low to moderate | Short | Contact-free; gentle processing; compatible with continuous flow | Requires precise instrumentation; low throughput | [105,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, Z.; Chai, Z.; Ma, S.; Li, A.; Li, Y. Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 8272. https://doi.org/10.3390/ijms26178272
Yu Y, Wang Z, Chai Z, Ma S, Li A, Li Y. Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(17):8272. https://doi.org/10.3390/ijms26178272
Chicago/Turabian StyleYu, Yiru, Zhen Wang, Zhen Chai, Shuyu Ma, Ang Li, and Ye Li. 2025. "Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 17: 8272. https://doi.org/10.3390/ijms26178272
APA StyleYu, Y., Wang, Z., Chai, Z., Ma, S., Li, A., & Li, Y. (2025). Central Nervous System-Derived Extracellular Vesicles as Biomarkers in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(17), 8272. https://doi.org/10.3390/ijms26178272





