Metabolic Signatures in Lung Cancer: Prognostic Value of Acid–Base Disruptions and Serum Indices
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
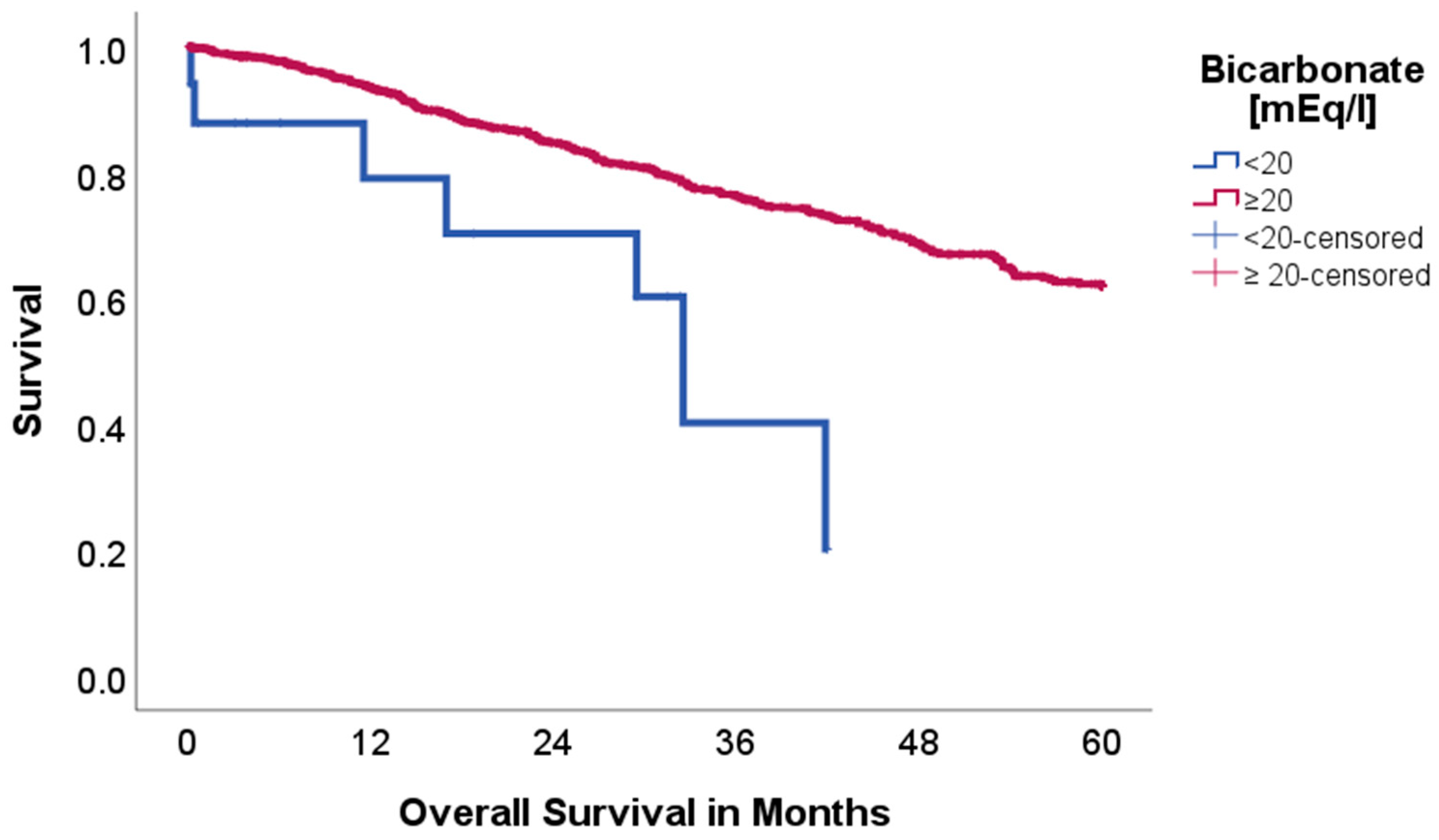
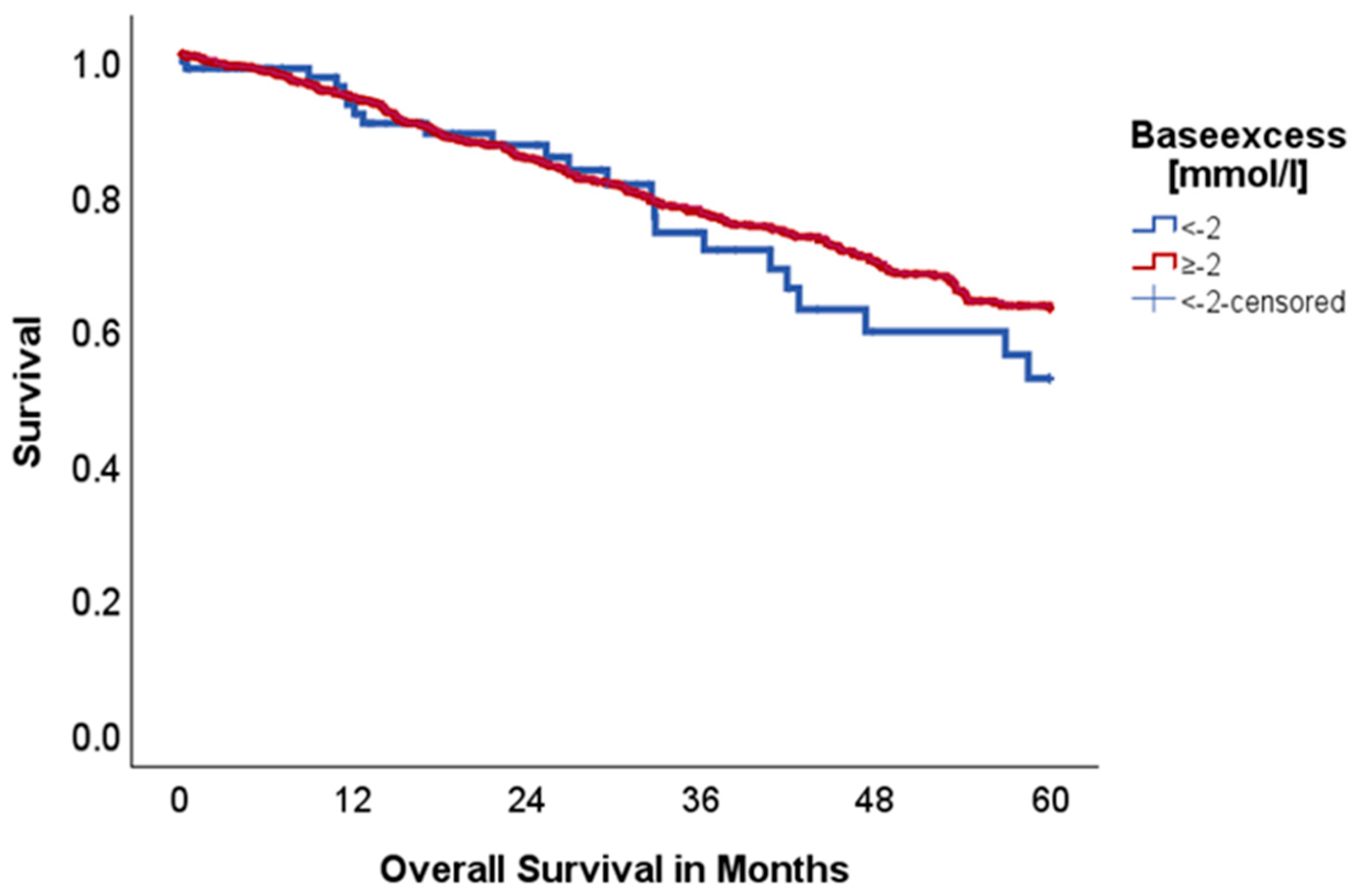
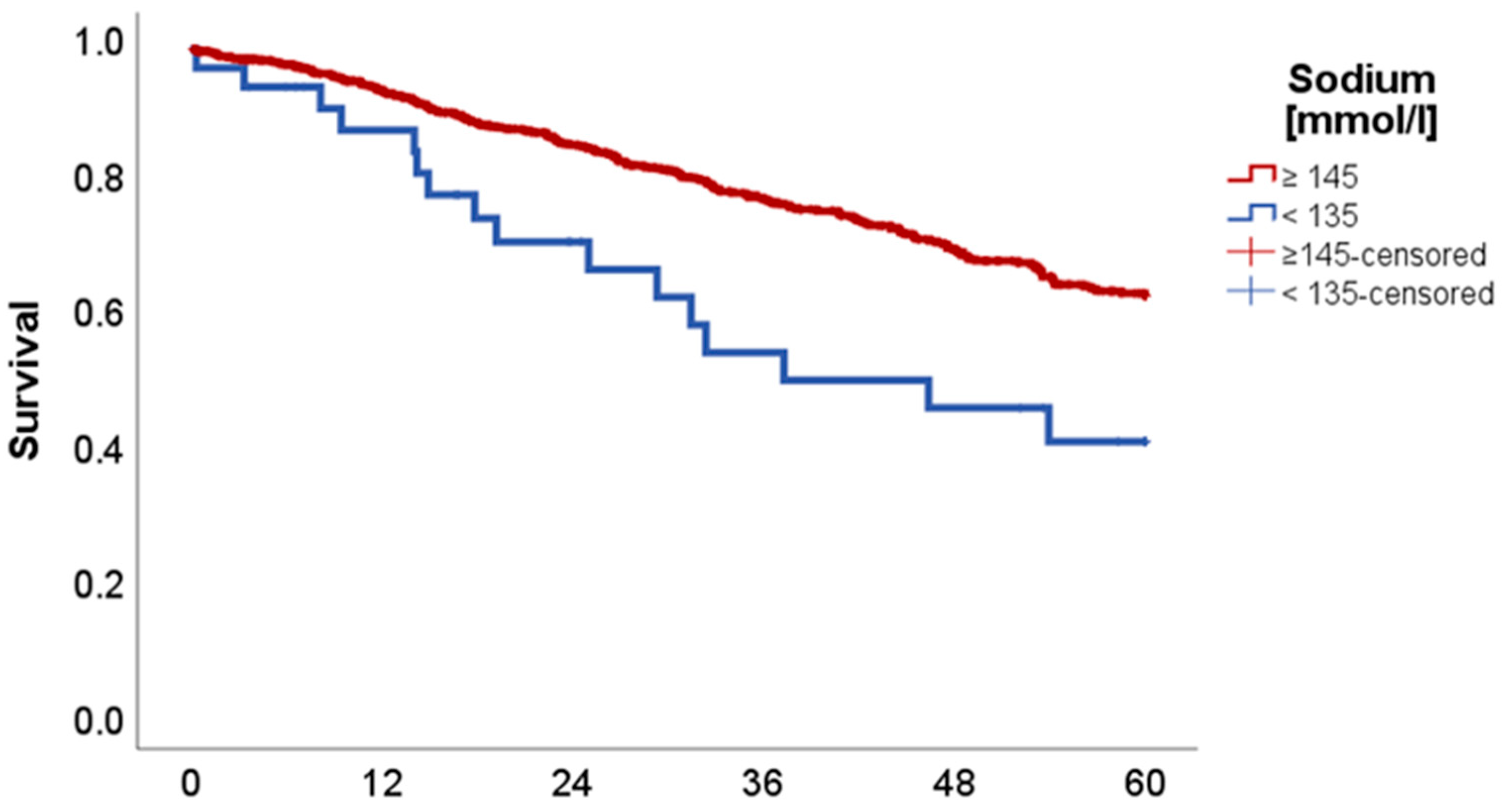
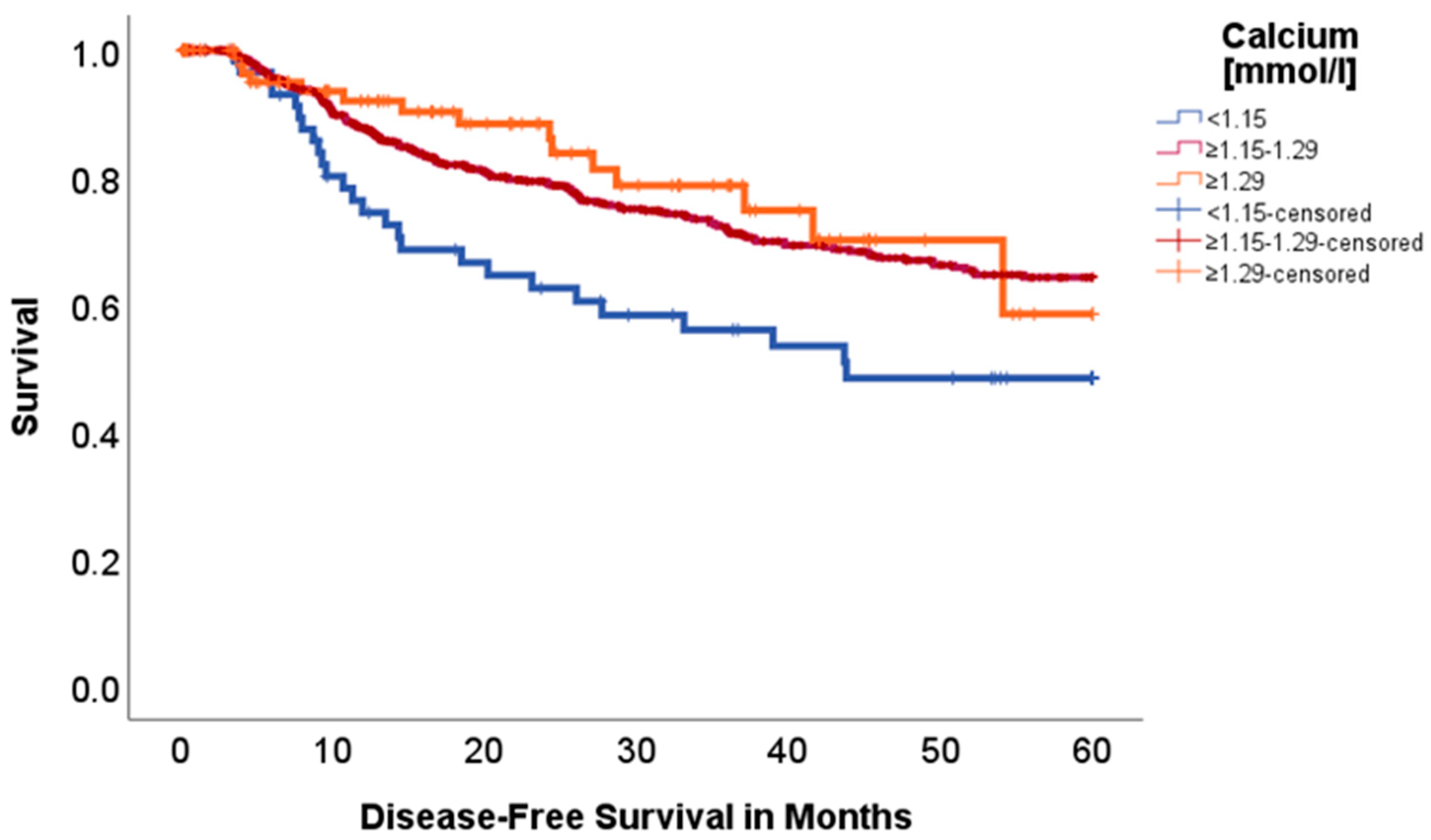
2.2. Effect of Acid–Base and Serum Indices on 5-Year Overall and Disease-Free Survival
3. Materials and Methods
3.1. Patient Selection and Data Collection
3.2. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18 (Suppl. 2), s41–s46. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in: Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The warburg effect 97 years after its discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatenby, R.A.; et al. Bicarbonate increases tumor pH and inhibits spon-taneous metastases. Cancer Res. 2009, 69, 2260–2268. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microen-vironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Faes, S.; Duval, A.P.; Planche, A.; Uldry, E.; Santoro, T.; Pythoud, C.; Stehle, J.C.; Horlbeck, J.; Letovanec, I.; Riggi, N.; et al. Acidic tumor microenvironment abrogates the efficacy of mTORC1 inhibitors. Mol. Cancer 2016, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, W.; Lu, X.; Wang, J.; Zhang, C.; Xu, Y. Acid-base Homeostasis and Implications to the Phenotypic Behaviors of Cancer. Genom. Proteom. Bioinform. 2023, 21, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yao, K.; Zhu, X.; Chen, N.; Xiao, N.; Wang, Y.; Peng, B.; Yao, L.; Li, P.; Zhang, P.; et al. Evolutionary metabolic landscape from preneoplasia to invasive lung adenocarcinoma. Nat. Commun. 2021, 12, 6479. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Guggino, S.E.; Solaiyappan, M.; Pathak, A.P.; Ichikawa, Y.; Bhujwalla, Z.M. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 2003, 5, 533–545. [Google Scholar] [CrossRef]
- Sebastian, N.; Wu, T.; Driscoll, E.; Willers, H.; Kelly, S.; Musunuru, H.B.; Mo, X.; Tan, Y.; Bazan, J.; Haglund, K.; et al. Pre-treatment serum bicarbonate predicts for primary tumor control after stereotactic body radiation therapy in patients with localized non-small cell lung cancer. Radiother. Oncol. 2019, 140, 26–33. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human mel-anoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699. [Google Scholar] [CrossRef] [PubMed]
- Shie, W.-Y.; Chu, P.-H.; Kuo, M.Y.; Chen, H.-W.; Lin, M.-T.; Su, X.-J.; Hong, Y.-L.; Chou, H.-Y.E. Acidosis promotes the metastatic colonization of lung cancer via remodeling of the extracellular matrix and vasculogenic mimicry. Int. J. Oncol. 2023, 63, 136. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 25 February 2025).
- Kassambara, A.; Kosinski, M.; Biecek, P.; Scheipl, F. Drawing Survival Curves Using ‘ggplot2’. 2024 [cited 2024 Aug 7]. Available online: https://cran.r-project.org/web/packages/survminer/survminer.pdf (accessed on 25 February 2025).
- Kreisberg, R.A. Lactate homeostasis and lactic acidosis. Ann. Intern. Med. 1980, 92, 227–237. [Google Scholar] [CrossRef]
- de La Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar]
- Bartalis, E.; Gergics, M.; Tinusz, B.; Földi, M.; Kiss, S.; Németh, D.; Solymár, M.; Szakács, Z.; Hegyi, P.; Mezösi, E.; et al. Prevalence and prognostic significance of hyponatremia in patients with lung cancer: Systematic review and meta-analysis. Front. Med. 2021, 8, 671951. [Google Scholar] [CrossRef]
- Fiordoliva, I.; Meletani, T.; Baleani, M.G.; Rinaldi, S.; Savini, A.; Paolo, M.D.P.; Berardi, R. Managing hyponatremia in lung cancer: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 711–719. [Google Scholar] [CrossRef]
- Corona, G.; Giuliani, C.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Hyponatremia improvement is associated with a reduced risk of mortality: Evidence from a meta-analysis. PLoS ONE 2015, 10, e0124105, Erratum in: PLoS ONE 2016, 11, e0152846. [Google Scholar] [CrossRef]
- Gastanaga, V.M.; Schwartzberg, L.S.; Jain, R.K.; Pirolli, M.; Quach, D.; Quigley, J.M.; Mu, G.; Stryker, W.S.; Liede, A. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016, 5, 2091–2100. [Google Scholar] [CrossRef]
- Gonçalves, J.A.F.; Costa, T.; Rema, J.; Pinto, C.; Magalhães, M.; Esperança, A.; Sousa, L. Hypocalcemia in cancer patients: An ex-ploratory study. Porto Biomed. J. 2019, 4, e45. [Google Scholar]
- Li, Y.; Chen, X.; Shen, Z.; Wang, Y.; Hu, J.; Xu, J.; Shen, B.; Ding, X. Electrolyte and acid-base disorders in cancer patients and its impact on clinical outcomes: Evidence from a real-world study in China. Ren. Fail. 2020, 42, 234–243. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, P.; Visnick, H.; Balasubramanian, S. Accuracy of commercial blood gas analyzers for monitoring ionized calcium at low concentrations. Clin. Chim. Acta 2016, 461, 34–40. [Google Scholar] [CrossRef]
- Kelly, A.; Levine, M.A. Hypocalcemia in the critically ill patient. J. Intensive Care Med. 2013, 28, 166–177. [Google Scholar] [CrossRef]
- Aoe, K.; Hiraki, A.; Maeda, T.; Katayama, H.; Fujiwara, K.; Tabata, M.; Kiura, K.; Ueoka, H.; Tanimoto, M. Serum hemoglobin level determined at the first presen-tation is a poor prognostic indicator in patients with lung cancer. Intern. Med. 2005, 44, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Shimizu, T.; Hara, M.; Ayabe, T.; Onitsuka, T. Impact of preoperative hemoglobin level on survival of non-small cell lung cancer patients. Anticancer Res. 2008, 28, 1947–1950. [Google Scholar] [PubMed]
- Zhang, Y.; Lu, Y.; Lu, H.; Zhang, M.; Zhou, Y.; Li, X.; Lv, P.; Zhao, X. Pre-treatment hemoglobin levels are an independent prognostic factor in patients with non-small cell lung cancer. Mol. Clin. Oncol. 2018, 9, 44–49. [Google Scholar] [CrossRef]
- Sorensen, M.B.; Jacobsen, E. Pulmonary hemodynamics during induction of anesthesia. Anesthesiology 1977, 46, 246–251. [Google Scholar] [CrossRef]
- Madias, N.E. Renal acidification responses to respiratory acid-base disorders. J. Nephrol. 2010, 23 (Suppl. 16), S85–S91. [Google Scholar]
- Franchini, S. Physiological approach to assessment of acid-base disturbances. N. Engl. J. Med. 2015, 372, 193–194. [Google Scholar]
- Alarcón, O.M.; Reinosa, J.; Medina de Caraballo, M.I.; Silva, T. Halothane anesthesia and serum electrolytes. J. Trace Elem. Med. Biol. 1996, 10, 46–49. [Google Scholar] [CrossRef] [PubMed]
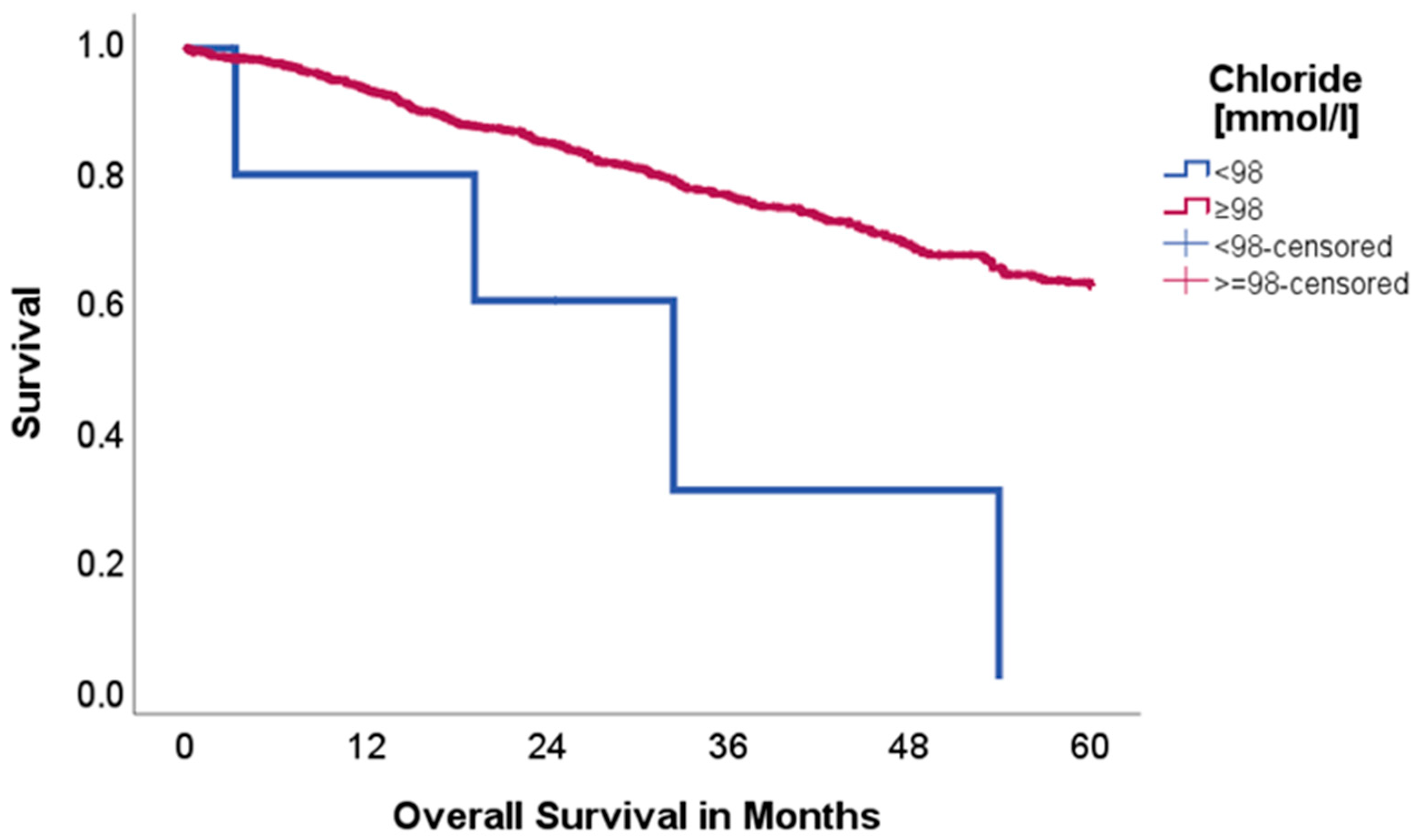
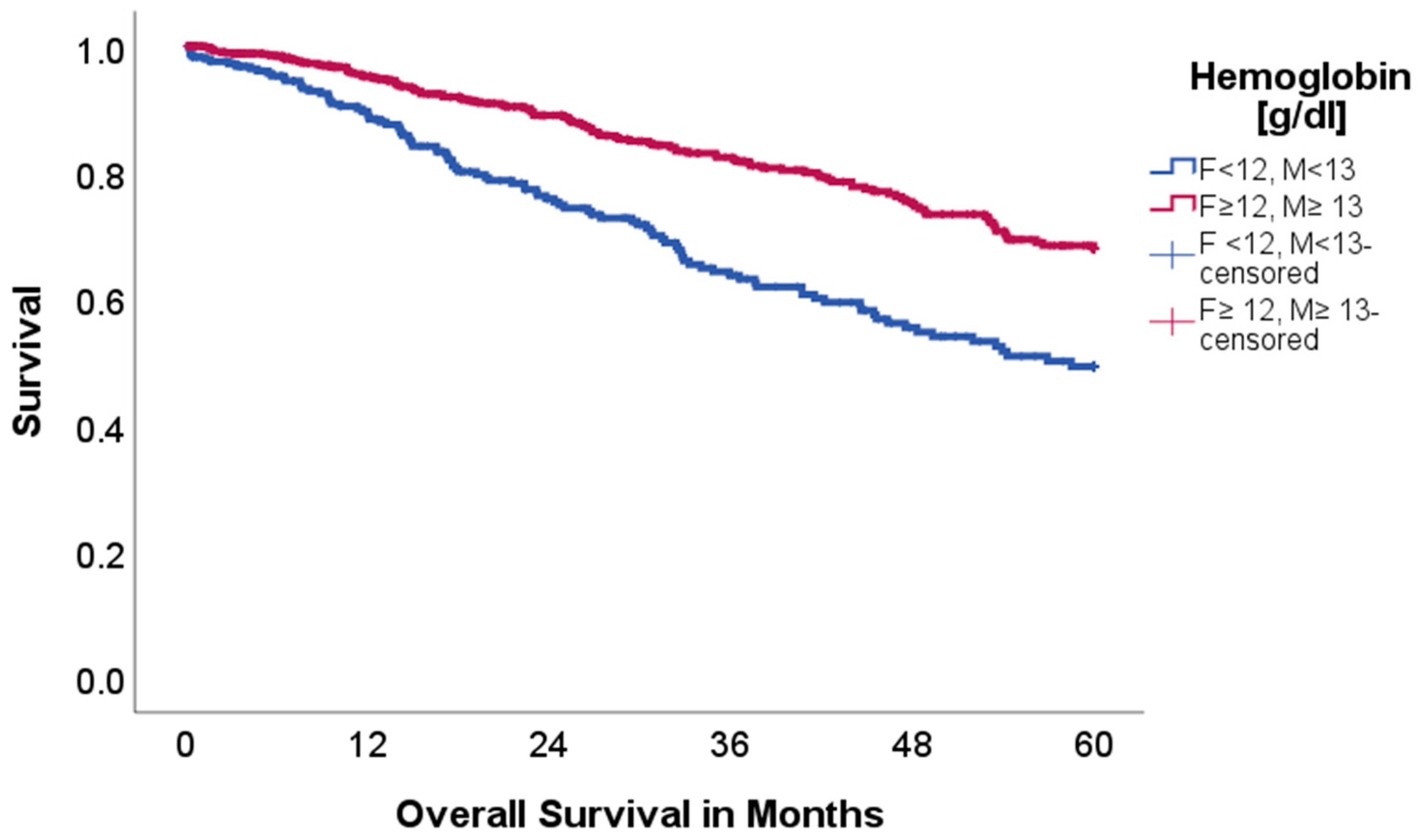
| Factor | n = 937 | % |
|---|---|---|
| Sex | ||
| Females | 448 | 47.8 |
| Males | 489 | 52.2 |
| Mean Age [y] (median, IQR *) | 64.34 (65.0, 14) | |
| Mean BMI [kg/m2] (median, IQR) | 25.38 (24.9, 5.77) | |
| Mean Length of Stay [days] (median, IQR) | 10.24 (8.0, 5) | |
| Age-Adjusted Charlson Comorbidity Index ** | ||
| 0 | 47 | 5.0 |
| 1 | 83 | 8.9 |
| 2 | 196 | 20.9 |
| 3 | 233 | 24.9 |
| 4 | 169 | 18.0 |
| 5 | 102 | 10.9 |
| ≥6 | 107 | 11.4 |
| pT | ||
| 0 | 12 | 1.3 |
| 1 | 559 | 59.7 |
| 2 | 254 | 27.1 |
| 3 | 97 | 10.4 |
| 4 | 15 | 1.6 |
| pN | ||
| 0 | 683 | 73.3 |
| 1 | 134 | 14.3 |
| 2 | 115 | 12.3 |
| pM | ||
| 0 | 911 | 97.5 |
| 1 | 22 | 2.3 |
| 2 | 1 | 0.1 |
| UICC | ||
| I | 570 | 60.8 |
| II | 198 | 21.1 |
| III | 135 | 14.4 |
| IV | 25 | 2.7 |
| Pathology | ||
| Adeno | 657 | 70.1 |
| Squamous | 161 | 17.2 |
| Neuroendocrine | 74 | 7.9 |
| Mixed | 17 | 1.8 |
| Large-Cell | 11 | 1.2 |
| Small-Cell Lung Cancer | 9 | 1.0 |
| Others | 8 | 0.9 |
| Clavien Dindo *** | ||
| <III | 824 | 90.4 |
| ≥III | 87 | 9.6 |
| Neoadjuvant therapy | 91 | 9.7 |
| Analyte [ASTRUP] | Range/Limit | |
|---|---|---|
| Hemoglobin | ||
| Female | 12.0–15.7 g/dL | |
| Male | 13.0–17.7 g/dL | |
| Bicarbonate | 20.0–26.0 mmol/L | |
| Baseexcess (BE) | ≥−2–+3 mmol/L | |
| Sodium | 136–146 mmol/L | |
| Calcium2+ (ionized) | 1.15–1.29 mmol/L | |
| Chloride | 98–106 mmol/L | |
| Lactate | 4–20 mg/dL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponholzer, F.; Neuschmid, M.-C.; Komi, H.; Bogensperger, C.; Ng, C.; Maier, H.; Lucciarini, P.; Schneeberger, S.; Augustin, F. Metabolic Signatures in Lung Cancer: Prognostic Value of Acid–Base Disruptions and Serum Indices. Int. J. Mol. Sci. 2025, 26, 8231. https://doi.org/10.3390/ijms26178231
Ponholzer F, Neuschmid M-C, Komi H, Bogensperger C, Ng C, Maier H, Lucciarini P, Schneeberger S, Augustin F. Metabolic Signatures in Lung Cancer: Prognostic Value of Acid–Base Disruptions and Serum Indices. International Journal of Molecular Sciences. 2025; 26(17):8231. https://doi.org/10.3390/ijms26178231
Chicago/Turabian StylePonholzer, Florian, Marie-Christin Neuschmid, Helga Komi, Christina Bogensperger, Caecilia Ng, Herbert Maier, Paolo Lucciarini, Stefan Schneeberger, and Florian Augustin. 2025. "Metabolic Signatures in Lung Cancer: Prognostic Value of Acid–Base Disruptions and Serum Indices" International Journal of Molecular Sciences 26, no. 17: 8231. https://doi.org/10.3390/ijms26178231
APA StylePonholzer, F., Neuschmid, M.-C., Komi, H., Bogensperger, C., Ng, C., Maier, H., Lucciarini, P., Schneeberger, S., & Augustin, F. (2025). Metabolic Signatures in Lung Cancer: Prognostic Value of Acid–Base Disruptions and Serum Indices. International Journal of Molecular Sciences, 26(17), 8231. https://doi.org/10.3390/ijms26178231






