1. Introduction
Alzheimer’s disease (AD) and major depressive disorder (MDD) are prevalent neuro-psychiatric conditions that often present overlapping clinical features, particularly in cognitive decline and mood disturbances. Growing evidence suggests that this clinical overlap may reflect shared neurobiological mechanisms and a substantial, though complex, genetic relationship between the two disorders [
1]. This symptomatic convergence complicates accurate diagnosis and may lead to suboptimal treatment strategies. Therefore, identifying reliable biomarkers that can distinguish between AD and MDD is crucial for improving diagnostic precision and therapeutic outcomes. Recent bioinformatics analyses have highlighted several shared immune-related genes and pathways, underscoring the potential of integrative molecular approaches to differentiate these conditions [
2].
MicroRNAs (miRNAs), small non-coding RNA molecules approximately 22 nucleotides in length, have emerged as significant regulators of gene expression at the post-transcriptional level. They achieve this regulation mainly through translational repression and mRNA destabilization, impacting a wide range of biological processes [
3]. Importantly, miRNAs play crucial roles in neuronal development, synaptic plasticity, and neuroinflammatory pathways—mechanisms directly relevant to the pathophysiology of neurodegenerative and neuropsychiatric disorders such as AD and MDD. Their dysregulation can disrupt neural circuitry and cognitive processes, underscoring their potential as diagnostic biomarkers and therapeutic targets [
4,
5]. Altered miRNA expression profiles have been implicated in the progression of these disorders, suggesting their potential as diagnostic biomarkers [
6]. Specifically, circulating levels of miR-125b and miR-181c were significantly down-regulated in AD patients, highlighting their role in disease mechanisms. These findings support the notion that serum-based miRNA signatures could provide a cost-effective, rapid, and noninvasive approach for early diagnosis and monitoring of AD [
7].
In AD, specific miRNAs such as miR-132 and miR-212 are found to be dysregulated, affecting pathways related to amyloid-beta production and tau phosphorylation, which are hallmark features of the disease. Notably, downregulation of the miR-132/212 cluster occurs early in disease progression and shows a strong correlation with increased Tau pathology. These findings highlight the crucial role of this miRNA cluster in modulating neurodegenerative processes and its potential as a therapeutic target [
8]. Similarly, in MDD, miRNAs like miR-1202 and miR-124 have been associated with neuroplastic changes and stress response mechanisms. Notably, miR-1202 regulates the expression of the metabotropic glutamate receptor 4 (mGluR4), influencing glutamatergic transmission and predicting antidepressant response [
9]. Furthermore, miR-124 has been shown to modulate synaptic plasticity and the HPA axis, contributing to the regulation of neurogenesis and stress-induced neuronal remodeling in depression [
10]. Furthermore, neuroinflammatory pathways, which are increasingly recognized as shared pathophysiological mechanisms in both disorders, have been linked to miRNA-mediated regulation of cytokines and immune signaling molecules. For instance, miRNAs can modulate NF-κB and NLRP3 inflammasome activation, thereby influencing the production of pro-inflammatory cytokines such as IL-1β and TNF-α. These regulatory effects highlight the therapeutic potential of targeting miRNA-driven neuroinflammatory cascades in neurodegenerative and neuropsychiatric diseases [
11].
Recent studies suggest that depression may act as both a prodromal symptom and a risk factor for AD, further blurring the diagnostic boundaries between the two disorders. Notably, late-life depression has been shown to correlate with increased amyloid-beta deposition and tau pathology, which are hallmark features of AD [
12].
In patients with late-life depression (LLD), circulating levels of hsa-miR-184 were found to be significantly downregulated. Knockdown of miR-184 in
Drosophila melanogaster resulted in decreased locomotor activity and impaired memory performance, indicating a role in age-associated cognitive decline. These findings suggest that miR-184 dysregulation in LLD may contribute to molecular mechanisms relevant to AD pathology [
13].
Furthermore, in the prefrontal cortex of individuals with LLD, several differentially expressed microRNAs—such as miR-124-3p and miR-9-5p—were identified, both of which are involved in regulating synaptic function and neuronal development. These miRNAs were enriched in pathways related to Wnt signaling, neurogenesis, and synaptic plasticity, all of which are essential processes implicated in cognitive resilience and AD. The observed miRNA alterations may therefore reflect early neuropathological changes shared between LLD and AD [
14].
Moreover, other studies have identified several miRNAs as potential biomarkers linking LLD to neurodegenerative processes. These miRNAs modulate key molecular pathways, including neuroinflammation, neurotrophic signaling, and neuronal plasticity. Dysregulation of these pathways may increase vulnerability to cognitive decline and facilitate progression from LLD to mild cognitive impairment and ultimately to AD [
15].
Taken together, the convergence of dysregulated miRNAs in LLD on key biological processes—such as synaptic remodeling, hippocampal neurogenesis, and immune activation—supports a mechanistic overlap with early AD pathology [
13,
14,
15]. Rather than reflecting an isolated affective disorder, LLD may constitute a prodromal phase within the broader neurodegenerative continuum [
15]. miRNAs such as miR-124 and miR-184, due to their roles in regulating memory, synaptic integrity, and neuronal adaptation, may serve not only as biomarkers but also as active molecular mediators driving the progression from LLD to mild cognitive impairment and eventually to AD [
13,
14]. This perspective underscores the importance of miRNA-based profiling in LLD as a means of identifying individuals at elevated risk for subsequent neurodegenerative decline [
15].
Additionally, individuals with a history of depression have a significantly higher likelihood of developing AD, underscoring the need for early screening and integrated management approaches [
16]. This bidirectional relationship underscores the necessity of identifying molecular tools that can distinguish the early pathological processes unique to each condition. In this context, miRNAs emerge not only as passive biomarkers but also as active players in the neurodegenerative cascade, modulating key elements of neuronal integrity, neurogenesis, and synaptic remodeling. Recent findings highlight that dysregulated miRNAs can both reflect and drive disease progression through pathways affecting synaptic function and neuroinflammation [
17]. Moreover, novel structural insights into ion channel modulation suggest that targeting these processes may offer precise intervention strategies for halting neurodegenerative decline [
18].
Furthermore, miRNAs are present in a variety of biofluids, including serum, plasma, cerebrospinal fluid (CSF), and saliva, making them ideal candidates for non-invasive biomarker discovery. Their stability in circulation and specificity to cell type or disease state enhance their translational value for clinical application. Notably, endogenous plasma miRNAs remain stable even after multiple freeze–thaw cycles or prolonged room temperature incubation, demonstrating exceptional resilience [
19]. Additionally, cell-free miRNAs can be packaged within exosomes or bound to proteins such as Argonaute 2, which further protects them from degradation and supports their reliable detection in clinical samples [
20]. In addition to diagnosis, miRNAs are increasingly explored as potential therapeutic targets through miRNA mimics or inhibitors designed to restore aberrant expression patterns. Notably, recent strategies include the development of locked nucleic acid (LNA) anti-miRs and synthetic mimics that enhance stability and targeting specificity in vivo [
21]. Moreover, combination approaches employing miRNA-based therapeutics with conventional drugs have shown synergistic effects, highlighting their promise for integrated cancer therapy [
22].
Despite these advances, most current studies investigating miRNA roles in AD and MDD have focused on isolated conditions. Comparative analyses that explore differential expression patterns across both disorders in parallel are limited. Therefore, integrative bioinformatic approaches using publicly available datasets offer a promising strategy to uncover unique and shared molecular signatures with diagnostic relevance.
This study builds on this rationale by applying differential expression analysis and downstream bioinformatic enrichment to identify miRNAs with potential discriminative value between AD and MDD. The findings aim to provide a molecular framework for refining diagnostic accuracy, informing clinical decision-making, and ultimately guiding personalized medicine initiatives in neuropsychiatry.
3. Discussion
The present study investigated microRNA (miRNA) expression profiles in AD and MDD across both central (cortical tissue) and peripheral (serum, whole-blood) compartments using publicly available Gene Expression Omnibus datasets. We identified a panel of 10 overlapping miRNAs between the disorders, with particular emphasis on hsa-miR-24-3p and hsa-miR-1202, and performed functional enrichment and network analyses to explore their potential mechanistic roles. Below, our findings are interpreted in light of existing literature, highlighting concordances and discrepancies, possible biological underpinnings, and clinical implications.
3.1. Overall Interpretation of Differentially Expressed miRNAs in AD and MDD
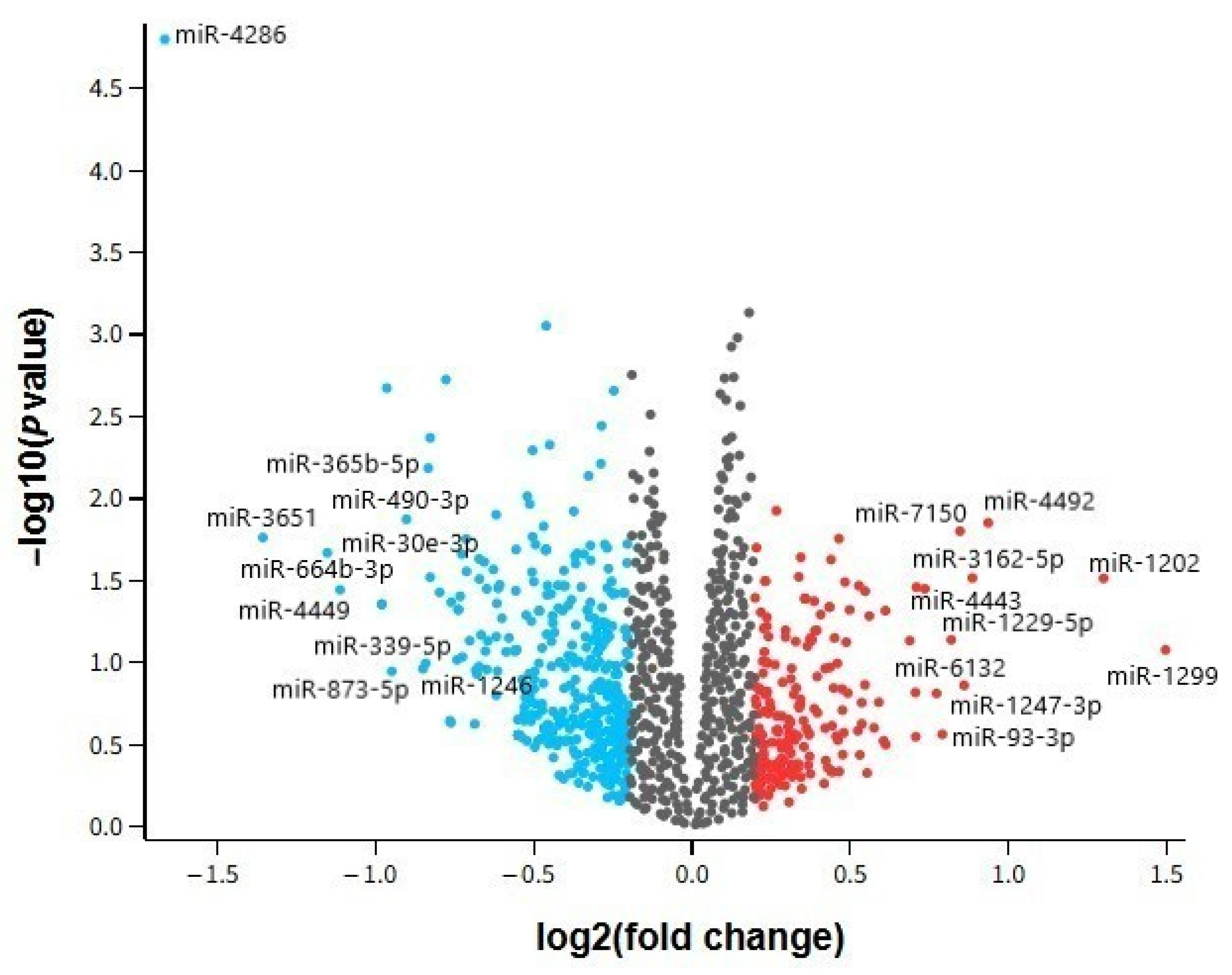
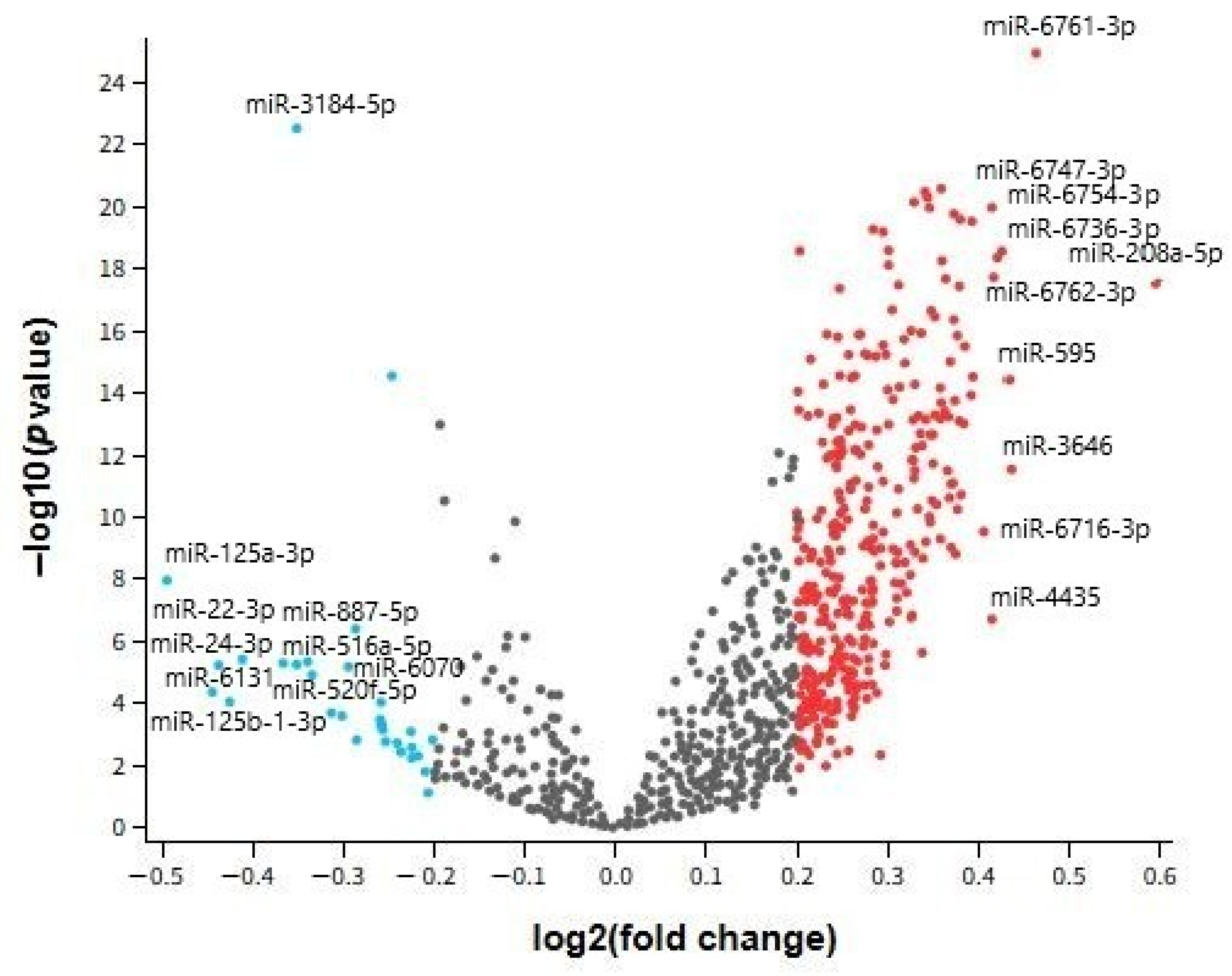
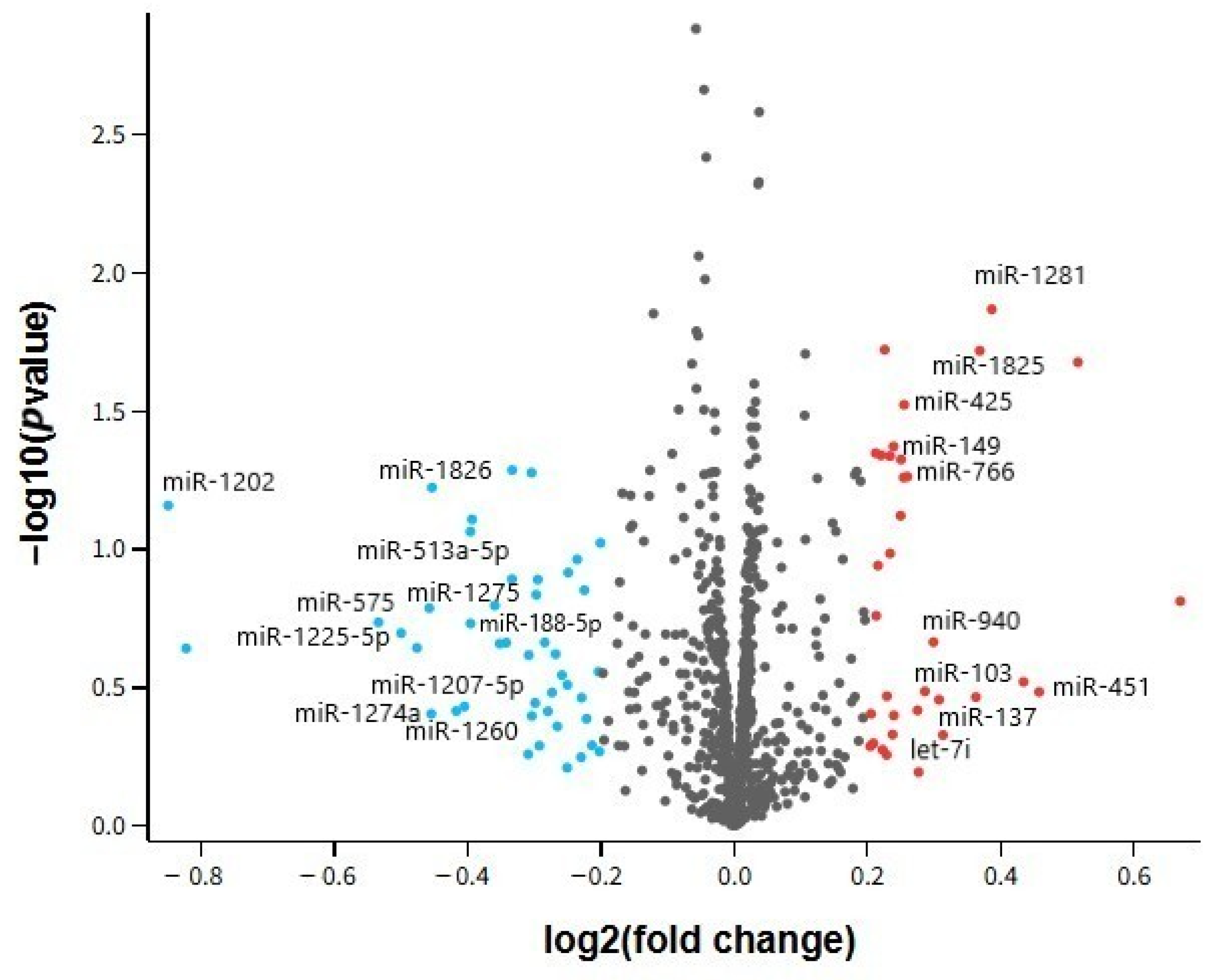
In our analysis, several miRNAs demonstrated dysregulation in both AD and MDD, suggesting shared molecular pathways despite their distinct clinical phenotypes. Notably, hsa-miR-24-3p exhibited consistent downregulation across all examined tissues, while hsa-miR-1202 displayed contrasting patterns—upregulated in AD brain tissue but downregulated in MDD cortex. Other miRNAs, such as hsa-miR-125a-3p and hsa-miR-664b-3p, were altered in multiple datasets but without uniform directionality.
These results are in agreement with studies reporting overlapping miRNA perturbations in neurodegenerative and psychiatric disorders [
37,
38]. For example, Lopez et al. [
9] identified altered cortical expression of miR-1202 in MDD, while Lau et al. [
8] demonstrated miRNA involvement in amyloid precursor protein processing in AD. However, unlike some previous reports that described uniform regulation of candidate miRNAs across biofluids and brain tissue, our findings revealed tissue-dependent variation, possibly reflecting compartment-specific transcriptional control or differential release mechanisms into circulation [
36].
Clinically, the convergence of certain miRNAs across disorders reinforces their potential as multi-disease biomarkers, but our results also caution against assuming systemic reflection of brain changes without tissue-specific validation [
39].
3.2. Tissue-Specific and Systemic miRNA Signatures
Our dataset comparison showed that hsa-miR-24-3p was robustly downregulated in both brain and peripheral compartments, suggesting a systemic disturbance potentially accessible for non-invasive biomarker development. In contrast, hsa-miR-1202 presented opposing regulation between AD and MDD brain samples, and several other miRNAs displayed inconsistent changes between peripheral and central samples.
This divergence aligns with reports that circulating miRNA profiles often only partially mirror CNS expression patterns [
40,
41]. Such differences can result from the selective packaging of miRNAs into exosomes, the influence of peripheral inflammation, or varying stability in biofluids [
42]. Our finding that miR-24-3p maintained consistent downregulation across compartments strengthens its candidacy for diagnostic purposes, while the discordant patterns observed for miR-1202 emphasize the necessity of context-specific interpretation.
From a biological perspective, these tissue-dependent differences may indicate that certain miRNAs serve specialized roles within the CNS that are not replicated peripherally, or that peripheral expression is modulated by systemic factors independent of brain pathology [
43,
44,
45].
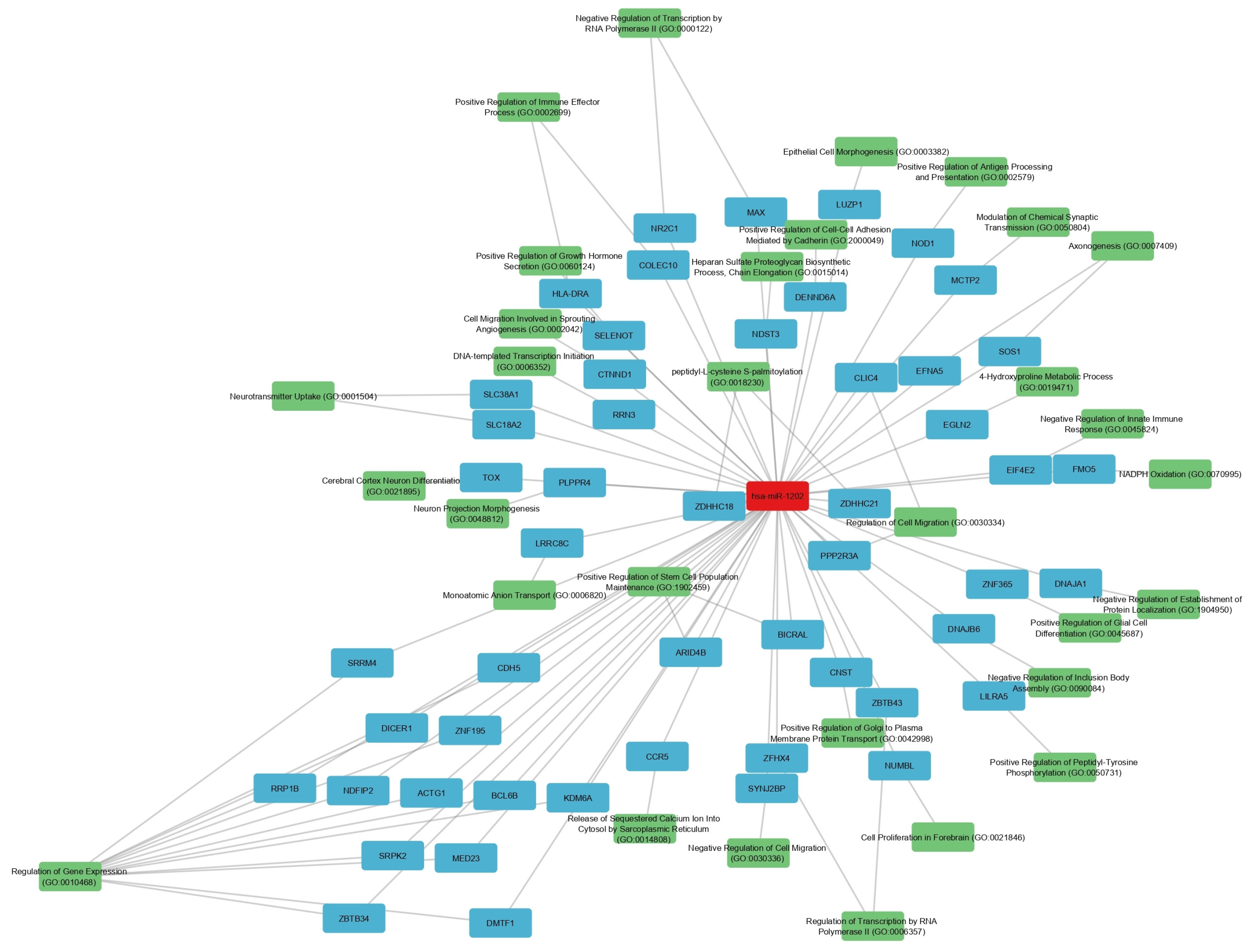
3.3. Biological Significance of Shared miRNAs: Focus on hsa-miR-24-3p and hsa-miR-1202
In our study, hsa-miR-24-3p emerged as the most consistent cross-disease marker, being downregulated in all datasets. Literature supports its role in modulating neuronal apoptosis, microglial activation, and endothelial function [
25,
26]. Our consistent finding across brain and blood is in line with Liu et al., who reported reduced serum miR-24-3p levels in AD patients [
46], and with Marchetti et al., who observed that inhibition of miR-24-3p in ischemic limb models led to dysfunctional vessel formation, supporting its role in vascular integrity [
47].
hsa-miR-1202, on the other hand, showed disease- and tissue-specific regulation. Its upregulation in AD cortex may represent a compensatory mechanism to sustain glutamatergic signaling amid synaptic degeneration, while its downregulation in MDD cortex could exacerbate glutamatergic hypoactivity, a known feature of depression [
9,
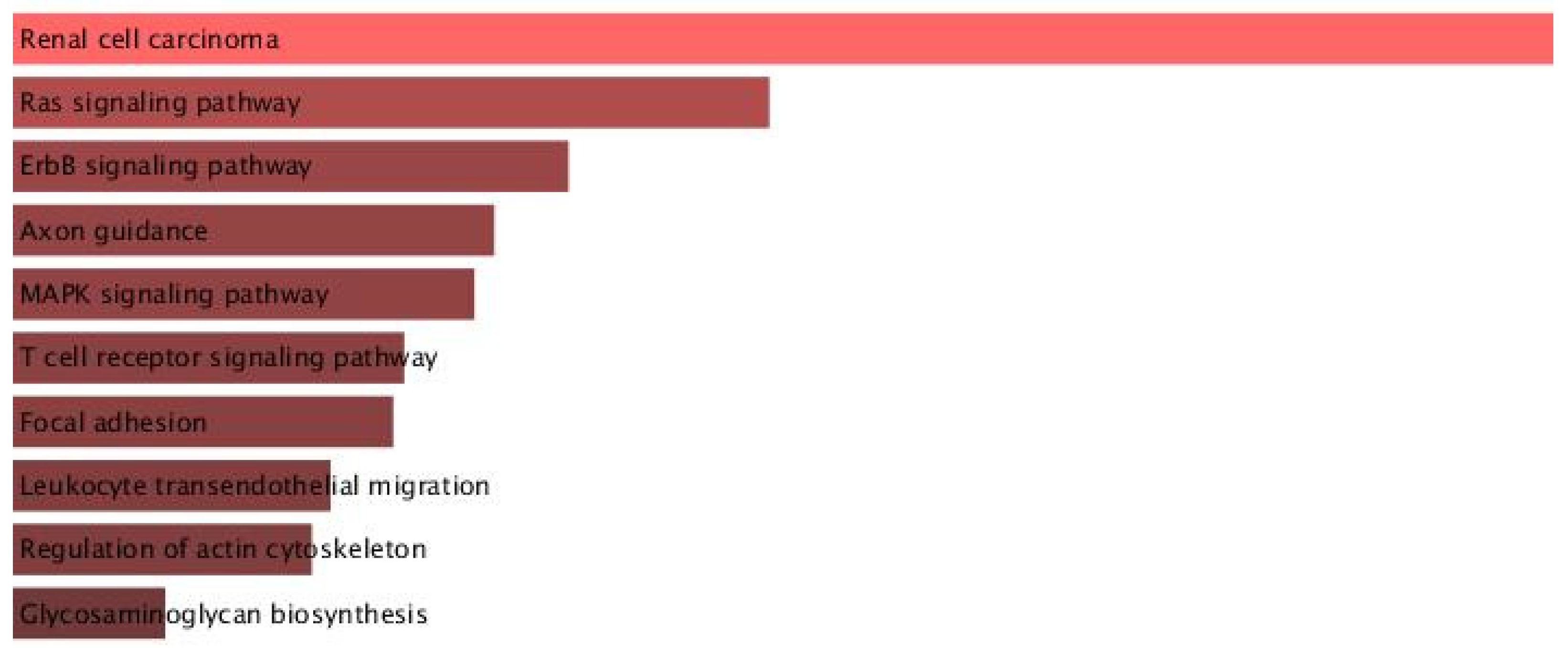
31]. Our network analysis linking miR-1202 to MAPK, Ras, and ErbB signaling pathways supports its involvement in both synaptic plasticity and inflammatory regulation [
48,
49,
50].
These results, when viewed alongside experimental data on antidepressant-induced normalization of miR-1202 [
5], highlight its potential as both a disease-specific and treatment-responsive biomarker.
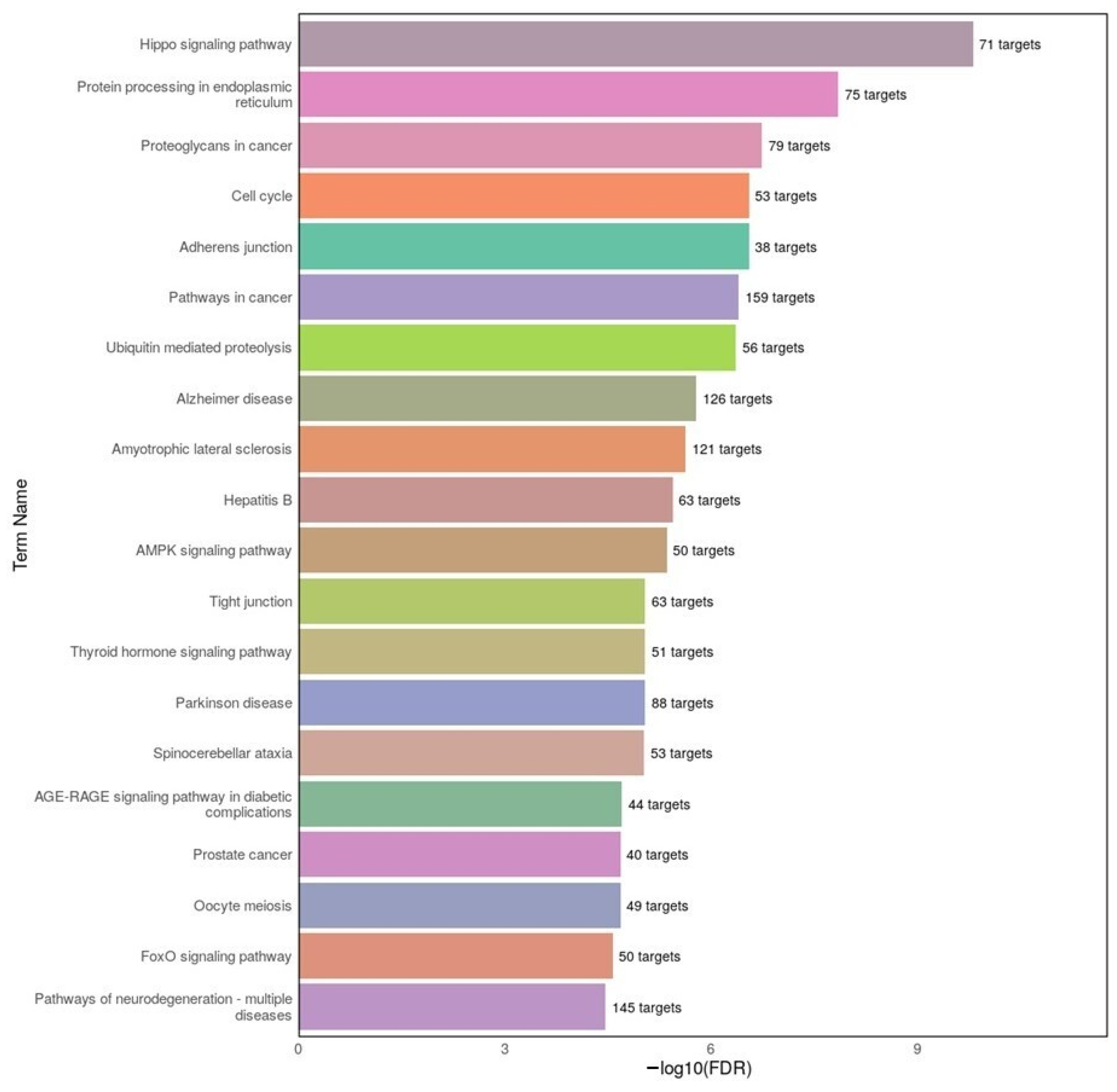
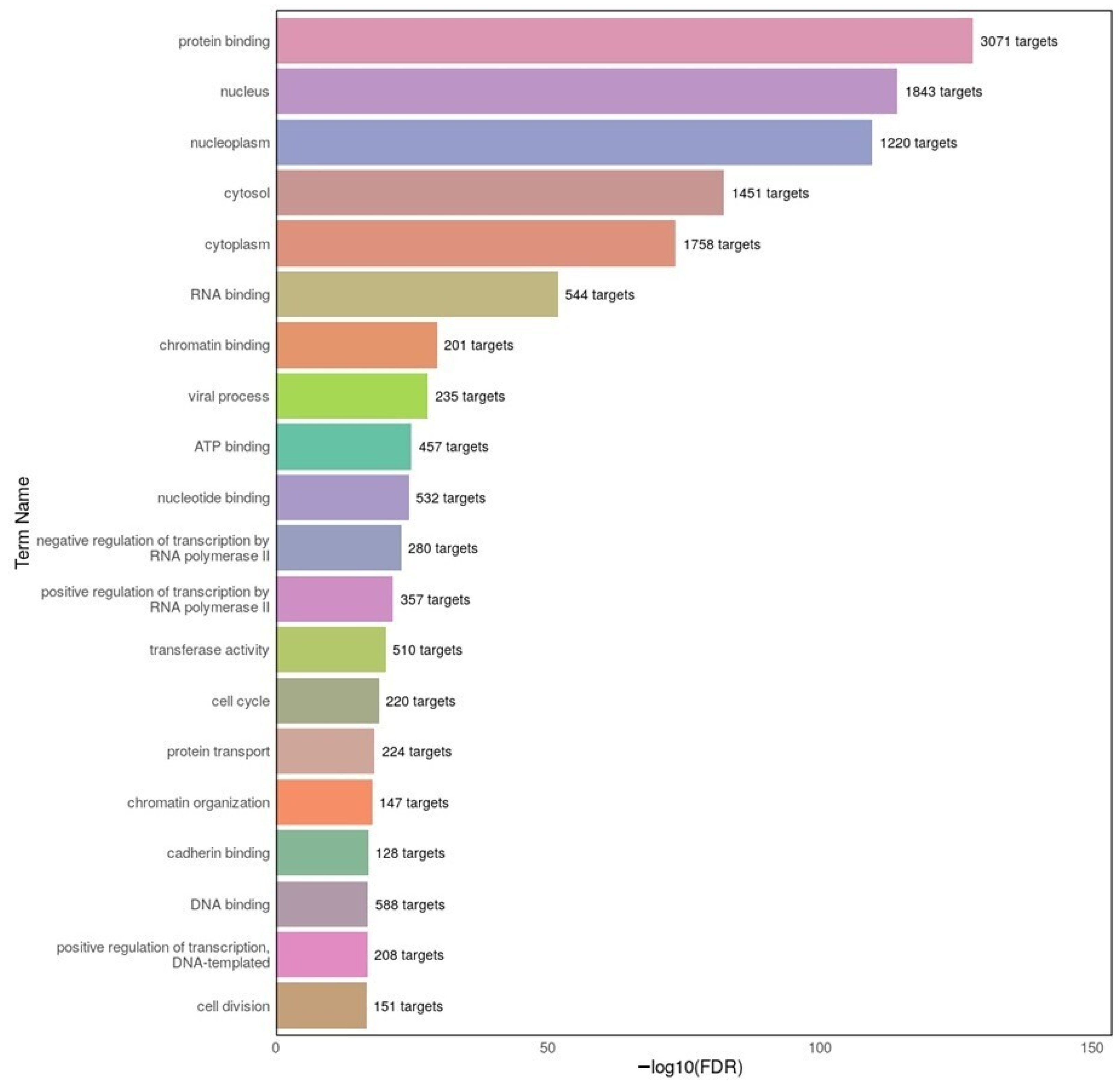
3.4. Functional Implications from GO and KEGG Enrichment Analyses
Our GO enrichment analysis revealed that dysregulated miRNAs in both AD and MDD target genes involved in protein binding, RNA binding, and nuclear localization. These categories suggest a strong influence on transcriptional regulation and post-transcriptional processing—echoing previous findings in the CNS context, such as the role of miR-124 in modulating alternative splicing and nuclear gene regulation in neuronal cells [
43], and the broader post-transcriptional regulatory functions of miRNAs in CNS trauma and degeneration [
51].
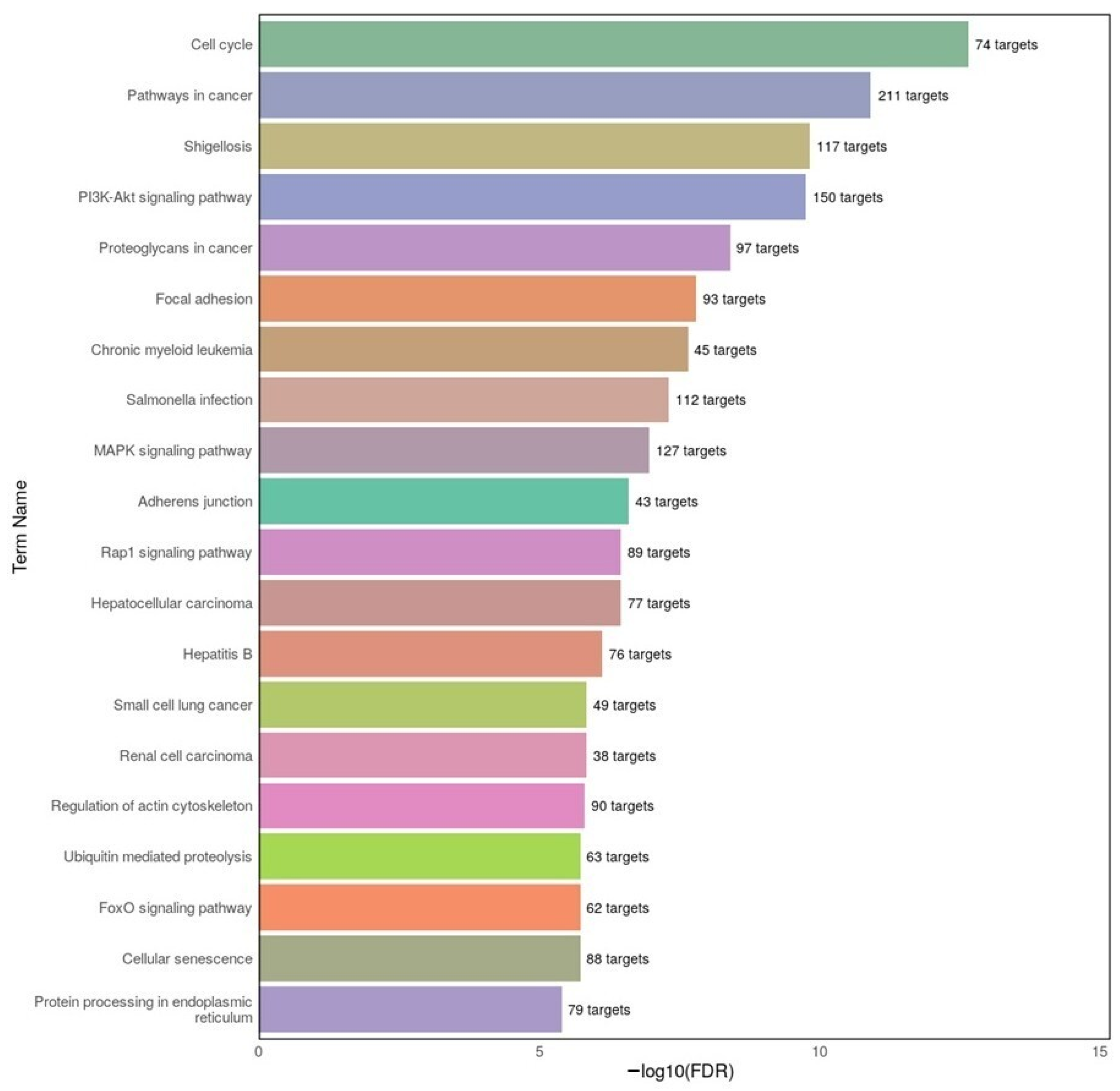
KEGG pathway analysis in our study showed significant overlap between disorders in cell cycle regulation, PI3K–Akt signaling, and focal adhesion. In AD datasets, we found enrichment of MAPK and PI3K–Akt signaling, consistent with prior studies linking these pathways to tau phosphorylation and neuroinflammation [
50,
52]. In MDD datasets, enrichment in Hippo signaling and ubiquitin-mediated proteolysis points toward altered neurogenesis and protein homeostasis, consistent with evidence that chronic stress activates the Hippo–YAP pathway in depression models [
53], and that dysregulation of ubiquitin–proteasome subunit expression is observed in the post-mortem brains of MDD patients [
54].
By directly linking our differentially expressed miRNAs to these pathways, our results not only validate earlier work but also extend it by identifying specific miRNA–pathway associations that may underlie shared and divergent aspects of AD and MDD.
3.5. Overlap Between Neurodegenerative and Psychiatric Pathways
Our KEGG enrichment results demonstrated that MDD-associated miRNAs also map to pathways typically associated with neurodegenerative diseases, including Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis. This finding aligns with epidemiological evidence that depression is both a risk factor for and a prodrome of dementia [
48,
55].
While previous studies have suggested shared mechanisms such as mitochondrial dysfunction and oxidative stress [
56,
57], our data provide a direct molecular link via miRNA regulatory networks. For example, the appearance of AD pathway enrichment in MDD miRNA targets reinforces the possibility of overlapping early pathogenic events [
58,
59].
Clinically, these overlaps underscore the potential for shared therapeutic targets and for miRNA-based tools to aid in early detection of patients at risk for transitioning from psychiatric to neurodegenerative conditions [
6,
15,
60].
3.6. Potential Clinical Applications: Biomarker and Therapeutic Prospects
Our identification of hsa-miR-24-3p as a consistently downregulated miRNA across tissues supports its candidacy as a non-invasive biomarker. Meanwhile, hsa-miR-1202’s regulation pattern and responsiveness to antidepressants make it attractive for monitoring treatment effects in MDD and potentially tracking disease progression in AD [
9,
61].
The use of circulating miRNAs as biomarkers is supported by their stability in biofluids and their detectability with standard molecular methods [
62]. Therapeutically, targeting these miRNAs with mimics or inhibitors could theoretically restore dysregulated pathways, though delivery challenges remain—particularly for CNS diseases where the blood–brain barrier presents a major obstacle [
63,
64,
65,
66].
3.7. Strengths and Limitations of the Present Study
A strength of our work is the integration of multi-tissue data from both AD and MDD, enabling direct comparison of central and peripheral miRNA signatures. Our combined approach of differential expression, functional enrichment, and network analysis provided mechanistic insights beyond mere list comparisons. However, our study is limited by several factors. Heterogeneity in microarray platforms and potential batch effects could influence comparability across datasets [
67].
Cross-dataset and cross-tissue comparisons in our study should be interpreted with caution. Public GEO cohorts differ in platform technology (e.g., probe content and miRBase annotation versions), sample sizes, demographic and clinical composition, tissue source (post-mortem cortex vs. whole blood/serum), RNA isolation and pre-analytical handling, and preprocessing pipelines. These factors—together with potential batch effects—can yield apparent discrepancies in the direction of change for the same miRNA across compartments [
35,
68]. Accordingly, our cross-condition summary prioritizes recurrence of candidates rather than assuming conserved regulation across tissues. To strengthen generalizability, future work should validate these in silico findings in well-matched, deeply phenotyped cohorts using harmonized protocols, include tissue-matched brain and biofluid specimens (e.g., EV-enriched plasma/CSF), and apply cross-platform normalization strategies [
44,
69]. Such designs will be essential to determine whether candidate miRNAs function as systemic biomarkers or reflect compartment-specific biology.
The cross-sectional nature of the data precludes conclusions about causality. Furthermore, our analysis was limited by the lack of access to detailed demographic and clinical information, such as sex, age, number of depressive episodes, and comorbidities, which precluded the assessment of potential moderating effects on miRNA expression patterns. Such variables are known to influence both the prevalence and molecular presentation of AD and MDD, and their absence may obscure important subgroup-specific associations [
70,
71]. In addition, the absence of peripheral biomarker data—particularly amyloid-beta concentrations in blood, serum, or brain tissue—restricted our ability to directly investigate mechanistic links between circulating or brain-expressed miRNAs and established pathological hallmarks. Inclusion of such data in future studies would enable more precise modeling of disease pathways and strengthen causal inference.
3.8. Future Research Directions
Future research should aim to validate the candidate miRNAs identified in this study—particularly hsa-miR-24-3p and hsa-miR-1202—in longitudinal cohorts to assess their predictive value for disease onset and progression. Integrating additional omics layers such as transcriptomics, proteomics, and metabolomics will provide a more comprehensive understanding of the molecular networks underlying both AD and MDD. Advanced approaches, including cell type–specific profiling and spatial transcriptomics, could help to map miRNA–mRNA interactions within distinct neural circuits, offering insights into cell-specific regulatory mechanisms. Furthermore, preclinical studies modulating the expression of these miRNAs will be crucial to evaluate their therapeutic potential and safety. Finally, combining miRNA biomarkers with neuroimaging, cognitive testing, and other clinical measures could lead to more accurate and disease-specific diagnostic tools, paving the way for precision medicine strategies in neuropsychiatric disorders.