Impact of Aflatoxins on the Digestive, Immune, and Nervous Systems: The Role of Microbiota and Probiotics in Toxicity Protection
Abstract
1. Introduction
1.1. General Background
1.2. Literature Overview
1.3. Study Rationale
1.4. Objectives and Method of Review
1.5. Significance
2. Regulatory Framework
- Feed materials: 20 μg/kg;
- Complementary and complete feed mixtures: 10 μg/kg;
- Feed mixtures for dairy cattle and calves, dairy sheep and lambs, dairy goats and kids, piglets, and young poultry: 5 μg/kg;
- General feed mixtures for other categories not specified above: 20 μg/kg [25].
3. Effects of Aflatoxins on the Digestive System
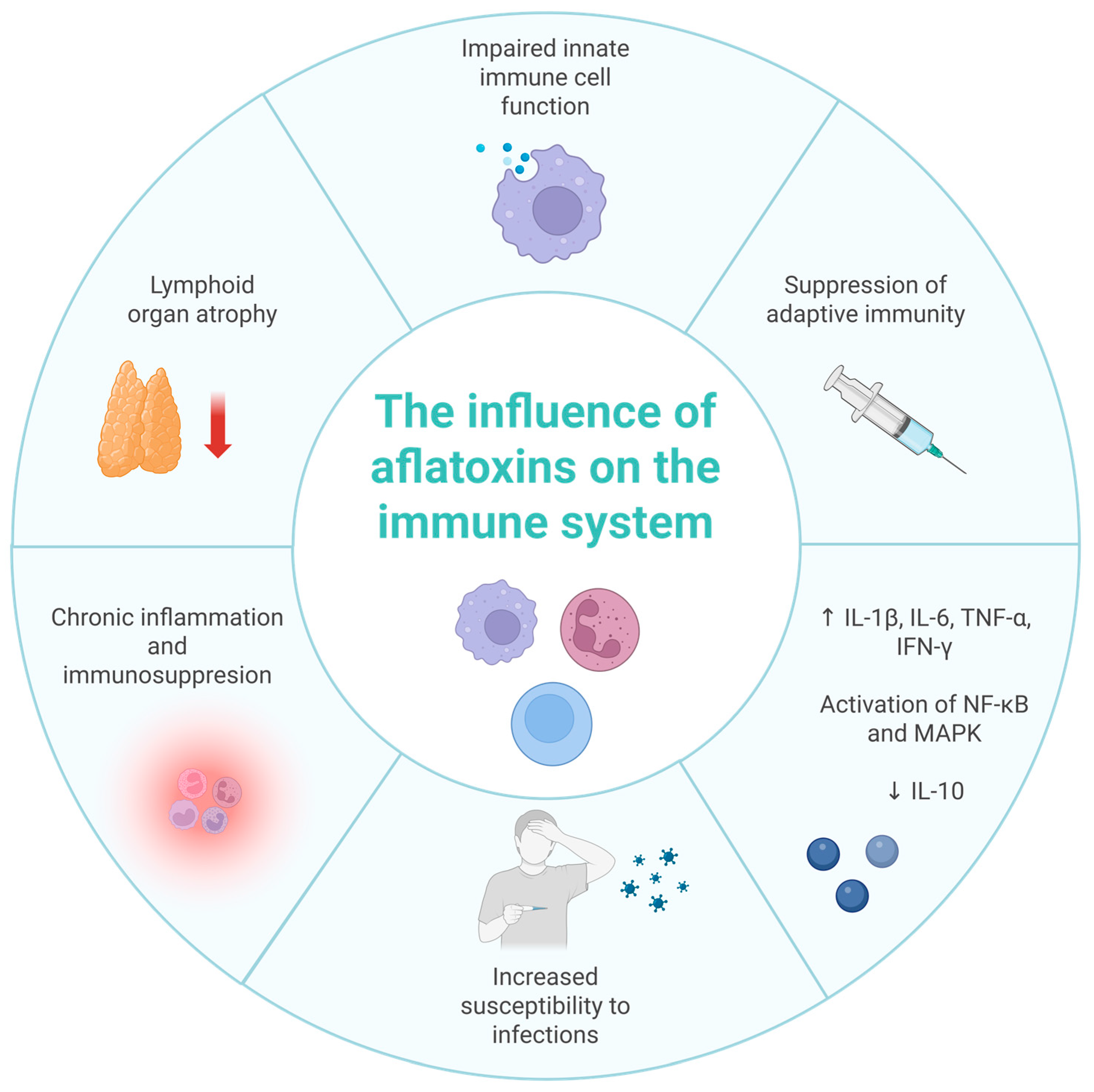
4. Effect of Aflatoxins on the Immune System
5. Overview of Gut Microbiota Types and Role in Immune Homeostasis and Inflammation Control
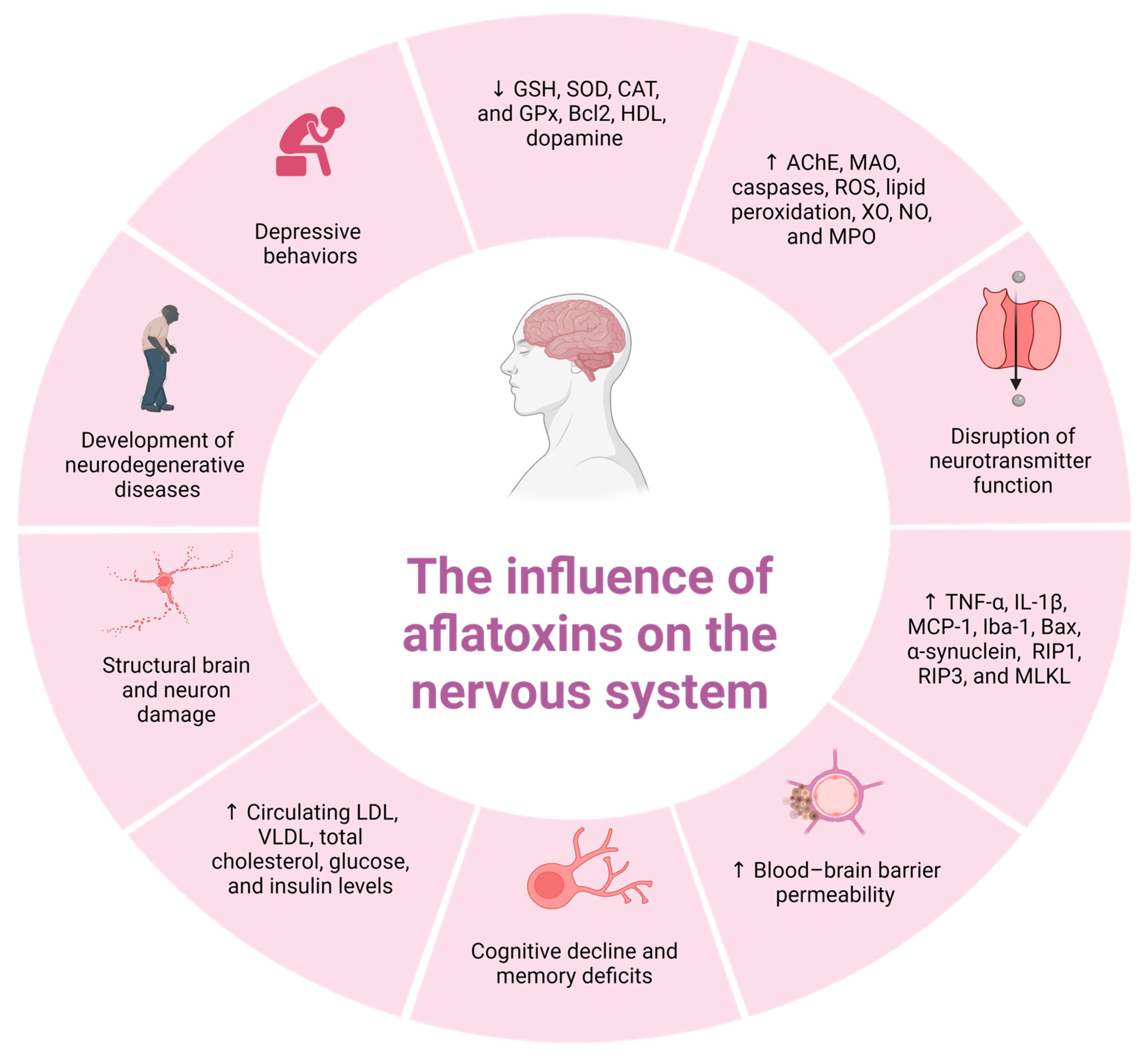
6. Neurotoxicity of Aflatoxin B1
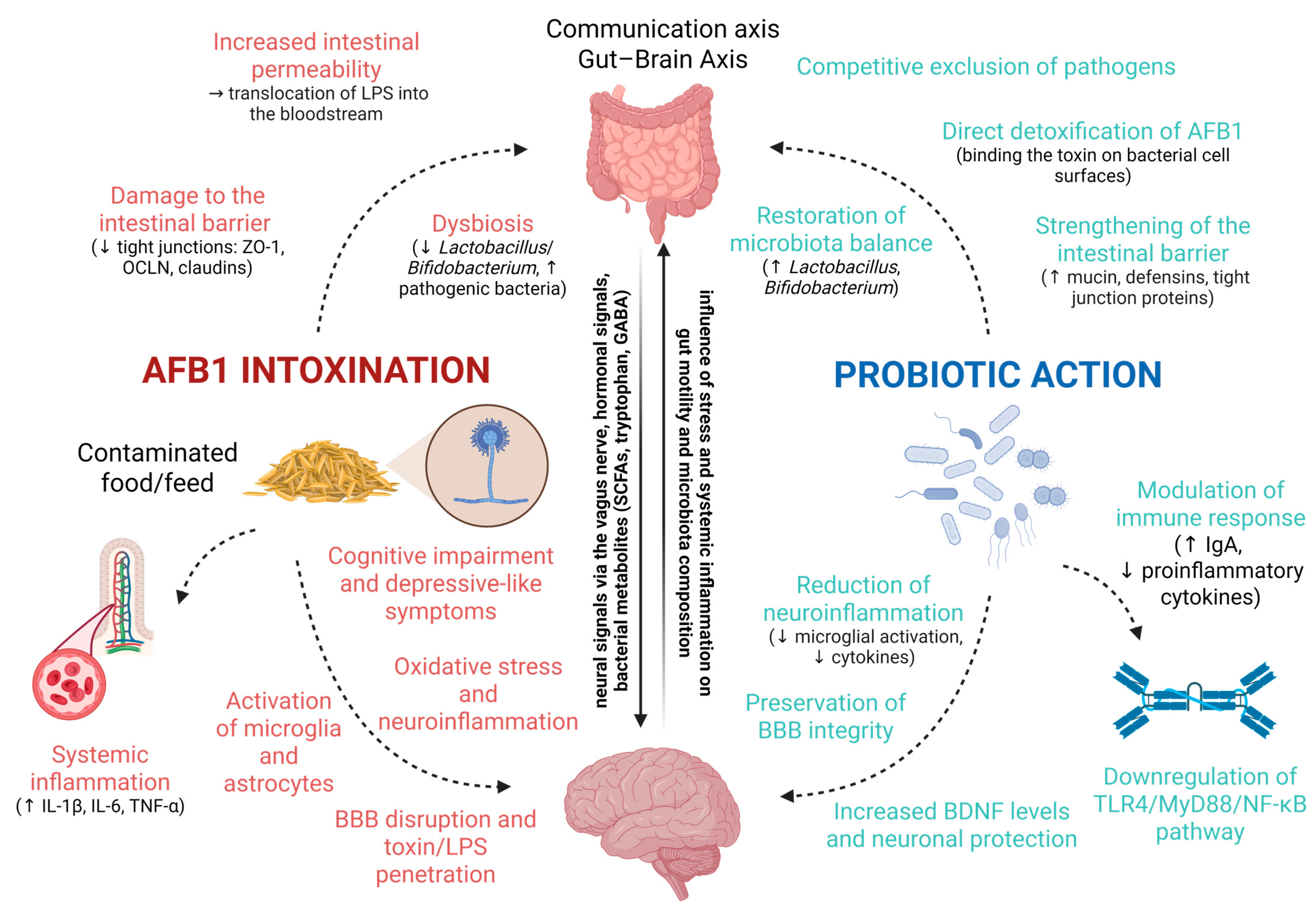
7. Microbiota–Gut–Brain Axis and Aflatoxin Neurotoxicity and Protective Effects of Probiotics
Therapeutic Potential and Clinical Application
- Human health: Probiotic supplementation may serve as an adjunct strategy for populations at high risk of aflatoxin exposure (e.g., in endemic regions), potentially reducing systemic inflammation, protecting liver and neural function, and supporting immune resilience.
- Veterinary practice: In livestock, targeted probiotic feed additives could limit the bioavailability of AFB1, protect gut and liver health, and improve productivity by maintaining nutrient absorption and immune competence. Importantly, in poultry farming, the use of probiotics has also been associated with a reduction in antibiotic consumption, supporting both animal welfare and antimicrobial stewardship.
- Neuroprotection: Given their ability to modulate the gut–brain axis, selected probiotic strains hold promise for mitigating neurotoxicity and preserving cognitive function in both humans and animals.
- Strain specificity: Probiotic effects are highly strain-dependent, and not all strains confer equal protection against AFB1 toxicity.
- Standardization: Variability in formulation, dosing, and delivery methods complicates reproducibility and cross-study comparisons.
- Colonization efficiency: Survival through the gastrointestinal tract and long-term persistence in the host microbiota remain uncertain for many strains.
- Host factors: Age, health status, baseline microbiota composition, and diet can all influence efficacy.
- Safety considerations: While generally regarded as safe, probiotics may induce transient dysbiosis or unwanted immune modulation in immunocompromised individuals.
8. Detection of Aflatoxins in Biological Samples
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AFB1 | Aflatoxin B1 |
| AFM1 | Aflatoxin M1 |
| AFG1 | Aflatoxin G1 |
| AFB2 | Aflatoxin B2 |
| AFM2 | Aflatoxin M2 |
| AFG2 | Aflatoxin G2 |
| EFSA | European Food Safety Authority |
| FAO | Food and Agriculture Organization of the United Nations |
| LD50 | Lethal Dose |
| CYP450 | Cytochrome P450 |
| AFBO | AFB1-exo-8,9-epoxide |
| WHO | World Health Organization |
| DT50 | Dissipation Time |
| FDA | U.S. Food and Drug Administration |
| BIS | Bureau of Indian Standards |
| SOD | Superoxide Dismutase |
| SGLT1 | Sodium-Glucose Cotransporter 1 |
| SLC7A1 | Solute Carrier Family 7 Member 1 |
| ZO-1 | Zonula Occludens-1 |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| CAT | Catalase |
| GPx | Glutathione Peroxidase |
| TAC | Total Antioxidant Capacity |
| TBARS | Thiobarbituric Acid Reactive Substances |
| Ca2+ | Calcium |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| ALB | Albumin |
| TP | Total Protein |
| ALP | Alkaline Phosphatase |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| T-SOD | Total SOD |
| GGT | γ-glutamyltransferase |
| GST | Glutathione S-transferase |
| NO | Nitric Oxide |
| GSH | Glutathione |
| SPF | Specific Pathogen-Free |
| DMSO | Dimethyl Sulfoxide |
| b.w. | Body Weight |
| H2O2 | Hydrogen Peroxide |
| GR | Glutathione Reductase |
| I-FABP | Intestinal Fatty Acid-Binding Protein |
| VH | Villus Height |
| CD | Crypt Depth |
| DAO | Diamine Oxidase |
| PCoA | Principal Coordinate Analysis |
| VSA | Villus Surface Area |
| ROS | Reactive Oxygen Species |
| LPS | Lipopolysaccharide |
| HCC | Hepatocellular Carcinoma |
| HBV | Hepatitis B Virus |
| PAS | Periodic Acid–Schiff |
| IFN-γ | Interferon-gamma |
| TLR4 | Toll-Like Receptor 4 |
| HIV | Human Immunodeficiency Virus |
| HDAC | Histone Deacetylase |
| NOS2 | Inducible Nitric Oxide Synthase |
| APCA | Alternative Pathway of Complement Activation |
| ACP | Acid Phosphatase |
| LOAEL | Lowest Observed Adverse Effect Level |
| BBB | Blood–Brain Barrier |
| AChE | Acetylcholinesterase |
| DG | Dentate Gyrus |
| MAO | Monoamine Oxidase |
| XO | Xanthine Oxidase |
| UA | Uric Acid |
| TH | Tyrosine Hydroxylase |
| NOR | Novel Object Recognition |
| GFAP | Glial Fibrillary Acidic Protein |
| MPO | Myeloperoxidase |
| RONS | Reactive Oxygen And Nitrogen Species |
| LPO | Lipid Peroxidation |
| TSH | Thyroid-Stimulating Hormone |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| CNS | Central Nervous System |
| MAMP | Microbe-Associated Molecular Pattern |
| SCFA | Short-Chain Fatty Acid |
| ADHD | Attention-Deficit Hyperactivity Disorder |
| BDNF | Brain-Derived Neurotrophic Factor |
| SAMP8 | Senescence-Accelerated Prone 8 |
| EPS | Exopolysaccharides |
| Aβ | Amyloid Beta |
| MPP+ | 1-methyl-4-phenylpyridinium |
| PPA | Propionic Acid |
| LDH | Lactate Dehydrogenase |
| AA | Acrylamide |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| LFIA | Lateral Flow Immunoassay |
| HPLC-FLD | High-Performance Liquid Chromatography with Fluorescence Detection |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| SPE | Solid-Phase Extraction |
| QuEChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
References
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef]
- Spencer Smith, J.; Williams, W.P.; Windham, G.L. Aflatoxin in maize: A review of the early literature from “moldy-corn toxicosis” to the genetics of aflatoxin accumulation resistance. Mycotoxin Res. 2019, 35, 111–128. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Outcome of a public consultation on the draft risk assessment of aflatoxins in food. EFSA Support. Publ. 2020, 17, 1798E. [Google Scholar] [CrossRef]
- Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 2022, 14, 853. [Google Scholar] [CrossRef]
- Byarugaba, I.; Faizan, A. Aflatoxins: A one health concern. J. Biomed. Eng. Curr. Res. 2022, 4, 23–25. [Google Scholar]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- FAO; OIE; WHO. Taking a Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries; FAO: Rome, Italy, 2019. [Google Scholar]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin contamination: An overview on health issues, detection and management strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Humboldt-Dachroeden, S.; Mantovani, A. Assessing environmental factors within the One Health approach. Medicina 2021, 57, 240. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Saha Turna, N.; Wu, F. Aflatoxin M1 in milk: A global occurrence, intake, & exposure assessment. Trends Food Sci. Technol. 2021, 110, 183–192. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Eaton, D.L.; Williams, D.E.; Coulombe, R.A. Species differences in the biotransformation of aflatoxin B1: Primary determinants of relative carcinogenic potency in different animal species. Toxins 2025, 17, 30. [Google Scholar] [CrossRef]
- Fouché, T.; Claassens, S.; Maboeta, M. Aflatoxins in the soil ecosystem: An overview of its occurrence, fate, effects and future perspectives. Mycotoxin Res. 2020, 36, 303–309. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. The Aspergilli and their mycotoxins: Metabolic interactions with plants and the soil biota. Front. Microbiol. 2020, 10, 2921. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125. [Google Scholar] [CrossRef]
- Albert, J.; Muñoz, K. Kinetics of microbial and photochemical degradation of aflatoxin B1 in a sandy loam and clay soil. Sci. Rep. 2022, 12, 16849. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, K.; Sun, X.; Tang, L.; Wang, J.-S. Multi-toxic endpoints of the foodborne mycotoxins in nematode Caenorhabditis elegans. Toxins 2015, 7, 5224–5235. [Google Scholar] [CrossRef]
- Fouché, T.; Claassens, S.; Maboeta, M.S. Ecotoxicological effects of aflatoxins on earthworms under different temperature and moisture conditions. Toxins 2022, 14, 75. [Google Scholar] [CrossRef]
- Zhao, X. Effect of aflatoxin B1 on development, survival, and fecundity of Ahasverus advena (Waltl). J. Stored Prod. Res. 2018, 77, 225–230. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Medina, Á.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and Aspergillus flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–180. [Google Scholar] [CrossRef]
- de Freitas, C.H.; Gonçalves, C.L.; da Silva Nascente, P. Aflatoxins B1 and M1: Risks related to milk produced in Brazil. Ann. Microbiol. 2018, 68, 793–802. [Google Scholar] [CrossRef]
- European Commission. Directive (2002/32/EC) of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. J. Eur. Union 2002, C221, 232. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Aflatoxin Information. 2024. Available online: https://agriculture.mo.gov/plants/feed/aflatoxin.php (accessed on 28 October 2024).
- Hao, W.H.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin contamination of feeds and raw materials in China in year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Resolução RDC N. 7, de 18 de fevereiro de 2011. Diário Of. Rep. Fed. Bras. 2011, 46, 66. [Google Scholar]
- Thakur, S.; Singh, R.K.; De, P.S.; Dey, A. Aflatoxins in feeds: Issues and concerns with safe food production. Indian J. Anim. Health 2022, 61, 1–13. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. A short guide to the tight junction. J. Cell Sci. 2024, 137, jcs261776. [Google Scholar] [CrossRef]
- Abebe, B.; Abriham, K.; Yobsan, T. Review on aflatoxin and its impacts on livestock. J. Dairy Vet. Sci. 2018, 6, 555685. [Google Scholar] [CrossRef]
- Pu, J.; Yuan, Q.; Yan, H.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of chronic exposure to low levels of dietary aflatoxin B1 on growth performance, apparent total tract digestibility and intestinal health in pigs. Animals 2021, 11, 336. [Google Scholar] [CrossRef]
- Sui, Y.; Lu, Y.; Zuo, S.; Wang, H.; Bian, X.; Chen, G.; Huang, S.; Dai, H.; Liu, F.; Dong, H. Aflatoxin B1 exposure in sheep: Insights into hepatotoxicity based on oxidative stress, inflammatory injury, apoptosis, and gut microbiota analysis. Toxins 2022, 14, 840. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Q.; Lin, L.X.; Xu, T.T.; Lu, Y.; Zhang, C.D.; Yue, K.; Huang, S.C.; Dong, H.J.; Jian, F.C. Aflatoxin B1 alters meat quality associated with oxidative stress, inflammation, and gut-microbiota in sheep. Ecotoxicol. Environ. Saf. 2021, 225, 112754. [Google Scholar] [CrossRef]
- Elgioushy, M.M.; Elgaml, S.A.; El-Adl, M.M.; Hegazy, A.M.; Hashish, E.A. Aflatoxicosis in cattle: Clinical findings and biochemical alterations. Environ. Sci. Pollut. Res. 2020, 27, 35526–35534. [Google Scholar] [CrossRef]
- Guo, H.; Wang, P.; Liu, C.; Zhou, T.; Chang, J.; Yin, Q.; Wang, L.; Jin, S.; Zhu, Q.; Lu, F. Effects of compound mycotoxin detoxifier on alleviating aflatoxin B1-induced inflammatory responses in intestine, liver and kidney of broilers. Toxins 2022, 14, 665. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, M.; He, Y.; Lei, J.; Han, Y.; Wu, Y.; Bai, D.; Guo, Y.; Zhang, B. Mycotoxins binder supplementation alleviates aflatoxin B1 toxic effects on the immune response and intestinal barrier function in broilers. Poult. Sci. 2022, 101, 101683. [Google Scholar] [CrossRef]
- Chen, X.; Ishfaq, M.; Wang, J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune responses, and Salmonella Pullorum infection resistance of broilers challenged with aflatoxin B1. Poult. Sci. 2022, 101, 101651. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Q.; Ma, S.; Zhang, J.; Jia, R.; Ji, C.; Zhao, L. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins 2017, 9, 1. [Google Scholar] [CrossRef]
- El-Sheshtawy, S.M.; El-Zoghby, A.F.; Shawky, N.A.; Samak, D.H. Aflatoxicosis in Pekin duckling and the effects of treatments with lycopene and silymarin. Vet. World 2021, 14, 788–793. [Google Scholar] [CrossRef]
- Altyar, A.E.; Kensara, O.A.; Sayed, A.A.; Aleya, L.; Almutairi, M.H.; Zaazouee, M.S.; Elshanbary, A.A.; El-Demerdash, F.M.; Abdel-Daim, M.M. Acute aflatoxin B1-induced hepatic and cardiac oxidative damage in rats: Ameliorative effects of morin. Heliyon 2023, 9, e21837. [Google Scholar] [CrossRef]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolaena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Hathout, A.S.; Salman, A.S.; Abdel-Aziem, S.H.; Sabry, B.A.; Hassan, N.S.; Abdel-Aziz, M.S.; Aly, S.E.; Jaswir, I. Bioactive compounds from Aspergillus niger extract enhance the antioxidant activity and prevent the genotoxicity in aflatoxin B1-treated rats. Toxicon 2020, 181, 57–68. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, H.I.; Al-Kahtani, M.A.; Shati, A.A.; Alshehri, M.A.; Al-Doaiss, A.A.; Elmansi, A.A.; Ahmed, A.E. Black tea and curcumin synergistically mitigate the hepatotoxicity and nephropathic changes induced by chronic exposure to aflatoxin-B1 in Sprague–Dawley rats. J. Food Biochem. 2020, 44, e13346. [Google Scholar] [CrossRef]
- Gündüz, A.; Yalçın, E.; Çavuşoğlu, K. Combined toxic effects of aflatoxin B2 and the protective role of resveratrol in Swiss albino mice. Sci. Rep. 2021, 11, 18081. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Wang, J.; Sun, J.; Xiang, Y.; Jin, X. Aflatoxin B1 disrupts the intestinal barrier integrity by reducing junction protein and promoting apoptosis in pigs and mice. Ecotoxicol. Environ. Saf. 2022, 247, 114250. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bao, X.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. Aflatoxin B1 and aflatoxin M1 induce compromised intestinal integrity through clathrin-mediated endocytosis. Toxins 2021, 13, 184. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, S.; Tang, Y.; Chen, W.; Zong, Y.; Geng, J.; Zhao, Y.; He, Z.; Du, R. Dihydromyricetin attenuates aflatoxin B1-induced IEC-6 cell damage and intestinal damage in mice by activating the Nrf2/HO-1 signaling pathway and modulation of gut microbiota. J. Funct. Foods 2025, 127, 106716. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Taherpour, K.; Ghasemi, H.A.; Fakharzadeh, S.; Nooreh, Z.; Kalanaky, S. Efficacy of advanced chelate technology-based 7-mineral supplementation in mitigating aflatoxin B1 induced impairments in broiler chicken performance and intestinal health. Microb. Pathog. 2025, 200, 107350. [Google Scholar] [CrossRef]
- Cao, X.; Cheng, J.; Yang, Y.; Wang, J.; Wang, Y. Arginine-derived carbon dots with antioxidant activity for treating aflatoxin B1-induced liver injury via Nrf2/Keap1 and NLRP3 pathways in mice. Life Sci. 2025, 364, 123430. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Qi, Z.; Ma, L.; Hu, G.; Zou, C.; Chen, T. Engineered S. cerevisiae-pYD1-ScFv-AFB1 mitigates aflatoxin B1 toxicity via bio-binding and intestinal microenvironment repair. Food Chem. Toxicol. 2025, 196, 115232. [Google Scholar] [CrossRef] [PubMed]
- Mayada, R.M.R.; Farag, H.S.A.; Gharib, K.; El-Naggar, B.M.; Hendam, E.A.M.; Ahmad, M.; Alagawany, M.; El-Ghazali, H.M. Origanum majorana essential oil ameliorated the behavioral, biochemical, physiological and performance perturbations induced by aflatoxin B1 in growing rabbits. Ann. Anim. Sci. 2023, 23, 1201–1210. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Chen, G.; Kou, R.; Zhang, C.; Wang, Y.; Wang, J.; Huang, Y.; Chen, C. Mechanistic insights into ferroptosis and apoptosis pathways: Synergistic effects of multi-organ toxicity and transgenerational effects induced by co-exposure of epoxiconazole and aflatoxin B1 in zebrafish. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Mehrzad, J.; Hosseinkhani, S.; Malvandi, A.M. Human microglial cells undergo proapoptotic induction and inflammatory activation upon in vitro exposure to a naturally occurring level of aflatoxin B1. Neuroimmunomodulation 2018, 25, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Bahari, A.; Bassami, M.R.; Mahmoudi, M.; Dehghani, H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunol. Lett. 2018, 197, 44–52. [Google Scholar] [CrossRef]
- Githang’a, D.; Wangia, R.N.; Mureithi, M.W.; Wandiga, S.O.; Mutegi, C.; Ogutu, B.; Agweyu, A.; Wang, J.-S.; Anzala, O. The effects of aflatoxin exposure on hepatitis B vaccine-induced immunity in Kenyan children. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 117–130. [Google Scholar] [CrossRef]
- Jolly, P.E.; Akinyemiju, T.F.; Sakhuja, S.; Sheth, R. Association of aflatoxin B1 levels with mean CD4 cell count and uptake of ART among HIV-infected patients: A prospective study. PLoS ONE 2022, 17, e0260873. [Google Scholar] [CrossRef]
- Mehrzad, J.; Fazel, F.; Pouyamehr, N.; Hosseinkhani, S.; Dehghani, H. Naturally occurring level of aflatoxin B1 injures human, canine and bovine leukocytes through ATP depletion and caspase activation. Int. J. Toxicol. 2020, 39, 30–38. [Google Scholar] [CrossRef]
- Shirani, K.; Zanjani, B.R.; Mahmoudi, M.; Jafarian, A.H.; Hassani, F.V.; Giesy, J.P.; Karimi, G. Immunotoxicity of aflatoxin M1: As a potent suppressor of innate and acquired immune systems in a subacute study. J. Sci. Food Agric. 2018, 98, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, Y.; Liu, J.; Chen, H.; Zhao, R. Glucocorticoid receptor-targeting antagomirs alleviates AFB1-induced hepatotoxicity in mice. Ecotoxicol. Environ. Saf. 2025, 292, 117935. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef]
- Tian, Y.; Dong, P.-Y.; Liang, S.-L.; Li, L.; Zhang, S.-E.; Klinger, F.G.; Shen, W.; Yan, Y.-Y.; Zhang, X.-F. Aflatoxin B1 affects porcine alveolar macrophage growth through the calcium signaling pathway mediated by the ceRNA regulatory network. Mol. Biol. Rep. 2023, 50, 8237–8247. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, I.; Koynarski, T.; Sotirov, L.; Nikolov, Y.; Petkov, P. Effect of aflatoxin B1 on Moulard duck’s natural immunity. Pak. Vet. J. 2014, 35, 67–70. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, S.; Fang, J.; Cui, H.; Zuo, Z.; Deng, J. Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Int. J. Environ. Res. Public Health 2014, 11, 13130–13143. [Google Scholar] [CrossRef]
- Nemati, Z.; Karimi, A.; Besharati, M. Impact of aflatoxin contaminated feed and yeast cell wall supplementation on immune system in broiler chickens. In Proceedings of the International Conference on Innovations in Chemical & Agricultural Engineering (ICICAE’2015), Kuala Lumpur, Malaysia, 8–9 February 2015. [Google Scholar]
- Zhao, M.; Chen, L.; Zhao, Y.; Liu, J.; Chen, H.; Zhao, R. miR124a-3p inhibitor alleviates AFB1-induced hepatoxicity via targeting chicken glucocorticoid receptor mRNA. Poult. Sci. 2025, 104, 104841. [Google Scholar] [CrossRef]
- Mahmood, S.; Younus, M.; Aslam, A.; Anjum, A.A. Toxicological effect of aflatoxin B1 on growth performance, humoral immune response and blood profile of Japanese quail. J. Anim. Plant Sci. 2017, 27, 833–840. [Google Scholar]
- Zeng, Z.-Z.; Jiang, W.-D.; Wu, P.; Liu, Y.; Zeng, Y.-Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q.; Feng, L. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2019, 500, 1–17. [Google Scholar] [CrossRef]
- He, X.-N.; Zeng, Z.-Z.; Wu, P.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Feng, L.; Zhou, X.-Q. Dietary aflatoxin B1 attenuates immune function of immune organs in grass carp (Ctenopharyngodon idella) by modulating NF-κB and the TOR signaling pathway. Front. Immunol. 2022, 13, 1027064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Liu, M.; Jiang, K.; Wang, M.; Wang, L. Comparative transcriptome analysis reveals the different roles between hepatopancreas and intestine of Litopenaeus vannamei in immune response to aflatoxin B1 (AFB1) challenge. Comp. Biochem. Physiol. C 2019, 222, 1–10. [Google Scholar] [CrossRef]
- Chang, T.T.; Chang, C.H.; Liao, V.H.C. Early life long-term exposure to aflatoxin B1 induces aging and alters innate immunity associated with SKN-1/Nrf2 in Caenorhabditis elegans. Chem. Biol. Interact. 2025, 406, 111349. [Google Scholar] [CrossRef]
- Cui, X.; Cong, Y. Role of Gut Microbiota in the Development of Some Autoimmune Diseases. J. Inflamm. Res. 2025, 18, 4409–4419. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Kim, C.H. Control of Lymphocyte Functions by Gut Microbiota-Derived Short-Chain Fatty Acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, K.; Wei, J.; Ding, Y.; Wang, X.; Hou, H.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut Microbiota-Derived Short-Chain Fatty Acids Regulate Gastrointestinal Tumor Immunity: A Novel Therapeutic Strategy? Front. Immunol. 2023, 14, 1158200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, Y.; Mu, L.; Yang, M.; Ai, X. Gut Microbiota Contributes to the Intestinal and Extraintestinal Immune Homeostasis by Balancing Th17/Treg Cells. Int. Immunopharmacol. 2024, 143 Pt 3, 113570. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Yan, X.; Shi, L.; Zhu, X.; Zhao, Y.; Luo, J.; Li, Q.; Xu, Z.; Zhao, J. From Microbial Homeostasis to Systemic Pathogenesis: A Narrative Review on Gut Flora’s Role in Neuropsychiatric, Metabolic, and Cancer Disorders. J. Inflamm. Res. 2025, 18, 8851–8873. [Google Scholar] [CrossRef]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef]
- Singh, K.; Saso, K. Oxidative stress: Role and response of short guanine tracts at genomic locations. Int. J. Mol. Sci. 2019, 20, 4258. [Google Scholar] [CrossRef]
- Naeini, A.H.; Nassireslami, E.; Shariatpanahi, M.; Chamanara, M.; Abdolali, A.; Aghsami, M. Necroptosis signaling in spatial memory impairment caused by aflatoxin B1 in male mice; involvement of the nitric oxide pathway. Toxicon 2025, 256, 108272. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.Y.; You, S.; Song, G.; Lim, W. Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. J. Hazard. Mater. 2020, 386, 121639. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Liu, S.; Liang, Z.; Zheng, Q.; Luo, K.; Li, J.; Du, Z.; Yu, J.; Yang, L.; et al. Aflatoxin B1 exposure induces Alzheimer’s disease-like pathology by disrupting redox homeostasis and activating ferroptotic signals in C57BL/6 J mice. Sci. Total Environ. 2025, 970, 179049. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A.; Yousef, M.I.; Ghareeb, D.A.; Augustyniak, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; El Wakil, A. Artichoke leaf extract-mediated neuroprotection against effects of aflatoxin in male rats. Biomed. Res. Int. 2022, 2022, 4421828. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Hesperetin protects hippocampal neurons from the neurotoxicity of aflatoxin B1 in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115782. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Wagner, K.M.; Lee, R.D.; Hwang, S.H.; Morisseau, C.; Wulff, H.; Hammock, B.D. Aflatoxin B1 increases soluble epoxide hydrolase in the brain and induces neuroinflammation and dopaminergic neurotoxicity. Int. J. Mol. Sci. 2023, 24, 9938. [Google Scholar] [CrossRef]
- Sahoo, M.; Thakor, J.C.; Kumar, P.; Singh, R.; Kumar, P.; Singh, K.; Puvvala, B.; Kumar, A.; Gopinathan, A.; Palai, S.; et al. AFB1-induced free radicals cause encephalopathy in goat kids via intrinsic pathway of apoptosis: Pathological and immunohistochemical confirmation of non-hepatic neuroaflatoxicosis. Vet. Res. Commun. 2024, 48, 317–327. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sabran, M.R.; Stanslas, J.; Kirby, B.P. Effect of aflatoxin B1 exposure on the progression of depressive-like behavior in rats. Front. Nutr. 2022, 9, 1032810. [Google Scholar] [CrossRef]
- Sahin, G.A.; Karabulut, D.; Unal, G.; Sayan, M.; Sahin, H. Effects of probiotic supplementation on very low dose AFB1-induced neurotoxicity in adult male rats. Life Sci. 2022, 306, 120798. [Google Scholar] [CrossRef]
- Adedara, I.A.; Owumi, S.E. Neurobehavioral and biochemical responses to artemisinin-based drug and aflatoxin B1 co-exposure in rats. Mycotoxin Res. 2023, 39, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Okoro, N.; Alilonu, D.O.; Eze, M.C.; Ebokaiwe, A.P. Aflatoxin B1-induced redox imbalance in the hippocampus and cerebral cortex of male Wistar rats is accompanied by altered cholinergic, indoleaminergic, and purinergic pathways: Abatement by dietary rutin. Toxicon 2024, 239, 107595. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.; Chimezie, J.; Salami, M.O.; Ishaya, J.A.; Onyemuwa, C.V.; Nnamdi, M.; Owoeye, O. Lutein and zeaxanthin abated neurobehavioral, neurochemical and oxido-inflammatory derangement in rats intoxicated with aflatoxin B1. Toxicon 2025, 258, 108345. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, T.; Liao, G.; Gu, J.; Hou, R.; Qiu, J. Lipidomic profiling study on neurobehavior toxicity in zebrafish treated with aflatoxin B1. Sci. Total Environ. 2023, 898, 165553. [Google Scholar] [CrossRef]
- Umbrello, G.; Esposito, S. Microbiota and neurologic diseases: Potential effects of probiotics. J. Transl. Med. 2016, 14, 298. [Google Scholar] [CrossRef]
- Lv, G.; Cheng, N.; Wang, H. The gut microbiota, tumorigenesis, and liver diseases. Engineering 2017, 3, 110–114. [Google Scholar] [CrossRef]
- Abd Mutalib, N.; Syed Mohamad, S.A.; Jusril, N.A.; Hasbullah, N.I.; Mohd Amin, M.C.I.; Ismail, N.H. Lactic acid bacteria (LAB) and neuroprotection, what is new? An up-to-date systematic review. Pharmaceutics 2023, 16, 712. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Al-Orf, N.; El-Ansary, A.; Bjørklund, G.; Moubayed, N.; Bhat, R.S.; Bacha, A.B. Therapeutic effects of probiotics on neurotoxicity induced by clindamycin and propionic acid in juvenile hamsters. Metab. Brain Dis. 2018, 33, 1811–1820. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J., Eds.; Springer: New York, NY, USA, 2014; Volume 817, pp. 1–19. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.; Talley, N. The mucosal immune system: Master regulator of bidirectional gut–brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The gut-brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Gritsenko, V.A.; Martins, A.C.; Tizabi, Y.; Korobeinikova, T.V.; Paoliello, M.M.B.; Tinkov, A.A. Modulation of gut microbiota with probiotics as a strategy to counteract endogenous and exogenous neurotoxicity. Adv. Neurotoxicol. 2024, 11, 133–176. [Google Scholar] [CrossRef]
- Jameson, K.G.; Olson, C.A.; Kazmi, S.A.; Hsiao, E.Y. Toward understanding microbiome-neuronal signaling. Mol. Cell 2020, 78, 577–583. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef]
- Winnie-Pui-Pui, L.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Guerre, P. Mycotoxin and gut microbiota interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Bonfili, L.; Gong, C.; Lombardi, F.; Cifone, M.G.; Eleuteri, A.M. Strategic modification of gut microbiota through oral bacteriotherapy influences hypoxia-inducible factor-1α: Therapeutic implication in Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 357. [Google Scholar] [CrossRef]
- Balaguer-Trias, J.; Deepika, D.; Schuhmacher, M.; Kumar, V. Impact of contaminants on microbiota: Linking the gut-brain axis with neurotoxicity. Int. J. Environ. Res. Public Health 2022, 19, 1368. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Raeis, S.N.; Ghorbani-Hasan Saraei, A.; Roozbeh Nasiraii, L. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Malvandi, A.M.; Alipour, M.; Hosseinkhani, S. Environmentally relevant level of aflatoxin B1 elicits toxic pro-inflammatory response in murine CNS-derived cells. Toxicol. Lett. 2017, 279, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Li, H.; Guptae, S.D.; Hao, Z.; Wang, Z.; Velkov, T.; Shen, J. Molecular mechanisms of aflatoxin neurotoxicity and potential neuroprotective agents. Food Sci. Hum. Wellness 2024, 13, 2445–2455. [Google Scholar] [CrossRef]
- Pop, O.L.; Suharoschi, R.; Gabbianelli, R. Biodetoxification and protective properties of probiotics. Microorganisms 2022, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Javanshir, N.; Hosseini, G.N.G.; Sadeghi, M.; Esmaeili, R.; Satarikia, F.; Ahmadian, G.; Allahyari, N. Evaluation of the function of probiotics, emphasizing the role of their binding to the intestinal epithelium in the stability and their effects on the immune system. Biol. Proced. Online 2021, 23, 23. [Google Scholar] [CrossRef]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Lim, S.-M.; Lee, N.-K.; Paik, H.-D. Potential neuroprotective effects of heat-killed Lactococcus lactis KC24 using SH-SY5Y cells against oxidative stress induced by hydrogen peroxide. Food Sci. Biotechnol. 2020, 29, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Aslim, B. Protective effect of exopolysaccharides from lactic acid bacteria against amyloid beta1-42-induced oxidative stress in SH-SY5Y cells: Involvement of the AKT, MAPK, and NF-κB signaling pathway. Process Biochem. 2021, 106, 50–59. [Google Scholar] [CrossRef]
- del Milagro Teran, M.; de Moreno de LeBlanc, A.; de Giori, G.S.; LeBlanc, J.G. Thiamine-producing lactic acid bacteria and their potential use in the prevention of neurodegenerative diseases. Appl. Microbiol. Biotechnol. 2021, 105, 2097–2107. [Google Scholar] [CrossRef]
- Perez Visñuk, D.; del Milagro Teran, M.; Savoy de Giori, G.; LeBlanc, J.G.; de Moreno de LeBlanc, A. Neuroprotective effect of riboflavin-producing lactic acid bacteria in Parkinsonian models. Neurochem. Res. 2022, 47, 1269–1279. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X.; Liu, Q.; Jiang, Y.; Li, Y.; Feng, W.; Xu, H.; Shao, M. Protective effect of Lactobacillus plantarum ATCC8014 on acrylamide-induced oxidative damage in rats. Appl. Biol. Chem. 2020, 63, 43. [Google Scholar] [CrossRef]
- Ugbaja, R.N.; Okedairo, O.M.; Oloyede, A.R.; Ugwor, E.I.; Akinloye, D.I.; Ojo, O.P.; Ademuyiwa, O. Probiotics consortium synergistically ameliorates aflatoxin B1-induced disruptions in lipid metabolism of female albino rats. Toxicon 2020, 186, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, Z.; Cheng, Z.; Wang, T.; Li, P.; Wu, A.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 inhibits aflatoxin B1-induced cecal inflammation in mice by regulating their intestinal flora. Food Chem. Toxicol. 2021, 156, 112438. [Google Scholar] [CrossRef] [PubMed]
- Ziemniczak, H.M.; Conceição, L.M.A.; Godoy, A.C.; Neu, D.H.; Rodrigues, A.T.; de Campos, C.M.; Acunha, R.M.G.; Gandra, J.R.; Saturnino, K.C.; de Pádua Pereira, U.; et al. Probiotic-based adsorbent mitigates aflatoxin B1 toxicity in Piaractus mesopotamicus: Assessing well-being via changes in tissue architecture and digestive enzyme activity. Vet. Res. Commun. 2025, 49, 94. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, T.; Ji, J.; Wang, J.-S.; Zhang, Y.; Sun, X. Bifidobacterium breve alleviates gut-liver-axis injury caused by high-fat diet and aflatoxin B1 in mice. Food Sci. Hum. Wellness 2025, 14, 9250039. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Gençer Bingöl, F.; Çelik, E.; Cemali, Ö.; Özenir, Ç.; Özoğul, F.; Capasso, R. Recent developments in probiotics as live biotherapeutic products (LBPs) as modulators of gut-brain axis related neurological conditions. J. Transl. Med. 2022, 20, 460. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent Advances in Immunoassays and Biosensors for Mycotoxins Detection in Feedstuffs and Foods. J. Anim. Sci. Biotechnol. 2021, 12, 108. [Google Scholar] [CrossRef]
- Hattimare, D.; Shakya, S.; Patyal, A.; Chandrakar, C.; Kumar, A. Occurrence and Exposure Assessment of Aflatoxin M1 in Milk and Milk Products in India. J. Food Sci. Technol. 2022, 59, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Kortei, N.K.; Annan, T.; Kyei-Baffour, V.; Essuman, E.K.; Boakye, A.A.; Tettey, C.O.; Boadi, N.O. Exposure Assessment and Cancer Risk Characterization of Aflatoxin M1 (AFM1) through Ingestion of Raw Cow Milk in Southern Ghana. Toxicol. Rep. 2022, 9, 1189–1197. [Google Scholar] [CrossRef]
- Du, X.; Schrunk, D.E.; Imerman, P.M.; Tahara, J.; Tkachenko, A.; Guag, J.; Reimschuessel, R.; Rumbeiha, W.K. Extensive Evaluation of a Method for Quantitative Measurement of Aflatoxins B1 and M1 in Animal Urine Using High-Performance Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2023, 106, 645–651. [Google Scholar] [CrossRef]
- Zitomer, N.C.; Rybak, M.E.; Sternberg, M.R. Assessing the Impacts of Preanalytical Field Sampling Challenges on the Reliability of Serum Aflatoxin B1-Lysine Measurements by Use of LC–MS/MS. Toxins 2022, 14, 612. [Google Scholar] [CrossRef]
- Renaud, J.B.; Walsh, J.P.; Sumarah, M.W. Optimization of Aflatoxin B1-Lysine Analysis for Public Health Exposure Studies. Toxins 2022, 14, 672. [Google Scholar] [CrossRef]
- Bellamri, M.; Yao, L.; Tomar, R.; Vartanian, V.; Rizzo, C.J.; Stone, M.P.; Groopman, J.D.; Lloyd, R.S.; Turesky, R.J. Mass Spectrometry-Based Method to Measure Aflatoxin B1 DNA Adducts in Formalin-Fixed Paraffin-Embedded Tissues. Chem. Res. Toxicol. 2024, 37, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kępka-Borkowska, K.; Chałaśkiewicz, K.; Ogłuszka, M.; Borkowski, M.; Lepczyński, A.; Pareek, C.S.; Starzyński, R.R.; Lichwiarska, E.; Sultana, S.; Kalra, G.; et al. Current Approaches to Aflatoxin B1 Control in Food and Feed Safety: Detection, Inhibition, and Mitigation. Int. J. Mol. Sci. 2025, 26, 6534. [Google Scholar] [CrossRef] [PubMed]
- Pantano, L.; La Scala, L.; Olibrio, F.; Galluzzo, F.G.; Bongiorno, C.; Buscemi, M.D.; Macaluso, A.; Vella, A. QuEChERS LC–MS/MS Screening Method for Mycotoxin Detection in Cereal Products and Spices. Int. J. Environ. Res. Public Health 2021, 18, 3774. [Google Scholar] [CrossRef] [PubMed]
- Mbisana, M.; Rebagamang, T.; Mogopodi, D.; Chibua, I. Development and Validation of a QuEChERS-LC–MS/MS Method for Determination of Multiple Mycotoxins in Maize and Sorghum from Botswana. Front. Fungal Biol. 2023, 4, 1141427. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Ng, D.K.; Alvarez, C.S.; Egner, P.A.; Burke, S.M.; Chen, J.-G.; Kensler, T.W.; Koshiol, J.; Rivera-Andrade, A.; Kroker-Lobos, M.F.; et al. Assessing the Validity of Normalizing Aflatoxin B1-Lysine Albumin Adduct Biomarker Measurements to Total Serum Albumin Concentration across Multiple Human Population Studies. Toxins 2022, 14, 162. [Google Scholar] [CrossRef] [PubMed]

| AF Level [μg/kg] | Class of Animal | Commodities |
|---|---|---|
| 20 | Dairy animals, animals not specified in other categories, or animals with unknown use | For corn, peanut products, cottonseed meal, and other animal feeds and feed ingredients |
| 20 | Immature animals | For corn, peanut products, and other animal feeds and feed ingredients, excluding cottonseed meal |
| 20 | Pets of all ages (e.g., dogs, cats, rabbits) | For corn, peanut products, cottonseed meal, other food ingredients, and complete pet food |
| 100 | Breeding cattle, breeding swine, and mature poultry (e.g., laying hens) | For corn and peanut products |
| 200 | Finishing swine (weighing 100 pounds or more) | For corn and peanut products |
| 300 | Beef cattle, swine, and poultry (regardless of age or breeding status) | For cotton seed meal |
| 300 | Finishing beef cattle (e.g., feedlot cattle) | For corn and peanut products |
| Feedstuff | AB1 Level [μg/kg] | |
|---|---|---|
| Raw materials | Maize processing products, peanut meal | ≤50 |
| Vegetable oil | ≤10 | |
| Maize oil, peanut oil | ≤20 | |
| Other plant-based feed materials | ≤30 | |
| Products | Concentrated feed for piglets and young birds | ≤10 |
| Concentrated feed for meat ducks, growing ducks, and ducks for egg production | ≤15 | |
| Other concentrated feed | ≤20 | |
| Calf and lamb concentrate supplement | ≤20 | |
| Concentrate supplement for lactation | ≤10 | |
| Other concentrate supplements | ≤30 | |
| Compound feed for piglets and young birds | ≤10 | |
| Compound feed for meat ducks, growing ducks, and laying ducks | ≤15 | |
| Other formula feed | ≤20 |
| Animal Model | AFB1/Toxins Dose and Route | Probiotic Strain(s)/Formulation | Main Outcomes | Ref. |
|---|---|---|---|---|
| SAMP8 mice | Age-related decline model | ProBiotic-4 (B. lactis, L. casei, B. bifidum, L. acidophilus) | Improved memory, reduced neuroinflammation, restored BBB and gut barrier integrity, altered microbiota composition | [106] |
| SH-SY5Y cells (in vitro) | H2O2-induced oxidative stress | L. lactis KC24-CM, L. rhamnosus GG, L. delbrueckii, L. plantarum | Increased BDNF, reduced apoptosis (Bax/Bcl-2), enhanced cell viability | [126] |
| N2a cells + C57BL/6 mice (MPP+ PD model) | MPP+ neurotoxin | L. plantarum CRL2130 | Reduced ROS and IL-6, improved motor function, increased IL-10 | [129] |
| Syrian golden hamsters | PPA 250 mg/kg ×3 days or clindamycin 30 mg/kg (oral) | Multi-strain mix (B. infantis, B. breve, L. acidophilus, L. bulgaricus, L. casei, L. rhamnosus, S. thermophilus) | Restored microbiota balance, improved oxidative stress markers, reduced lipid peroxidation | [103] |
| Albino female rats | AFB1 40 µg/kg feed ×8 weeks | Lactic acid bacteria preparation | Reduced brain lipid accumulation, partial normalization of jejunal and ileal lipid profiles | [131] |
| Sprague Dawley rats | Acrylamide 40 mg/kg/day | L. plantarum ATCC8014 | ↑ SOD, CAT, GSH; ↓ lipid peroxidation; improved histology of brain and gut | [130] |
| Adult male rats | AFB1 25 µg/kg/week ×8 weeks (oral) | VSL#3 (multi-strain, 2.5 × 1010 CFU/day) | ↑ GSH, GPx, GST, SOD; ↓ MDA, TNF-α, IL-6; fewer anxiety/depression-like symptoms | [94] |
| Male mice | AFB1 51.8 µL/day ×28 days (oral) | B. amyloliquefaciens B10 | ↑ Occludin, claudin-1, ZO-1; ↓ MyD88, TNF-α, IL-6, NF-κB; improved microbiota profile | [132] |
| Piaractus mesopotamicus (fish) | AFB1 25 or 400 µg/kg feed ×15 days | Probiotic-based adsorbent (PBA) | Improved digestive enzyme activity, reduced gut histopathology | [133] |
| C57BL/6J mice (HFD + AFB1) | AFB1 200 µg/kg/day ×9 weeks (oral) | B. breve (107 CFU/day) | ↓ Weight gain, improved liver histology, ↑ SCFAs, reduced inflammation, modulated lipid metabolism | [134] |
| Target Analyte | Matrix | Method | LOD | Advantages | Limitations |
|---|---|---|---|---|---|
| AFB1–albumin adducts | Serum/Plasma | LC–MS/MS after enzymatic digest | pg/mL | High sensitivity/specificity | Requires specialized equipment |
| Urinary AFM1 | Urine | ELISA | ng/mL | Fast, high throughput | Less specific vs. chromatographic methods |
| Free AFB1/AFM1 | Serum/Milk | HPLC–FLD after SPE cleanup | ng/mL | Established workflow | Time-consuming |
| Multiple metabolite profiling | Plasma/Urine | LC–MS/MS | Low pg/mL | Multiplex capability | High cost, technical requirements |
| AFM1 screening | Urine | LFIA | μg/mL | Portable and rapid | Minimum sensitivity and scope |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chałaśkiewicz, K.; Kępka-Borkowska, K.; Starzyński, R.R.; Ogłuszka, M.; Borkowski, M.; Poławska, E.; Lepczyński, A.; Lichwiarska, E.; Sultana, S.; Kalra, G.; et al. Impact of Aflatoxins on the Digestive, Immune, and Nervous Systems: The Role of Microbiota and Probiotics in Toxicity Protection. Int. J. Mol. Sci. 2025, 26, 8258. https://doi.org/10.3390/ijms26178258
Chałaśkiewicz K, Kępka-Borkowska K, Starzyński RR, Ogłuszka M, Borkowski M, Poławska E, Lepczyński A, Lichwiarska E, Sultana S, Kalra G, et al. Impact of Aflatoxins on the Digestive, Immune, and Nervous Systems: The Role of Microbiota and Probiotics in Toxicity Protection. International Journal of Molecular Sciences. 2025; 26(17):8258. https://doi.org/10.3390/ijms26178258
Chicago/Turabian StyleChałaśkiewicz, Katarzyna, Katarzyna Kępka-Borkowska, Rafał Radosław Starzyński, Magdalena Ogłuszka, Mateusz Borkowski, Ewa Poławska, Adam Lepczyński, Elżbieta Lichwiarska, Sharmin Sultana, Garima Kalra, and et al. 2025. "Impact of Aflatoxins on the Digestive, Immune, and Nervous Systems: The Role of Microbiota and Probiotics in Toxicity Protection" International Journal of Molecular Sciences 26, no. 17: 8258. https://doi.org/10.3390/ijms26178258
APA StyleChałaśkiewicz, K., Kępka-Borkowska, K., Starzyński, R. R., Ogłuszka, M., Borkowski, M., Poławska, E., Lepczyński, A., Lichwiarska, E., Sultana, S., Kalra, G., Purohit, N., Pareek, C. S., & Pierzchała, M. (2025). Impact of Aflatoxins on the Digestive, Immune, and Nervous Systems: The Role of Microbiota and Probiotics in Toxicity Protection. International Journal of Molecular Sciences, 26(17), 8258. https://doi.org/10.3390/ijms26178258








