Beneficial Effects of Chymase Inhibition on Cardiac Diastolic Function and Remodeling Induced by Chronic Angiotensin II Stimulation
Abstract
1. Introduction
2. Results
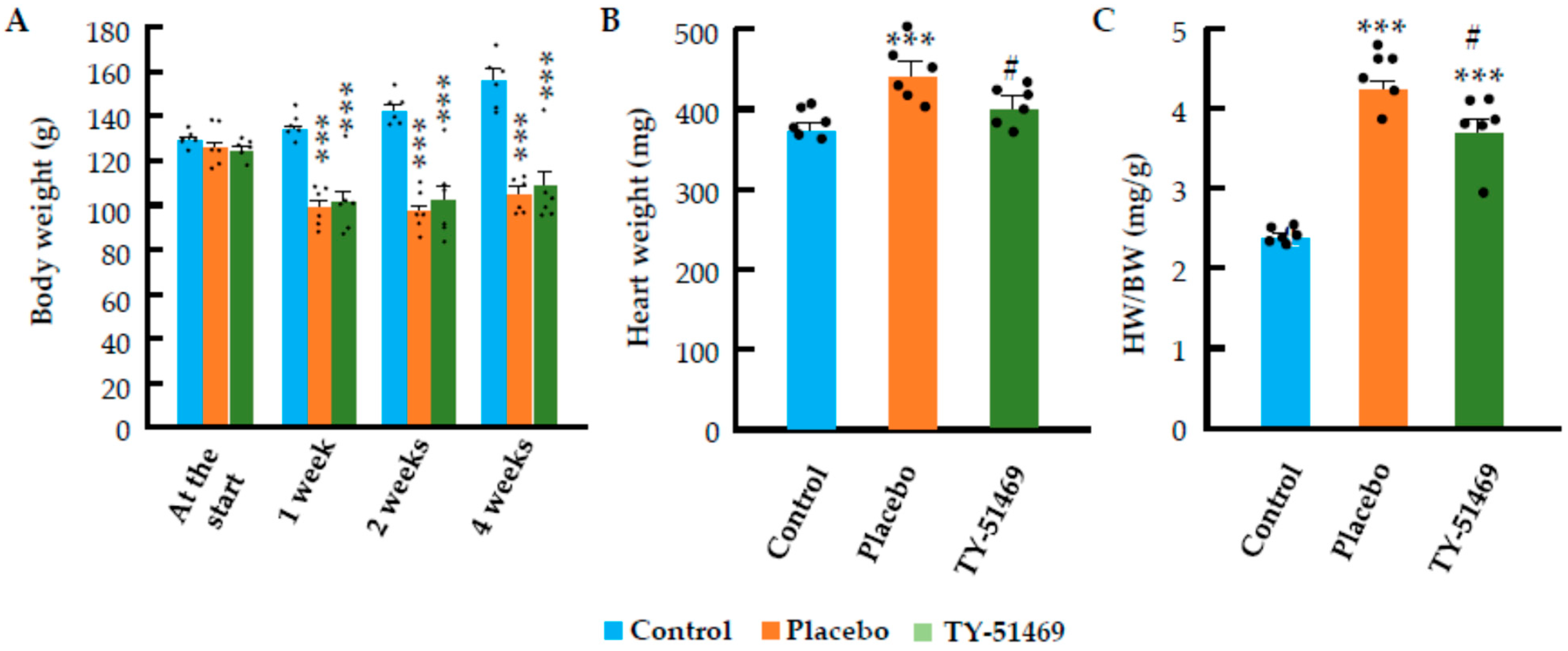
2.1. Changes in Body Weight, Heart Weight, and Systolic Blood Pressure Following Chronic Ang II Infusion
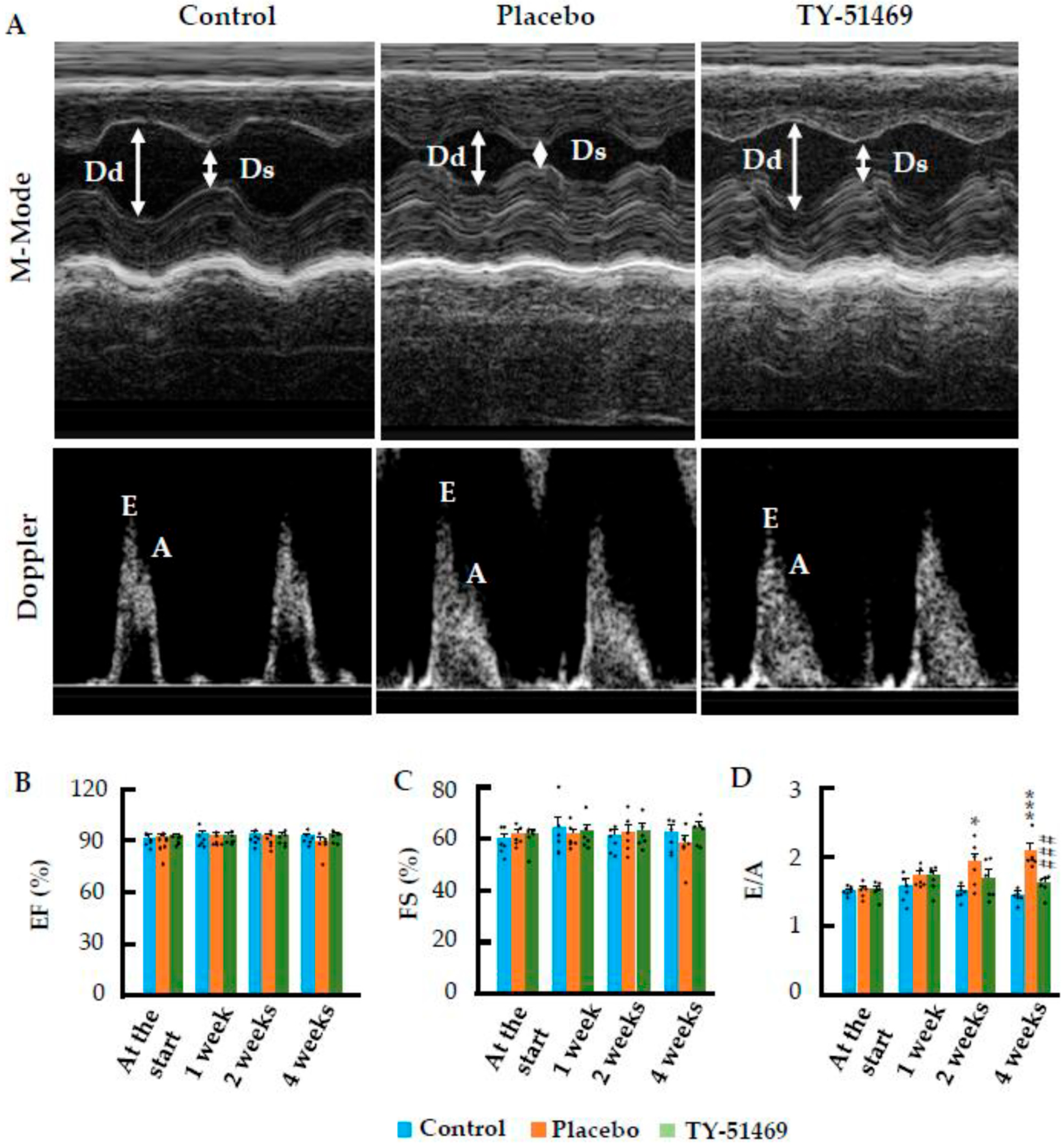
2.2. Beneficial Effects of Cardiac Chymase Inhibition on Cardiac Function Following Chronic Ang II Infusion
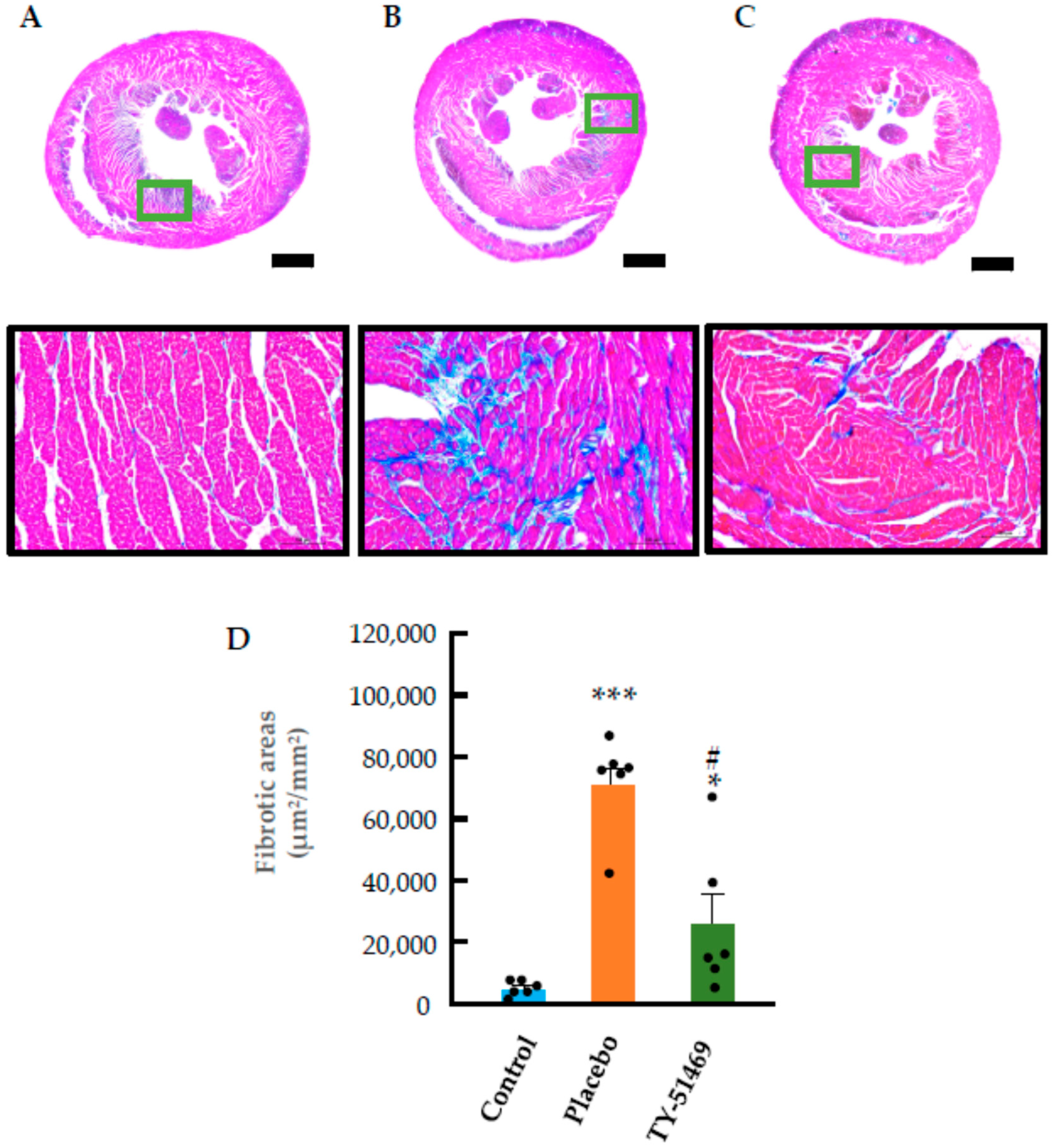
2.3. Characteristics of Cardiac Fibrosis Induced by Chronic Ang II Infusion
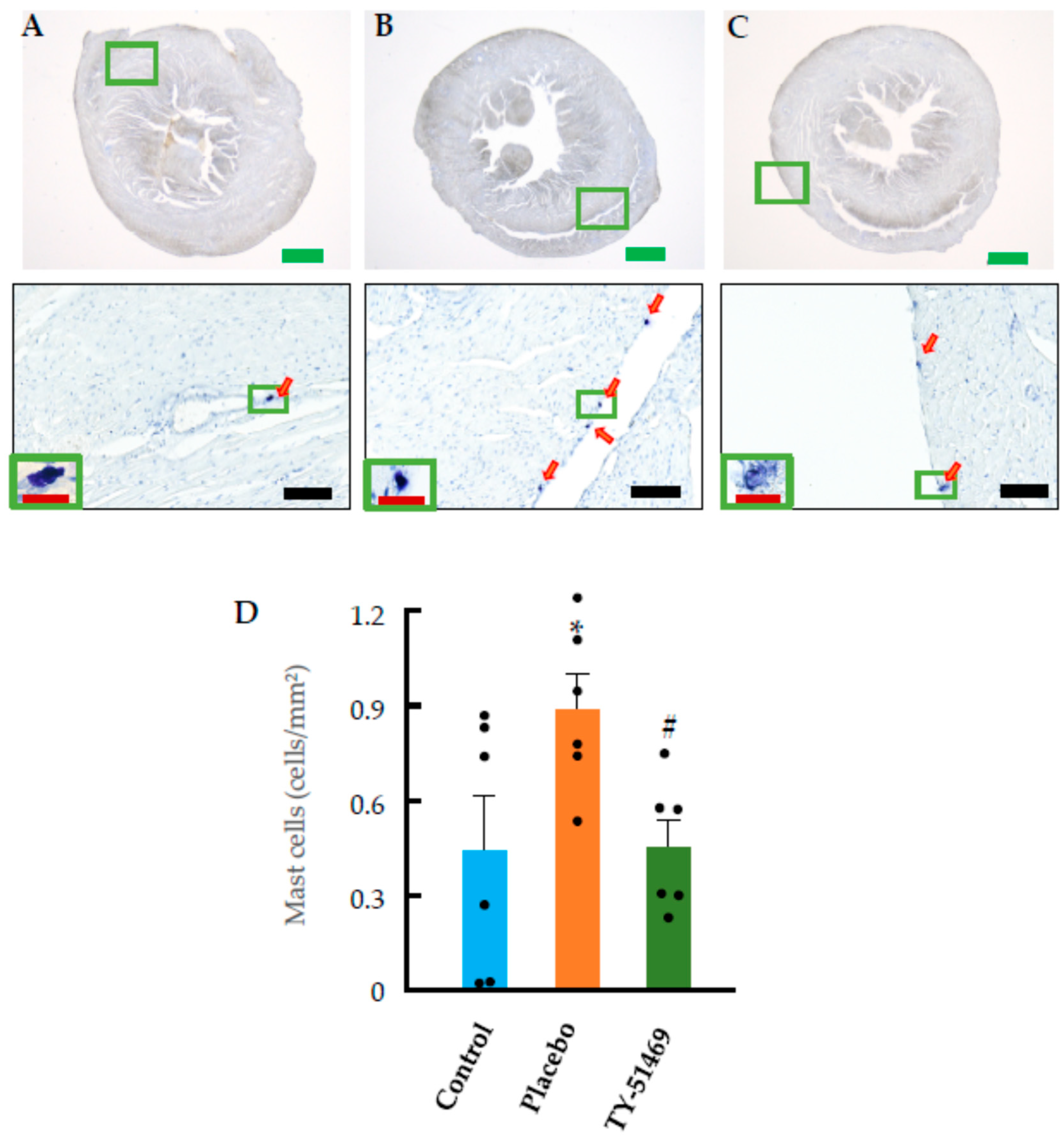
2.4. Changes in Cardiac Mast Cells, Chymase-Positive Cells, and Chymase mRNA Expression Following Chronic Ang II Infusion
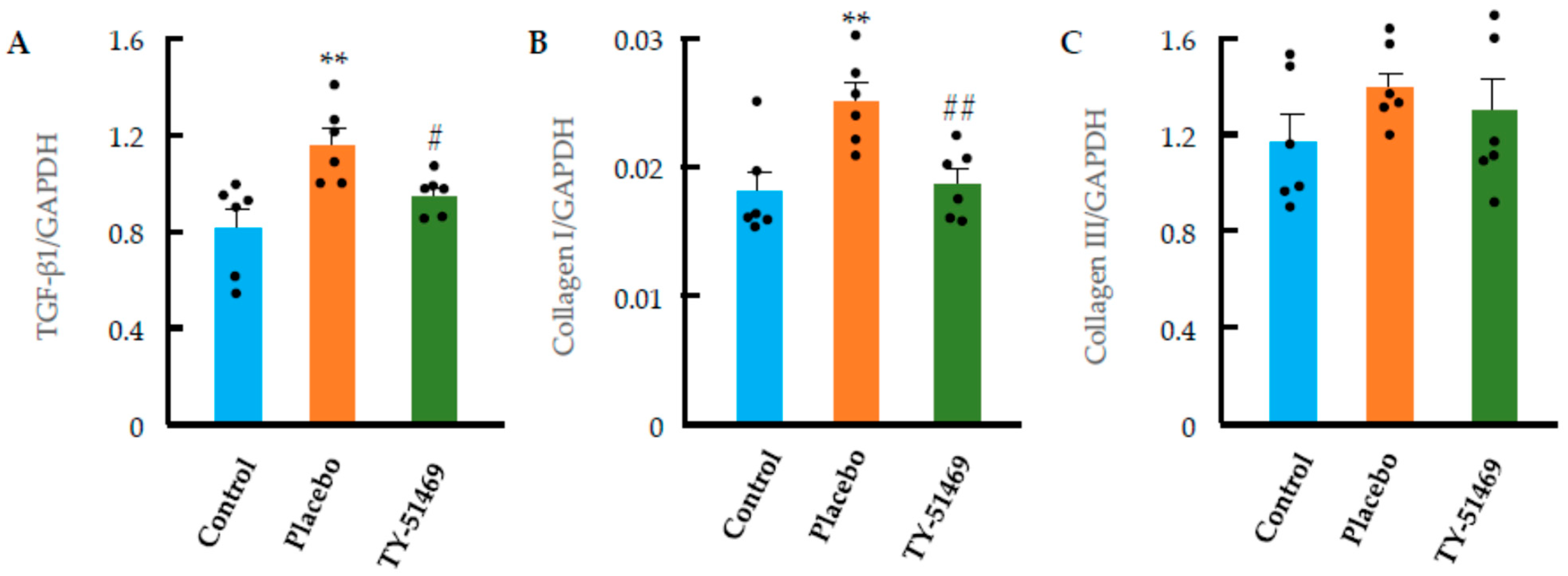
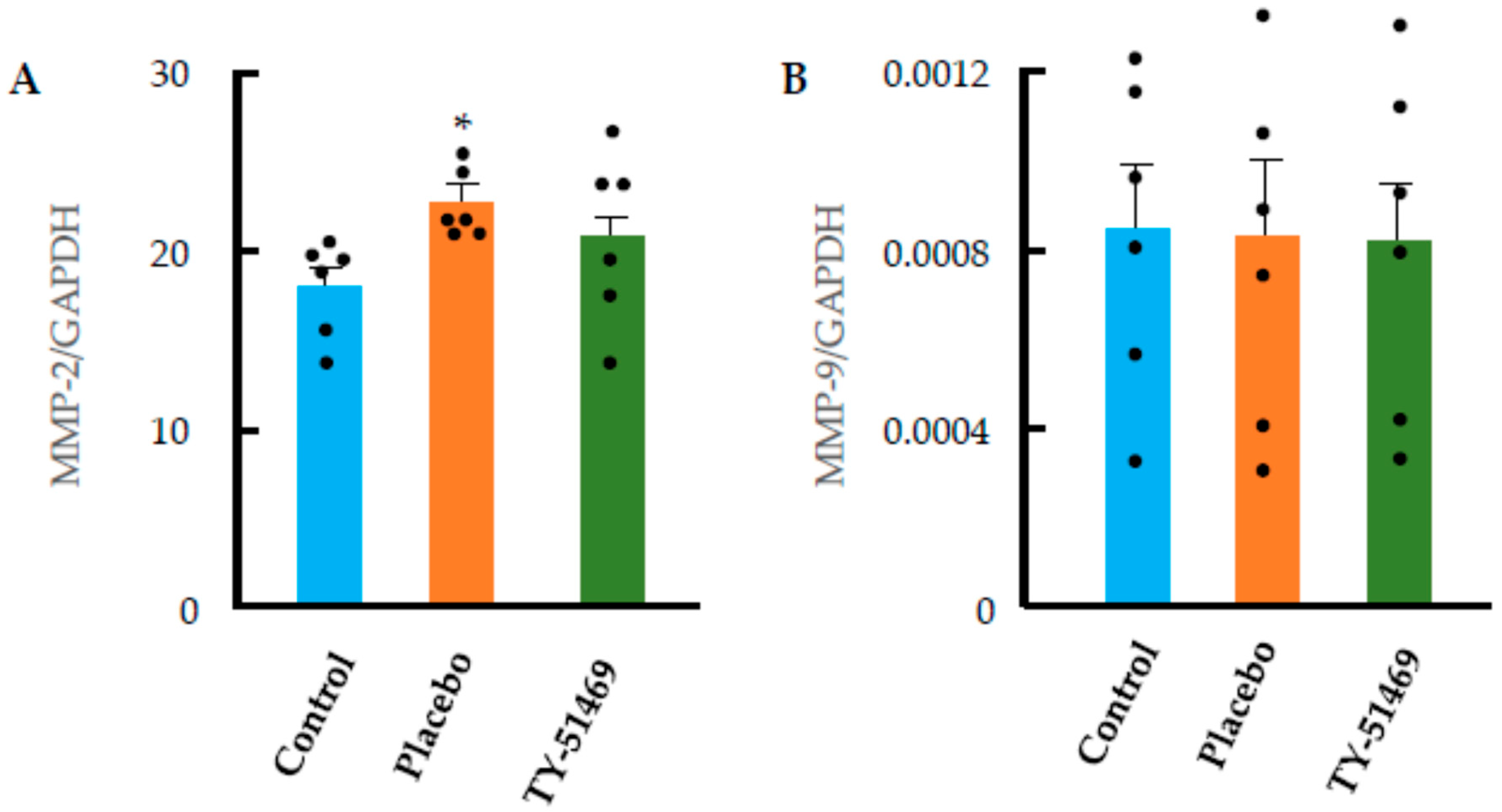
2.5. Cardiac Fibrosis Markers and ECM-Degrading Enzyme Expression Following Chronic Ang II Infusion
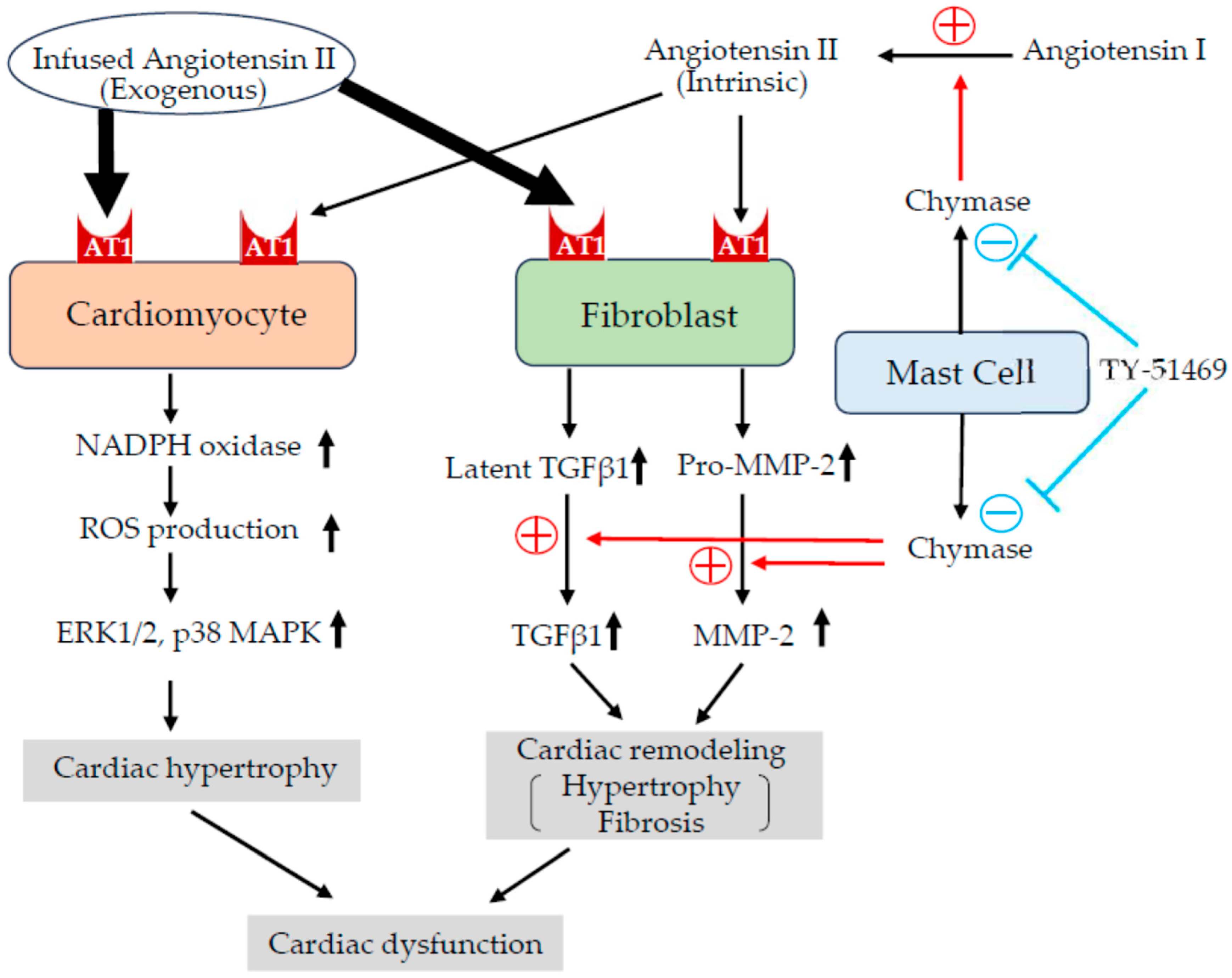
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Osmotic Mini-Pump Implantation and Grouping
4.3. Blood Pressure Measurement and Echocardiographic Study
4.4. Preparation of Tissue Samples
4.5. Histological Studies
4.6. Real-Time Polymerase Chain Reaction (RT-PCR)
- Chymase: Left: ggacaaaggggcctgtaaat; Right: gtatgctgatctgaccttcgtg.
- TGF-β1: Left: gctaccatgccaacttctgc; Right: ccaggaccttgctgtactgtg.
- MMP-2: Left: gggagctcaggccagaat; Right: ttggcggacagtgacactac.
- MMP-9: Left: cttcgacgacgacgagttg; Right: ttgcgtttccaaagtaagtgg.
- Collagen I: Left: tggaccttgttcacctctctc; Right: ccctgctggcaaagatgta.
- Collagen III: Left: caccacgctcgccattat; Right: tgggcctcaaggtattcaag.
- GAPDH: Left: agcttgtcatcaacgggaag; Right: gcatcaccccatttgatgtt.
- The probe sequences were as follows:
- Chymase: tgggcagc.
- TGF-β1: gagcctgg.
- MMP-2: ggccacca.
- MMP-9: ctgggcaa.
- Collagen I: cagcagga.
- Collagen III: ctggctcc.
- GAPDH: catcaca.
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Mechanisms and models in heart failure: A combinatorial approach. Circulation 2002, 100, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef]
- Kim, S.; Ohta, K.; Hamaguchi, A.; Yukimura, T.; Miura, K.; Iwao, H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension 1995, 25, 1252–1259. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Hasking, G.J.; Miller, E. Angiotensin II and sympathetic activity in patients with congestive heart failure. J. Am. Coll. Cardiol. 1993, 21, 1107–1113. [Google Scholar] [CrossRef]
- Swedberg, K.; Eneroth, P.; Kjekshus, J.; Wilhelmsen, L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation 1990, 82, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Carretero, O.A.; Vuljaj, N.; Liao, T.D.; Motivala, A.; Peterson, E.L.; Rhaleb, N.E. Angiotensin-converting enzyme inhibitors: A new mechanism of action. Circulation 2005, 112, 2436–2445. [Google Scholar] [CrossRef]
- Swedberg, K.; Kjekshus, J. Effects of enalapril on mortality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am. J. Cardiol. 1988, 62, 60A–66A. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol.-Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef]
- Urata, H.; Kinoshita, A.; Misono, K.S.; Bumpus, F.M.; Husain, A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J. Biol. Chem. 1990, 265, 22348–22357. [Google Scholar] [CrossRef]
- Urata, H.; Nishimura, H.; Ganten, D. Chymase-dependent angiotensin II forming systems in humans. Am. J. Hypertens. 1996, 9, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Shiota, N.; Yamamoto, D.; Okunishi, H.; Miyazaki, M. Purification and characterization of angiotensin II-generating chymase from hamster cheek pouch. Life Sci. 1996, 58, 591–597. [Google Scholar] [CrossRef]
- Jin, D.; Takai, S.; Yamada, M.; Sakaguchi, M.; Miyazaki, M. The functional ratio of chymase and angiotensin converting enzyme in angiotensin I-induced vascular contraction in monkeys, dogs and rats. Jpn. J. Pharmacol. 2000, 84, 449–454. [Google Scholar] [CrossRef]
- Lindstedt, K.A.; Wang, Y.; Shiota, N.; Saarinen, J.; Hyytiäinen, M.; Kokkonen, J.O.; Keski-Oja, J.; Kovanen, P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: A novel function for chymase. FASEB J. 2001, 15, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.C.; Raymond, W.W.; Lazarus, S.C.; Caughey, G.H. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J. Clin. Investig. 1996, 97, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Muramatsu, M.; Jin, D.; Furubayashi, K.; Takai, S.; Tamai, H.; Miyazaki, M. The effects of chymase on matrix metalloproteinase-2 activation in neointimal hyperplasia after balloon injury in dogs. Hypertens. Res. 2007, 30, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chung, A.C.; Huang, X.R.; Lan, H.Y. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: The role of Smad3. Hypertension 2009, 54, 877–884. [Google Scholar] [CrossRef]
- Odenbach, J.; Wang, X.; Cooper, S.; Chow, F.L.; Oka, T.; Lopaschuk, G.; Kassiri, Z.; Fernandez-Patron, C. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension 2011, 57, 123–130. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 2005, 115, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Izumo, S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993, 73, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rupérez, M.; Esteban, V.; Rodríguez-Vita, J.; Sánchez-López, E.; Carvajal, G.; Egido, J. Angiotensin II: A key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol. Dial. Transplant. 2006, 21, 16–20. [Google Scholar] [CrossRef]
- Gray, M.O.; Long, C.S.; Kalinyak, J.E.; Li, H.T.; Karliner, J.S. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc. Res. 1998, 40, 352–363. [Google Scholar] [CrossRef]
- Schultz, J.E.J.; Witt, S.A.; Glascock, B.J.; Nieman, M.L.; Reiser, P.J.; Nix, S.L.; Kimball, T.R.; Doetschman, T.J. TGFbeta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. Clin. Investig. 2002, 109, 787–796. [Google Scholar] [CrossRef]
- de Andrade, C.R.; Cotrin, P.; Graner, E.; Almeida, O.P.; Sauk, J.J.; Coletta, R.D. Transforming growth factor beta1 autocrine stimulation regulates fibroblast proliferation in hereditary gingival fibromatosis. J. Periodontol. 2001, 72, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Siddesha, J.M.; Valente, A.J.; Sakamuri, S.S.; Yoshida, T.; Gardner, J.D.; Somanna, N.; Takahashi, C.; Noda, M.; Chandrasekar, B. Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. J. Mol. Cell. Cardiol. 2013, 65, 9–18. [Google Scholar] [CrossRef]
- Fu, Y.; Li, M.; Zhang, H.; Dong, Y.F. Sophocarpine attenuates doxorubicin-induced heart injury through inhibition of fibrosis. Minerva Cardiol. Angiol. 2024, 72, 568–576. [Google Scholar] [CrossRef]
- Matsusaka, H.; Ide, T.; Matsushima, S.; Ikeuchi, M.; Kubota, T.; Sunagawa, K.; Kinugawa, S.; Tsutsui, H. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension 2006, 47, 711–717. [Google Scholar] [CrossRef]
- Jin, D.; Takai, S.; Nonaka, Y.; Yamazaki, S.; Fujiwara, M.; Nakamura, Y. A Chymase Inhibitory RNA Aptamer Improves Cardiac Function and Survival after Myocardial Infarction. Mol. Ther. Nucleic Acids 2019, 14, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Jin, D.; Kinoshita, I.; Taniuchi, M.; Higashino, M.; Terada, T.; Takai, S.; Kawata, R. Increased Chymase-Positive Mast Cells in High Grade Mucoepidermoid Carcinoma of the Parotid Gland. Int. J. Mol. Sci. 2023, 24, 8267. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, S.; Jin, D.; Morihara, H.; Yokoe, S.; Moriwaki, K.; Takai, S. Beneficial Effects of Chymase Inhibition on Cardiac Diastolic Function and Remodeling Induced by Chronic Angiotensin II Stimulation. Int. J. Mol. Sci. 2025, 26, 8236. https://doi.org/10.3390/ijms26178236
Taniguchi S, Jin D, Morihara H, Yokoe S, Moriwaki K, Takai S. Beneficial Effects of Chymase Inhibition on Cardiac Diastolic Function and Remodeling Induced by Chronic Angiotensin II Stimulation. International Journal of Molecular Sciences. 2025; 26(17):8236. https://doi.org/10.3390/ijms26178236
Chicago/Turabian StyleTaniguchi, Shiguma, Denan Jin, Hirofumi Morihara, Shunichi Yokoe, Kazumasa Moriwaki, and Shinji Takai. 2025. "Beneficial Effects of Chymase Inhibition on Cardiac Diastolic Function and Remodeling Induced by Chronic Angiotensin II Stimulation" International Journal of Molecular Sciences 26, no. 17: 8236. https://doi.org/10.3390/ijms26178236
APA StyleTaniguchi, S., Jin, D., Morihara, H., Yokoe, S., Moriwaki, K., & Takai, S. (2025). Beneficial Effects of Chymase Inhibition on Cardiac Diastolic Function and Remodeling Induced by Chronic Angiotensin II Stimulation. International Journal of Molecular Sciences, 26(17), 8236. https://doi.org/10.3390/ijms26178236





