CRISPR/Cas9 TCR-Edited NKp30 CAR T Cells Exhibit Superior Anti-Tumor Immunity to B7H6-Expressing Leukemia and Melanoma
Abstract
1. Introduction
2. Results
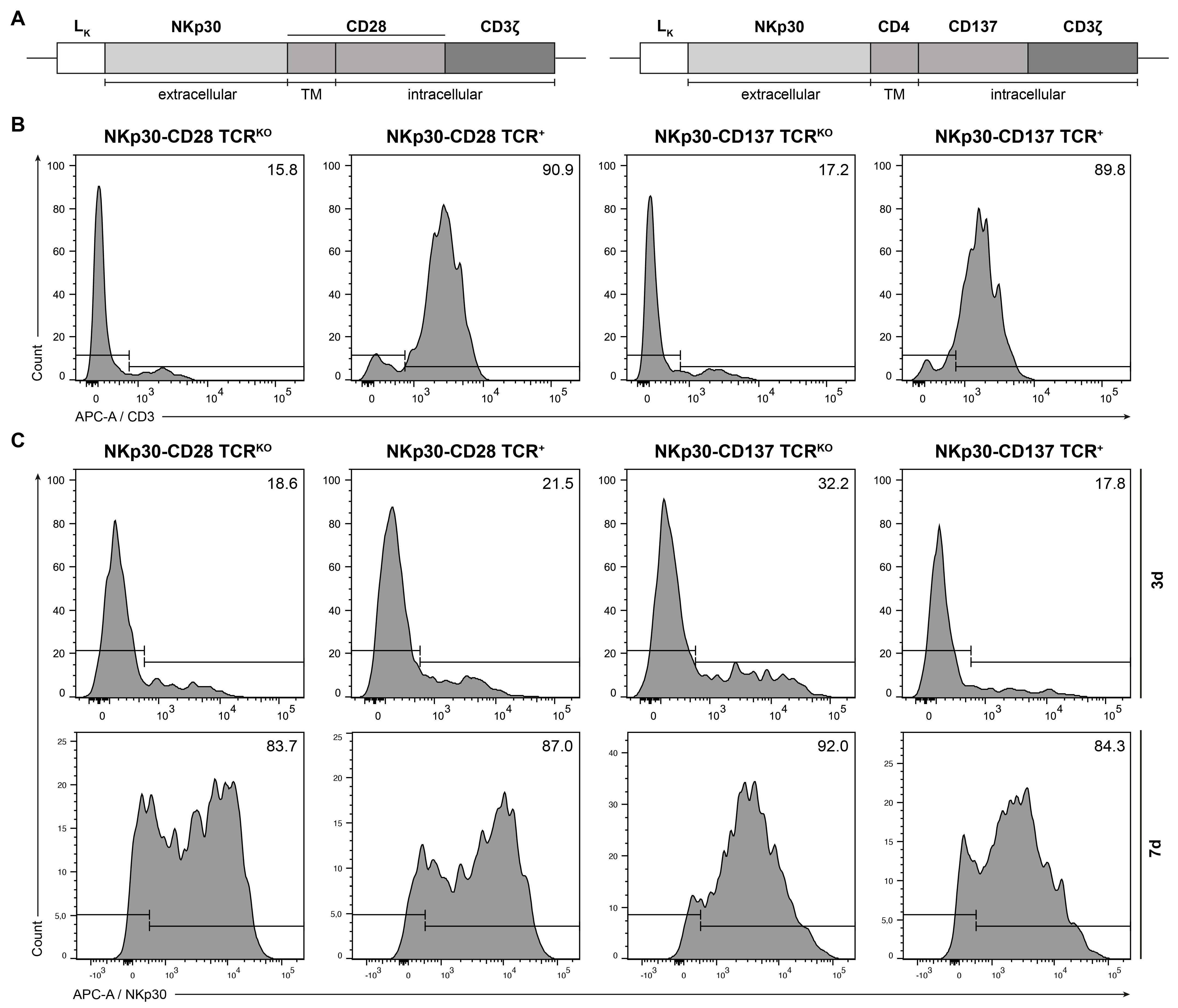
2.1. Generation of NKp30-Based CARs and Expression in Human TCRKO T Lymphocytes
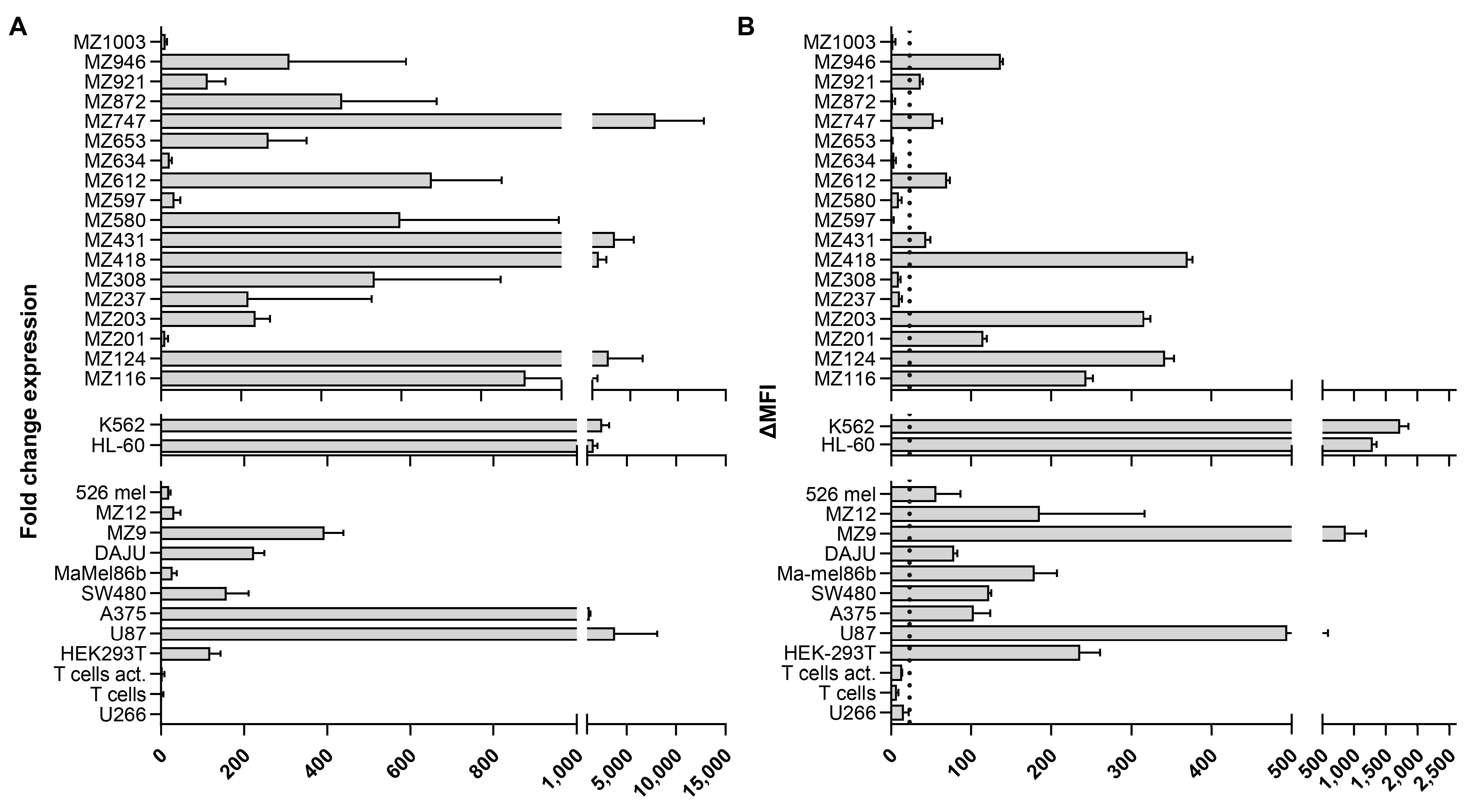
2.2. B7H6 Is Widely Expressed on the Surface of AML and Melanoma
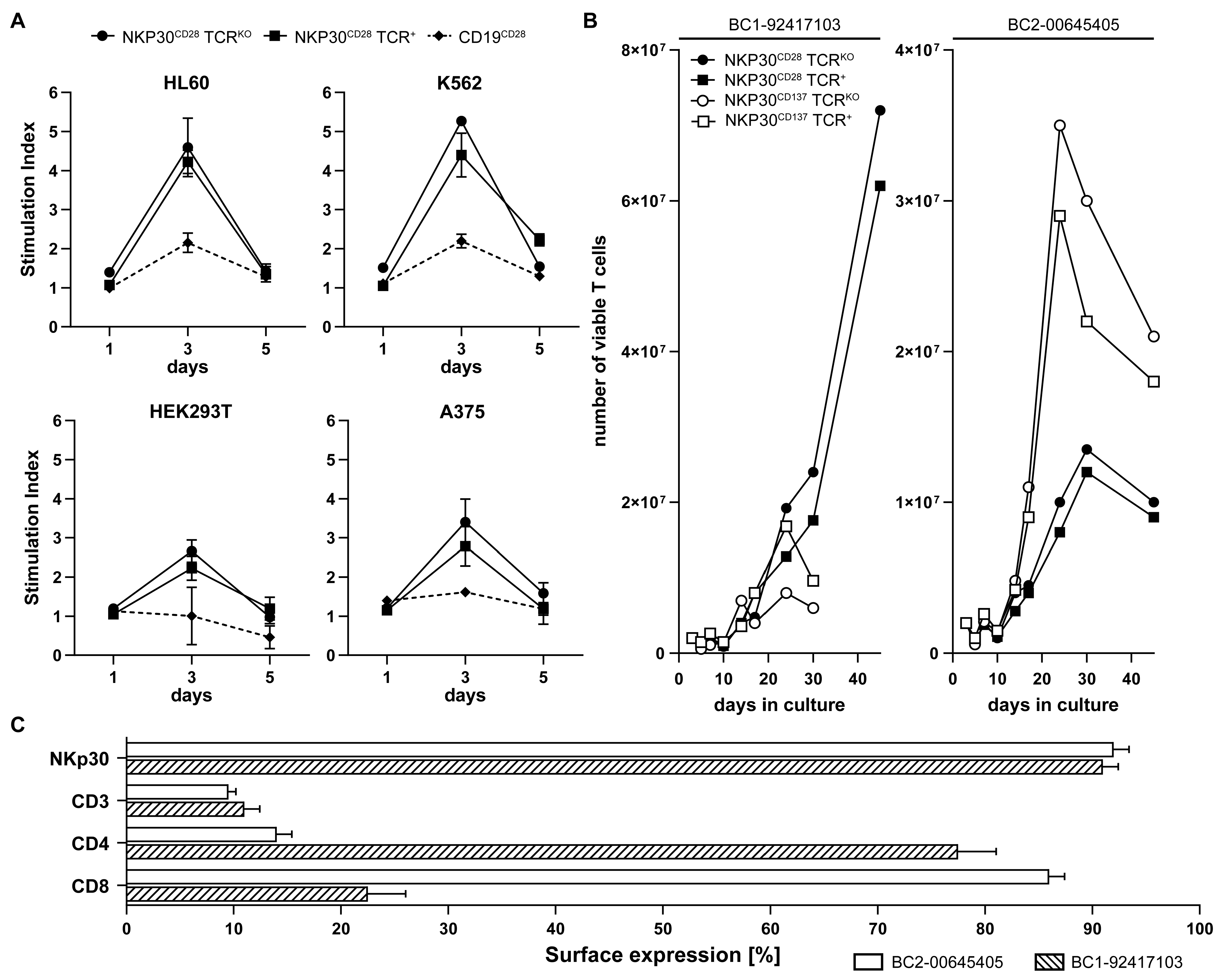
2.3. NKp30 CAR/TCRKO T Cells Are Effectively Stimulated with B7H6 Positive Tumor Targets
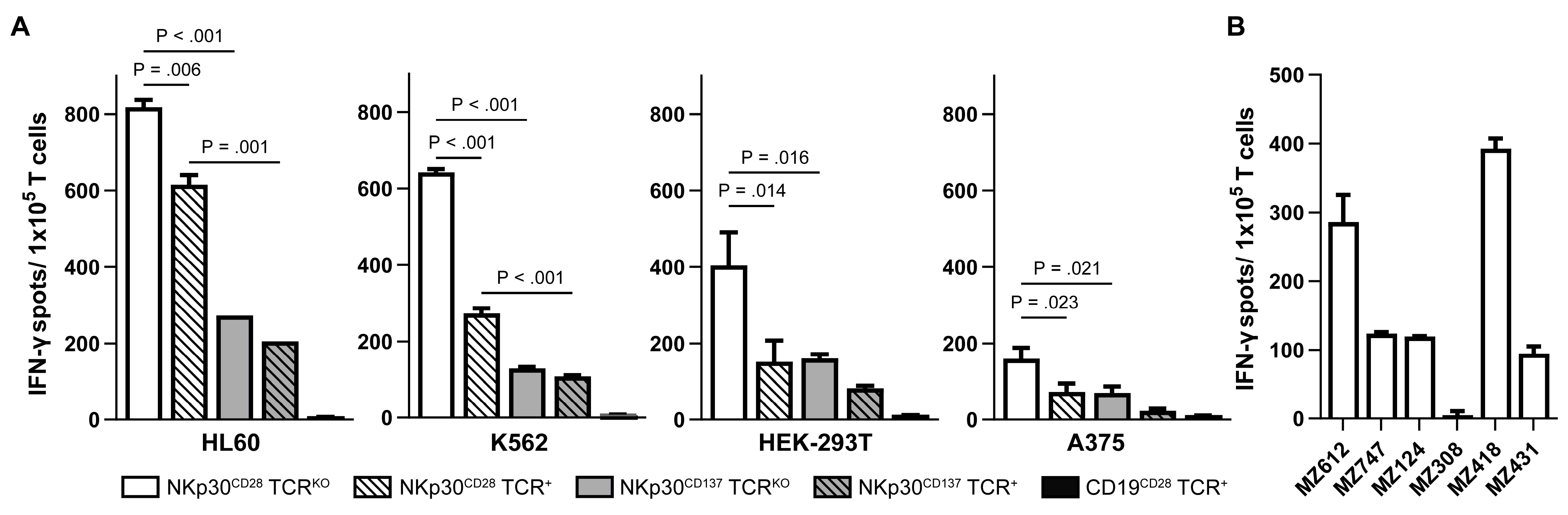
2.4. NKp30 CAR TCRKO T Cells Produce High Amounts of IFN-γ upon Recognition of B7H6
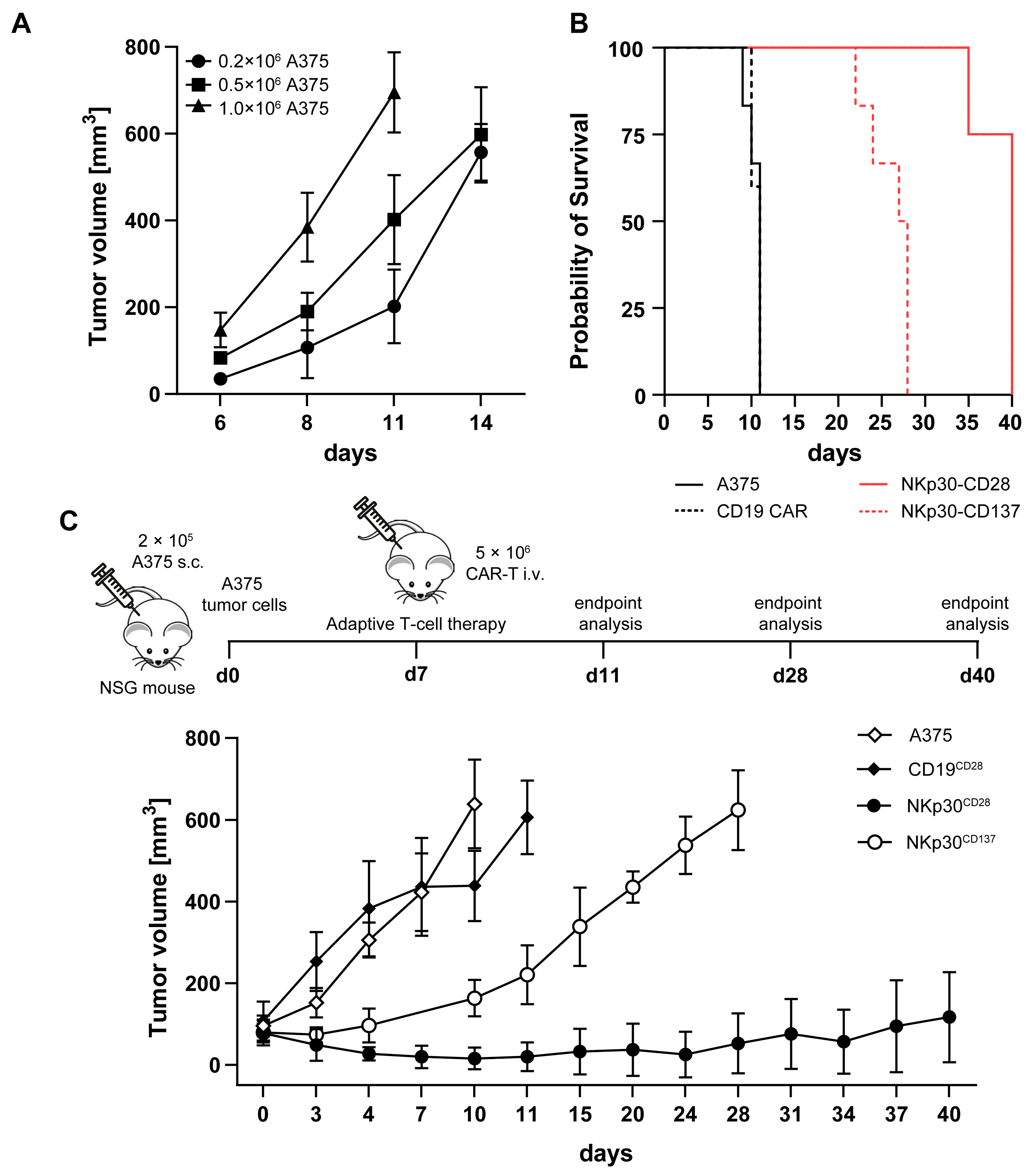
2.5. NKp30 CAR TCRKO T Cells Elicit Strong B7H6-Mediated Cytotoxicity In Vitro and in a NSG Melanoma Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Blood Samples, Primary Cells, and Cell Lines
4.2. Generation of Luciferase-Expressing Target Cells
4.3. Generation of NKp30-CAR TCRKO T Cells
4.4. Antigen-Specific Stimulation and Proliferation of CAR T Cells
4.5. Real-Time Quantitative PCR (qRT-PCR)
4.6. Flow Cytometry Analysis
4.7. Measuring IFN-γ Production by T Cells
4.8. Bioluminescence-Imaging-Based Cytotoxicity Assay
4.9. Treatment of Tumor-Bearing Mice with NKp30 CAR T Cells
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.T.; Carpenter, R.O.; Maryalice, S.S.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated with Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Hopewell, E.; Salman, H. CAR T Cell Therapy for Treatment of Acute Myeloid Leukemia, Advances and Outcomes. Mol. Ther. 2025, 33, 2441–2453. [Google Scholar] [CrossRef]
- Epperly, R.; Gottschalk, S.; Velasquez, M.P. A Bump in the Road: How the Hostile AML Microenvironment Affects CAR T Cell Therapy. Front. Oncol. 2020, 10, 262. [Google Scholar] [CrossRef]
- Damiani, D.; Tiribelli, M. Advancing Chimeric Antigen Receptor T-Cell Therapy for Acute Myeloid Leukemia: Current Limitations and Emerging Strategies. Pharmaceuticals 2024, 17, 1629. [Google Scholar] [CrossRef]
- Budde, L.; Song, J.Y.; Kim, Y.; Blanchard, S.; Wagner, J.; Stein, A.S.; Weng, L.; Del Real, M.; Hernandez, R.; Marcucci, E.; et al. Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood 2017, 130, 811. [Google Scholar] [CrossRef]
- Shah, N.N.; Tasian, S.K.; Kohler, M.E.; Hsieh, E.M.; Baumeister, S.H.C.; Summers, C.; Shalabi, H.; Pollard, J.A.; Yates, B.; Brazauskas, R.; et al. CD33 CAR T-Cells (CD33CART) for Children and Young Adults with Relapsed/Refractory AML: Dose-Escalation Results from a Phase I/II Multicenter Trial. Blood 2023, 142, 771. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, M.; Sun, R.; Lyu, H.; Xiao, X.; Zhang, X.; Li, F.; Xie, D.; Xiong, X.; Wang, J.; et al. First-in-Human Phase I Study of CLL-1 CAR-T Cells in Adults with Relapsed/Refractory Acute Myeloid Leukemia. J. Hematol. Oncol. 2022, 15, 88. [Google Scholar] [CrossRef]
- Sallman, D.A.; Kerre, T.; Havelange, V.; Poiré, X.; Lewalle, P.; Wang, E.S.; Brayer, J.B.; Davila, M.L.; Moors, I.; Machiels, J.P.; et al. CYAD-01, an Autologous NKG2D-Based CAR T-Cell Therapy, in Relapsed or Refractory Acute Myeloid Leukaemia and Myelodysplastic Syndromes or Multiple Myeloma (THINK): Haematological Cohorts of the Dose Escalation Segment of a Phase 1 Trial. Lancet Haematol. 2023, 10, e191–e202. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.S.; Neeson, P.J.; Khot, A.; Peinert, S.; Tai, T.; Tainton, K.; Chen, K.; Shin, M.; Wall, D.M.; Hönemann, D.; et al. Persistence and Efficacy of Second Generation CAR T Cell Against the LeY Antigen in Acute Myeloid Leukemia. Mol. Ther. 2013, 21, 2122–2129. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Li, S.; Liu, J.; Xing, Y.; Xing, H.; Tian, Z.; Tang, K.; Rao, Q.; Wang, M.; et al. Targeting FLT3 in Acute Myeloid Leukemia Using Ligand-Based Chimeric Antigen Receptor-Engineered T Cells. J. Hematol. Oncol. 2018, 11, 60. [Google Scholar] [CrossRef]
- Lynn, R.C.; Poussin, M.; Kalota, A.; Feng, Y.; Low, P.S.; Dimitrov, D.S.; Powell, D.J. Targeting of Folate Receptor β on Acute Myeloid Leukemia Blasts with Chimeric Antigen Receptor-Expressing T Cells. Blood 2015, 125, 3466–3476. [Google Scholar] [CrossRef]
- Casucci, M.; Di Robilant, B.N.; Falcone, L.; Camisa, B.; Norelli, M.; Genovese, P.; Gentner, B.; Gullotta, F.; Ponzoni, M.; Bernardi, M.; et al. CD44v6-Targeted T Cells Mediate Potent Antitumor Effects Against Acute Myeloid Leukemia and Multiple Myeloma. Blood 2013, 122, 3461–3472. [Google Scholar] [CrossRef]
- Lichtman, E.I.; Du, H.; Shou, P.; Song, F.; Suzuki, K.; Ahn, S.; Li, G.; Ferrone, S.; Su, L.; Savoldo, B.; et al. Preclinical Evaluation of B7-H3-Specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Scarfò, I.; Choi, B.D.; Larson, R.; Bouffard, A.A.; Castano, A.; Cabral, M.L.; Schmidts, A.; Frigault, M.J.; Maus, M.V. Use of CD70 Targeted Chimeric Antigen Receptor (CAR) T Cells for the Treatment of Acute Myeloid Leukemia (AML). Blood 2019, 134, 4443. [Google Scholar] [CrossRef]
- Gomes-Silva, D.; Atilla, E.; Atilla, P.A.; Mo, F.; Tashiro, H.; Srinivasan, M.; Lulla, P.; Rouce, R.H.; Cabral, J.M.S.; Ramos, C.A.; et al. CD7 CAR T Cells for the Therapy of Acute Myeloid Leukemia. Mol. Ther. 2019, 27, 272–280. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 Family Member B7-H6 Is a Tumor Cell Ligand for the Activating Natural Killer Cell Receptor NKp30 in Humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Cao, G.; Wang, J.; Zheng, X.; Wei, H.; Tian, Z.; Sun, R. Tumor Therapeutics Work as Stress Inducers to Enhance Tumor Sensitivity to Natural Killer (NK) Cell Cytolysis by up-Regulating NKp30 Ligand B7-H6. J. Biol. Chem. 2015, 290, 29964–29973. [Google Scholar] [CrossRef] [PubMed]
- Obiedat, A.; Charpak-Amikam, Y.; Tai-Schmiedel, J.; Seidel, E.; Mahameed, M.; Avril, T.; Stern-Ginossar, N.; Springuel, L.; Bolsée, J.; Gilham, D.E.; et al. The Integrated Stress Response Promotes B7H6 Expression. J. Mol. Med. 2020, 98, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Jang, I.H.; Jo, S.; Lee, S.Y.; Oh, S.C.; Kim, S.M.; Kong, L.; Ko, J.; Kim, T.D. Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 10326. [Google Scholar] [CrossRef]
- Lee, S.; Chae, S.J.; Jang, I.-H.; Oh, S.-C.; Kim, S.-M.; Lee, S.Y.; Kim, J.H.; Ko, J.; Kim, H.J.; Song, I.-C.; et al. B7H6 Is the Predominant Activating Ligand Driving Natural Killer Cell-Mediated Killing in Patients with Liquid Tumours: Evidence from Clinical, in Silico, in Vitro, and in Vivo Studies. EbioMedicine. 2024, 110, 105459. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; Ma, L.; Sun, X.; Ge, J.; Yu, Y.; Ma, J.; Zhang, M. B7-H6 as an Efficient Target for T Cell-Induced Cytotoxicity in Haematologic Malignant Cells. Investig. New Drugs 2021, 39, 24–33. [Google Scholar] [CrossRef]
- Baragaño Raneros, A.; Rodriguez, R.M.; Bernardo Flórez, A.; Palomo, P.; Colado, E.; Minguela, A.; Suárez Álvarez, B.; López-Larrea, C. Bromodomain Protein BRD4 Is an Epigenetic Activator of B7-H6 Expression in Acute Myeloid Leukemia. Oncoimmunology 2021, 10, 1897294. [Google Scholar] [CrossRef]
- Messaoudene, M.; Fregni, G.; Enot, D.; Jacquelot, N.; Neves, E.; Germaud, N.; Garchon, H.J.; Boukouaci, W.; Tamouza, R.; Chanal, J.; et al. NKp30 Isoforms and NKp46 Transcripts in Metastatic Melanoma Patients: Unique NKp30 Pattern in Rare Melanoma Patients with Favorable Evolution. Oncoimmunology 2016, 5, e1154251. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.-R.; Sentman, C.L. An NKp30-Based Chimeric Antigen Receptor Promotes T Cell Effector Functions and Antitumor Efficacy In Vivo. J. Immunol. 2012, 189, 2290–2299. [Google Scholar] [CrossRef]
- Wu, M.R.; Zhang, T.; DeMars, L.R.; Sentman, C.L. B7H6-Specific Chimeric Antigen Receptors Lead to Tumor Elimination and Host Antitumor Immunity. Gene Ther. 2015, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Friedrich, M.J.; Hai-Ning Lu, K.; Vonhören, D.; Jansky, S.; Michel, J.; Keib, A.; Stange, S.; Hackert, N.; Kehl, N.; et al. The Immunoglobulin Superfamily Ligand B7H6 Subjects T Cell Responses to NK Cell Surveillance. Sci. Immunol. 2024, 9, eadj7970. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T Memory Stem Cells in Health and Disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Shuford, W.W.; Klussman, K.; Tritchler, D.D.; Loo, D.T.; Chalupny, J.; Siadak, A.W.; Brown, T.J.; Emswiler, J.; Raecho, H.; Larsen, C.P.; et al. 4-1BB Costimulatory Signals Preferentially Induce CD8+ T Cell Proliferation and Lead to the Amplification in Vivo of Cytotoxic T Cell Responses. J. Exp. Med. 1997, 186, 47–55. [Google Scholar] [CrossRef]
- Fiegler, N.; Textor, S.; Arnold, A.; Rölle, A.; Oehme, I.; Breuhahn, K.; Moldenhauer, G.; Witzens-Harig, M.; Cerwenka, A. Downregulation of the Activating NKp30 Ligand B7-H6 by HDAC Inhibitors Impairs Tumor Cell Recognition by NK Cells. Blood 2013, 122, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef]
- Textor, S.; Bossler, F.; Henrich, K.O.; Gartlgruber, M.; Pollmann, J.; Fiegler, N.; Arnold, A.; Westermann, F.; Waldburger, N.; Breuhahn, K.; et al. The Proto-Oncogene Myc Drives Expression of the NK Cell-Activating NKp30 Ligand B7-H6 in Tumor Cells. Oncoimmunology 2016, 5, e1116674. [Google Scholar] [CrossRef]
- Kiuchi, N.; Nakajima, K.; Ichiba, M.; Fukada, T.; Narimatsu, M.; Mizuno, K.; Hibi, M.; Hirano, T. STAT3 Is Required for the Gp130-Mediated Full Activation of the c-Myc Gene. J. Exp. Med. 1999, 189, 63–73. [Google Scholar] [CrossRef]
- Shastri, A.; Choudhary, G.; Teixeira, M.; Gordon-Mitchell, S.; Ramachandra, N.; Bernard, L.; Bhattacharyya, S.; Lopez, R.; Pradhan, K.; Giricz, O.; et al. Antisense STAT3 Inhibitor Decreases Viability of Myelodysplastic and Leukemic Stem Cells. J. Clin. Investig. 2018, 128, 5479–5488. [Google Scholar] [CrossRef]
- Hu, J.; Gong, J.; Shu, X.; Dai, X.; Cai, D.; Zhao, Z.; Li, J.; Zhong, G.; Gong, J. SNRPA Promotes Hepatocellular Carcinoma Proliferation and Lenvatinib Resistance via B7-H6-STAT3/AKT Axis by Facilitating B7-H6 Pre-MRNA Maturation. Biosci. Trends 2025, 19, 337–350. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; Von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-Mediated Tumor Cell Shedding of B7-H6, the Ligand of the Natural Killer Cell-Activating Receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Baratin, M.; Chiche, L.; Forel, J.M.; Cognet, C.; Thomas, G.; Farnarier, C.; Piperoglou, C.; Papazian, L.; Chaussabel, D.; et al. Induction of B7-H6, a Ligand for the Natural Killer Cell-Activating Receptor NKp30, in Inflammatory Conditions. Blood 2013, 122, 394–404. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, X.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. A Versatile System for Rapid Multiplex Genome-Edited CAR T Cell Generation. Oncotarget 2017, 8, 17002–17011. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, C.; Preece, R.; Nickolay, L.; Etuk, A.; Petrova, A.; Ladon, D.; Danyi, A.; Humphryes-Kirilov, N.; Ajetunmobi, A.; Kim, D.; et al. Long Terminal Repeat CRISPR-CAR-Coupled “Universal” T Cells Mediate Potent Anti-Leukemic Effects. Mol. Ther. 2018, 26, 1215–1227. [Google Scholar] [CrossRef]
- Ottaviano, G.; Georgiadis, C.; Gkazi, S.A.; Syed, F.; Zhan, H.; Etuk, A.; Preece, R.; Chu, J.; Kubat, A.; Adams, S.; et al. Phase 1 Clinical Trial of CRISPR-Engineered CAR19 Universal T Cells for Treatment of Children with Refractory B Cell Leukemia. Sci. Transl. Med. 2022, 14, eabq3010. [Google Scholar] [CrossRef]
- Frigault, M.J.; Lee, J.; Basil, M.C.; Carpenito, C.; Motohashi, S.; Scholler, J.; Kawalekar, O.U.; Guedan, S.; McGettigan, S.E.; Posey, A.D.; et al. Identification of Chimeric Antigen Receptors That Mediate Constitutive or Inducible Proliferation of T Cells. Cancer Immunol. Res. 2015, 3, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Muller, Y.D.; Nguyen, D.P.; Ferreira, L.M.R.; Ho, P.; Raffin, C.; Valencia, R.V.B.; Congrave-Wilson, Z.; Roth, T.L.; Eyquem, J.; Van Gool, F.; et al. The CD28-Transmembrane Domain Mediates Chimeric Antigen Receptor Heterodimerization with CD28. Front. Immunol. 2021, 12, 639818. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Ivey, R.G.; Kennedy, J.J.; Voillet, V.; Rajan, A.; Alderman, E.J.; Voytovich, U.J.; Lin, C.; Sommermeyer, D.; Liu, L.; et al. Phosphoproteomic Analysis of Chimeric Antigen Receptor Signaling Reveals Kinetic and Quantitative Differences That Affect Cell Function. Sci. Signal. 2018, 11, eaat6753. [Google Scholar] [CrossRef]
- Distler, E.; Albrecht, J.; Brunk, A.; Khan, S.; Schnürer, E.; Frey, M.; Mottok, A.; Jordán-Garrote, A.L.; Brede, C.; Beilhack, A.; et al. Patient-Individualized CD8+ Cytolytic T-Cell Therapy Effectively Combats Minimal Residual Leukemia in Immunodeficient Mice. Int. J. Cancer 2016, 138, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Santos, E.; Nikhamin, Y.; Yeh, R.; Matsushita, M.; La Perle, K.; Quintás-Cardama, A.; Larson, S.M.; Sadelain, M. Genetically Targeted T Cells Eradicate Systemic Acute Lymphoblastic Leukemia Xenografts. Clin. Cancer Res. 2007, 13, 5426–5435. [Google Scholar] [CrossRef]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, X.; Niu, X.; Liu, Z.; Li, M.; Liu, M.; Yan, D.; Zhang, G.; Wan, X. CD28 Is Superior to 4-1BB Costimulation in Generating CAR-NK Cells for Tumor Immunotherapy. Exp. Hematol. Oncol. 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of Large, Established Tumor Xenografts with Genetically Retargeted Human T Cells Containing CD28 and CD137 Domains. Proc. Natl. Acad. Sci. USA 2009, 106, 3360–3365. [Google Scholar] [CrossRef]
- Sun, C.; Shou, P.; Du, H.; Hirabayashi, K.; Chen, Y.; Herring, L.E.; Ahn, S.; Xu, Y.; Suzuki, K.; Li, G.; et al. THEMIS-SHP1 Recruitment by 4-1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer Cell 2020, 37, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ponath, V.; Hoffmann, N.; Bergmann, L.; Mäder, C.; Alhamwe, B.A.; Preußer, C.; Strandmann, E.P. von Secreted Ligands of the Nk Cell Receptor Nkp30: B7-h6 Is in Contrast to Bag6 Only Marginally Released via Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 2189. [Google Scholar] [CrossRef]
- Gacerez, A.T.; Hua, C.K.; Ackerman, M.E.; Sentman, C.L. Chimeric Antigen Receptors with Human ScFvs Preferentially Induce T Cell Anti-Tumor Activity Against Tumors with High B7H6 Expression. Cancer Immunol. Immunother. 2018, 67, 749–759. [Google Scholar] [CrossRef]
- Hua, C.K.; Gacerez, A.T.; Sentman, C.L.; Ackerman, M.E. Development of Unique Cytotoxic Chimeric Antigen Receptors Based on Human ScFv Targeting B7H6. Protein Eng. Des. Sel. 2017, 30, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, C.; Drexler, I.; Van Pel, A.; Thres, T.; Leister, N.; Herr, W.; Sutter, G.; Huber, C.; Wölfel, T. Transporter (TAP)- and Proteasome-Independent Presentation of a Melanoma-Associated Tyrosinase Epitope. Int. J. Cancer. 2000, 88, 432–438. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Givi, S.; Lohnes, B.J.; Ebrahimi, S.; Riedel, S.; Khokhali, S.; Khan, S.A.; Keller, M.; Wölfel, C.; Echchannaoui, H.; Bockamp, E.; et al. CRISPR/Cas9 TCR-Edited NKp30 CAR T Cells Exhibit Superior Anti-Tumor Immunity to B7H6-Expressing Leukemia and Melanoma. Int. J. Mol. Sci. 2025, 26, 8235. https://doi.org/10.3390/ijms26178235
Givi S, Lohnes BJ, Ebrahimi S, Riedel S, Khokhali S, Khan SA, Keller M, Wölfel C, Echchannaoui H, Bockamp E, et al. CRISPR/Cas9 TCR-Edited NKp30 CAR T Cells Exhibit Superior Anti-Tumor Immunity to B7H6-Expressing Leukemia and Melanoma. International Journal of Molecular Sciences. 2025; 26(17):8235. https://doi.org/10.3390/ijms26178235
Chicago/Turabian StyleGivi, Sedigheh, Benedikt J. Lohnes, Saber Ebrahimi, Sophie Riedel, Sneha Khokhali, Shamsul A. Khan, Maximilian Keller, Catherine Wölfel, Hakim Echchannaoui, Ernesto Bockamp, and et al. 2025. "CRISPR/Cas9 TCR-Edited NKp30 CAR T Cells Exhibit Superior Anti-Tumor Immunity to B7H6-Expressing Leukemia and Melanoma" International Journal of Molecular Sciences 26, no. 17: 8235. https://doi.org/10.3390/ijms26178235
APA StyleGivi, S., Lohnes, B. J., Ebrahimi, S., Riedel, S., Khokhali, S., Khan, S. A., Keller, M., Wölfel, C., Echchannaoui, H., Bockamp, E., Andre, M. C., Abken, H., Theobald, M., & Hartwig, U. F. (2025). CRISPR/Cas9 TCR-Edited NKp30 CAR T Cells Exhibit Superior Anti-Tumor Immunity to B7H6-Expressing Leukemia and Melanoma. International Journal of Molecular Sciences, 26(17), 8235. https://doi.org/10.3390/ijms26178235






