Silent Inflammation, Loud Consequences: Decoding NLR Across Renal, Cardiovascular and Metabolic Disorders
Abstract
1. Introduction
2. Materials and Methods
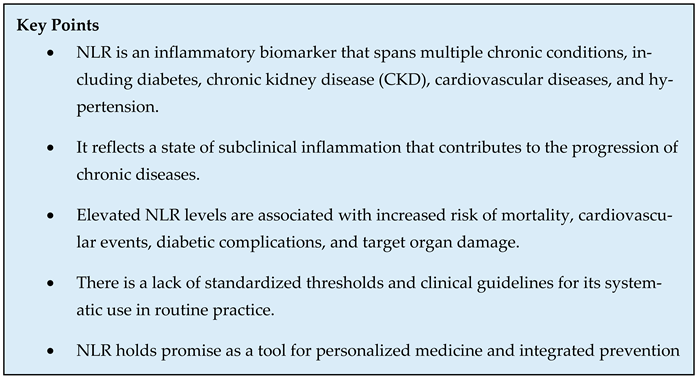
3. NLR and Inflammation: Biological Basis
4. NLR and Diabetes
5. NLR and CKD
6. NLR and Cardiovascular Disease
7. NLR and Hypertension
8. Current Limitations and Gaps
9. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Targeting Innate Immunity-Driven Inflammation in CKD and Cardiovascular Disease; Nature Reviews Nephrology. Available online: https://www.nature.com/articles/s41581-022-00621-9 (accessed on 21 July 2025).
- Margioris, A.N.; Dermitzaki, E.; Venihaki, M.; Tsatsanis, C. 4-Chronic low-grade inflammation. In Diet, Immunity and Inflammation; Calder, P.C., Yaqoob, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 105–120. ISBN 978-0-85709-037-9. [Google Scholar]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Plonsky-Toder, M.; Magen, D.; Pollack, S. Innate Immunity and CKD: Is There a Significant Association? Cells 2023, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Mechanisms of Vascular Inflammation and Potential Therapeutic Targets: A Position Paper from the ESH Working Group on Small Arteries. Hypertension. Available online: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.123.22483 (accessed on 22 July 2025).
- Savoia, C.; Sada, L.; Zezza, L.; Pucci, L.; Lauri, F.M.; Befani, A.; Alonzo, A.; Volpe, M. Vascular Inflammation and Endothelial Dysfunction in Experimental Hypertension. Int. J. Hypertens. 2011, 2011, 281240. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Ozturk, C.; Balta, I.; Demirkol, S.; Demir, M.; Celik, T.; Iyisoy, A. The Neutrophil–Lymphocyte Ratio and Inflammation. Angiology 2016, 67, 298–299. [Google Scholar] [CrossRef]
- Islam, M.M.; Satici, M.O.; Eroglu, S.E. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: An extensive literature review. Turk. J. Emerg. Med. 2024, 24, 8–19. [Google Scholar] [CrossRef]
- Alotiby, A. Immunology of Stress: A Review Article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef]
- Obeagu, E.I. Stress, neutrophils, and immunity: A dynamic interplay. Ann. Med. Surg. 2025, 87, 3573–3585. [Google Scholar] [CrossRef]
- Rosales, C.; Demaurex, N.; Lowell, C.A.; Uribe-Querol, E. Neutrophils: Their Role in Innate and Adaptive Immunity. J. Immunol. Res. 2016, 2016, 1469780. [Google Scholar] [CrossRef]
- Yang, L.; Shi, F.; Cao, F.; Wang, L.; She, J.; He, B.; Xu, X.; Kong, L.; Cai, B. Neutrophils in Tissue Injury and Repair: Molecular Mechanisms and Therapeutic Targets. MedComm 2025, 6, e70184. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef]
- Behaviour of Carbonyl Groups in Several Clinical Conditions: Analysis of Our Survey—Gregorio Caimi, Eugenia Hopps, Maria Montana, Caterina Carollo, Vincenzo Calandrino, Eleonora Gallà, Baldassare Canino, Rosalia Lo Presti; 2020. Available online: https://journals.sagepub.com/doi/abs/10.3233/CH-190689 (accessed on 22 July 2025).
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.Y.; Bae, Y.-S. Emerging roles of neutrophils in immune homeostasis. BMB Rep. 2022, 55, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Ren, H.; Jiang, J.; Shao, C.; Shi, Y.; Li, P. Dying to Defend: Neutrophil Death Pathways and their Implications in Immunity. Adv. Sci. 2023, 11, 2306457. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Elsayed, A.M.; Husseiny, M.I. Regulatory T-cells: The Face-off of the Immune Balance. Front. Biosci. (Landmark Ed.) 2024, 29, 377. [Google Scholar] [CrossRef] [PubMed]
- Lymphocyte Depletion and Subset Alteration Correlate to Renal Function in Chronic Kidney Disease Patients. Available online: https://www.tandfonline.com/doi/full/10.3109/0886022X.2015.1106871 (accessed on 22 July 2025).
- Nagasawa, M.; Spits, H.; Ros, X.R. Innate Lymphoid Cells (ILCs): Cytokine Hubs Regulating Immunity and Tissue Homeostasis. Cold Spring Harb. Perspect. Biol. 2018, 10, a030304. [Google Scholar] [CrossRef]
- Nouari, W.; Aribi, M. Innate lymphoid cells, immune functional dynamics, epithelial parallels, and therapeutic frontiers in infections. Int. Rev. Immunol. 2025, 1–28, ahead of print. [Google Scholar] [CrossRef]
- Kostenko, V.; Akimov, O.; Gutnik, O.; Kostenko, H.; Kostenko, V.; Romantseva, T.; Morhun, Y.; Nazarenko, S.; Taran, O. Modulation of redox-sensitive transcription factors with polyphenols as pathogenetically grounded approach in therapy of systemic inflammatory response. Heliyon 2023, 9, e15551. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J. Mol. Med. 2021, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, R.; Iorio, A.D. The neutrophil-to-lymphocyte ratio in aging and immunosenescence. Explor. Immunol. 2025, 5, 1003200. [Google Scholar] [CrossRef]
- Sarejloo, S.; Dehesh, M.; Fathi, M.; Khanzadeh, M.; Lucke-Wold, B.; Ghaedi, A.; Khanzadeh, S. Meta-analysis of differences in neutrophil to lymphocyte ratio between hypertensive and non-hypertensive individuals. BMC Cardiovasc. Disord. 2023, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Xiao, Z. Diagnostic Accuracy of Neutrophil-to-Lymphocyte Ratio in Type 2 Diabetic Nephropathy: A Meta-Analysis. Front. Endocrinol. 2025, 16, 1564170. [Google Scholar] [CrossRef]
- Aneez, F.A.; Shariffdeen, N.; Haleem, F.A.; Thangarajah, B.R.; Rasaratnam, K. Correlation between neutrophil to lymphocyte ratio and platelet to lymphocyte ratio with proteinuria in different stages of chronic kidney disease. Egypt. J. Intern. Med. 2024, 36, 6. [Google Scholar] [CrossRef]
- Zhao, W.-M.; Tao, S.-M.; Liu, G.-L. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2020, 42, 1059–1066. [Google Scholar] [CrossRef]
- Ghasempour Dabaghi, G.; Rabiee Rad, M.; Mortaheb, M.; Darouei, B.; Amani-Beni, R.; Mazaheri-Tehrani, S.; Izadan, M.; Touhidi, A. The Neutrophil-to-Lymphocyte Ratio Predicts Cardiovascular Outcomes in Patients With Diabetes: A Systematic Review and Meta-Analysis. Cardiol. Rev. 2025, 33, 202–211. [Google Scholar] [CrossRef]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef]
- Usman, M.S.; Khan, M.S.; Butler, J. The Interplay Between Diabetes, Cardiovascular Disease, and Kidney Disease. In Chronic Kidney Disease and Type 2 Diabetes; ADA Clinical Compendia Series; American Diabetes Association: Arlington, VA, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK571718/ (accessed on 22 July 2025).
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, H.; Zhu, X.; Mao, F.; Zhang, S.; Shi, H.; Li, Y.; Lu, B. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2017, 130, 90–97. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Chen, H.L.; Wu, C.; Cao, L.; Wang, R.; Zhang, T.Y.; He, Z. The association between the neutrophil-to-lymphocyte ratio and type 2 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 107. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Wang, H.; Yan, W.; Mu, Y. Neutrophil-lymphocyte ratio as a predictor of kidney function decline among individuals with diabetes and prediabetes: A 3-year follow-up study. J. Diabetes 2019, 11, 427–430. [Google Scholar] [CrossRef]
- Adane, T.; Melku, M.; Worku, Y.B.; Fasil, A.; Aynalem, M.; Kelem, A.; Getawa, S. The Association between Neutrophil-to-Lymphocyte Ratio and Glycemic Control in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2023, 2023, 3117396. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Chen, Q.; Xiong, X.; Zheng, M. The association of inflammatory biomarkers with clinical outcomes in diabetic retinopathy participants: Data from NHANES 2009-2018. Diabetol. Metab. Syndr. 2024, 16, 181. [Google Scholar] [CrossRef]
- Wang, J.-R.; Chen, Z.; Yang, K.; Yang, H.-J.; Tao, W.-Y.; Li, Y.-P.; Jiang, Z.-J.; Bai, C.-F.; Yin, Y.-C.; Duan, J.-M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Pei, G.; Jiang, Z.; Qin, A.; Tan, J.; Tang, Y.; Qin, W. High Neutrophil-To-Lymphocyte Ratio Is an Independent Risk Factor for End Stage Renal Diseases in IgA Nephropathy. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Lin, C.-H.; Li, Y.-H.; Wang, Y.-Y.; Chang, W.-D. Higher Neutrophil-To-Lymphocyte Ratio Was Associated with Increased Risk of Chronic Kidney Disease in Overweight/Obese but Not Normal-Weight Individuals. Int. J. Environ. Res. Public Health 2022, 19, 8077. [Google Scholar] [CrossRef] [PubMed]
- Ao, G.; Wang, Y.; Qi, X.; Wang, F.; Wen, H. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: A meta-analysis. Clin. Exp. Nephrol. 2021, 25, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Solak, Y.; Yilmaz, M.I.; Sonmez, A.; Saglam, M.; Cakir, E.; Unal, H.U.; Gok, M.; Caglar, K.; Oguz, Y.; Yenicesu, M.; et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 532–540. [Google Scholar] [CrossRef]
- Carollo, C.; Mancia, E.; Sorce, A.; Altieri, C.; Altieri, D.; Brunori, G.; Mulè, G. Prognostic impact of neutrophil-to-lymphocyte ratio and vascular access in patients on chronic hemodialysis. J. Nephrol. 2025, 38, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Wang, S.; Li, H. Neutrophil-to-Lymphocyte Ratio and Erythropoietin Resistance among Maintenance Hemodialysis Patients. Available online: https://dx.doi.org/10.1159/000519644 (accessed on 22 July 2025).
- Carollo, C.; Sorce, A.; Mancia, E.; Cirafici, E.; Ciuppa, M.E.; De Biasio, B.; Mulè, G.; Brunori, G. Assessing the Impact of Inflammation on Erythropoietin Resistance in Hemodialysis: The Role of the NLR. J. Clin. Med. 2025, 14, 3411. [Google Scholar] [CrossRef]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-Inflammatory Cytokine-Mediated Anemia: Regarding Molecular Mechanisms of Erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef]
- Pineault, J.; Lamarche, C.; Bell, R.; Lafrance, J.-P.; Ouellet, G.; Leblanc, M.; Pichette, V.; Bezzaoucha, S.; Vallée, M. Association of Neutrophil-to-Lymphocyte Ratio with Inflammation and Erythropoietin Resistance in Chronic Dialysis Patients. Can. J. Kidney Health Dis. 2017, 4, 2054358117735563. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.; Wu, J.; Li, C.; Zheng, L.; Zhu, B.; Min, Y.; Ling, T.; Liu, X. Association between systemic inflammatory indicators with the survival of chronic kidney disease: A prospective study based on NHANES. Front. Immunol. 2024, 15, 1365591. [Google Scholar] [CrossRef]
- Catabay, C.; Obi, Y.; Streja, E.; Soohoo, M.; Park, C.; Rhee, C.M.; Kovesdy, C.P.; Hamano, T.; Kalantar-Zadeh, K. Lymphocyte Cell Ratios and Mortality among Incident Hemodialysis Patients. Am. J. Nephrol. 2017, 46, 408–416. [Google Scholar] [CrossRef]
- Carollo, C.; Benfante, A.; Sorce, A.; Montalbano, K.; Cirafici, E.; Calandra, L.; Geraci, G.; Mulè, G.; Scichilone, N. Predictive Biomarkers of Acute Kidney Injury in COVID-19: Distinct Inflammatory Pathways in Patients with and Without Pre-Existing Chronic Kidney Disease. Life 2025, 15, 720. [Google Scholar] [CrossRef]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N. Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil-lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef]
- Altunoren, O.; Akkus, G.; Sezal, D.T.; Ciftcioglu, M.; Guzel, F.B.; Isiktas, S.; Torun, G.I.; Uyan, M.; Sokmen, M.F.; Sevim, H.A.; et al. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression? Int. Urol. Nephrol. 2019, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, J.; Peng, Z.; Zhou, Q.; Xiao, X.; Xie, Y.; Wang, W.; Huang, L.; Tang, W.; Sun, D.; et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J. Transl. Med. 2019, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Diao, J.; Qi, C.; Jin, J.; Li, L.; Gao, X.; Gong, L.; Wu, W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc Disord. 2018, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, Y.; Ji, H.; Jian, X. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: A retrospective cohort study. BMJ Open 2018, 8, e023459. [Google Scholar] [CrossRef]
- Ul Hussain, H.; Kumar, K.A.; Zahid, M.; Husban Burney, M.; Khan, Z.; Asif, M.; Rehan, S.T.; Ahmad Cheema, H.; Swed, S.; Yasmin, F.; et al. Neutrophil to lymphocyte ratio as a prognostic marker for cardiovascular outcomes in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention: A systematic review and meta-analysis. Medicine 2024, 103, e38692. [Google Scholar] [CrossRef]
- Caimi, G.; Lo Presti, R.; Canino, B.; Ferrera, E.; Hopps, E. Behaviour of the neutrophil to lymphocyte ratio in young subjects with acute myocardial infarction. Clin. Hemorheol. Microcirc. 2016, 62, 239–247. [Google Scholar] [CrossRef]
- Caimi, G.; Montana, M.; Andolina, G.; Hopps, E.; Lo Presti, R. Plasma Viscosity and NLR in Young Subjects with Myocardial Infarction: Evaluation at the Initial Stage and at 3 and 12 Months. Clin. Med. Insights Cardiol. 2019, 13, 1179546819849428. [Google Scholar] [CrossRef]
- Cho, J.H.; Cho, H.-J.; Lee, H.-Y.; Ki, Y.-J.; Jeon, E.-S.; Hwang, K.-K.; Chae, S.C.; Baek, S.H.; Kang, S.-M.; Choi, D.-J.; et al. Neutrophil-Lymphocyte Ratio in Patients with Acute Heart Failure Predicts In-Hospital and Long-Term Mortality. J. Clin. Med. 2020, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Sasmita, B.R.; Zhu, Y.; Gan, H.; Hu, X.; Xue, Y.; Xiang, Z.; Huang, B.; Luo, S. Prognostic value of neutrophil-lymphocyte ratio in cardiogenic shock complicating acute myocardial infarction: A cohort study. Int. J. Clin. Pract. 2021, 75, e14655. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Lai, X.; Wu, C.; Wang, L.; Shang, J.; Zhang, H.; Jia, S.; Xing, W.; Liu, H. The roles of neutrophils in cardiovascular diseases. Front. Cardiovasc. Med. 2025, 12, 1526170. [Google Scholar] [CrossRef]
- Peng, L.; Liu, L.; Chai, M.; Cai, Z.; Wang, D. Predictive value of neutrophil to lymphocyte ratio for clinical outcome in patients with atrial fibrillation: A systematic review and meta-analysis. Front Cardiovasc. Med. 2024, 11, 1461923. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Thomassen, J.Q.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Neutrophil counts and cardiovascular disease. Eur. Heart J. 2023, 44, 4953–4964. [Google Scholar] [CrossRef]
- Li, B.; Lai, X.; Yan, C.; Jia, X.; Li, Y. The associations between neutrophil-to-lymphocyte ratio and the Chinese Visceral Adiposity Index, and carotid atherosclerosis and atherosclerotic cardiovascular disease risk. Exp. Gerontol. 2020, 139, 111019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Wang, Y.; Peng, S.; Pi, J.; Yue, J.; Meng, Q.; Liu, J.; Zheng, L.; Chan, P.; et al. The Association of Lipoprotein(a) and Neutrophil-to-Lymphocyte Ratio Combination with Atherosclerotic Cardiovascular Disease in Chinese Patients. Int. J. Gen. Med. 2023, 16, 2805–2817. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wu, G. Relationship of Neutrophil-to-Lymphocyte Ratio with Carotid Plaque Vulnerability and Occurrence of Vulnerable Carotid Plaque in Patients with Acute Ischemic Stroke. Biomed Res. Int. 2021, 2021, 6894623. [Google Scholar] [CrossRef]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef]
- Immunomodulatory Activity of Cytokines in Hypertension: A Vascular Perspective. Hypertension. Available online: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.124.21712 (accessed on 21 July 2025).
- Sunbul, M.; Gerin, F.; Durmus, E.; Kivrak, T.; Sari, I.; Tigen, K.; Cincin, A. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin. Exp. Hypertens. 2014, 36, 217–221. [Google Scholar] [CrossRef]
- Belen, E.; Sungur, A.; Sungur, M.A.; Erdoğan, G. Increased Neutrophil to Lymphocyte Ratio in Patients with Resistant Hypertension. J. Clin. Hypertens. 2015, 17, 532–537. [Google Scholar] [CrossRef]
- Yu, X.; Xue, Y.; Bian, B.; Wu, X.; Wang, Z.; Huang, J.; Huang, L.; Sun, Y. NLR-A Simple Indicator of Inflammation for the Diagnosis of Left Ventricular Hypertrophy in Patients with Hypertension. Int. Heart J. 2020, 61, 373–379. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Shi, H.; Wang, C.; Xia, Y.; Guo, X.; Li, C.; et al. Blood Neutrophil to Lymphocyte Ratio as a Predictor of Hypertension. Am. J. Hypertens. 2015, 28, 1339–1346. [Google Scholar] [CrossRef]
- Xu, J.-P.; Zeng, R.-X.; Zhang, Y.-Z.; Lin, S.-S.; Tan, J.-W.; Zhu, H.-Y.; Mai, X.-Y.; Guo, L.-H.; Zhang, M.-Z. Systemic inflammation markers and the prevalence of hypertension: A NHANES cross-sectional study. Hypertens. Res. 2023, 46, 1009–1019. [Google Scholar] [CrossRef]
- Jhuang, Y.-H.; Kao, T.-W.; Peng, T.-C.; Chen, W.-L.; Li, Y.-W.; Chang, P.-K.; Wu, L.-W. Neutrophil to lymphocyte ratio as predictor for incident hypertension: A 9-year cohort study in Taiwan. Hypertens. Res. 2019, 42, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-N.; Huang, Y.-R.; Chen, X.; Liu, K.; Li, S.-J.; Yang, H.; Chen, W.; Ren, B.-Q.; Luo, Z.-H. Value of neutrophil-to-lymphocyte ratio as a marker of renal damage in patients with H-type hypertension. Biomark. Med. 2021, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.; Geng, Y.; Wu, H.; Chu, Y.; Liu, R.; Wei, Y.; Qiu, Z. The relationship between neutrophil to lymphocyte ratio and artery stiffness in subtypes of hypertension. J. Clin. Hypertens. 2017, 19, 780–785. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, R.; Wang, X.; Zhang, W.; Li, M.; Ni, T.; Weng, W.; Li, Q. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc. Diabetol. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; He, H.; Fang, P.; Liu, S.; Chen, C. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular and all-cause mortality in hypertension patients. Heliyon 2024, 10, e27517. [Google Scholar] [CrossRef] [PubMed]
- Bertouch, J.V.; Roberts-Thomson, P.J.; Bradley, J. Diurnal variation of lymphocyte subsets identified by monoclonal antibodies. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Population | Main Outcomes | Key Findings |
|---|---|---|---|
| Li et al., 2019 [43] | 3000+ T2DM patients | NLR correlation with renal function | NLR significantly higher in patients with diabetic nephropathy |
| Adane et al, 2023 [44] | 13 studies meta-analysis | Relationship between NLR and glycemic control | NLR positively correlated with HbA1c and poor glycemic control |
| Rui-Wang et al., 2020 [46] | 470 T2DM and controls | Association between NLR, PLR and DR | NLR independently associated with diabetic retinopathy as continuous and categorical variable |
| Si et al. 2024 [45] | 572 adults with diabetic retinopathy (NHANES 2009–2018) | All-cause mortality, diabetes-related cardiovascular mortality | Elevated NLR (≥1.516), MLR (≥0.309), and SIRI (≥0.756) independently associated with increased mortality risk. Combined marker (NLR + MLR + SIRI) improved prognostic performance. J-shaped mortality curves observed. |
| Study (Author, Year) | Population | Main Outcomes | Key Findings |
|---|---|---|---|
| Yoshitomi et al., 2019 [47] | 256 CKD stage 3–5, Japan | eGFR decline, renal replacement therapy | Higher NLR associated with faster renal decline and dialysis initiation |
| Wang et al., 2021 [48] | 966 IgA nephropathy patients | ESKD progression, histological severity | NLR ≥ 2.67 linked to glomerulosclerosis and fibrosis, higher progression risk |
| Muresan et al., 2023 [51] | ESKD Romanian patients (2016–2019) | NLR/MLR/PLR at admission vs. 30-day mortality | High NLR: 30-day mortality = 40.12% vs. 1.97% (p < 0.0001); independent predictor of early death |
| Yuan et al., 2021 [64] | 938 Chinese CKD patients, stages 1–4 | ESKD progression, CV and all-cause mortality | NLR associated with ESKD risk only in stage 4 CKD; no significant association with CVD events or all-cause mortality across all stages |
| Chen et al., 2024 [58] | 3500 NHANES CKD participants | All-cause mortality | Elevated NLR associated with higher 10-year mortality (HR > 1.5) |
| Carollo et al. 2025 [53] | 236 chronic hemodialysis patients | 12-month mortality, inflammation/nutrition markers | High NLR linked to increased mortality and worse nutritional profile |
| Lin et al., 2021 [49] | 3000+ patients, Taiwan | eGFR decline, albuminuria, inflammation | Higher NLR linked to progression in both diabetic and non-diabetic CKD |
| Ao, et al., 2021 [50] | 116,709 patients with CKD, including those on dialysis (13 studies) | CV and all-cause mortality | Elevated NLR was significantly associated with increased all-cause mortality (HR 1.93) and cardiovascular mortality (HR 1.45); among dialysis patients, all-cause mortality HR 1.94 |
| Catabay et al., 2017 [59] | Incident HD patients (2007–2011, USA) | Short- & long-term mortality | High NLR predicted mortality; improved AUROC and R2; PLR had no predictive value |
| Setting/Study | Design & Sample | NLR Cut-Off/Quartile | Outcome(s) | Key Prognostic Findings |
|---|---|---|---|---|
| Ul Hussain et al. 2024 [67] | STEMI patients post-PCI; 28,756 individuals (35 studies) | High vs. low NLR (varied) | In-hospital & long-term mortality, CV mortality, MACE | High NLR: in-hospital mortality RR = 3.52; long-term mortality HR ≈ 1.07; CV mortality RR ≈ 2.66; MACE RR ≈ 2.92 |
| Cho et al., 2018 [70] | Acute HF | Elevated NLR (thresholds unspecified) | In-hospital mortality, three years mortality | Elevated NLR independently associated with increased in-hospital mortality (OR ≈ 2.2) and three-year post-discharge mortality (OR ≈ 1.44) |
| Caimi et al. 2024 [68] | 123 young AMI patients (mean age 39.4 ± 5.8 years) | NLR ≥ 6.5 | STEMI/Non STEMI Long term outcome | NLR significantly elevated in AMI patients vs. controls (2.38 ± 0.87 vs. 1.82 ± 0.71, p < 0.0001). No difference in NLR between STEMI vs. non-STEMI or diabetic vs. non-diabetic. NLR not correlated with number of CV risk factors or CAD extent. Neutrophil count decreased over time leading to reduced NLR; lymphocyte counts stable. |
| Peng et al. 2024 [73] | AF cohort meta-analysis; ~59,000 patients | High NLR (varied) | AF recurrence, stroke, mortality, left atrial thrombus | High NLR predicted AF recurrence and stroke; linked to mortality and thrombus |
| Cho et al., 2020 [70] | Decompensated HF; 5580 patients | Quartile 4 (not specified) | In-hospital & 3-year mortality | Highest quartile NLR: OR ~2.2 for in-hospital mortality; OR ~1.44 at 3 years |
| Study (Author, Year) | Design/Population | Main Focus/Outcome | Key Findings |
|---|---|---|---|
| Zhang et al., 2024 [88] | NHANES 2009–2014; 3067 hypertensive adults | NLR and mortality | NLR > 3.5 associated with higher all-cause (HR = 1.96) and CV mortality (HR = 2.33); AUC 0.64–0.70 |
| Hong et al., 2024 [89] | NHANES (extended sample) | NLR thresholds and mortality | NLR > 2.0 linked to all-cause (HR~1.47) and CV mortality (HR~2.08) |
| Liu et al., 2023 [83] | Prospective cohort; 28,850 normotensives | Incident hypertension | Highest NLR quintile: HR = 1.23 for new-onset hypertension |
| Sarejiloo et al., 2021 [31] | 21 studies | NLR in hypertensive vs. normotensive | WMD = 0.40; Non-dippers showed higher NLR (WMD = 0.58) |
| Yu et al., 2020 [82] | Cross-sectional; hypertensive patients | NLR and LVH | NLR independently associated with LVH (OR = 1.506); AUC = 0.626 |
| Wang et al., 2017 [87] | 217 hypertensive + 132 controls | NLR and arterial stiffness (baPWV) | NLR higher in ISH and SDH; NLR independently predicted baPWV |
| Belen et al. [81] | Cohort of resistant hypertension | NLR and BP severity | NLR correlated with office and ambulatory BP; elevated in RHT |
| Chen et al. [86] | H-type hypertension patients | NLR and renal function | 1 unit ↑ NLR = 51% ↑ risk of renal dysfunction |
| NHANES 1999–2010 [84] | Cross-sectional; n = 22,290 | NLR and HTN prevalence | ln-NLR: OR = 1.087 for HTN; SII stronger; PLR and LMR not significant |
| Jhuang et al., 2019 [85] | 6278 adults | NLR and prevalent hypertension | HR = 1.28 overall; stronger in older adults (HR = 1.88) |
| Sunbul et al., 2014 [80] | 83 Non-dipper hypertensives | NLR and BP pattern | NLR > 2.7 predicted non-dipping (83% sens., 65% spec.) |
| Biomarker | Biological Significance | Biological Variability | Advantages | Limitations | Main Prognostic Evidence |
|---|---|---|---|---|---|
| NLR (Neutrophil-to-Lymphocyte Ratio) | Ratio between neutrophils (innate inflammation) and lymphocytes (adaptive response) | Influenced by circadian rhythm, infections, stress, corticosteroids, other therapies | Simple, inexpensive, derived from standard blood count; reflects immune balance | Non-specific, intra-individual variability; lack of standardized cut-off values | Associated with prognosis in sepsis, cardiovascular diseases, cancer, COVID-19 |
| CRP (C-reactive protein) | Acute-phase protein produced by the liver in response to IL-6 | Relatively stable; rises within 6–8 h after inflammatory stimulus | Widely validated; available in labs and point-of-care; standardized marker | Cannot differentiate acute vs. chronic inflammation; less informative on immune status | Strong marker of acute inflammatory severity and cardiovascular risk |
| IL-6 | Central pro-inflammatory cytokine, stimulates CRP production | High intra-individual variability; short half-life; rapid peaks | Sensitive marker of early inflammatory activation | Requires dedicated assays; costly; less accessible in routine clinical use | Strongly associated with severity in sepsis, ARDS, COVID-19 |
| TNF-α | Key cytokine in systemic inflammation and tumor necrosis | High variability; low basal levels; transient peaks | Early indicator of systemic inflammation | Measurement complex, less standardized; high cost | Involved in sepsis and autoimmune diseases; prognostic potential but less established compared with CRP/IL-6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, C.; Sorce, A.; Cirafici, E.; Ciuppa, M.E.; Mulè, G.; Caimi, G. Silent Inflammation, Loud Consequences: Decoding NLR Across Renal, Cardiovascular and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 8256. https://doi.org/10.3390/ijms26178256
Carollo C, Sorce A, Cirafici E, Ciuppa ME, Mulè G, Caimi G. Silent Inflammation, Loud Consequences: Decoding NLR Across Renal, Cardiovascular and Metabolic Disorders. International Journal of Molecular Sciences. 2025; 26(17):8256. https://doi.org/10.3390/ijms26178256
Chicago/Turabian StyleCarollo, Caterina, Alessandra Sorce, Emanuele Cirafici, Maria Elena Ciuppa, Giuseppe Mulè, and Gregorio Caimi. 2025. "Silent Inflammation, Loud Consequences: Decoding NLR Across Renal, Cardiovascular and Metabolic Disorders" International Journal of Molecular Sciences 26, no. 17: 8256. https://doi.org/10.3390/ijms26178256
APA StyleCarollo, C., Sorce, A., Cirafici, E., Ciuppa, M. E., Mulè, G., & Caimi, G. (2025). Silent Inflammation, Loud Consequences: Decoding NLR Across Renal, Cardiovascular and Metabolic Disorders. International Journal of Molecular Sciences, 26(17), 8256. https://doi.org/10.3390/ijms26178256







