2.2. Double Potential Step Chronoamperometry (DPSCA) Study
Double potential step chronoamperometric (DPSCA) experiments were performed to gain insight into the processes taking place at the GCE modified with SWCNTs. This method was chosen due to our focus on studying the cathodic reaction, as this reaction should have fewer degrees of freedom, as it is independent of the concentration of electroactive species in the bulk. This means that the investigation of the electrochemical behavior can only be restricted to the porous SWCNTs layer if parameters such as the concentration of the Fe(II) and the concentration of the supporting electrolyte are chosen properly. This is based on the fact that the electroactive species—Fe(III)—only originates from the electrochemical oxidation of the initial Fe(II) solution. Therefore, the concentration variation of Fe(III) and, accordingly, the chronoamperometric response should be more pronounced in the cathodic branch of the DPSCAs. This concentration can be controlled by several parameters. The first parameter is the effective potential difference. In order to keep the degrees of freedom as low as possible, the variation of the effective potential difference was achieved by using a supporting electrolyte with different ionic strengths. In this way, the same potential steps can be used during the DPSCA measurements. The second parameter is the Fe(II) solution concentration. The details of the experimental conditions are given in the Materials and Methods.
The analysis of the experimental data shows that the most meaningful DPSCA responses and their derivatives, which provide the best understanding of the electrochemical phenomena in the porous SWCNT electrode, were noticed in 0.001 M KCl. The recorded DPSCAs, as well as the Cottrell analysis at different ionic strengths, are given in
Supplementary Materials—Figure S3. As shown, the given dependence does not provide sufficient evidence to draw relevant conclusions.
However, an interesting behavior can be observed when an appropriate analysis is performed. This analysis includes the representation
Itotal/
Iblank vs.
t (
Figure 3), log(
I) vs. log(
t) (
Figure 4), d(log(
I))/d(log(
t)) vs. log(
t) (
Figure 5). The presentation in
Figure 3 is based on our previous use of this analysis to determine the ‘apparent catalytic rate constant’ [
33], which aims to elucidate mass transport phenomena within the porous layer. However, some changes have been made to the earlier presentation, since in this work, the dependence of
Itotal/
Iblank on
t, but not on
t1/2 as in the original equation by [
34], is shown.
Itotal and
Iblank represent the currents recorded in the presence and absence of the electroactive species, respectively. This simplification is made because the ratio
Itotal/
Iblank essentially represents the ratio between the pure faradaic current (
Ifarad), which originates from the electrochemical reaction of the Fe(II) and Fe(III) species, and the “blank” current, i.e., the capacitive current (
Iblank), as follows:
As will be shown, this representation enables a more distinctive approach and offers a deeper insight into the phenomena that occur during the DPSCA measurements.
For the sake of clarity, the representations of log(I) vs. log(t) and d(log(I))/d(log(t)) vs. log(t) for the cathodic branch of DPSCAs are also adapted to the relative time from 0 s (10 s in DPSCAs) to 10 s (20 s in DPSCAs).
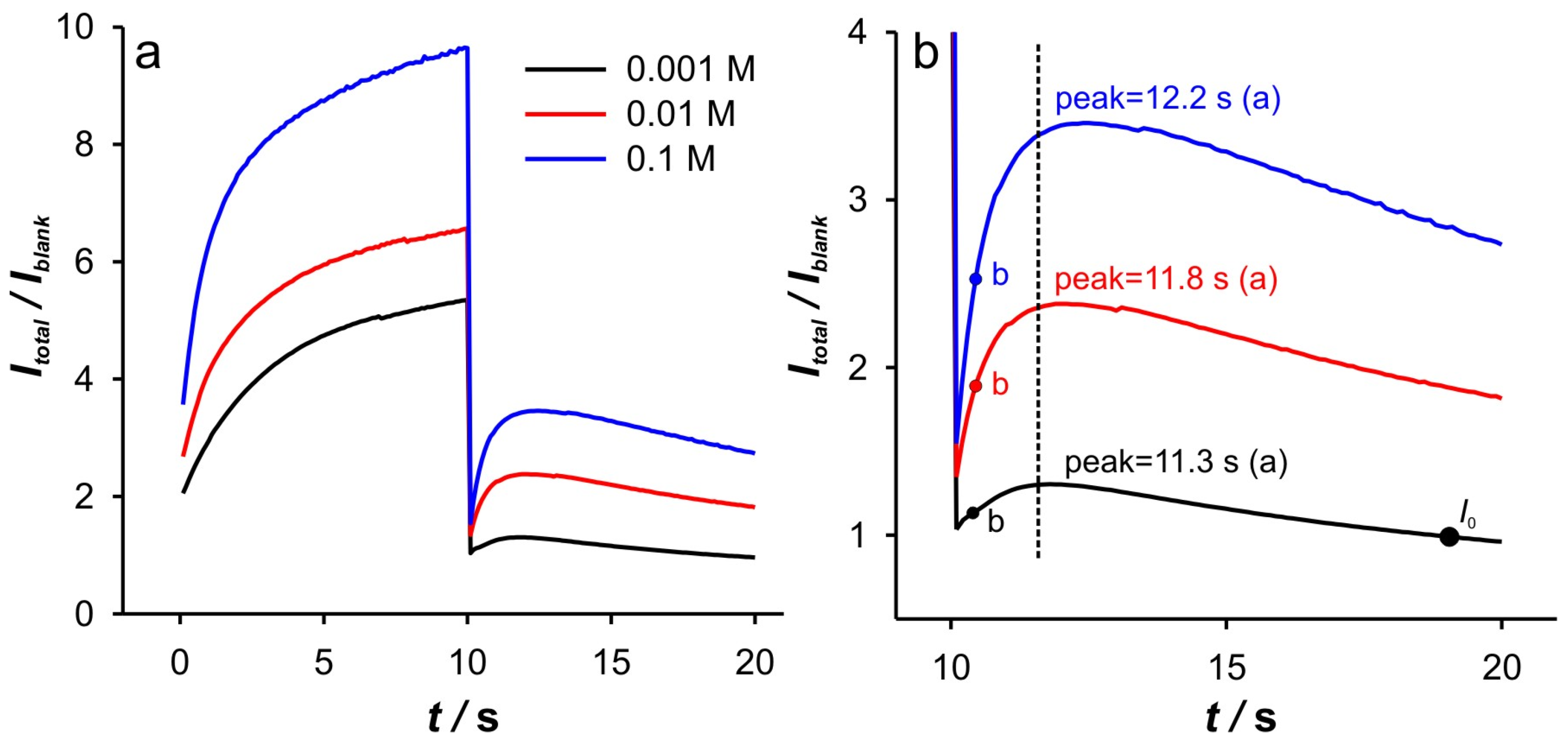
Since the cathodic response shows a more diverse behavior, the discussion of the analysis results will begin with the cathodic branch of DPSCAs. The
Itotal/
Iblank vs.
t branch of the DPSCAs is shown in
Figure 3a. The presented curve can be explained as follows. After switching the potential to –0.1 V, a double-layer charging current occurs, characterized by the decrease in the
Itotal/
Iblank ratio as the ionic strength of the supporting electrolyte decreases. This behavior is to be expected, as charging is particularly slow at a low ions concentration. The increase in the
Itotal/
Iblank ratio is then a consequence of the increase in faradaic currents. After the maximum value of the
Itotal/
Iblank ratio is achieved (peak), the
Itotal/
Iblank ratio begins to decrease. In the case of 0.001 M KCl, this ratio reaches the lowest value (
Itotal/
Iblank = 1, point “
I0” in
Figure 3b) at 17.8 s, indicating that no more Fe(III) is present in the system. In conclusion, the phenomena during the applied cathodic potential can be roughly divided into two regions.
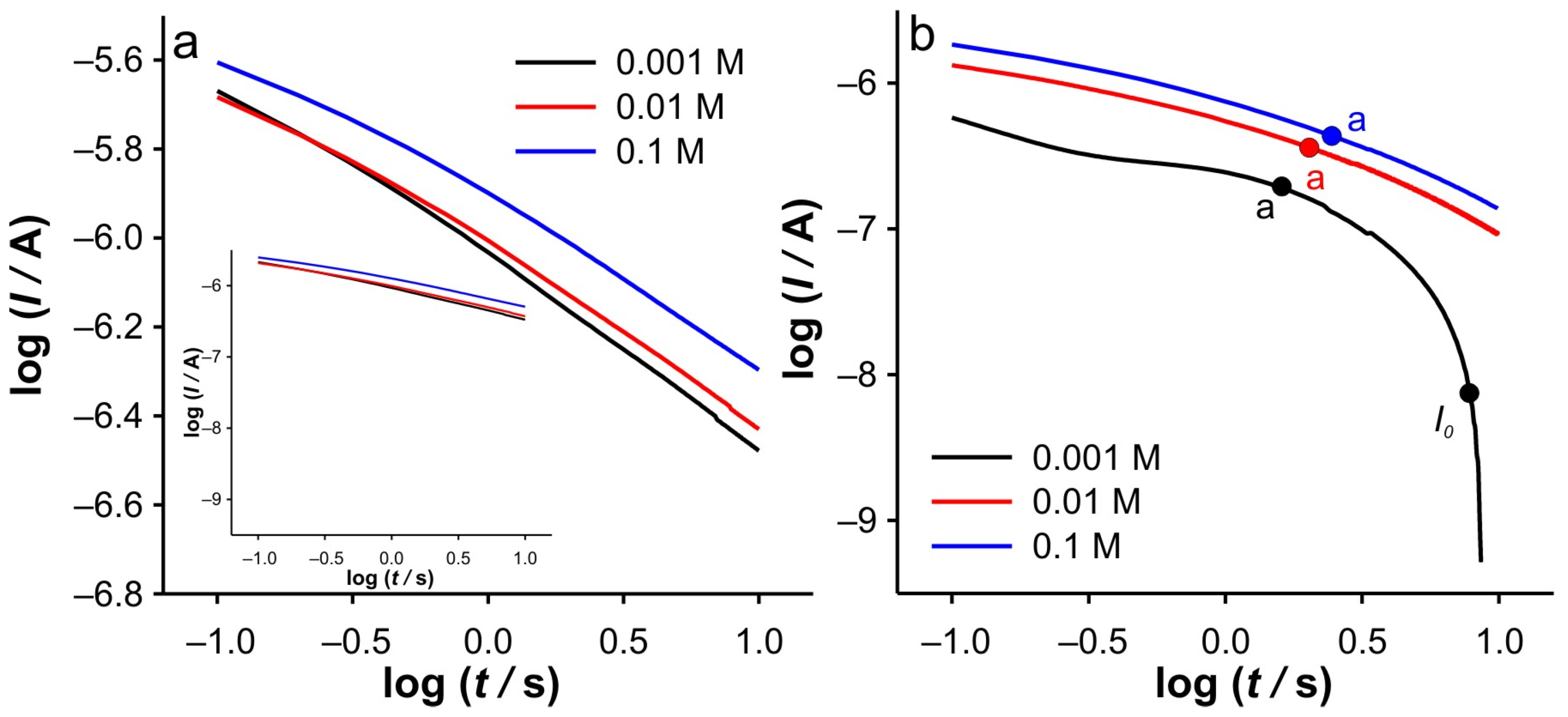
In order to elucidate the phenomena occurring in these two regions, the dependencies log(
I) vs. log(
t) and d(log(
I))/d(log(
t)) vs. log(
t) were examined in detail. At this point, it should be emphasized that all dependencies derived from the DPSCAs are based on faradaic currents. As presented in
Figure 4b, a significant variation of the dependencies of log(
I) vs. log(
t) was obtained in the case of the cathodic branch of DPSCAs in 0.001 M KCl. As previously concluded from
Figure 3b, the faradaic current disappears at 17.8 s (i.e., relative time in cathodic branch of DPSCAs of 7.8 s), which is consistent with the point “
I0” (log(
t) = 0.89) in
Figure 4b.
Accordingly, under these conditions, the system needs 7.8 s to totally depletion of the Fe(III). This strongly suggests that SWCNTs are not involved as an active center (at least not to an extent that has a significant impact on the overall current signal) for heterogeneous electron transfer, as their large specific surface area and porosity are expected to behave similarly to thin-layer cells. Accordingly, the electrochemical reaction seems to take place on the surface of the GCE. This assumption is strongly supported by the analysis of
Figure 5c at short times. As shown, the bell curve of d(log(
I))/d(log(
t)) vs. log(
t) was obtained during short times for 0.001 M KCl (black curve). The ascending part of the curve indicates the formation of the concentration profile of Fe(III) in the vicinity of glassy carbon. After the formation of the concentration profile near the GCE, a short plateau around log(
t) ≈ −0.1 can be observed, characterized by a minimum of the slope (about −0.2). This plateau reflects a steady-state current. This is possible at a low effective potential in 0.001 M KCl, as this condition favors the kinetic control of the electrochemical reaction. As the electrolysis proceeds, Cottrellian behavior occurs as a diffusion profile is formed and confined in the vicinity of the GCE. After this region, there is a sharp increase in the slope as the Fe(III) is hardly present in the SWCNTs layer. This is a consequence of the rapid depletion of the remaining Fe(III) in the SWCNTs layer. However, from this analysis, it is very difficult to distinguish when the SWCNTs layer is depleted of Fe(III). The boundary between the mass transport through the SWCNTs layer and where the mass transport through solution also occurs can be estimated by a comparative analysis of the dependencies presented in
Figure 2b. The peak that occurs in
Itotal/
Iblank vs.
t represents the threshold of these two regimes. At Fe(II) concentration of 0.1 mM and an ionic strength of 0.001 M KCl, the peak occurs at 11.3 s (point “a”). In
Figure 4b and
Figure 5c, it corresponds to the log(
t) of 0.16 (note: the plot includes the relative cathodic time, i.e., 1.3 s), which is marked as point “a”. From the
Figure 5c, the value of d(log(
I))/d(log(
t)) vs. log(
t) was estimated as −0.63. Accordingly, the region between points “
I0” and “a” can be characterized as the regime in which the change in flux also occurs through the solution.
Relied on the above, the cathodic branch of DPSCAs can be analyzed for higher KCl ion strengths. As shown in
Figure 4b and
Figure 5b,c, no significant deviation in dependences was observed, as it was noticed in the case of 0.001 M KCl. This can be attributed to a higher initial electrochemically generated Fe(III) amount in the system due to the higher effective potentials at higher KCl concentration. Furthermore, the higher effective potential difference has a significant influence on the reduction of Fe(III). This manifests itself in a lack of detection of the processes at short times. As shown in
Figure 3b, at time 10 s (relative cathodic time 0), the applied potential is such that the Fe(III) present in the vicinity of GCE is depleted (linear part of cathodic branch up to point “b”) on a time scale of about 0.3 s, afterwards Cottrellian behavior occurs in the SWCNTs layer. Due to the interplay of the higher effective potential and the electrochemically generated amount of Fe(III), this process takes somewhat longer than in the case of 0.001 M KCl. The times and related parameters determined from
Figure 3,
Figure 4 and
Figure 5 are listed in
Table 1. As shown, the determined values of slopes (points “a”) are around −0.65, which confirms that this value can be used as a threshold between two regimes.
This analysis is simpler when applied to the anodic branches of DPSCAs. As can be seen from
Figure 3a, the processes during the anodic branch of the DPSCAs take place mainly in the porous SWCNTs layer. This was to be expected as Fe(II) is initially present in the solution. As a consequence of the higher concentration in the bulk of the solution, the slope values tend toward Cottrellian behavior (approaching −0.45) at longer times (
Figure 5a). Of course, some differences can be observed for short times, as the values of the slope is lower for higher KCl concentrations. As explained in the case of the cathodic DPSCA signal, the lower effective potential leads to the establishment of a local diffusion in the vicinity of the electrode. However, with a higher ionic strength of the supporting electrolyte, the depletion of Fe(II) in the vicinity of the GCE is more emphasized. This means that the diffusion layer is wider than in the case of the 0.001 M KCl. As a result of the above-mentioned, slower expansion of the diffusion layer at a given effective potential difference can be expected.
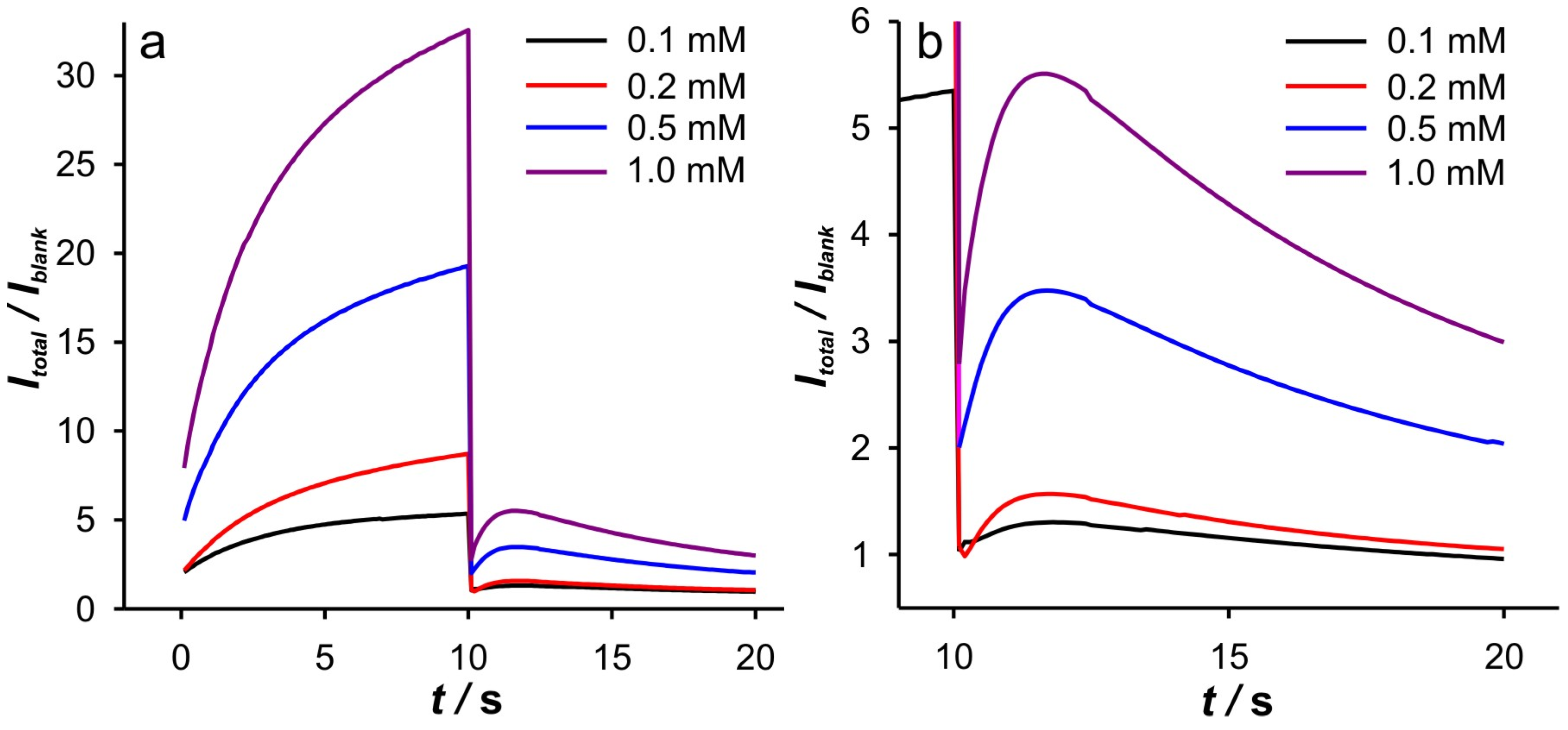
In a further experiment, DPSCA measurements were carried out at different Fe(II) concentrations in 0.001 M KCl. The obtained DPSCAs, as well as the Cottrell analysis, are included in the
Supplementary Materials—Figure S4, while the analysis of the cathodic branch of the DPSCAs is presented in
Figure 6,
Figure 7 and
Figure 8. As can be seen from
Figure 6a, the anodic branch of the DPSCAs shows the expected behavior with increasing Fe(II) concentration, as the peak is reached quickly at lower Fe(II) concentrations, indicating a slower expansion of the diffusion layer at higher Fe(II) concentrations. Accordingly, more information about the phenomena in the porous layer can be obtained from the cathodic branch of the DPSCAs.
Interestingly, similar behavior was observed at higher Fe(II) concentration, as in 0.01 M and 0.1 M KCl at 0.1 mM Fe(II) (
Figure 7). These trends can be explained by the self-support effect at low support concentration and a relatively high amount of electroactive species, especially in the case of the small and limited volume in the vicinity of the GCE. This conclusion can be supported by the dependence of log(
I) vs. log(
t) at higher Fe(II) concentration in different KCl solutions. As presented in
Supplementary Materials—Figure S5, no significant difference can be observed in the cathodic branch of the DPSCAs. Another interesting point is the observation of the steady-state current on the short time scale for 0.2 mM Fe(II) after the slope starts to increase and reaches values around −0.5 on the same time scale as in the case of 0.1 mM Fe(II) (
Figure 7 and
Figure 8). This behavior is most similar to that predicted by Yang et al. [
35] in analyzing the simulation of the dimensionless flux time transition to a planar electrode cover with different numbers of non-conducting layers. However, in contrast to the reported simulation, in our case, a rapid depletion in Fe(III) leads to a rapid decline of the slope with time. This happens due to the fact that it can be assumed that the concentration of the Fe(III) in the bulk of the solution is zero.
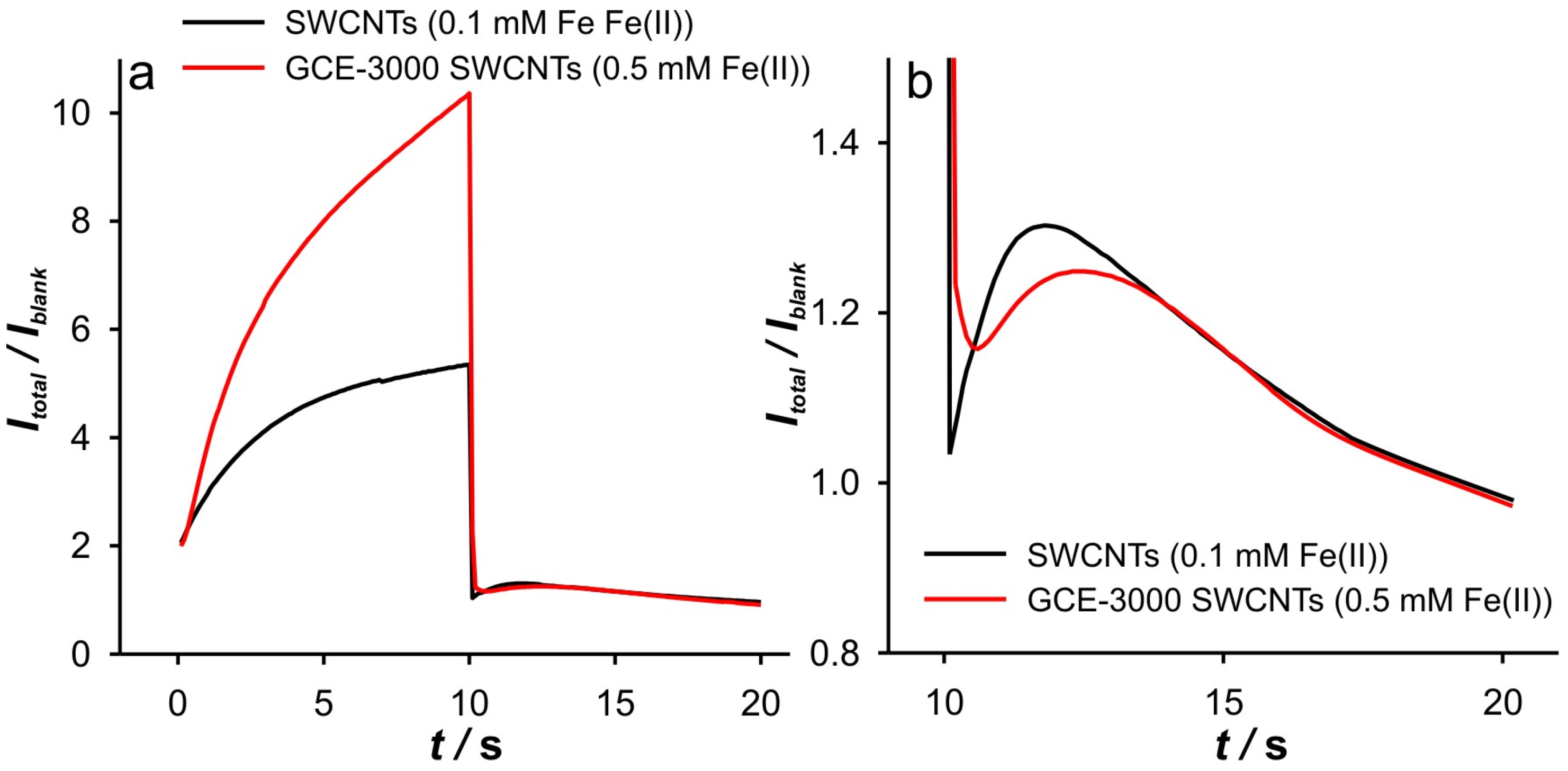
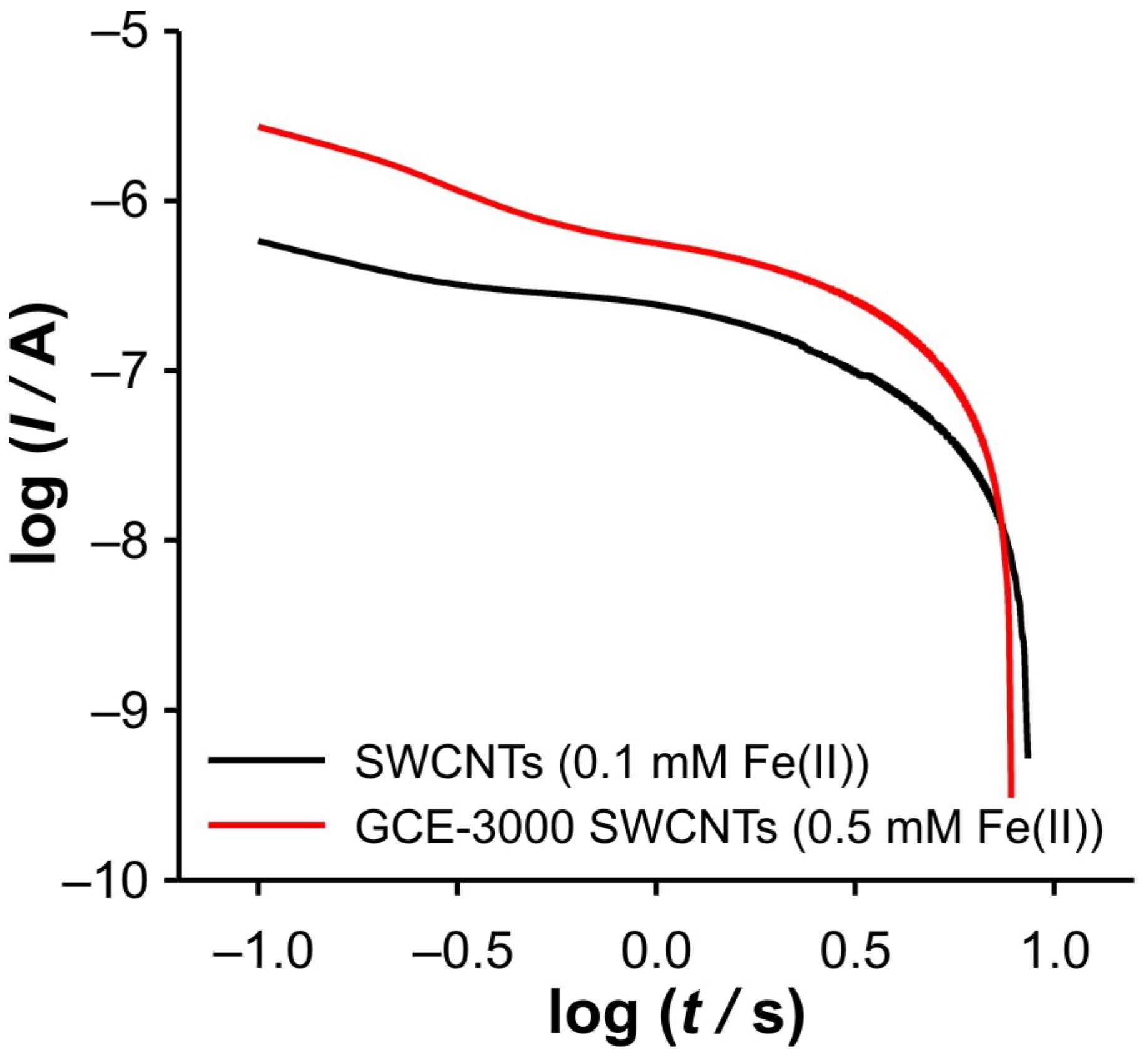
Additional evidence for the above thesis was found by enlarging the GCE surface area by grounding the GCE with paper 3000, after drop casting of SWCNTs was performed. DPSC experiments with this electrode (GCE-3000 SWCNTs) were performed at different concentrations of Fe(II) in 0.001 M KCl. Interestingly, no valuable data for the cathodic branch of the DPSCAs were obtained when the solution contained 0.1 mM Fe(II) (
Figure 9). However, at 0.5 mM, a signal more similar to that recorded with a SWCNT electrode (polished GCE) at 0.1 mM Fe(II) was obtained (see
Figure 10 and
Figure 11). In summary, as the GCE surface area increases, the described phenomena can be observed at higher Fe(II) concentration. The diversity of the signal presented in
Figure 11b is beyond the scope of this article, as the explanation for this dependence is to be found in the mass transport that takes place both at the tips and in the “valley” of the scratched GCE [
36,
37,
38].
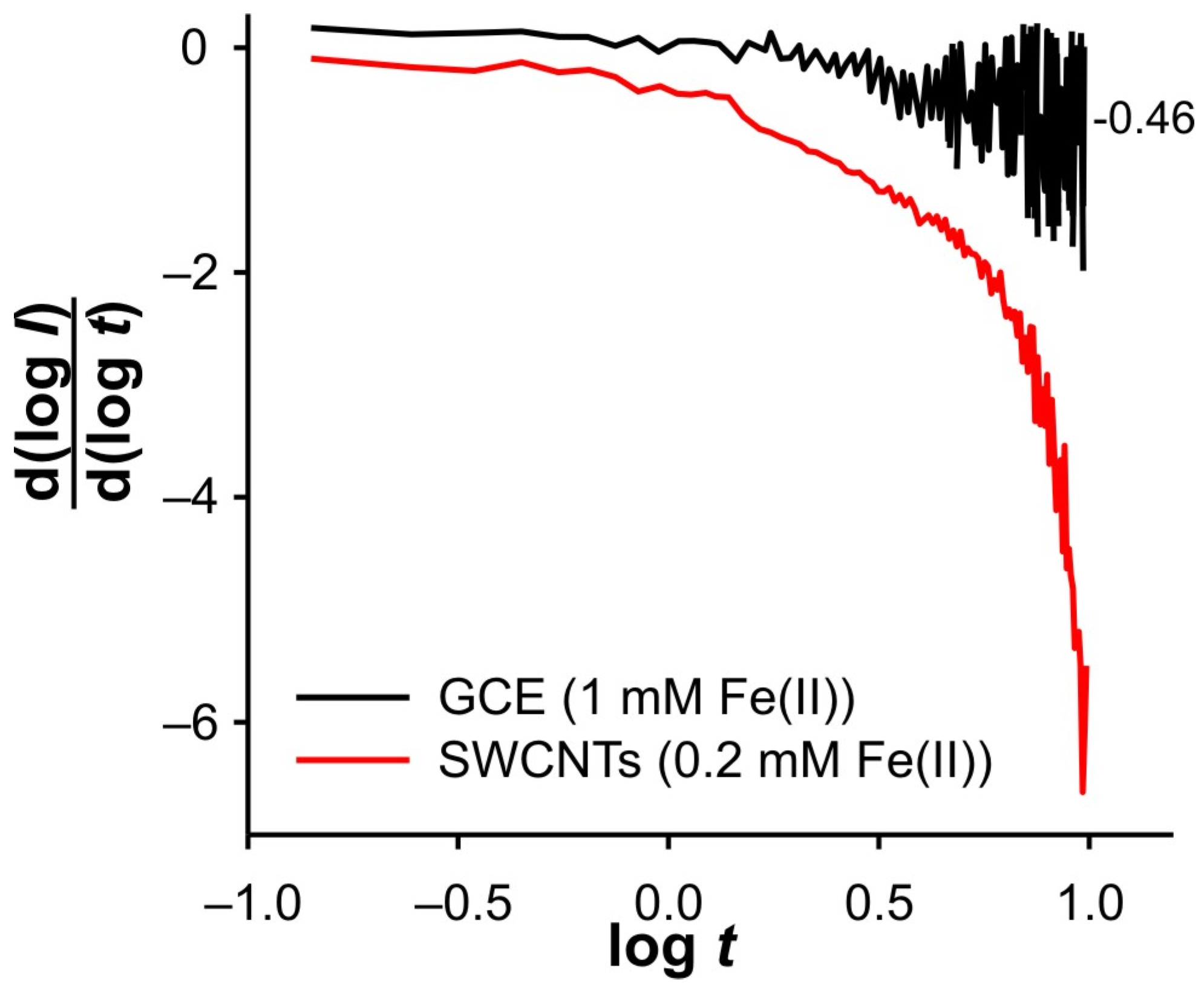
Finally,
Figure 12 shows a comparison of the DPSCAs result obtained with polished GCE and SWCNTs electrodes. The DPSCAs for GCE were performed in the presence of 1 mM Fe, which ensures a sufficient concentration of Fe(III) near the electrode. A lower Fe(II) concentration did not result in a valuable cathodic signal. The behavior in the cathodic branch of the DPSCAs shows a remarkable similarity with the results recorded at lower Fe(II) concentrations at the electrode modified with SWCNTs. All this supports the assumption that GCE is an electroactive surface in this system.
2.3. Reverse Pulse Voltammetry (RPV) Study
Although the detailed analysis in the previous Section indicates that the electrochemical reaction takes place at the GCE, it cannot provide sufficient evidence to clarify the voltametric behavior. This is mainly a consequence of the fundamental differences between these two techniques. Accordingly, reverse pulse voltammetry (RPV) is harnessed in this study to investigate the phenomena that occur during voltammogram scans. RPV was chosen in relation to the information obtained from the chronoamperometric analysis. In principle, this technique can be considered as a series of DPSC experiments at different second step potentials if appropriate parameters are adapted to the system under study. In this work, RPV was performed on the SWCNTs electrode and GCE in the presence of 0.5 mM Fe(II). The experiments were performed at different KCl concentrations. For simplicity, the influence of the pulse time and KCl concentration at a scan rate of 50 mV s−1 for the SWCNTs electrode and GCE are presented.
Figure 13 shows reverse pulse voltammograms (RPVs) with a pulse time of
tp = 0.05 s for the SWCNTs electrode and GCE. As shown, in low support (
Figure 13a), the SWCNTs electrode shows a continuous increase in cathodic current over the entire potential range. At low ionic strength of KCl, the charging currents are very high as the SWCNT layer acts as an electrochemical double-layer capacitor (EDLC). In combination with the short pulse time (
tp = 0.05 s), at anodic potentials, cathodic current is a consequence of the relaxation currents, while at more negative potentials, the charging currents dominate. This contribution is also expected to increase when the potential flows to more negative values due to a higher stimulus pulse. The voltammogram recorded at GCE in 0.001 M KCl will be commented on later (see discussion for
Figure 13d). For comparison, RPVs recorded, for both electrodes, in 0.1 M KCl with the same pulse time are shown in
Figure 13b. As expected, the significant lack of influence of the charging current on the current signal is observed at higher support. Another important point is that the reduction currents start to dominate at around 350 mV for both electrodes. This is a very important point when considering the electrocatalytic properties of SWCNTs. However, when the potential is further reduced, the current signals split in the same way as observed for the dependence of RPV signals on concentration. Accordingly, the difference in the cathodic signal can be attributed to the difference in Fe(III) concentration near the GCE.
This difference arises from the fact that during the electrochemical generation of Fe(III) at the bare GCE, the Fe(III) can diffuse into the bulk of the solution, decreasing the amount of Fe(III) near the GCE. On the contrary, in the case of the SWCNTs electrode, the generated Fe(III) remains trapped in the vicinity of the GCE, as well as in the porous SWCNTs layer. Finally, at potentials more negative than 125 mV, a significant change in the currents can now be observed, regardless of the type of electrode. With respect to the small tp, this is not surprising since similar current values were collected on a time scale where diffusion is mitigated. Accordingly, the collected signal currents are independent of a more negative potential.
Figure 13c,d show a comparison of the RPVs presented above, but for the same electrode in different support concentrations. An interesting observation was made for the GCE in 0.001 M KCl, where no charging currents at anodic potentials were observed at the measured pulse time. This strongly suggests that in the case of the polished GCE, the relaxation of the double layer due to a small potential perturbation is negligible compared with the SWCNTs electrode, due to its smaller charging electrode surface. When the base potential is applied (0.6 V for 0.45 s), the double layer is completely formed. Applying a short pulse with a potential step of 25 mV during the anodic branch of the RPV does not lead to any measurable relaxation current in the case of GCE due to their low total capacitance. The opposite is the case with the SWCNTs electrode.
Significant changes were obtained by increasing the pulse time (
tp = 0.4 s), as the contribution of the relaxation and charging currents in the total signal becomes negligible regardless of the support ionic strength. Accordingly, the anodic part of the RPVs is characterized by an anodic “plateau”, even for SWCNTs in 0.001 M KCl (see
Figure 14c). Since the system has enough time to relax the double layer, the current is only the consequence of the oxidation of the remaining Fe(II).
In addition, the cathodic current in the case of GCE starts at a more negative potential than in SWCNTs (
Figure 14a). In explaining the presented results, we rely on the influence of the applied surface electrode potential on the composition and potential profile across the diffuse or Gouy–Chapman (GC) layer in the Gouy–Chapman–Stern model of the double layer, and consequently, on Frumkin effects [
30,
31]. Accordingly, ion condensation in the GC layer near the GCE is more pronounced in the SWCNTs electrode due to the restricted electrolyte volume. Furthermore, this effect is more pronounced with increasing applied potential. As a consequence, the effective potential drop near the GCE can be considered somewhat large in the case of the modified electrode. As a result, the reduction starts at a slightly lower cathodic potential in the case of the SWCNTs electrode. In combination with the previously described diffusion of the electrochemically generated Fe(III) toward the bulk solution, the Fe(III) in the vicinity of the GCE for the SWCNTs electrode can be considered higher than in the case of the bare GCE. All this contributes to the shape of the recorded voltammograms. For instance, in the case of the SWCNTs electrode, the voltammograms show the maximum cathodic current at a lower cathodic potential than in the case of the GCE. Furthermore, the application of a higher effective potential and a long
tp results in electrolyte depletion near the GCE, which leads to the recording of a lower cathodic current than in the previous steps. As a result, the RPVs show a peak shape. This is not observed in the case of GCE, due to the lower effective potential that leads to a slower expansion of the diffusion layer. Moreover, the diffusion layer can be considered wider in the case of GCE than in the case of SWCNTs electrode (considering that the thickness of the diffusion layer can vary from 1 to 100 μm) [
39]. This is consistent with the micrograph presented in
Supplementary Materials—Figure S6 (the largest pore size is around 200 nm)
At higher support (
Figure 14b), due to a similar extent of the compression of the GC layer as a consequence, the cathodic currents start to dominate at the same potential (280 mV) for both electrodes, indicating that no electrocatalytic effects can be attributed to the SWCNTs. Interestingly, no difference in peak potential is observed between the SWCNTs electrode and the GCE in 0.1 M KCl. This clearly indicates that the reduction takes place on the same surface but at different amounts of Fe(III).
Figure 14c shows the comparison of the RPVs of SWCNTs electrodes recorded in different ionic strengths of KCl at
tp = 0.4 s. There is no significant difference in the RPVs up to a potential of 200 mV. Following the previous discussion, this is the consequence of the formation of a compressed and ion-condensed GC layer near the GCE. Accordingly, the potential drop can be considered similar to that in 0.1 M KCl. However, at a more negative potential, the more pronounced cathodic currents were recorded for RPV in 0.001 M?! Based on
tp = 0.4 s, the assumption of complete reduction of Fe(III) near the electrode seems reasonable, especially considering the results of the DPSCAs analysis. Accordingly, during the base potential (i.e., during 0.1 s of Fe(II) oxidation), the formation of a lower amount of Fe(III) is expected in the case of 0.001 M KCl. This excludes the possibility of an influence of the Fe(III) concentration on this phenomenon and can be attributed to the delayed behavior (i.e., rate) of the faradaic reaction in low support concentration. Also, during the cathodic pulse time, at a more cathodic potential, compression of the GC layer simultaneously occurs. As a result, the maximum effective potential difference, in the plane of the electron transfer, is formed later than in the case of 0.1 M KCl. As the GC layer is compressed, the effective potential drop also increases, which increases the reduction rate. However, the elapsed pulse time and the cathodic current are being collected at a higher value. The time consumed in compressing the GC layer results in the peak of the cathodic current occurring at a slightly higher cathodic potential (−12 mV relative to the peak observed in 0.1 M KCl). We find that this is a low cost considering that the signal is more than 50% higher in the 0.001 M case. Here, we must emphasize that the presented discussion is based on the assumption that the plane of the electron transfer, where quantum tunneling occurs, can reach 20 Å [
40].
2.4. Cyclic Voltammetry—Discussion
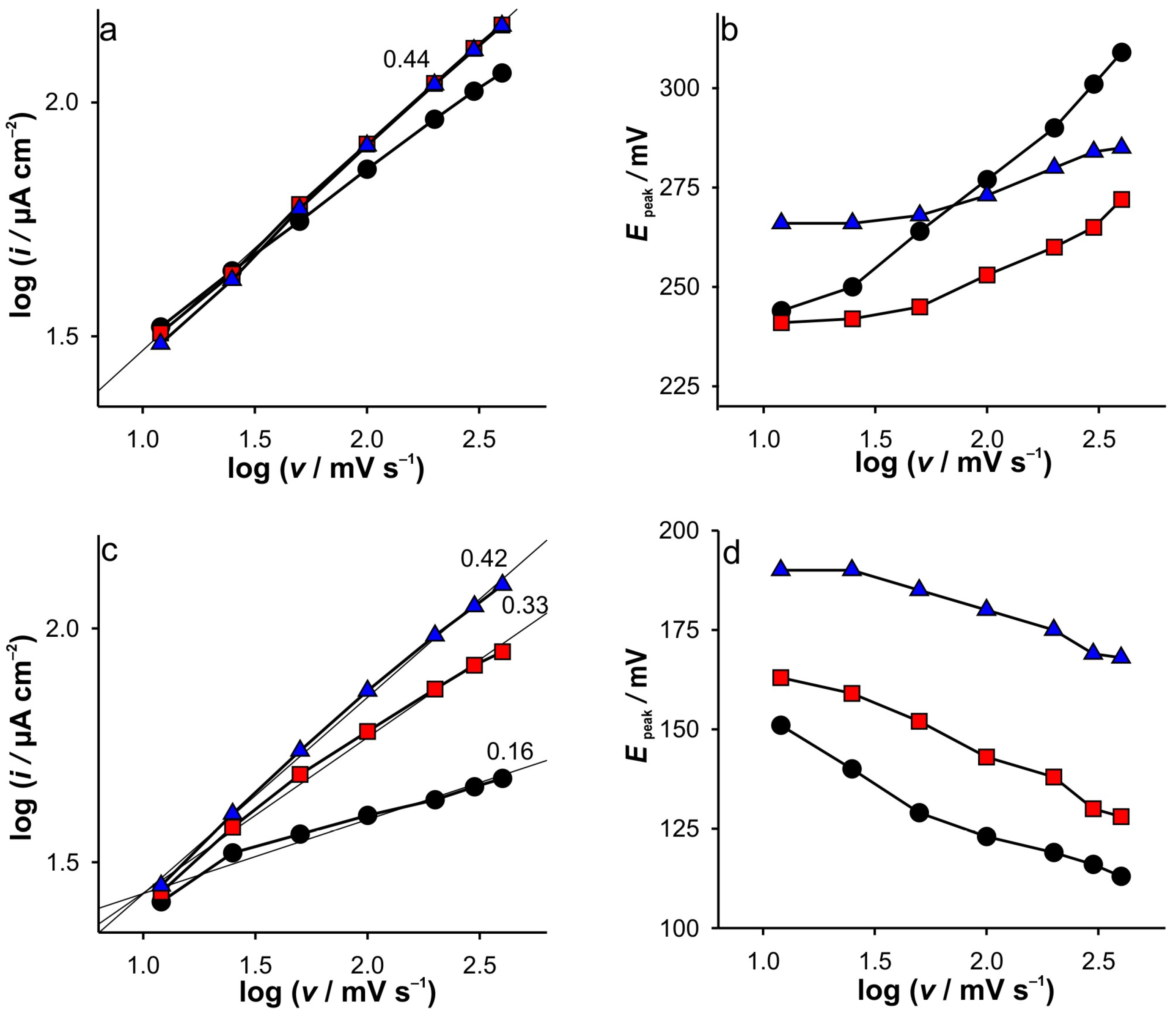
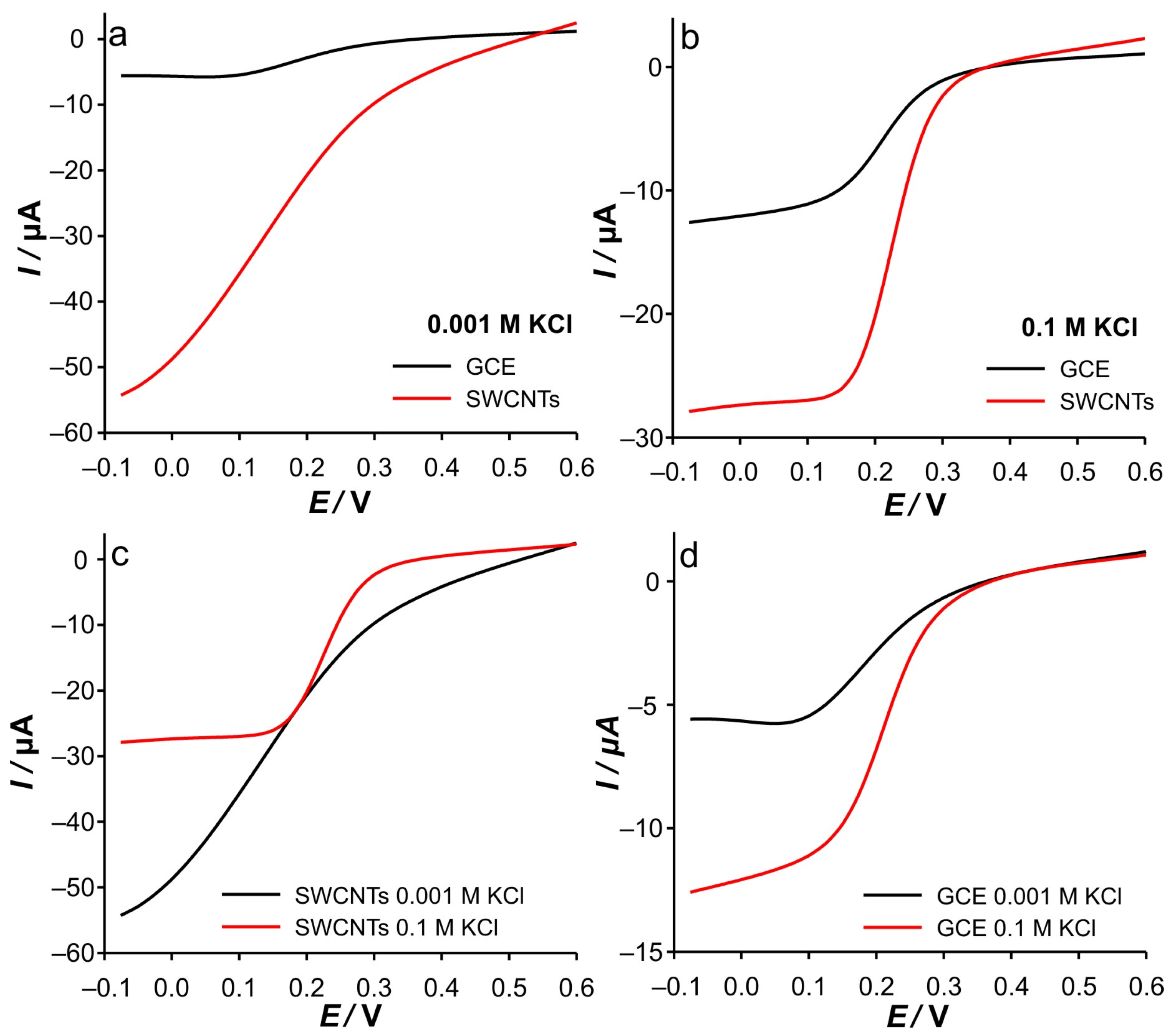
Based on all that was discussed above, the electrochemical behavior of Fe(II) on SWCNTs as well as on GCE can be easily elucidated. The most interesting observation of the CVs is that for GCE in 0.001 M KCl. As shown in
Figure 1a, the dependence of the anodic current peak on the scan rate shows a behavior suggesting that the reaction proceeds under diffusion control. Small discrepancies can be observed for CVs recorded in 0.001 M KCl at higher scan rates. However, differences in the potential of the current peaks vs. scan rate can be observed in the whole range. The latter is to be expected if we rely on the discussion of the RPV results with low support. However, the cathodic current peaks do not follow this trend (
Figure 1c). Consequently, the cathodic behavior cannot be rationalized only by the low support and ion condensation arguments, but rather by the presence of electroactive species in the bulk solution.
Due to the technical principles of staircase voltammetry, the GC layer can be considered compressed near the potentials of the current peaks at scan rates up to 100 mV s
−1 (
Figure 1a) when measurements are performed in 0.001 M KCl. Another aspect relevant to this discussion concerns the spreading of the electroactive species towards the bulk. As already mentioned, this is particularly important for GCE. When the diffusion layer is “reached” with electroactive species, the spreading (expansion or spontaneous diffusion) of the electroactive species towards the bulk during the half-cycle is less pronounced. This happens in the case of the anodic branch of the CV because Fe(II) is present in the bulk. Of course, if the scan rate is fast enough, this spreading should be slower. Accordingly, the effective potential near the peak potential is lower, resulting in the lower current being collected.
At a slow scan rate (12.5 mV s−1), the step time during staircase voltammetry is sufficient to establish diffusion conditions independent of the ionic strength of KCl (see explanation in the RPV experiments), which leads to the formation of the same current peak heights (when comparing cathodic and anodic). In fact, no differences were observed between peak heights when changing KCl concentrations, as no significant influence of GC layer compression is expected.
A further increase in the scan rates results in the significant splitting of the cathodic current peak heights when compared with the anodic ones. In fact, at high scan rates, the cathodic branch of CVs is characterized by constant current values independent of the potential. This may be a consequence of the following:
The phenomena during cathodic scanning related to the compression of the GC layer can be considered identical to those in the anodic branch. Accordingly, the first claim is directly related to the effective potential (i.e., the phenomena of GC layer compression) and should also be observed in the anodic branch of CVs at 0.001 M KCl. Since this was not observed, it can be excluded from the discussion.
In contrast to the anodic process, the concentration gradient of the electrochemically generated Fe(III) during the cathodic branch is directed towards the bulk (c(Fe(III)bulk = 0), which indicates an uneven spatial distribution of Fe(III) in the double layer. This indicates that the diffusion zone is larger than in the case of the anodic branch, when the electroactive species is present in the bulk of the solution. At this point, we must emphasize that when analyzing the charge transferred during oxidation (area under the peak), almost equal charges were transferred when the measurements were performed at the same scan rate, regardless of the concentration of the support. This suggests that equal concentration of Fe(III) is present in the system at the beginning of the cathodic branch of CV.
Accordingly, during cathodic scanning in the case of a kinetically limited reaction, a steady state is to be expected due to the wider diffusion zone (and thus a longer diffusion path), as observed in the case of 0.001 M KCl. As a result, lower cathodic currents were recorded at higher scan rates, followed by their independence upon potential (see
Supplementary Materials—Figure S2a).
This behavior is characterized by the slope of log(i) vs. log(υ) of 0.16. In the case of the higher support, the depletion of the diffusion zone is directly correlated with the increase in the cathodic potential due to the high effective potential. Accordingly, no steady state condition can be observed, and the peak shape is obtained for the cathodic branch even at the highest scan rate. This is manifested by the slope of the log(i) vs. log(υ) slope of 0.42. Here we must emphasize that the explanation is based on the fact that the potentiostat changes do not change the step potential with the changes in scan rates (but obviously the step time).
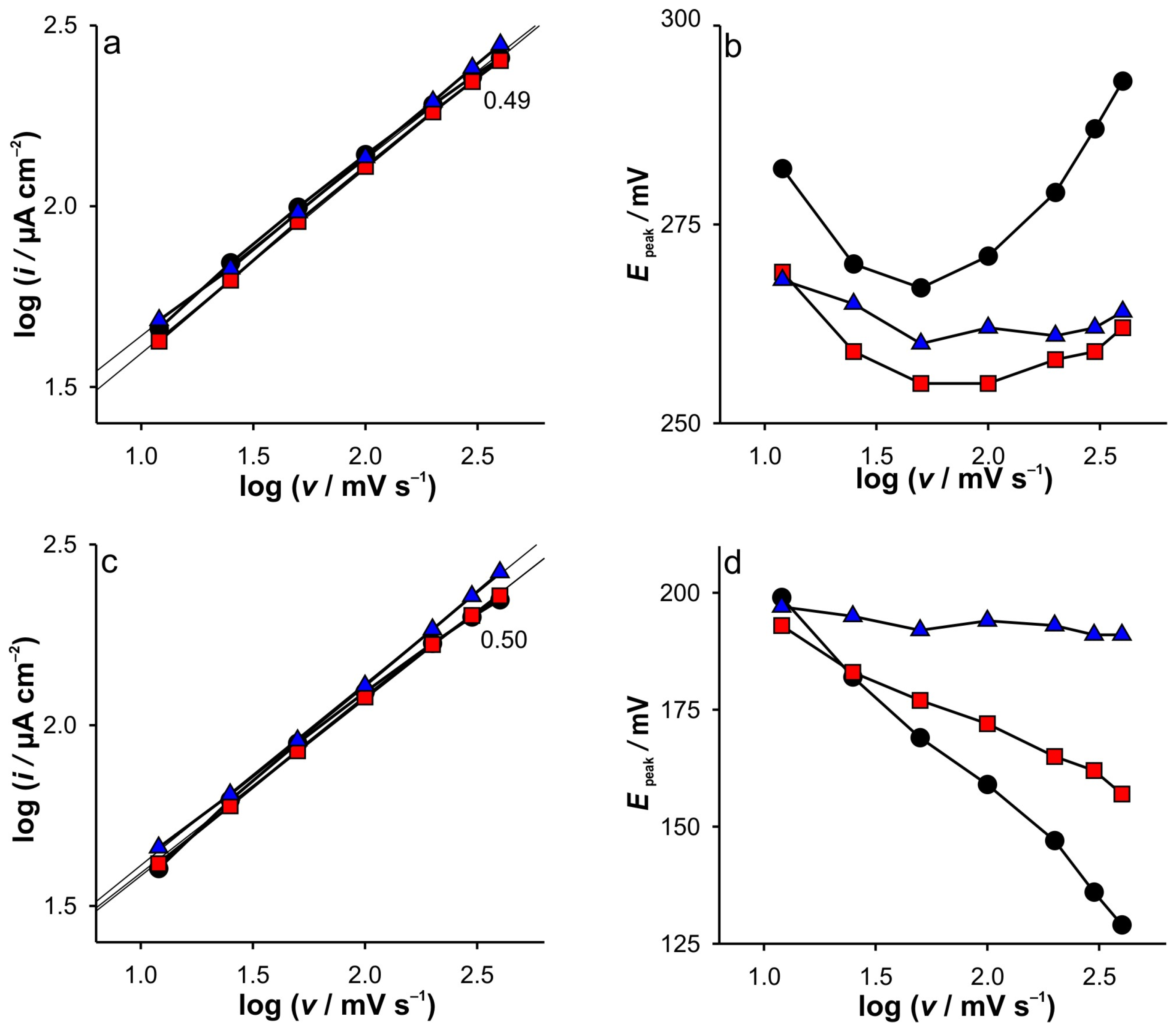
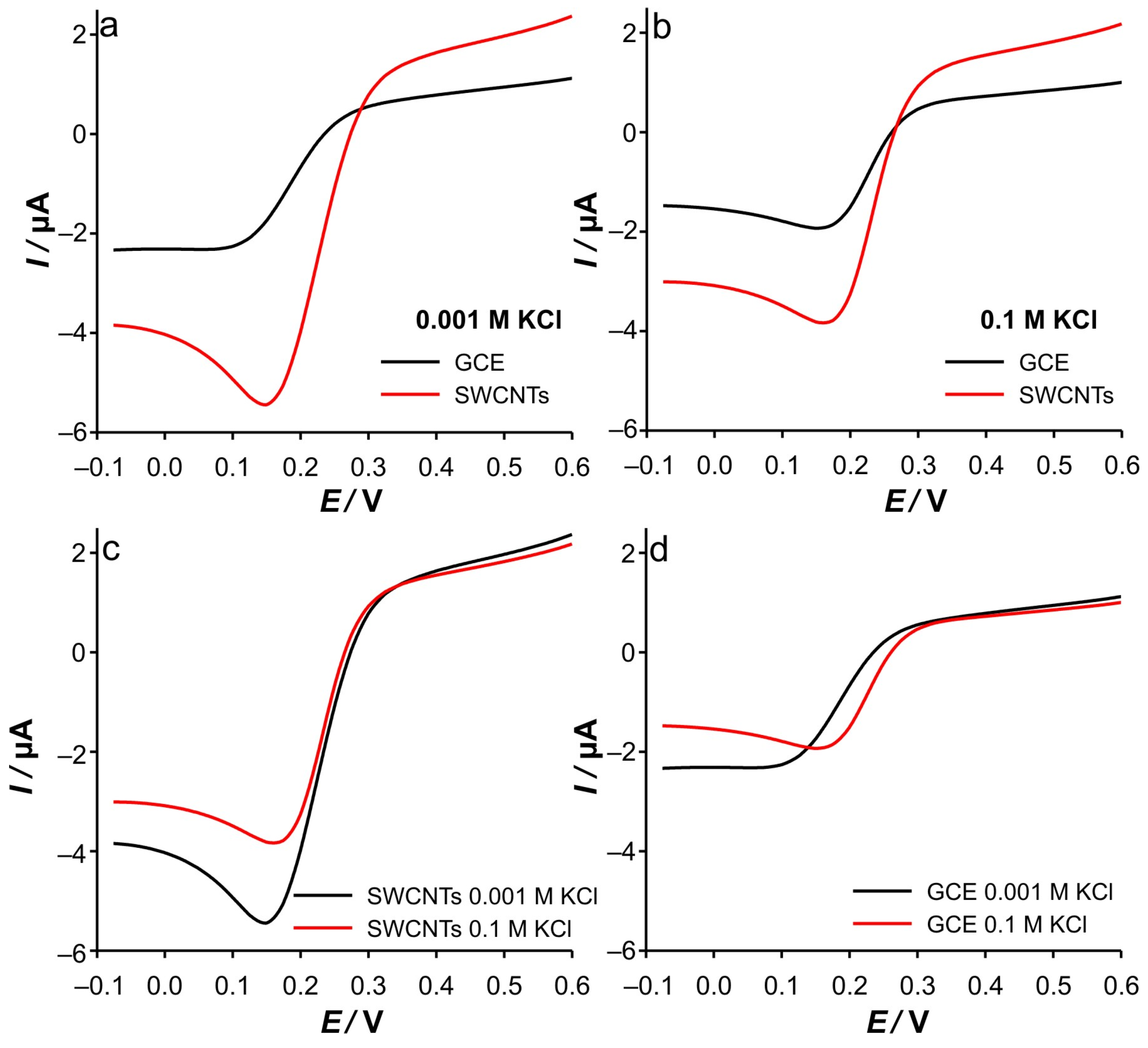
Finally, the analysis of the experimental data extracted from the CVs recorded at the SWCNTs (
Supplementary Materials—Figure S3), dependent on the scan rate (
Figure 2), confirms all assumptions and explains the phenomena based on the DPSCA, RPV, and CV on the GCE electrode results. Since the SWCNTs act as a blocking electrode not only towards the GCE electrode but also as a barrier to the diffusion of Fe(III) into the bulk of the solution, a thinner diffusion zone results. Consequently, the dependence of the cathodic and anodic current peaks on the scan rate suggests a diffusion-controlled reaction at all ionic strengths of KCl, indicating that the diffusion layer is depleted in SWCNTs at the same rate. This is possible due to the limited amount of Fe(III) trapped in the small volume near the GCE, but it is not the case for the anodic branch, as the expansion of the diffusion layer is highly influenced by the presence of Fe(II) in the bulk and correspondingly in the porous layer of the SWCNTs. It is also important to point out the unusual changes in the position of the anodic current peaks when changing the scan rate, which are more pronounced when the KCl concentration is decreased. This inherently counterintuitive behavior is predicted by the simulation of the voltammetric behavior of electrodes covered with porous inactive materials by Kathelon et al. [
41]. This behavior is more pronounced at low support concentration and slow scan rates, as it fits to the time frame of the processes that occur in SWCNTs during cycling.