α-Cyclodextrin/Moringin Impacts Actin Cytoskeleton Dynamics with Potential Implications for Synaptic Organization: A Preliminary Transcriptomic Study in NSC-34 Motor Neurons
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of α-CD/MOR Against Control Group
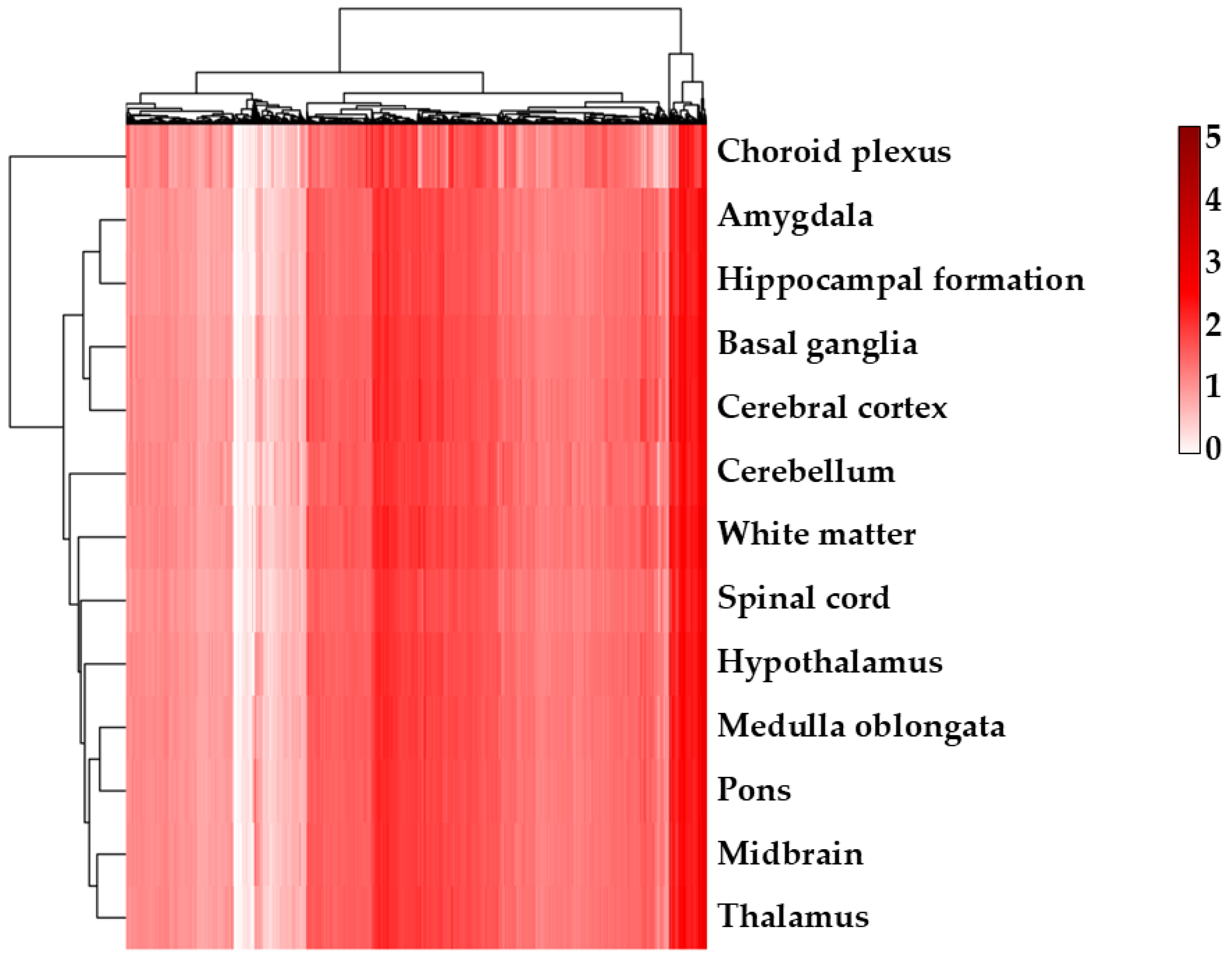
2.2. α-CD/MOR Modulated Synaptic Ontologies
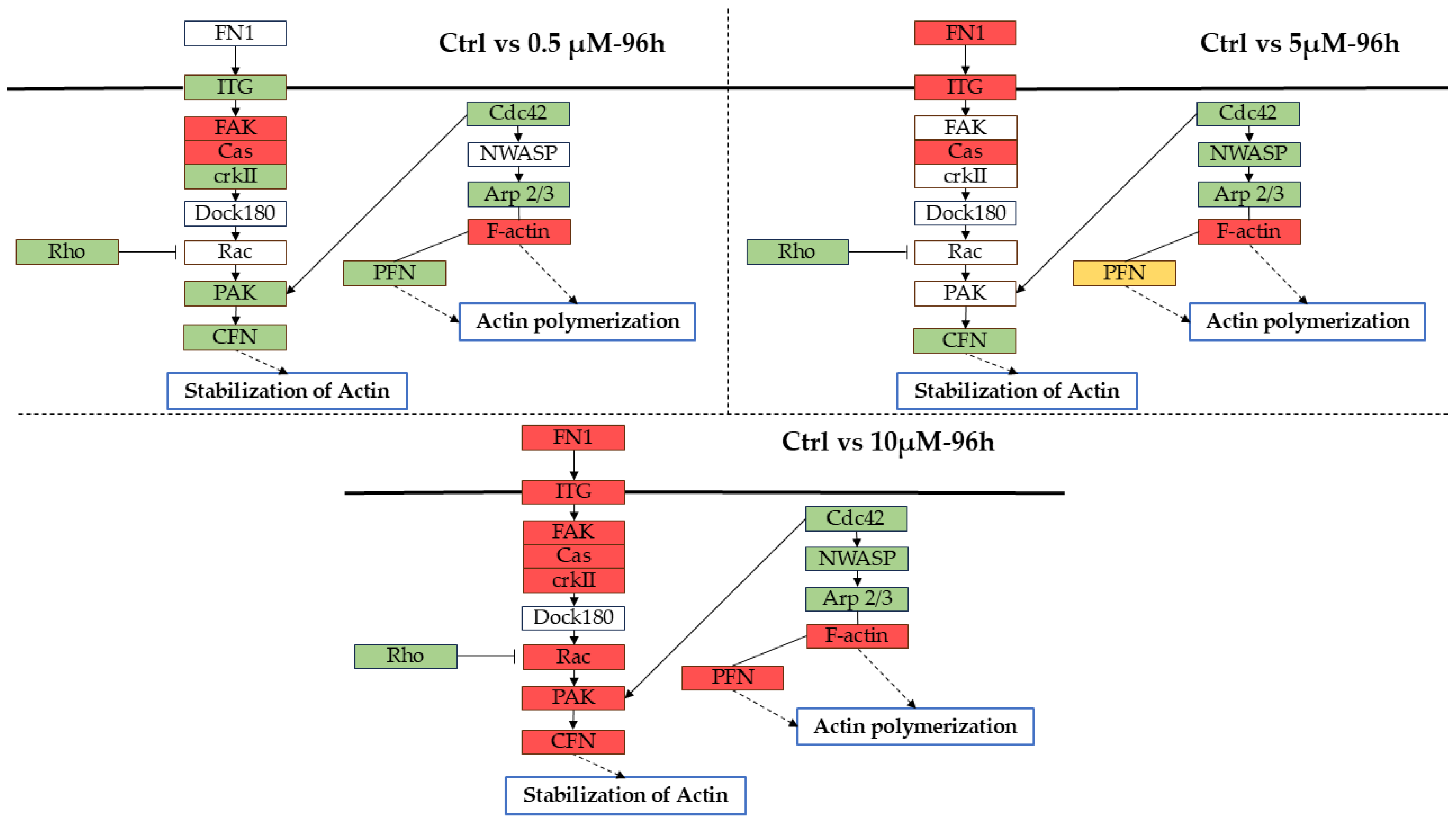
2.3. α-CD/MOR Modulated Synaptic Pathways
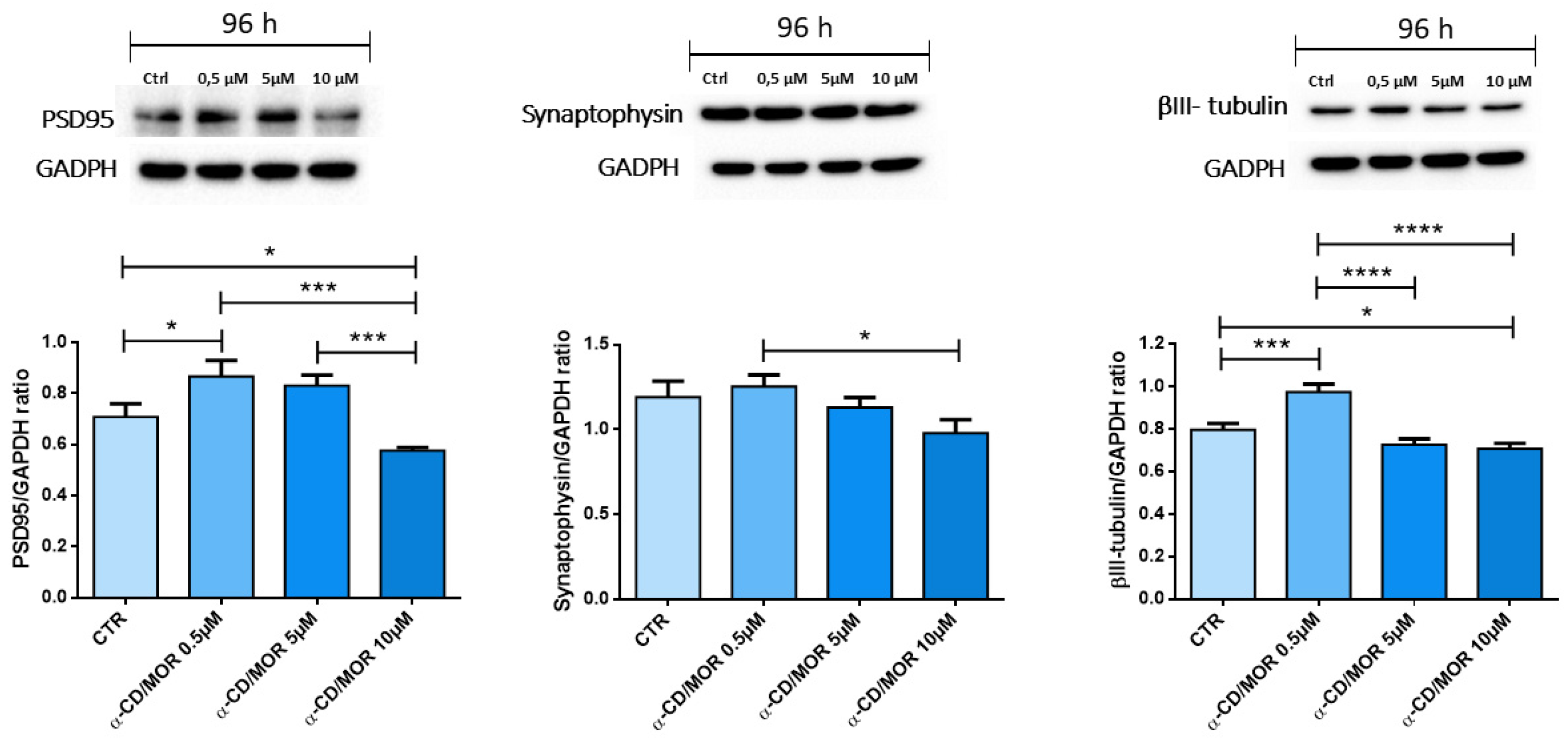
2.4. α-CD/MOR-Treated Cells Expressed Neuronal Markers
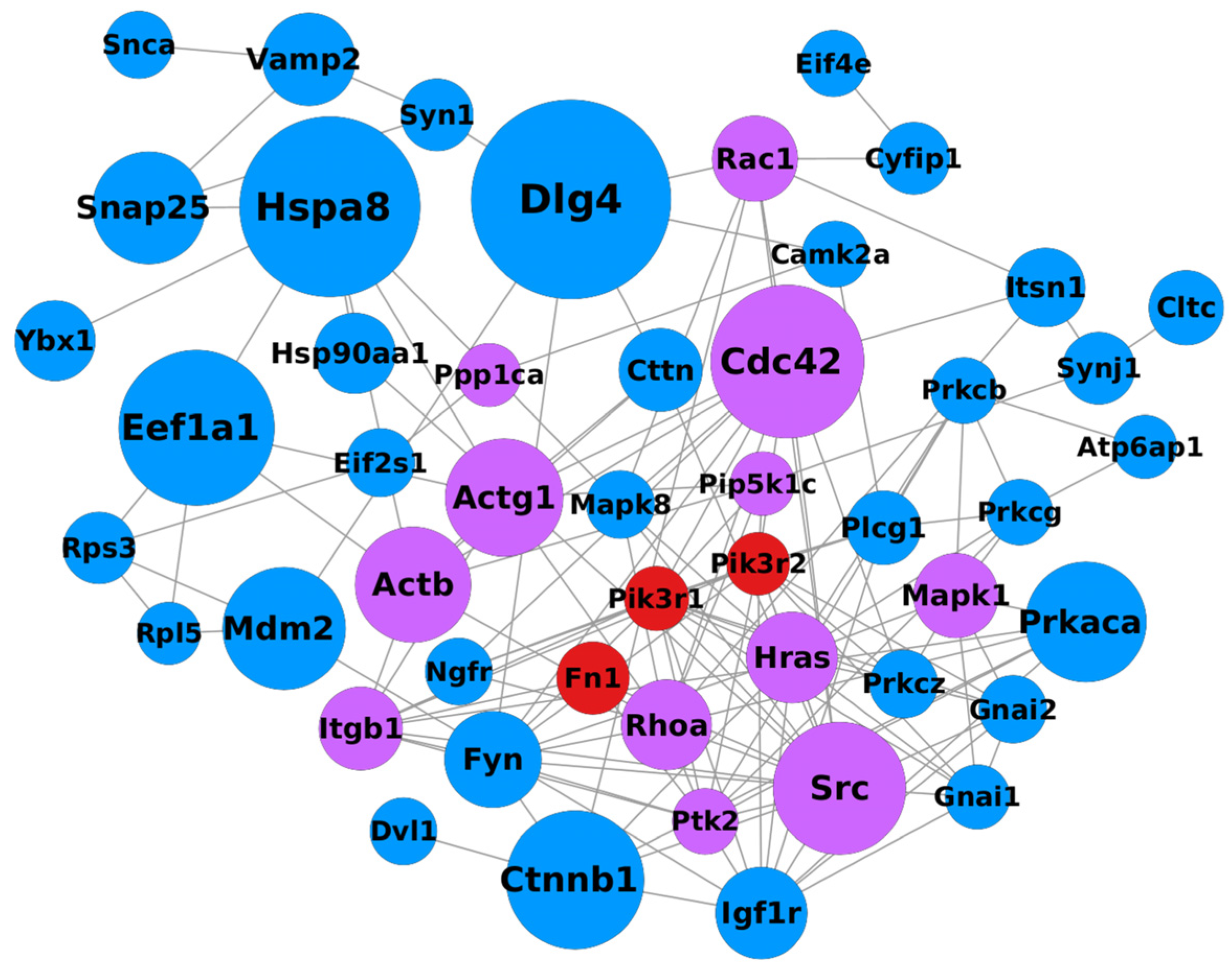
2.5. Integration of Synaptic Genes into Cytoskeleton Regulation
2.6. Transcription Factors Involved in α-CD/MOR Effects and Analysis of DEGs Associated with Neurodegenerative, Neurological, and Neuropsychiatric Diseases
3. Discussion
4. Materials and Methods
4.1. Synthesis of the α-CD/MOR Complex
4.2. NSC-34 Culture, Differentiation, and Treatment
4.3. Library Preparation and Sequencing
4.4. Comparative Transcriptomic and In Silico Analysis
4.5. Protein Extraction and WTestern Blot
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITCs | Isothiocyanates |
| GLs | Glucosinolates |
| GRA | Glucoraphanin |
| SFN | Sulforaphane |
| GMG | Glucomoringin |
| MOR | Moringin |
| ALS | Amyotrophic lateral sclerosis |
| AD | Alzheimer’s disease |
| CD | α-cyclodextrin |
| CTRL | Control |
| DEGs | Differentially expressed genes |
References
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Azlan, U.K.; Khairul Annuar, N.A.; Mediani, A.; Aizat, W.M.; Damanhuri, H.A.; Tong, X.; Yanagisawa, D.; Tooyama, I.; Wan Ngah, W.Z.; Jantan, I.; et al. An insight into the neuroprotective and anti-neuroinflammatory effects and mechanisms of Moringa oleifera. Front. Pharmacol. 2022, 13, 1035220. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Su, R.; Yang, M.; Zhang, N.; Li, X.; Li, L.; Sheng, J.; Tian, Y. Multi-Target Antibacterial Mechanism of Moringin From Moringa oleifera Seeds Against Listeria monocytogenes. Front. Microbiol. 2022, 13, 925291. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Nordin, N.; Shaari, K.; Rosli, R.; Abdull Razis, A.F. Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells. PLoS ONE 2018, 13, e0196403. [Google Scholar] [CrossRef]
- Manjunath, S.H.; Nataraj, P.; Swamy, V.H.; Sugur, K.; Dey, S.K.; Ranganathan, V.; Daniel, S.; Leihang, Z.; Sharon, V.; Chandrashekharappa, S.; et al. Development of Moringa oleifera as functional food targeting NRF2 signaling: Antioxidant and anti-inflammatory activity in experimental model systems. Food Funct. 2023, 14, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The alpha-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/alpha-CD Pretreatment Induces Neuroprotection in an In Vitro Model of Alzheimer’s Disease: A Transcriptomic Study. Curr. Issues Mol. Biol. 2021, 43, 197–214. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The Isothiocyanate Isolated from Moringa oleifera Shows Potent Anti-Inflammatory Activity in the Treatment of Murine Subacute Parkinson’s Disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef]
- Galuppo, M.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. Administration of 4-(alpha-L-rhamnosyloxy)-benzyl isothiocyanate delays disease phenotype in SOD1(G93A) rats: A transgenic model of amyotrophic lateral sclerosis. BioMed Res. Int. 2015, 2015, 259417. [Google Scholar] [CrossRef]
- Giacoppo, S.; Soundara Rajan, T.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. Moringin activates Wnt canonical pathway by inhibiting GSK3beta in a mouse model of experimental autoimmune encephalomyelitis. Drug Des. Dev. Ther. 2016, 10, 3291–3304. [Google Scholar] [CrossRef]
- Verma, H.; Kaur, S.; Kaur, S.; Gangwar, P.; Dhiman, M.; Mantha, A.K. Role of Cytoskeletal Elements in Regulation of Synaptic Functions: Implications Toward Alzheimer’s Disease and Phytochemicals-Based Interventions. Mol. Neurobiol. 2024, 61, 8320–8343. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.; Marcut, C.; Fonseca, R. Actin remodeling, the synaptic tag and the maintenance of synaptic plasticity. IUBMB Life 2020, 72, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hlushchenko, I.; Koskinen, M.; Hotulainen, P. Dendritic spine actin dynamics in neuronal maturation and synaptic plasticity. Cytoskeleton 2016, 73, 435–441. [Google Scholar] [CrossRef]
- Gordon-Weeks, P.R.; Fournier, A.E. Neuronal cytoskeleton in synaptic plasticity and regeneration. J. Neurochem. 2014, 129, 206–212. [Google Scholar] [CrossRef]
- Pelucchi, S.; Stringhi, R.; Marcello, E. Dendritic Spines in Alzheimer’s Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure. Int. J. Mol. Sci. 2020, 21, 908. [Google Scholar] [CrossRef]
- Theunissen, F.; West, P.K.; Brennan, S.; Petrovic, B.; Hooshmand, K.; Akkari, P.A.; Keon, M.; Guennewig, B. New perspectives on cytoskeletal dysregulation and mitochondrial mislocalization in amyotrophic lateral sclerosis. Transl. Neurodegener. 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, M.; Wallin, A. Pathophysiological aspects of frontotemporal dementia--emphasis on cytoskeleton proteins and autoimmunity. Mech. Ageing Dev. 2001, 122, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, M.; Huang, X.; Liu, X.; Li, W. Inhibition of cytoskeletal protein carbonylation may protect against oxidative damage in traumatic brain injury. Exp. Ther. Med. 2016, 12, 4107–4112. [Google Scholar] [CrossRef]
- Richardson, B.; Goedert, T.; Quraishe, S.; Deinhardt, K.; Mudher, A. How do neurons age? A focused review on the aging of the microtubular cytoskeleton. Neural Regen. Res. 2024, 19, 1899–1907. [Google Scholar] [CrossRef]
- Mathiron, D.; Iori, R.; Pilard, S.; Soundara Rajan, T.; Landy, D.; Mazzon, E.; Rollin, P.; Djedaini-Pilard, F. A Combined Approach of NMR and Mass Spectrometry Techniques Applied to the alpha-Cyclodextrin/Moringin Complex for a Novel Bioactive Formulation (dagger). Molecules 2018, 23, 1714. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Cali, G.; Muscara, C.; Artimagnella, O.; Rollin, P.; Perenzoni, D.; Iori, R.; Mazzon, E.; Chiricosta, L. alpha-Cyclodextrin/Moringin Induces an Antioxidant Transcriptional Response Activating Nrf2 in Differentiated NSC-34 Motor Neurons. Antioxidants 2024, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Feng, J.; Huang, F.; Salissou, M.T.M.; Wang, J.; Liu, R.; Zhang, B.; Li, H.; Zhu, F.; Wang, X. Moringa Oleifera Alleviates Abeta Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients 2022, 14, 4284. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Y.; Yang, W.; Ge, Y.; Xu, L.; Ren, T.; Zhang, H.; Zhuo, R.; Peng, L.; Chen, C.; et al. Moringa oleifera seed extract protects against brain damage in both the acute and delayed stages of ischemic stroke. Exp. Gerontol. 2019, 122, 99–108. [Google Scholar] [CrossRef]
- Schneider, F.; Metz, I.; Rust, M.B. Regulation of actin filament assembly and disassembly in growth cone motility and axon guidance. Brain Res. Bull. 2023, 192, 21–35. [Google Scholar] [CrossRef]
- Zang, Y.; Chaudhari, K.; Bashaw, G.J. New insights into the molecular mechanisms of axon guidance receptor regulation and signaling. Curr. Top. Dev. Biol. 2021, 142, 147–196. [Google Scholar] [CrossRef]
- Davis-Lunn, M.; Goult, B.T.; Andrews, M.R. Clutching at Guidance Cues: The Integrin-FAK Axis Steers Axon Outgrowth. Biology 2023, 12, 954. [Google Scholar] [CrossRef]
- Cui, Y.; Rolova, T.; Fagerholm, S.C. The role of integrins in brain health and neurodegenerative diseases. Eur. J. Cell Biol. 2024, 103, 151441. [Google Scholar] [CrossRef]
- Handwerk, C.J.; Denzler, C.J.; Kalinowski, A.R.; Cook, H.N.; Rodriguez, H.V.; Bland, K.M.; Brett, C.A.; Swinehart, B.D.; Vinson, E.C.; Vidal, G.S. Integrin beta3 regulates apical dendritic morphology of pyramidal neurons throughout hippocampal CA3. Hippocampus 2023, 33, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yazdani, U.; Thompson-Peer, K.L.; Kolodkin, A.L.; Terman, J.R. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development 2007, 134, 2337–2347. [Google Scholar] [CrossRef]
- Monje, F.J.; Kim, E.J.; Pollak, D.D.; Cabatic, M.; Li, L.; Baston, A.; Lubec, G. Focal adhesion kinase regulates neuronal growth, synaptic plasticity and hippocampus-dependent spatial learning and memory. Neuro-Signals 2012, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Auer, M.; Hausott, B.; Klimaschewski, L. Rho GTPases as regulators of morphological neuroplasticity. Ann. Anat.-Anat. Anz. Off. Organ Anat. Ges. 2011, 193, 259–266. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Pena, C.; Bouchard, R.J.; Linseman, D.A. Dysregulation of Rac or Rho elicits death of motor neurons and activation of these GTPases is altered in the G93A mutant hSOD1 mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2020, 136, 104743. [Google Scholar] [CrossRef]
- Pan, X.; Chang, X.; Leung, C.; Zhou, Z.; Cao, F.; Xie, W.; Jia, Z. PAK1 regulates cortical development via promoting neuronal migration and progenitor cell proliferation. Mol. Brain 2015, 8, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, C.; Li, J.; Zhou, Z.; Ju, X.; Xia, S.; Li, Y.; Liu, A.; Teng, H.; Zhang, K.; et al. PAK2 Haploinsufficiency Results in Synaptic Cytoskeleton Impairment and Autism-Related Behavior. Cell Rep. 2018, 24, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.W.; Gupton, S.L.; Gertler, F.B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011, 3, a001800. [Google Scholar] [CrossRef]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef]
- Namme, J.N.; Bepari, A.K.; Takebayashi, H. Cofilin Signaling in the CNS Physiology and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 10727. [Google Scholar] [CrossRef] [PubMed]
- Witke, W.; Podtelejnikov, A.V.; Di Nardo, A.; Sutherland, J.D.; Gurniak, C.B.; Dotti, C.; Mann, M. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 1998, 17, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Alkam, D.; Feldman, E.Z.; Singh, A.; Kiaei, M. Profilin1 biology and its mutation, actin(g) in disease. Cell. Mol. Life Sci. CMLS 2017, 74, 967–981. [Google Scholar] [CrossRef]
- Strasser, G.A.; Rahim, N.A.; VanderWaal, K.E.; Gertler, F.B.; Lanier, L.M. Arp2/3 is a negative regulator of growth cone translocation. Neuron 2004, 43, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Duly, A.M.P.; Kao, F.C.L.; Teo, W.S.; Kavallaris, M. betaIII-Tubulin Gene Regulation in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 851542. [Google Scholar] [CrossRef]
- Radwitz, J.; Hausrat, T.J.; Heisler, F.F.; Janiesch, P.C.; Pechmann, Y.; Rubhausen, M.; Kneussel, M. Tubb3 expression levels are sensitive to neuronal activity changes and determine microtubule growth and kinesin-mediated transport. Cell. Mol. Life Sci. CMLS 2022, 79, 575. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, Y.; Zhang, M. Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 2016, 17, 209–223. [Google Scholar] [CrossRef]
- Keith, D.; El-Husseini, A. Excitation Control: Balancing PSD-95 Function at the Synapse. Front. Mol. Neurosci. 2008, 1, 4. [Google Scholar] [CrossRef]
- Merlo, L.; Cimino, F.; Angileri, F.F.; La Torre, D.; Conti, A.; Cardali, S.M.; Saija, A.; Germano, A. Alteration in synaptic junction proteins following traumatic brain injury. J. Neurotrauma 2014, 31, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef]
- Hou, X.Y.; Zhang, G.Y.; Wang, D.G.; Guan, Q.H.; Yan, J.Z. Suppression of postsynaptic density protein 95 by antisense oligonucleotides diminishes postischemic pyramidal cell death in rat hippocampal CA1 subfield. Neurosci. Lett. 2005, 385, 230–233. [Google Scholar] [CrossRef]
- Le, S.; Xu, L.; Wen, C.; Li, X.; Wei, L.; Xue-rong, Y.; Yu-guang, H. PSD95 gene specific siRNAs attenuate neuropathic pain through modulating neuron sensibility and postsynaptic CaMKIIalpha phosphorylation. Chin. Med. Sci. J. = Chung-Kuo I Hsueh K’o Hsueh Tsa Chih 2011, 26, 201–207. [Google Scholar] [CrossRef]
- Cousin, M.A. Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-Mechanism and function. J. Neurochem. 2021, 159, 78–89. [Google Scholar] [CrossRef]
- Lonze, B.E.; Ginty, D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- He, Y.; Casaccia-Bonnefil, P. The Yin and Yang of YY1 in the nervous system. J. Neurochem. 2008, 106, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Zurkirchen, L.; Varum, S.; Giger, S.; Klug, A.; Hausel, J.; Bossart, R.; Zemke, M.; Cantu, C.; Atak, Z.K.; Zamboni, N.; et al. Yin Yang 1 sustains biosynthetic demands during brain development in a stage-specific manner. Nat. Commun. 2019, 10, 2192. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhang, P.; Liu, Y.; Wang, Y.; Sun, W.; Yu, Z.; Cheng, Z.; Wang, Y. Runx2 was Correlated with Neurite Outgrowth and Schwann Cell Differentiation, Migration After Sciatic Nerve Crush. Neurochem. Res. 2018, 43, 2423–2434. [Google Scholar] [CrossRef]
- Li, Q.; Fadoul, G.; Ikonomovic, M.; Yang, T.; Zhang, F. Sulforaphane promotes white matter plasticity and improves long-term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic. Biol. Med. 2022, 193, 292–303. [Google Scholar] [CrossRef]
- Sunkaria, A.; Bhardwaj, S.; Yadav, A.; Halder, A.; Sandhir, R. Sulforaphane attenuates postnatal proteasome inhibition and improves spatial learning in adult mice. J. Nutr. Biochem. 2018, 51, 69–79, Erratum in J. Nutr. Biochem. 2018, 53, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hwang, E.S.; Choi, G.Y.; Kim, H.B.; Park, K.S.; Sul, J.Y.; Hwang, Y.; Choi, G.W.; Kim, B.I.; Park, H.; et al. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021, 238, 113467. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. Anti-inflammatory and anti-apoptotic effects of (RS)-glucoraphanin bioactivated with myrosinase in murine sub-acute and acute MPTP-induced Parkinson’s disease. Bioorganic Med. Chem. 2013, 21, 5532–5547. [Google Scholar] [CrossRef]
- Pawlik, A.; Szczepanski, M.A.; Klimaszewska, A.; Gackowska, L.; Zuryn, A.; Grzanka, A. Phenethyl isothiocyanate-induced cytoskeletal changes and cell death in lung cancer cells. Food Chem. Toxicol. 2012, 50, 3577–3594. [Google Scholar] [CrossRef]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A. Moringin, A Stable Isothiocyanate from Moringa oleifera, Activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, M.J.; Chaouni, R.; Biller, T.M.; Gilbert, R.M.; Paisan-Ruiz, C. A novel TRPA1 variant is associated with carbamazepine-responsive cramp-fasciculation syndrome. Clin. Genet. 2018, 93, 164–168. [Google Scholar] [CrossRef]
- Bali, A.; Schaefer, S.P.; Trier, I.; Zhang, A.L.; Kabeche, L.; Paulsen, C.E. Molecular mechanism of hyperactivation conferred by a truncation of TRPA1. Nat. Commun. 2023, 14, 2867. [Google Scholar] [CrossRef]
- Piciu, F.; Balas, M.; Badea, M.A.; Cucu, D. TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1327. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, W.; Jiang, P. CREB1 and ATF1 Negatively Regulate Glutathione Biosynthesis Sensitizing Cells to Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 698264. [Google Scholar] [CrossRef]
- Maier, O.; Bohm, J.; Dahm, M.; Bruck, S.; Beyer, C.; Johann, S. Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem. Int. 2013, 62, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P.; et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33, D433–D437. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
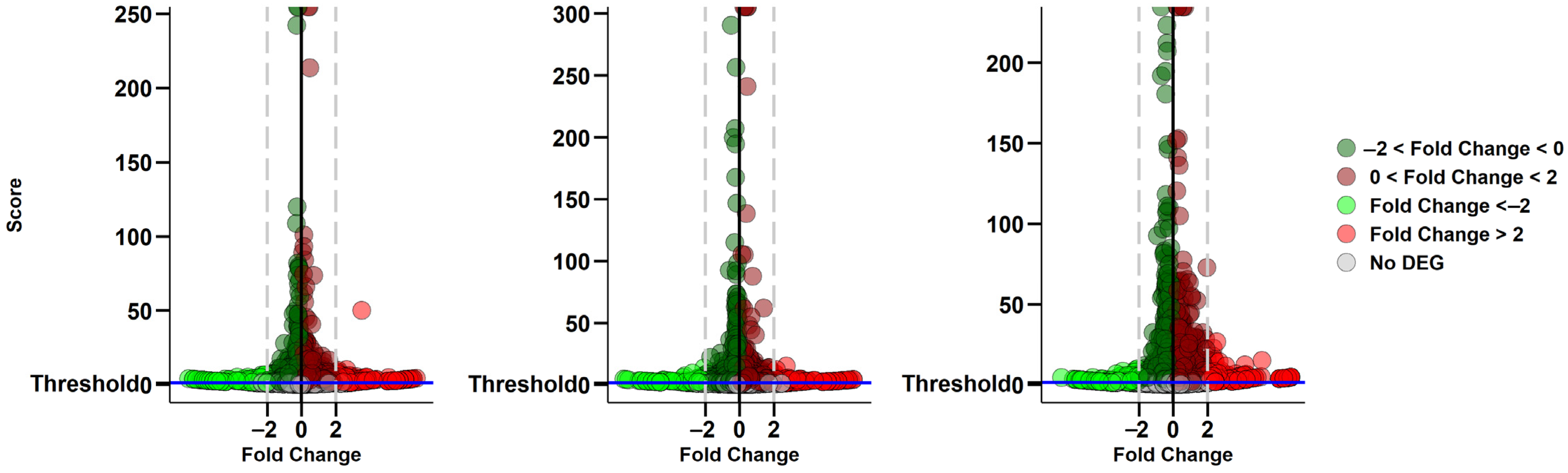
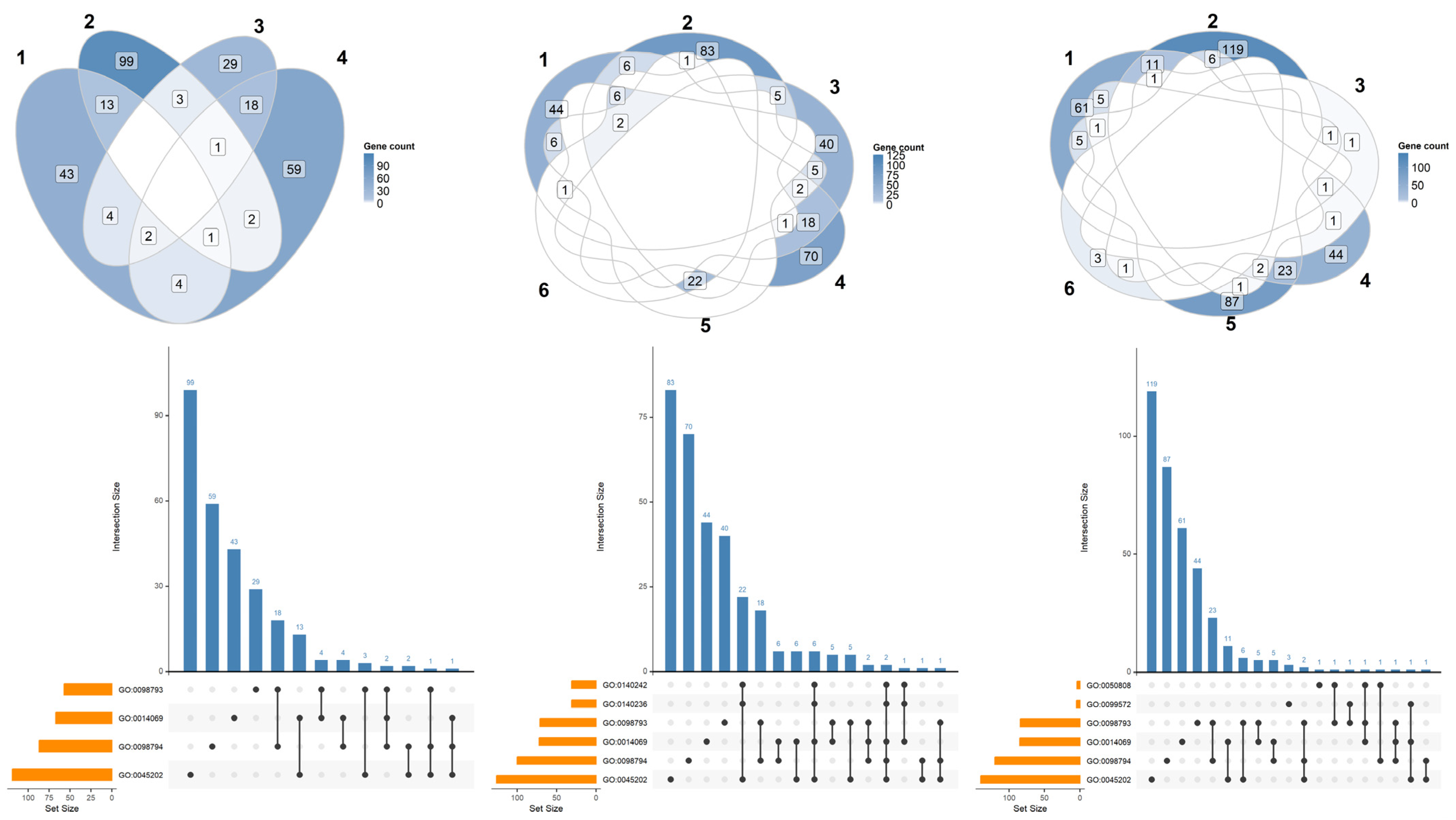
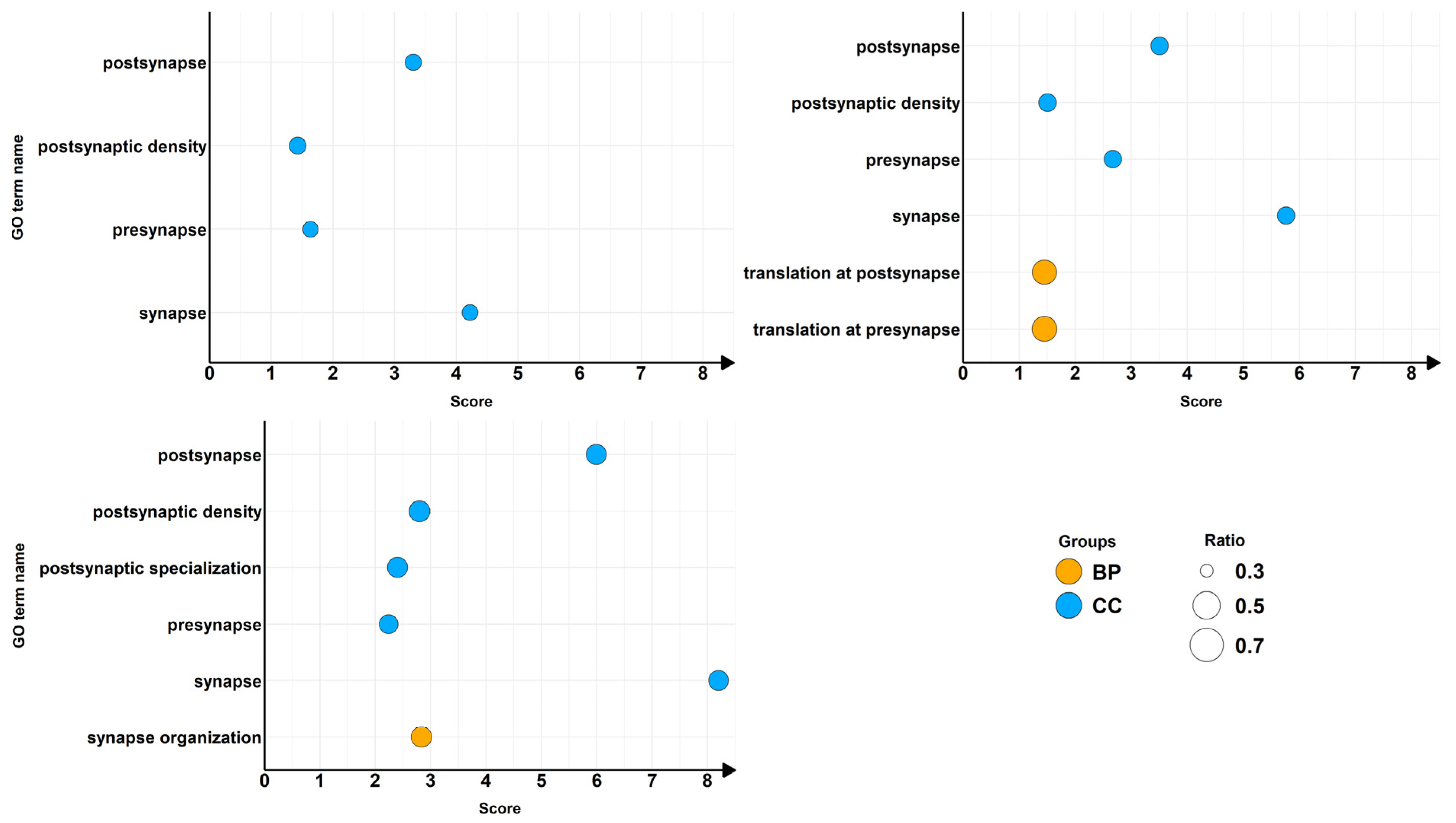
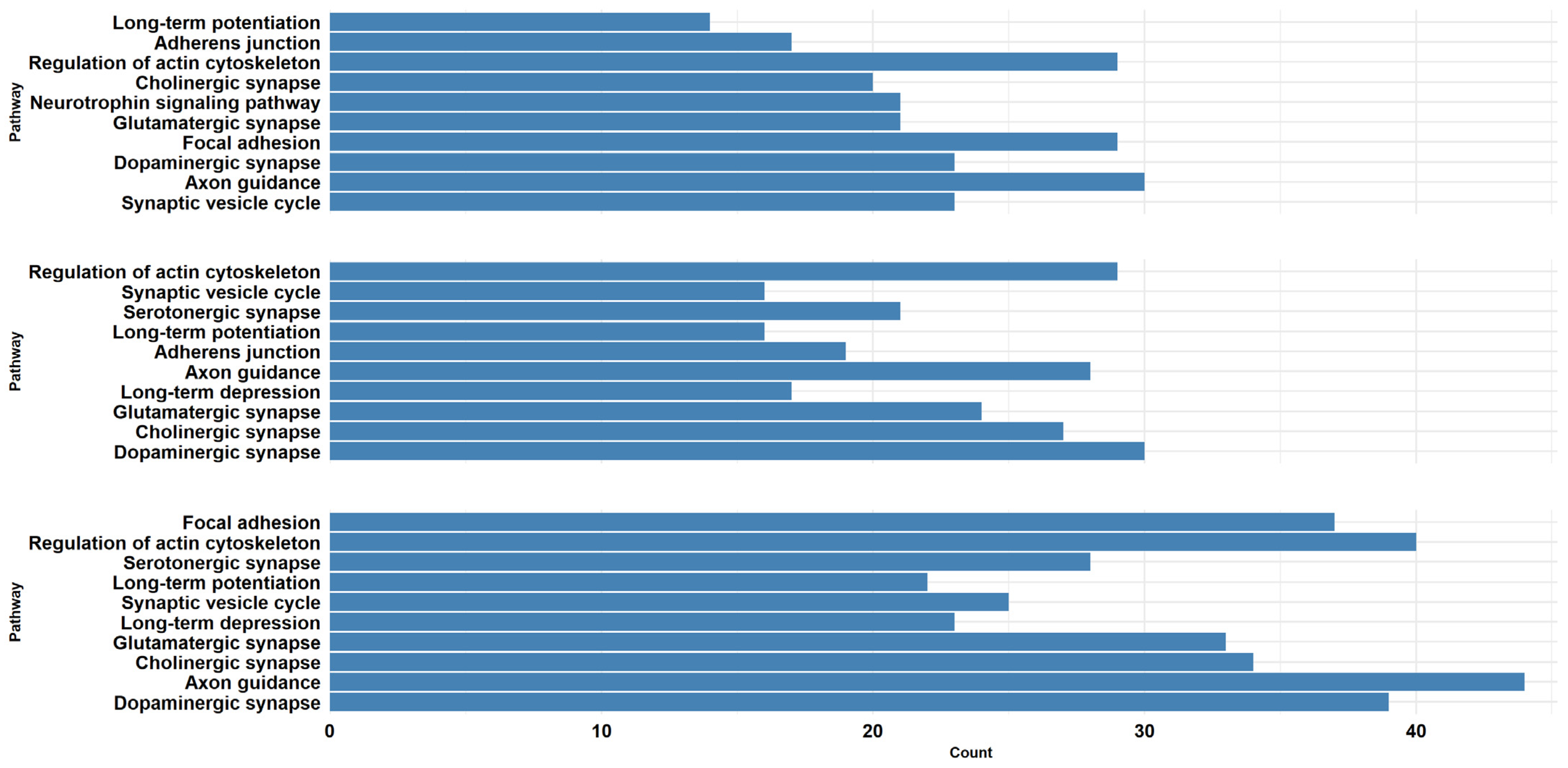
| GO Term ID | GO Domain | GO Term Name | GSEA Count Background | GSEA Count Input | Comparisons |
|---|---|---|---|---|---|
| GO:0045202 | CC | synapse | 1478 | 506 | CTRL vs. α-CD/MOR0.5-96 |
| 551 | CTRL vs. α-CD/MOR5-96 | ||||
| 639 | CTRL vs. α-CD/MOR10-96 | ||||
| GO:0098793 | CC | presynapse | 692 | 233 | CTRL vs. α-CD/MOR0.5-96 |
| 258 | CTRL vs. α-CD/MOR5-96 | ||||
| 282 | CTRL vs. α-CD/MOR10-96 | ||||
| GO:0098794 | CC | postsynapse | 911 | 321 | CTRL vs. α-CD/MOR0.5-96 |
| 341 | CTRL vs. α-CD/MOR5-96 | ||||
| 405 | CTRL vs. α-CD/MOR10-96 | ||||
| GO:0014069 | CC | postsynaptic density | 348 | 125 | CTRL vs. α-CD/MOR0.5-96 |
| 132 | CTRL vs. α-CD/MOR5-96 | ||||
| 162 | CTRL vs. α-CD/MOR10-96 | ||||
| GO:0140236 | BP | translation at presynapse | 51 | 31 | CTRL vs. α-CD/MOR5-96 |
| GO:0140242 | BP | translation at postsynapse | 56 | 33 | CTRL vs. α-CD/MOR5-96 |
| GO:0099572 | CC | postsynaptic specialization | 415 | 183 | CTRL vs. α-CD/MOR10-96 |
| GO:0050808 | BP | synapse organization | 424 | 195 | CTRL vs. α-CD/MOR10-96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, A.; Chiricosta, L.; Calì, G.; Rollin, P.; Perenzoni, D.; Iori, R.; Mazzon, E.; D’Angiolini, S. α-Cyclodextrin/Moringin Impacts Actin Cytoskeleton Dynamics with Potential Implications for Synaptic Organization: A Preliminary Transcriptomic Study in NSC-34 Motor Neurons. Int. J. Mol. Sci. 2025, 26, 8220. https://doi.org/10.3390/ijms26178220
Gugliandolo A, Chiricosta L, Calì G, Rollin P, Perenzoni D, Iori R, Mazzon E, D’Angiolini S. α-Cyclodextrin/Moringin Impacts Actin Cytoskeleton Dynamics with Potential Implications for Synaptic Organization: A Preliminary Transcriptomic Study in NSC-34 Motor Neurons. International Journal of Molecular Sciences. 2025; 26(17):8220. https://doi.org/10.3390/ijms26178220
Chicago/Turabian StyleGugliandolo, Agnese, Luigi Chiricosta, Gabriella Calì, Patrick Rollin, Daniele Perenzoni, Renato Iori, Emanuela Mazzon, and Simone D’Angiolini. 2025. "α-Cyclodextrin/Moringin Impacts Actin Cytoskeleton Dynamics with Potential Implications for Synaptic Organization: A Preliminary Transcriptomic Study in NSC-34 Motor Neurons" International Journal of Molecular Sciences 26, no. 17: 8220. https://doi.org/10.3390/ijms26178220
APA StyleGugliandolo, A., Chiricosta, L., Calì, G., Rollin, P., Perenzoni, D., Iori, R., Mazzon, E., & D’Angiolini, S. (2025). α-Cyclodextrin/Moringin Impacts Actin Cytoskeleton Dynamics with Potential Implications for Synaptic Organization: A Preliminary Transcriptomic Study in NSC-34 Motor Neurons. International Journal of Molecular Sciences, 26(17), 8220. https://doi.org/10.3390/ijms26178220






