Shaping Orthodontics of the Future: Concepts and Implications from a Cellular and Molecular Perspective
Abstract
1. Introduction
2. Methods
3. Orthodontic Tooth Movement: Cellular and Molecular Mechanisms, Clinical Implications, and Future Perspectives for Precision Therapy and Innovation
3.1. Clinical and Biological Framing of Orthodontic Tooth Movement (OTM)
3.2. The Models of OTM and Pilon’s Model of the 4 Phases
3.3. The Cellular and Molecular Processes Which Form the Basis of OTM
3.3.1. OTM-Related Periodontal Stress, Cell Death, Inflammation and PDL Hyalinization
3.3.2. The Phenomenon of Macrophage Polarization (MP) and Its Role in OTM-Induced OEARR
| Macrophage Type | Inducers | Secreted Mediators | Function in OTM | References |
|---|---|---|---|---|
| M1 | IFN-γ, GM-CSF, LPS | IL-1β, TNF-α, CXCL10/11 | Pro-inflammatory; promotes OEARR | [45,52,53] |
| M2 | IL-4, IL-10, M-CSF | IL-10, IL-1RA, CCL17/22 | Anti-inflammatory; tissue repair | [45,53] |
| IL-34 | Compressive force induced | Stimulates odontoclast formation | Linked to cementum resorption | [46,54] |
3.3.3. The RANK RANKL OPG System Orchestrates OTM-Related Bone and Root Resorption
3.3.4. Summary of the Cellular and Molecular Basis of OTM
3.4. Approaches for Shaping Orthodontics of the Future Concerning Gene Polymorphisms as Well as Concepts Based on Cells, Biomaterials and Molecules and Small Molecules, Respectively
3.4.1. The Role of Gene Polymorphisms in Inflammation per Se and Inflammation-Related OEARR
3.4.2. Conclusion on Gene Polymorphisms and Future Perspectives in the Context of Orthodontic Treatment and OTM
3.4.3. Strategies for PDL Regeneration as a Result of Hyalinization
3.4.4. Summary Highlighting Key Strategies to Overcome Hyalinization
3.4.5. Biomaterial Concepts for Spatio-Temporally Directed Release of RANKL and Osteoclasts from Inducible Pluripotent Stem Cells (iPCs) via Injectable Hydrogels
3.4.6. Bone Remodeling, Osteoclastogenesis, Root Resorption and Its Prevention in OTM
3.4.7. PROTACs: Targeted Protein Degradation for Controlling OTM
3.4.8. Biologics: Infliximab and Caspase-1 Inhibition
3.4.9. Topical Administration of JAK Inhibitors and Further Approaches in Controlling Inflammation During OTM
3.4.10. Semaphorin 3A: A Dual Regulator of Osteoblast Maturation and Osteoclast Inhibition in OTM
| Agent | Target | Effect | Delivery Mode | References |
|---|---|---|---|---|
| Infliximab | TNF-α | Reduces RANKL, suppresses NF-κB | Systemic/injectable | [115,116] |
| Anakinra | IL-1R | Blocks IL-1β signaling | Local/systemic | [122,123] |
| Tofacitinib | JAK1/3 | Inhibits STAT phosphorylation | Topical/gel | [119,120] |
| Denosumab | RANKL | Prevents osteoclast maturation | Injectable | [127] |
| IL-10 | Anti-inflammatory cytokine | Inhibits NFATc1, reduces TRAP/cathepsin K | Hydrogel/nanoparticle | [51,124,125] |
3.4.11. The Role of Artificial Intelligence (AI) Exemplified by Cancer Research and OTM
3.4.12. Summary of the Section 3.4.5, Section 3.4.6, Section 3.4.7, Section 3.4.8, Section 3.4.9, Section 3.4.10 and Section 3.4.11
3.4.13. Open Research Questions in the Future of Orthodontic Treatment
- Cellular Therapies and Gene Polymorphisms
- Biomaterials, Hydrogels, and MMP Inhibitors
- Molecular Therapies and Small Molecules
- Artificial Intelligence and Personalized Treatment
- Interdisciplinary Collaboration and Integration
4. Integrating Cellular, Molecular, and Digital Innovation to Shape the Future of Orthodontic Treatment
Clinical Translation: Opportunities, Limitations, and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| AKT | Protein Kinase B |
| ALP | Alkaline Phosphatase |
| AP-1 | Activator Protein 1 |
| ATP | Adenosine Triphosphate |
| Bcl6 | B-cell Lymphoma 6 |
| BMP2 | Bone Morphogenetic Protein 2 |
| CCL17 | Chemokine (C-C Motif) Ligand 17 |
| CCL22 | Chemokine (C-C Motif) Ligand 22 |
| CD36 | Cluster of Differentiation 36 |
| CD80 | Cluster of Differentiation 80 |
| CD86 | Cluster of Differentiation 86 |
| CD200R | Cluster of Differentiation 200 Receptor |
| CD206 | Cluster of Differentiation 206 |
| COL1 | Collagen Type I |
| COL2 | Collagen Type II |
| COL3 | Collagen Type III |
| COL4 | Collagen Type IV |
| COL5 | Collagen Type V |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCL11 | C-X-C Motif Chemokine Ligand 11 |
| CYLD | Cylindromatosis |
| ECM | Extracellular Matrix |
| ERK | Extracellular Signal-Regulated Kinase |
| FAK | Focal Adhesion Kinase |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| HSP | Heat Shock Protein |
| IKK | IκB Kinase |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-23 | Interleukin-23 |
| IL-34 | Interleukin-34 |
| JAK | Janus Kinase |
| LPS | Lipopolysaccharide |
| MHCII | Major Histocompatibility Complex II |
| miR-125a | MicroRNA-125a |
| MMP-1 | Matrix Metalloproteinase 1 |
| MMP-8 | Matrix Metalloproteinase 8 |
| MMP-9 | Matrix Metalloproteinase 9 |
| MMP-13 | Matrix Metalloproteinase 13 |
| mTOR | Mechanistic Target of Rapamycin |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NF-κB | Nuclear Factor Kappa B |
| NFATc1 | Nuclear Factor of Activated T-Cells 1 |
| NEMO | NF-κB Essential Modulator |
| OSX | Osterix |
| p38 | p38 Mitogen-Activated Protein Kinase |
| PDL | Periodontal Ligament |
| PROTAC | Proteolysis-Targeting Chimera |
| Rac1 | Ras-Related C3 Botulinum Toxin Substrate 1 |
| RANK | Receptor Activator of NF-κB |
| RANKL | Receptor Activator of NF-κB Ligand |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SEMA3A | Semaphorin 3A |
| SOST | Sclerostin |
| TGF-β | Transforming Growth Factor Beta |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRAF6 | TNF Receptor-Associated Factor 6 |
| TRAP | Tartrate-Resistant Acid Phosphatase |
| VEGF | Vascular Endothelial Growth Factor |
References
- Swanson, W.B.; Yao, Y.; Mishina, Y. Novel approaches for periodontal tissue engineering. Genesis 2022, 60, e23499. [Google Scholar] [CrossRef]
- Handoko, H.; Yohana, N. Speech production and malocclusion: A review. J. Arbitrer 2023, 10, 107–115. [Google Scholar] [CrossRef]
- Zanon, G.; Contardo, L.; Reda, B. The impact of orthodontic treatment on masticatory performance: A literature review. Cureus 2022, 14, e30453. [Google Scholar] [CrossRef]
- Maltha, J.C.; Krishnan, V.; Kuijpers-Jagtman, A.M. Cellular and molecular biology of orthodontic tooth movement. Biol. Mech. Tooth Mov. 2021, 33–48. [Google Scholar]
- Tsolakis, I.A.; Christopoulou, I.; Sitaras, S.; Lyros, I.; Rontogianni, A.; Dalampira, M.; Tsolakis, A.I. Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review. Bioengineering 2023, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Nile, M.; Folwaczny, M.; Wichelhaus, A.; Baumert, U.; Janjic Rankovic, M. Fluid flow shear stress and tissue remodeling—An orthodontic perspective: Evidence synthesis and differential gene expression network analysis. Front. Bioeng. Biotechnol. 2023, 11, 1256825. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.; Röder, K. Chemical bonds in collagen rupture selectively under tensile stress. Phys. Chem. Chem. Phys. 2023, 25, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, T.; Lu, W.; Yi, X.; Zhao, Z.; Liu, J. Proteomic analysis of human periodontal ligament cells under hypoxia. Proteome Sci. 2019, 17, 3. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Lin, F.; Zheng, Q.; Xu, X.; Mei, L. Dynamic study into autophagy and apoptosis during orthodontic tooth movement. Exp. Ther. Med. 2021, 21, 430. [Google Scholar] [CrossRef]
- Yong, J.; von Bremen, J.; Groeger, S.; Ruiz-Heiland, G.; Ruf, S. Hypoxia-inducible factor 1-alpha acts as a bridge factor for crosstalk between ERK1/2 and caspases in hypoxia-induced apoptosis of cementoblasts. J. Cell. Mol. Med. 2021, 25, 9710–9723. [Google Scholar] [CrossRef]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef]
- Schröder, A.; Käppler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of compressive and tensile strain on macrophages during simulated orthodontic tooth movement. Mediat. Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Mah, S.-J.; Lee, Y.; Chun, Y.-S.; Lim, W.H. Expression of MMP-9 and-13 on the Pressure Side under Orthodontic Loading. Open J. Stomatol. 2014, 4, 412–417. [Google Scholar] [CrossRef]
- Rosenblum, G.; Van den Steen, P.E.; Cohen, S.R.; Bitler, A.; Brand, D.D.; Opdenakker, G.; Sagi, I. Direct visualization of protease action on collagen triple helical structure. PLoS ONE 2010, 5, e11043. [Google Scholar] [CrossRef]
- Behm, C.; Zhao, Z.; Andrukhov, O. Immunomodulatory activities of periodontal ligament stem cells in orthodontic forces-induced inflammatory processes: Current views and future perspectives. Front. Oral. Health 2022, 3, 877348. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.; Sathe, A.A.; Jin, Z.; Guan, J.; Sun, W.; Xing, C.; Zhang, H.; Yan, B. CCR2+ Macrophages promote orthodontic tooth movement and alveolar bone remodeling. Front. Immunol. 2022, 13, 835986. [Google Scholar] [CrossRef] [PubMed]
- Klein, Y.; David, E.; Pinto, N.; Khoury, Y.; Barenholz, Y.; Chaushu, S. Breaking a dogma: Orthodontic tooth movement alters systemic immunity. Prog. Orthod. 2024, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-L.; Wang, W.; Ma, W.; Guo, S.; Shen, B.-G.; Liu, H.-X. A novel Nb–TiNb nanocomposite with single-phase BCC structure for bio-implant applications. Rare Met. 2025, 44, 543–550. [Google Scholar] [CrossRef]
- Zwiri, A.; Alam, M.K.; Hajeer, M.Y.; Alserhani, E.D.M.; Alanazi, D.A.; Alanazi, J.M.; Noor, N.; Islam, Z. Exploring the Role of Exosomes in Accelerating Orthodontic Tooth Movement. J. Pharm. Bioallied Sci. 2025, 17, S1267–S1269. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Peng, C.; Du, Y.; Li, Q.; Yang, K. Effect of autophagy on aging-related changes in orthodontic tooth movement in rats. BMC Oral. Health 2024, 24, 785. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R.; Fields, H.; Larson, B.; Sarver, D.M. Contemporary Orthodontics-E-Book: Contemporary Orthodontics-E-Book; Elsevier Health Sciences: St. Louis, MO, USA, 2018. [Google Scholar]
- Burstone, C.J. The Biomechanics of Tooth Movement. In Vistas in Orthodontics; Kraus, B.S., Riedel, R.A., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1962; pp. 197–213. [Google Scholar]
- Kumar, A.A.; Saravanan, K.; Kohila, K.; Kumar, S.S. Biomarkers in orthodontic tooth movement. J. Pharm. Bioallied Sci. 2015, 7, S325–S330. [Google Scholar] [CrossRef] [PubMed]
- Pilon, J.J.; Kuijpers-Jagtman, A.M.; Maltha, J.C. Magnitude of orthodontic forces and rate of bodily tooth movement. An experimental study. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Maltha, J.C.; Kuijpers-Jagtman, A.M. Mechanobiology of orthodontic tooth movement: An update. J. World Fed. Orthod. 2023, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Rajpar, I.; Kumar, G.; Fortina, P.; Tomlinson, R.E. Toll-like receptor 4 signaling in osteoblasts is required for load-induced bone formation in mice. bioRxiv 2022, 2022.2008.2005.502963. [Google Scholar] [CrossRef]
- Marciniak, J.; Lossdörfer, S.; Knaup, I.; Bastian, A.; Craveiro, R.B.; Jäger, A.; Wolf, M. Orthodontic cell stress modifies proinflammatory cytokine expression in human PDL cells and induces immunomodulatory effects via TLR-4 signaling in vitro. Clin. Oral. Investig. 2020, 24, 1411–1419. [Google Scholar] [CrossRef]
- Roth, C.E.; Craveiro, R.B.; Niederau, C.; Malyaran, H.; Neuss, S.; Jankowski, J.; Wolf, M. Mechanical compression by simulating orthodontic tooth movement in an in vitro model modulates phosphorylation of AKT and MAPKs via TLR4 in human periodontal ligament cells. Int. J. Mol. Sci. 2022, 23, 8062. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.G.; Raghunath, N.; Kiran, H. RANK-RANKL-OPG: A current trends in orthodontic tooth movement and its role in accelerated orthodontics. Int. J. Appl. Dent. Sci. 2022, 8, 630–635. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar] [CrossRef]
- Vijayaraj, S.L.; Feltham, R.; Rashidi, M.; Frank, D.; Liu, Z.; Simpson, D.S.; Ebert, G.; Vince, A.; Herold, M.J.; Kueh, A. The ubiquitylation of IL-1β limits its cleavage by caspase-1 and targets it for proteasomal degradation. Nat. Commun. 2021, 12, 2713. [Google Scholar] [CrossRef]
- Zhen, H.; Hu, Y.; Liu, X.; Fan, G.; Zhao, S. The protease caspase-1: Activation pathways and functions. Biochem. Biophys. Res. Commun. 2024, 717, 149978. [Google Scholar] [CrossRef]
- Baidya, S.K.; Banerjee, S.; Guti, S.; Jha, T.; Adhikari, N. Matrix metalloproteinase-8 (MMP-8) and its inhibitors: A minireview. Eur. J. Med. Chem. Rep. 2024, 10, 100130. [Google Scholar] [CrossRef]
- Inanc, S.; Keles, D.; Oktay, G. An Improved Collagen Zymography Approach for Evaluating the Collagenases MMP-1, MMP-8, and MMP-13. BioTechniques 2017, 63, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Páez, J.; Rodríguez-Cavallo, E.; Díaz-Caballero, A.; Méndez-Cuadro, D. Quantification of matrix metalloproteinases MMP-8 and MMP-9 in gingival overgrowth. Saudi Dent. J. 2021, 33, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Moscalu, M.; Goriuc, A.; Nucci, L.; Tatarciuc, M.; Martu, I.; Covasa, M. Using salivary MMP-9 to successfully quantify periodontal inflammation during orthodontic treatment. J. Clin. Med. 2021, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Charzewski, Ł.; Krzyśko, K.A.; Lesyng, B. Structural characterisation of inhibitory and non-inhibitory MMP-9–TIMP-1 complexes and implications for regulatory mechanisms of MMP-9. Sci. Rep. 2021, 11, 13376. [Google Scholar] [CrossRef]
- Mohan, M.J.; Seaton, T.; Mitchell, J.; Howe, A.; Blackburn, K.; Burkhart, W.; Moyer, M.; Patel, I.; Waitt, G.M.; Becherer, J.D. The Τumor necrosis factor-α converting enzyme (TACE): A unique metalloproteinase with highly defined substrate selectivity. Biochemistry 2002, 41, 9462–9469. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Wilson, J.; Rock, P.; Chapple, I. Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic tooth movement. Eur. J. Orthod. 2013, 35, 644–651. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs expression levels in the periodontal ligament during orthodontic tooth movement: A systematic review of in vitro and in vivo studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Chen, G.; Shen, Y.; Huang, X.; Peng, J.; Wang, J.; Yin, Y.; Song, W.; Xie, M. The osteoclastic activity in apical distal region of molar mesial roots affects orthodontic tooth movement and root resorption in rats. Int. J. Oral. Sci. 2024, 16, 19. [Google Scholar] [CrossRef]
- He, D.; Kou, X.; Luo, Q.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; Zeng, M.; Gan, Y. Enhanced M1/M2 macrophage ratio promotes orthodontic root resorption. J. Dent. Res. 2015, 94, 129–139. [Google Scholar] [CrossRef]
- Kohno, R.; Yamaguchi, M.; Hikida, T.; Kikuta, J.; Shimizu, M.; Takahashi-Hikida, M.; Murakami, Y.; Kasai, K. Expression of IL-34 in Root Resorption by Excessive Orthodontic Force. Int. J. Oral-Med. Sci. 2017, 16, 8–15. [Google Scholar] [CrossRef]
- Du, M.; Wang, Y.; Liu, Z.; Wang, L.; Cao, Z.; Zhang, C.; Hao, Y.; He, H. Effects of IL-1β on MMP-9 Expression in Cementoblast-Derived Cell Line and MMP-Mediated Degradation of Type I Collagen. Inflammation 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed]
- de Ávila Andrade, A.L.C.; de Almeida Pinto, Y.D.; Maia, B.E.B.; Corrêa, J.D.; de Azevedo Miranda, D.; Manzi, F.R.; de Abreu Lima, I.L. Genetic polymorphisms in external apical root resorption and orthodontic tooth movements: A systematic review. Korean J. Orthod. 2024, 54, 284–302. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Lavado, N.; Nogueira, L.; Lopez, M.; Abreu, J.; Silva, H. Polymorphisms of genes encoding P 2 X 7R, IL-1 B, OPG and RANK in orthodontic-induced apical root resorption. Oral. Dis. 2014, 20, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-Y.; Chen, Z.-H.; Fu, Y.-F.; Li, Y.-Y.; Chen, M.-N.; Wu, J.-J.; Yuan, Z.-D.; Ye, J.-X.; Li, X.; Yuan, F.-L. Cilengitide inhibits osteoclast adhesion through blocking the αvβ3-mediated FAK/Src signaling pathway. Heliyon 2023, 9, e17841, Erratum in Heliyon 2024, 10, e22629. [Google Scholar] [CrossRef]
- de la Rica, L.; García-Gómez, A.; Comet, N.R.; Rodríguez-Ubreva, J.; Ciudad, L.; Vento-Tormo, R.; Company, C.; Álvarez-Errico, D.; García, M.; Gómez-Vaquero, C. NF-κB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol. 2015, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.; Yoo, J.; Kim, J.J.; Lee, J.B.; Choi, W.; Park, C.G.; Seo, J.-Y. Viperin deficiency promotes polarization of macrophages and secretion of M1 and M2 cytokines. Immune Netw. 2018, 18. [Google Scholar] [CrossRef]
- Lelios, I.; Cansever, D.; Utz, S.G.; Mildenberger, W.; Stifter, S.A.; Greter, M. Emerging roles of IL-34 in health and disease. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Nishida, D.; Arai, A.; Zhao, L. RANKL/OPG ratio regulates odontoclastogenesis in damaged dental pulp. Scientif. Rep. 2021, 11, 4575. [Google Scholar] [CrossRef]
- AlQranei, M.S.; Aljohani, H.; Majumdar, S.; Senbanjo, L.T.; Chellaiah, M.A. C-phycocyanin attenuates RANKL-induced osteoclastogenesis and bone resorption in vitro through inhibiting ROS levels, NFATc1 and NF-κB activation. Sci. Rep. 2020, 10, 2513. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Park-Min, K.H.; Lee, E.Y.; Moskowitz, N.K.; Lim, E.; Lee, S.K.; Lorenzo, J.A.; Huang, C.; Melnick, A.M.; Purdue, P.E.; Goldring, S.R. Negative regulation of osteoclast precursor differentiation by CD11b and β 2 integrin-B-cell lymphoma 6 signaling. J. Bone Miner. Res. 2013, 28, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Omata, Y.; Tachibana, H.; Aizaki, Y.; Mimura, T.; Sato, K. Essentiality of Nfatc1 short isoform in osteoclast differentiation and its self-regulation. Sci. Rep. 2023, 13, 18797. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Wada, S.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S. Compression and tension variably alter Osteoprotegerin expression via miR-3198 in periodontal ligament cells. BMC Mol. Cell Biol. 2019, 20, 6. [Google Scholar] [CrossRef]
- Lane, D.; Matte, I.; Rancourt, C.; Piché, A. Osteoprotegerin (OPG) protects ovarian cancer cells from TRAIL-induced apoptosis but does not contribute to malignant ascites-mediated attenuation of TRAIL-induced apoptosis. J. Ovarian Res. 2012, 5, 34. [Google Scholar] [CrossRef]
- Loreto, C.; Musumeci, G.; Castorina, S.; Valentino, J.; Giunta, S.; Leonardi, R. TRAIL Immunolocalisation in the Rat Periodontal Ligament during Experimental Tooth Movement. A Preliminary Study. Open J. Apoptosis 2013, 2, 31–36. [Google Scholar] [CrossRef]
- Amaro, E.R.; Ortiz, F.R.; Dorneles, L.S.; de Souza Santos, M.; Barrioni, B.R.; Miranda, R.M.; Garlet, G.P.; Teixeira, M.M.; Szawka, R.E.; Silva, T.A. Estrogen protects dental roots from orthodontic-induced inflammatory resorption. Arch. Oral. Biol. 2020, 117, 104820. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Optimum force magnitude for orthodontic tooth movement: A systematic literature review. Angle Orthod. 2003, 73, 86–92. [Google Scholar] [PubMed]
- Jamali, S.; Khosravi, S.; Shadmanpour, M.; Gharibpour, F.; Payahoo, S.; Darvish, M. Hyalinization and Molecular Pathways Involved in Orthodontic Tooth Movement: A Systematic Review and Meta-Analysis. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2020, 20, e5408. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, J.; Weinkamer, R.; Darendeliler, M.A.; Swain, M.V.; Sue, A.; Zheng, K.; Li, Q. In vivo effects of different orthodontic loading on root resorption and correlation with mechanobiological stimulus in periodontal ligament. J. R. Soc. Interface 2019, 16, 20190108. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fukasawa, S. Is inflammation a friend or foe for orthodontic treatment?: Inflammation in orthodontically induced inflammatory root resorption and accelerating tooth movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.-C. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J. Med. Sci. 2018, 34, 207–214. [Google Scholar] [CrossRef]
- Berger, P.; McConnell, J.P.; Nunn, M.; Kornman, K.S.; Sorrell, J.; Stephenson, K.; Duff, G.W. C-reactive protein levels are influenced by common IL-1 gene variations. Cytokine 2002, 17, 171–174. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A. C-reactive protein: The quintessential marker of systemic inflammation in coronary artery disease—Advancing toward precision medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef]
- Shirodaria, S.; Smith, J.; McKay, I.; Kennett, C.; Hughes, F. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1α protein in gingival crevicular fluid of teeth with severe periodontal disease. J. Dent. Res. 2000, 79, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Khazim, K.; Azulay, E.E.; Kristal, B.; Cohen, I. Interleukin 1 gene polymorphism and susceptibility to disease. Immunol. Rev. 2018, 281, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Mann, M.; Stelzig, J.; Bödeker, R.; Meyle, J. Single-nucleotide polymorphisms in the IL-4 and IL-13 promoter region in aggressive periodontitis. J. Clin. Periodontol. 2007, 34, 473–479. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kapley, A.; Yeltiwar, R.K.; Purohit, H.J. Assessment of single nucleotide polymorphism at IL-1A+ 4845 and IL-1B+ 3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J. Periodontol. 2006, 77, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Linhartová, P.; Cernochova, P.; Izakovicova Holla, L. IL 1 gene polymorphisms in relation to external apical root resorption concurrent with orthodontia. Oral. Dis. 2013, 19, 262–270. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Yañez-Vico, R.; Ballesta-Mudarra, S.; Ortiz-Ariza, E.; Ortega-Rivera, H.; Mendoza-Mendoza, A.; Solano-Reina, E.; Perea-Pérez, E. Postorthodontic external root resorption is associated with IL1 receptor antagonist gene variations. Oral. Dis. 2012, 18, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Choi, Y.J.; Choi, S.-H.; Jung, H.-S.; Lee, J.H.; Cha, J.-Y. The effect of genetic polymorphisms on treatment duration following premolar extraction. Sci. Rep. 2021, 11, 15942. [Google Scholar] [CrossRef]
- Ingman, T.; Apajalahti, S.; Mäntylä, P.; Savolainen, P.; Sorsa, T. Matrix metalloproteinase-1 and-8 in gingival crevicular fluid during orthodontic tooth movement: A pilot study during 1 month of follow-up after fixed appliance activation. Eur. J. Orthod. 2005, 27, 202–207. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, X.; Li, Y.; Xu, M.; Yao, Y.; Liu, K.; Ma, C.; Zhang, Y.; Ru, J.; He, Y. Macrophage-mediated extracellular matrix remodeling after fat grafting in nude mice. FASEB J. 2022, 36, e22550. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xue, Y.; Wang, P.; Lin, L.; Liu, Q.; Li, N.; Xu, J.; Cao, X. IFN-γ primes macrophage activation by increasing phosphatase and tensin homolog via downregulation of miR-3473b. J. Immunol. 2014, 193, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, J.; Yang, Y.; Cheng, G.; Guo, S.; Liu, C.; Ding, Y. Advances of hydrogel therapy in periodontal regeneration—A materials perspective review. Gels 2022, 8, 624. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Hefti, A.F.; Novak, M.J.; Michalowicz, B.S.; Pihlstrom, B.L.; Schoor, R.; Trummel, C.L.; Dean, J.; Van Dyke, T.E.; Walker, C.B. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: A multicenter trial. J. Periodontol. 2004, 75, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Gossage, D.L.; Cieslarová, B.; Ap, S.; Zheng, H.; Xin, Y.; Lal, P.; Chen, G.; Smith, V.; Sundy, J.S. Phase 1b study of the safety, pharmacokinetics, and disease-related outcomes of the matrix metalloproteinase-9 inhibitor andecaliximab in patients with rheumatoid arthritis. Clin. Ther. 2018, 40, 156–165.e155. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Zouein, F.A.; Tian, Y.; Padmanabhan Iyer, R.; de Castro Brás, L.E. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can. J. Physiol. Pharmacol. 2015, 93, 879–886. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Li, X.-Y.; Keller, E.T.; Yang, J.; Cho, J.-S.; Feinberg, T.Y.; Weiss, S.J. Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases. Sci. Transl. Med. 2020, 12, eaaw6143. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.; Nilforoushan, D.; Alghasi, H.; Dehpour, A.-R. The role of nitric oxide in orthodontic tooth movement in rats. Angle Orthod. 2002, 72, 211–215. [Google Scholar]
- Rangiani, A.; Jing, Y.; Ren, Y.; Yadav, S.; Taylor, R.; Feng, J.Q. Critical roles of periostin in the process of orthodontic tooth movement. Eur. J. Orthod. 2016, 38, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.G.; Wang, J.; Walker, J.T.; Michelsons, S.; Dunmore-Buyze, J.; Drangova, M.; Leask, A.; Hamilton, D.W. Periostin and CCN2 scaffolds promote the wound healing response in the skin of diabetic mice. Tissue Eng. Part A 2019, 25, 1326–1339. [Google Scholar] [CrossRef]
- Walker, J.T.; McLeod, K.; Kim, S.; Conway, S.J.; Hamilton, D.W. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res. 2016, 365, 453–465. [Google Scholar] [CrossRef]
- Abdolalian, F.; Bayani, M.; Afzali, S.; Nakhostin, A.; Almasi-Hashiani, A. Periostin level in gingival crevicular fluid in periodontal disease: A systematic review and meta-analysis. BMC Oral. Health 2023, 23, 284. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, Z.; Yuan, D.; Yu, M.; Min, J. Tissue engineering applications of recombinant human collagen: A review of recent progress. Front. Bioeng. Biotechnol. 2024, 12, 1358246. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, O.; Posen, Y.; Grynspan, F. Human collagen produced in plants: More than just another molecule. Bioengineered 2014, 5, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Chen, P.-J.; Arul, M.R.; Dutra, E.H.; Nanda, R.; Kumbar, S.G.; Yadav, S. Injectable RANKL sustained release formulations to accelerate orthodontic tooth movement. Eur. J. Orthod. 2020, 42, 317–325. [Google Scholar] [CrossRef]
- Xing, J.Z.; Lu, L.; Unsworth, L.D.; Major, P.W.; Doschak, M.R.; Kaipatur, N.R. RANKL release from self-assembling nanofiber hydrogels for inducing osteoclastogenesis in vitro. Acta Biomater. 2017, 49, 306–315. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Fu, W.; Liu, M.; Wang, M.; Hu, L.; Wang, L.; Zhao, X.; Ding, Z.; Dong, J. An induced pluripotent stem cells line (ZZUNEUi022-A) derived from urine cells of healthy male human. Stem Cell Res. 2021, 51, 102191. [Google Scholar] [CrossRef]
- Chen, I.-P. Differentiation of human induced pluripotent stem cells (hiPSCs) into osteoclasts. Bio-Protocol 2020, 10, e3854. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Vela Ramirez, J.E.; Haddadin, O.M.; Ross, K.A.; Narasimhan, B.; Peppas, N.A. pH-responsive microencapsulation systems for the oral delivery of polyanhydride nanoparticles. Biomacromolecules 2018, 19, 793–802, Erratum in Biomacromolecules 2018, 19, 3904. [Google Scholar] [CrossRef]
- Blümke, A.; Ijeoma, E.; Simon, J.; Wellington, R.; Purwaningrum, M.; Doulatov, S.; Leber, E.; Scatena, M.; Giachelli, C.M. Comparison of osteoclast differentiation protocols from human induced pluripotent stem cells of different tissue origins. Stem Cell Res. Ther. 2023, 14, 319. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, X.; Zhou, W.; Feng, G.; Zhang, Y.; Dai, H.; Zhou, J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am. J. Transl. Res. 2020, 12, 361. [Google Scholar]
- Yang, T.; Li, Y.; Hong, Y.; Chi, L.; Liu, C.; Lan, Y.; Wang, Q.; Yu, Y.; Xu, Q.; Teng, W. The construction of biomimetic cementum through a combination of bioskiving and fluorine-containing biomineralization. Front. Bioeng. Biotechnol. 2020, 8, 341. [Google Scholar] [CrossRef]
- Dolgos, H.; Freisleben, A.; Wimmer, E.; Scheible, H.; Krätzer, F.; Yamagata, T.; Gallemann, D.; Fluck, M. In vitro and in vivo drug disposition of cilengitide in animals and human. Pharmacol. Res. Perspect. 2016, 4, e00217. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yi, M.; Liu, C.; Jin, Y.; Liu, B.; Hu, G.; Yuan, X. Cilengitide, an αvβ3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model. Bioengineered 2022, 13, 4557–4572. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Saberi, N.; Pimental, T.; Teng, P.H. Present status and future directions: Root resorption. Int. Endod. J. 2022, 55, 892–921. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Waletzko-Hellwig, J.; Sellin, M.-L.; Seyfarth-Sehlke, A.; Wolfien, M.; Prehn, F.; Bader, R.; Jonitz-Heincke, A. Effects of the Interleukin-6 Receptor Blocker Sarilumab on Metabolic Activity and Differentiation Capacity of Primary Human Osteoblasts. Pharmaceutics 2022, 14, 1390. [Google Scholar] [CrossRef] [PubMed]
- Steemers, E.; Talbi, W.M.I.; Hogervorst, J.M.A.; Schoenmaker, T.; de Vries, T.J. IL-1 Receptor Antagonist Anakinra Inhibits the Effect of IL-1β- Mediated Osteoclast Formation by Periodontal Ligament Fibroblasts. Biology 2025, 14, 250. [Google Scholar] [CrossRef]
- Sincere, N.I.; Anand, K.; Ashique, S.; Yang, J.; You, C. PROTACs: Emerging targeted protein degradation approaches for advanced druggable strategies. Molecules 2023, 28, 4014. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Kaniskan, H.U.; Xie, L.; Chen, X.; Jin, J.; Wei, W. TF-PROTACs enable targeted degradation of transcription factors. J. Am. Chem. Soc. 2021, 143, 8902–8910. [Google Scholar] [CrossRef]
- Verzella, D.; Cornice, J.; Arboretto, P.; Vecchiotti, D.; Di Vito Nolfi, M.; Capece, D.; Zazzeroni, F.; Franzoso, G. The NF-κB pharmacopeia: Novel strategies to subdue an intractable target. Biomedicines 2022, 10, 2233. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Liu, Y.; Shen, Y.; Meng, F.; Kaniskan, H.U.; Jin, J.; Wei, W. Cancer selective target degradation by folate-caged PROTACs. J. Am. Chem. Soc. 2021, 143, 7380–7387. [Google Scholar] [CrossRef]
- Maneiro, M.a.; Forte, N.; Shchepinova, M.M.; Kounde, C.S.; Chudasama, V.; Baker, J.R.; Tate, E.W. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem. Biol. 2020, 15, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Gan, L.; Yang, B.; Wu, Y.; Meng, B.; Wu, D.; Zheng, J.; Cao, Y. Inhibitory effect of infliximab on orthodontic tooth movement in male rats. Arch. Oral. Biol. 2022, 144, 105573. [Google Scholar] [CrossRef]
- Perpétuo, I.P.; Caetano-Lopes, J.; Rodrigues, A.M.; Campanilho-Marques, R.; Ponte, C.; Canhão, H.; Ainola, M.; Fonseca, J.E. Effect of tumor necrosis factor inhibitor therapy on osteoclasts precursors in rheumatoid arthritis. BioMed Res. Int. 2017, 2017, 2690402. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, H.; Zhang, M.; Li, X.-L.; Yue, L.-T.; Zhang, P.; Zhao, Y.; Wang, S.; Duan, R.-N.; Li, Y.-B. Caspase-1 inhibitor ameliorates experimental autoimmune myasthenia gravis by innate dendric cell IL-1-IL-17 pathway. J. Neuroinflammation 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Palmroth, M.; Kuuliala, K.; Peltomaa, R.; Virtanen, A.; Kuuliala, A.; Kurttila, A.; Kinnunen, A.; Leirisalo-Repo, M.; Silvennoinen, O.; Isomäki, P. Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associates with treatment response. Front. Immunol. 2021, 12, 738481. [Google Scholar] [CrossRef] [PubMed]
- Irey, E.A.; Lassiter, C.M.; Brady, N.J.; Chuntova, P.; Wang, Y.; Knutson, T.P.; Henzler, C.; Chaffee, T.S.; Vogel, R.I.; Nelson, A.C. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proc. Natl. Acad. Sci. USA 2019, 116, 12442–12451. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, S.; Qiao, J.; Ji, Z.; Zhou, B.; Xu, W. CX3CL1 promotes M1 macrophage polarization and osteoclast differentiation through NF-κB signaling pathway in ankylosing spondylitis in vitro. J. Transl. Med. 2023, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, R.; Strashimirov, D.; Grozdeva, R.; Ivanov, D.; Trifonova, I.; Yancheva, N.; Tcherveniakova, T. Dynamics of Biomarkers in COVID-19 Patients Treated with Anakinra. Biomedicines 2024, 12, 2690. [Google Scholar] [CrossRef]
- Kooistra, E.J.; Waalders, N.J.; Grondman, I.; Janssen, N.A.; de Nooijer, A.H.; Netea, M.G.; van de Veerdonk, F.L.; Ewalds, E.; van der Hoeven, J.G.; Kox, M. Anakinra treatment in critically ill COVID-19 patients: A prospective cohort study. Crit. Care 2020, 24, 688. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 1–9. [Google Scholar] [CrossRef]
- Araújo, A.A.d.; Varela, H.; Brito, G.A.d.C.; Medeiros, C.A.C.X.d.; Araújo, L.d.S.; do Nascimento, J.H.O.; de Araújo Júnior, R.F. Azilsartan increases levels of IL-10, down-regulates MMP-2, MMP-9, RANKL/RANK, Cathepsin K and up-regulates OPG in an experimental periodontitis model. PLoS ONE 2014, 9, e96750. [Google Scholar] [CrossRef]
- Gao, Y.; Min, Q.; Li, X.; Liu, L.; Lv, Y.; Xu, W.; Liu, X.; Wang, H. Immune system acts on orthodontic tooth movement: Cellular and molecular mechanisms. Biomed. Res. Int. 2022, 2022, 9668610. [Google Scholar] [CrossRef]
- El-Masri, B.M.; Andreasen, C.M.; Laursen, K.S.; Kofod, V.B.; Dahl, X.G.; Nielsen, M.H.; Thomsen, J.S.; Brüel, A.; Sørensen, M.S.; Hansen, L.J. Mapping RANKL-and OPG-expressing cells in bone tissue: The bone surface cells as activators of osteoclastogenesis and promoters of the denosumab rebound effect. Bone Res. 2024, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Huang, D.; Huang, X. The effects of semaphorin 3A in bone and cartilage metabolism: Fundamental mechanism and clinical potential. Front. Cell Dev. Biol. 2023, 11, 1321151. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Ishii, T.; Nishii, Y. Semaphorin 3A regulates alveolar bone remodeling on orthodontic tooth movement. Sci. Rep. 2022, 12, 9243. [Google Scholar] [CrossRef]
- Hamidouche, Z.; Haÿ, E.; Vaudin, P.; Charbord, P.; Schüle, R.; Marie, P.J.; Fromigué, O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2 expression. FASEB J. 2008, 22, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Park, H.; Yang, H.-J.; Lee, S.; Lee, K.-Y.; Kim, T.S.; Jung, J.; Shin, J.-M. Cancer drug response profile scan (CDRscan): A deep learning model that predicts drug effectiveness from cancer genomic signature. Sci. Rep. 2018, 8, 8857. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Degn, K.; Saksager, A.; Tiberti, M.; Papaleo, E. Prediction of cancer driver genes and mutations: The potential of integrative computational frameworks. Brief. Bioinform. 2024, 25, bbad519. [Google Scholar] [CrossRef]
- Dhudum, R.; Ganeshpurkar, A.; Pawar, A. Revolutionizing drug discovery: A comprehensive review of AI applications. Drugs Drug Candidates 2024, 3, 148–171. [Google Scholar] [CrossRef]
- Papavassiliou, K.A.; Sofianidi, A.A.; Gogou, V.A.; Papavassiliou, A.G. The Promise of Artificial Intelligence in Reshaping Anticancer Drug Development. Cells 2024, 13, 1709. [Google Scholar] [CrossRef]
- Santagati, R.; Aspuru-Guzik, A.; Babbush, R.; Degroote, M.; González, L.; Kyoseva, E.; Moll, N.; Oppel, M.; Parrish, R.M.; Rubin, N.C. Drug design on quantum computers. Nat. Phys. 2024, 20, 549–557. [Google Scholar] [CrossRef]
- Sinha, S.; Vegesna, R.; Mukherjee, S.; Kammula, A.V.; Dhruba, S.R.; Wu, W.; Kerr, D.L.; Nair, N.U.; Jones, M.G.; Yosef, N. PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors. Nat. Cancer 2024, 5, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, I.; Kim, Y.J.; Kim, M.; Cho, J.H.; Hong, M.; Kang, K.H.; Lim, S.H.; Kim, S.J.; Kim, Y.H. Accuracy of automated identification of lateral cephalometric landmarks using cascade convolutional neural networks on lateral cephalograms from nationwide multi-centres. Orthod. Craniofacial Res. 2021, 24, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Chaurasia, A.; Arsiwala, L.; Lee, J.-H.; Elhennawy, K.; Jost-Brinkmann, P.-G.; Demarco, F.; Krois, J. Deep learning for cephalometric landmark detection: Systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 4299–4309. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, F.; Syben, C.; Roser, P.; Mill, L.; Maier, A. Cephalogram synthesis and landmark detection in dental cone-beam CT systems. Med. Image Anal. 2021, 70, 102028. [Google Scholar] [CrossRef] [PubMed]
- Adel, S.M.; Vaid, N.R.; El-Harouni, N.; Kassem, H.; Zaher, A.R. Digital model superimpositions: Are different software algorithms equally accurate in quantifying linear tooth movements? BMC Oral. Health 2022, 22, 103. [Google Scholar] [CrossRef]
- Li, R.; Zhu, C.; Chu, F.; Yu, Q.; Fan, D.; Ouyang, N.; Jin, Y.; Guo, W.; Xia, L.; Feng, Q. Deep learning for virtual orthodontic bracket removal: Tool establishment and application. Clin. Oral. Investig. 2024, 28, 121. [Google Scholar] [CrossRef]
- Park, J.H.; Hamimi, M.; Choi, J.J.E.; Figueredo, C.M.S.; Cameron, M.A. Comparisons of AI automated segmentation techniques to manual segmentation techniques of the maxilla and maxillary sinus for CT or CBCT scans—A Systematic review. Dentomaxillofacial Radiol. 2025, twaf042. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, Y.; Chen, J.; Wang, X.; Zhou, M.; Li, H.; Lin, J.; Zhang, W.; Chen, X. Automatic multimodal registration of cone-beam computed tomography and intraoral scans: A systematic review and meta-analysis. Clin. Oral. Investig. 2025, 29, 97. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Zou, B.; Chaudhari, P.K.; Adel, S.M.; Vaiid, N. Artificial Intelligence Applications in Orthodontics. In Artificial Intelligence for Oral Health Care: Applications and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2025; pp. 81–97. [Google Scholar]
- Reduwan, N.H.; Aziz, A.A.; Mohd Razi, R.; Abdullah, E.R.M.F.; Mazloom Nezhad, S.M.; Gohain, M.; Ibrahim, N. Application of deep learning and feature selection technique on external root resorption identification on CBCT images. BMC Oral. Health 2024, 24, 252, Erratum in BMC Oral. Health 2025, 25, 167. [Google Scholar] [CrossRef]
- Evangelista, K.; de Freitas Silva, B.S.; Yamamoto-Silva, F.P.; Valladares-Neto, J.; Silva, M.A.G.; Cevidanes, L.H.S.; de Luca Canto, G.; Massignan, C. Accuracy of artificial intelligence for tooth extraction decision-making in orthodontics: A systematic review and meta-analysis. Clin. Oral. Investig. 2022, 26, 6893–6905. [Google Scholar] [CrossRef]
- Ingle, N.A.; Alabsi, N.F.; Al-Hashimi, H.; Albuolayan, N.A.; Alburidy, F.; Alanazi, F.; Alhammad, A.T. The Use of Artificial Intelligence in Orthodontic Treatment Planning: A Systematic Review and Meta-analysis. Adv. Hum. Biol. 2025, 15, 158–166. [Google Scholar] [CrossRef]
- Nordblom, N.; Büttner, M.; Schwendicke, F. Artificial intelligence in orthodontics: Critical review. J. Dent. Res. 2024, 103, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Motmaen, I.; Xie, K.; Schönbrunn, L.; Berens, J.; Grunert, K.; Plum, A.M.; Raufeisen, J.; Ferreira, A.; Hermans, A.; Egger, J. Insights into predicting tooth extraction from panoramic dental images: Artificial intelligence vs. dentists. Clin. Oral. Investig. 2024, 28, 381. [Google Scholar] [CrossRef]
- Bhai, K.; Judge, R.; Abduo, J.; Palamara, J. Measuring tooth movement with treatment using the Dahl principle: An observational study. J. Prosthet. Dent. 2023, 129, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, N.; Kazimierczak, W.; Serafin, Z.; Nowicki, P.; Nożewski, J.; Janiszewska-Olszowska, J. AI in Orthodontics: Revolutionizing Diagnostics and Treatment Planning—A Comprehensive Review. J. Clin. Med. 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Shan, Z. Application of artificial intelligence in orthodontics: Current state and future perspectives. Healthcare 2023, 11, 2760. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Doken, S.; Zhuang, T. Correction to Lancet Digital Health 2019; 1: e106–07. Corrections 2019, 1, E160. [Google Scholar]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Montero Jiménez, O.G.; Dib Kanán, A.; Dipp Velázquez, F.A.; Aristizábal Pérez, J.F.; Moyaho Bernal, M.d.l.Á.; Salas Orozco, M.F.; Casillas Santana, M.A. Use of hydrogels to regulate orthodontic tooth movement in animal models: A systematic review. Appl. Sci. 2022, 12, 6683. [Google Scholar] [CrossRef]
- Vitale, M.; Ligorio, C.; McAvan, B.; Hodson, N.W.; Allan, C.; Richardson, S.M.; Hoyland, J.A.; Bella, J. Hydroxyapatite-decorated Fmoc-hydrogel as a bone-mimicking substrate for osteoclast differentiation and culture. Acta Biomater. 2022, 138, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Wang, Y.; Xin, Y.; Li, X.; Li, T.; Zhang, H.; Quan, L.; Li, Y.; Arya, D.K.; Rajinikanth, P. Bioinspired core-shell nanofiber drug-delivery system modulates osteogenic and osteoclast activity for bone tissue regeneration. Mater. Today Bio 2024, 26, 101088. [Google Scholar] [CrossRef]
- Liu, S.; Manshaii, F.; Chen, J.; Wang, X.; Wang, S.; Yin, J.; Yang, M.; Chen, X.; Yin, X.; Zhou, Y. Unleashing the potential of electroactive hybrid biomaterials and self-powered systems for bone therapeutics. Nano-Micro Lett. 2025, 17, 44. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J. Cyclic RGD pentapeptide cilengitide enhances efficacy of gefitinib on TGF-β1-induced epithelial-to-mesenchymal transition and invasion in human non-small cell lung cancer cells. Front. Pharmacol. 2021, 12, 639095. [Google Scholar] [CrossRef]
- Huang, J.; Ma, Z.; Peng, X.; Yang, Z.; Wu, Y.; Zhong, G.; Ouyang, T.; Chen, Z.; Liu, Y.; Wang, Q. Discovery of Novel Potent and Fast BTK PROTACs for the Treatment of Osteoclasts-Related Inflammatory Diseases. J. Med. Chem. 2024, 67, 2438–2465. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Ren, H.; Li, X.; Yu, D.; Mu, S.; Chen, Z.; Fu, Q. Effects of intermedin on proliferation, apoptosis and the expression of OPG/RANKL/M-CSF in the MC3T3-E1 osteoblast cell line. Mol. Med. Rep. 2015, 12, 6711–6717. [Google Scholar] [CrossRef]
- Shrestha, Y.R.; Krishna, V.; von Krogh, G. Augmenting organizational decision-making with deep learning algorithms: Principles, promises, and challenges. J. Bus. Res. 2021, 123, 588–603. [Google Scholar] [CrossRef]
- Li, Y.-H.; Li, Y.-L.; Wei, M.-Y.; Li, G.-Y. Innovation and challenges of artificial intelligence technology in personalized healthcare. Sci. Rep. 2024, 14, 18994. [Google Scholar] [CrossRef] [PubMed]
- Halme, D.G.; Kessler, D.A. FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 2006, 355, 1730. [Google Scholar] [CrossRef] [PubMed]
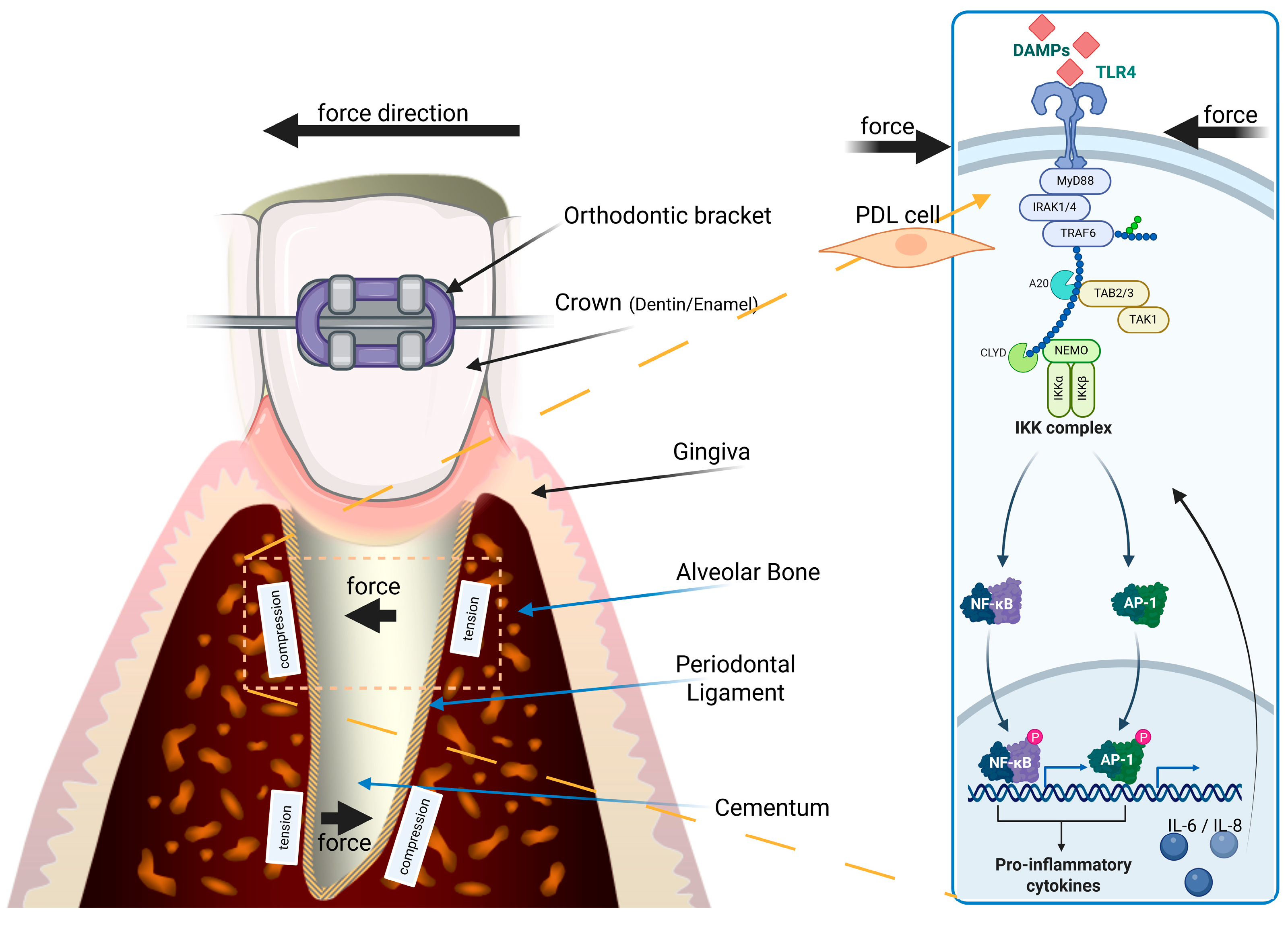
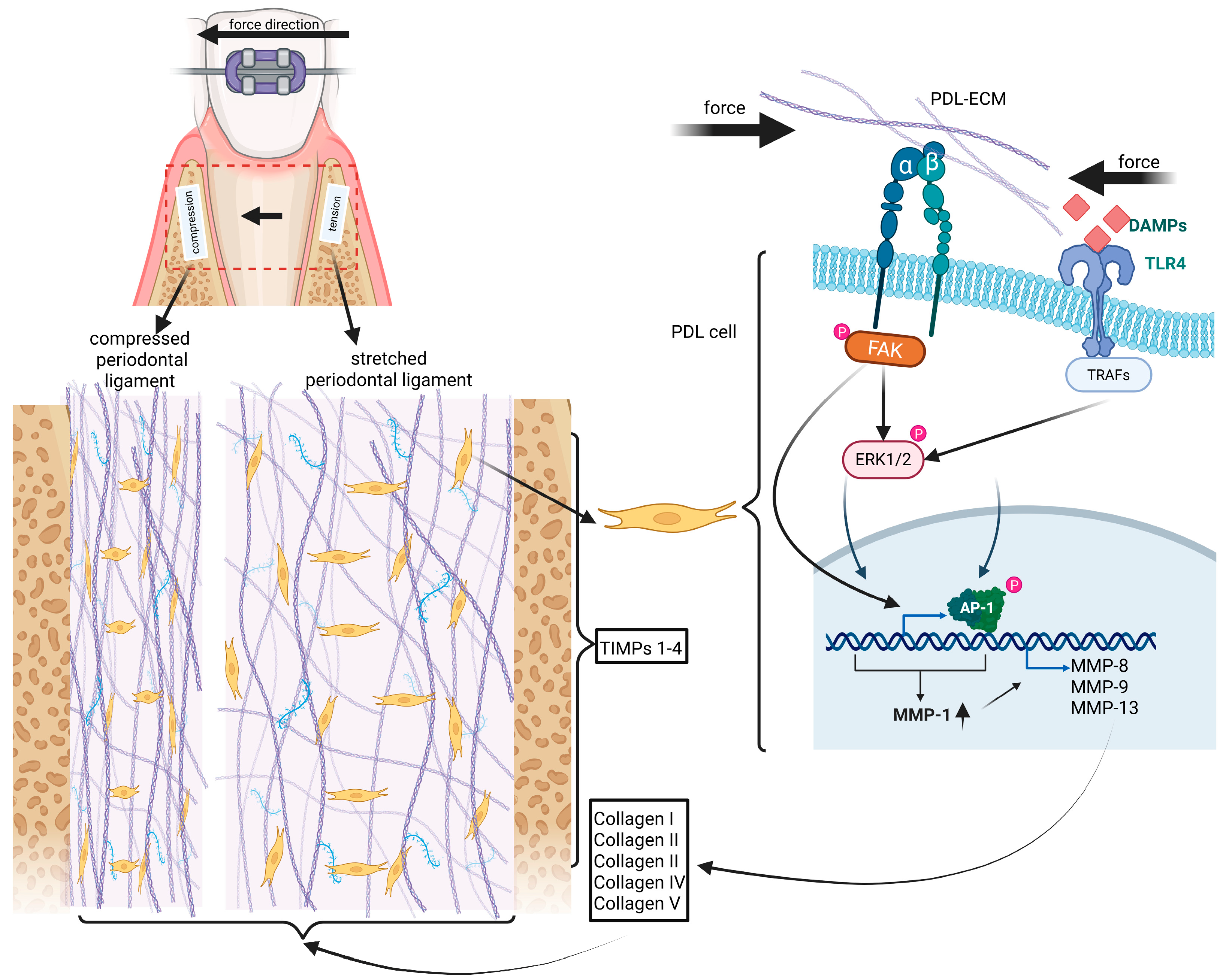


| Pathway/ Factor | Role in OTM | Mechanism | Implication | References |
|---|---|---|---|---|
| Hypoxia/HIF-1α | Triggers cell death | Mitochondrial apoptosis via Bax, caspase cascade | Zone clearance delay (lag phase) | [9,10,11] |
| MMPs (MMP-8, -9, -13) | ECM degradation | Collagen proteolysis | Hyalinization, loss of PDL structure | [13,14,38] |
| IL-1β, TNF-α, IL-6 | Drive sterile inflammation | TLR4 → MyD88/IRAK/TRAF6 → NF-κB | Stimulate osteoclastogenesis, pain response | [28,29,30,32] |
| TIMPs (TIMP-1/2/4) | Inhibit MMPs | Protease inhibition | Potential target to balance matrix turnover | [39,41,42,43] |
| Component | Function | Regulators | Impact | References |
|---|---|---|---|---|
| RANKL | Induces osteoclast fusion/differentiation | TNF-α, IL-1β, miR-125a | Bone resorption, OTM progression | [48,56,57] |
| RANK | Osteoclast precursor receptor | RANKL, miR-125a | Activates NF-κB, c-Fos, NFATc1 | [52,56] |
| OPG | Decoy receptor, inhibits RANKL-RANK | miR-3198 (represses OPG) | Protective against bone loss/OEARR | [62,63,64] |
| Gene | Polymorphism | Effect | Clinical Relevance | References |
|---|---|---|---|---|
| IL-1β | +3954 C/T | Elevated IL-1β in GCF, ↑ inflammatory risk | Higher OEARR susceptibility | [76,78] |
| P2RX7 | rs208294 | Impaired ATP receptor function | Strongly linked to root resorption | [49,50] |
| IL-1RN | VNTR alleles | Alters IL-1 antagonist levels | May exacerbate inflammatory cascades | [78] |
| Intervention | Mechanism | Clinical Benefit | References |
|---|---|---|---|
| rhCol1 + BMP hydrogels | Matrix replacement + growth factor delivery | PDL matrix regeneration | [94,95] |
| MMP inhibitors (Periostat) | Blocks ECM degradation | Shortens lag phase, preserves collagen | [85,86] |
| Arginine/Citrulline | ↑ NO → vasodilation → ↑ perfusion | Enhanced macrophage activity and matrix repair | [89] |
| Periostin + CCN2 | Stimulates fibroblast migration and adhesion | Speeds wound healing | [90,91,92] |
| Component | Function | Advantage | Limitation | References |
|---|---|---|---|---|
| RANKL Hydrogels | Stimulate osteoclast activity | Spatio-temporal control of bone resorption | Requires optimal dosing and biocompatibility | [96,97,98,101] |
| iPC-derived Osteoclasts | Personalized resorption units | Non-invasive, patient-specific therapies | Safety, regulatory barriers | [99,100,102] |
| Strategy | Mechanism | Target | References |
|---|---|---|---|
| Cilengitide | Blocks integrin-mediated adhesion | Osteoclasts/Odontoclasts | [51,105,106,107] |
| Biomimetic Cementum | Artificial cementum regeneration | Cementoblasts | [104] |
| Cytokine Inhibition | IL-1R/IL-6R blockade reduces osteoclastogenesis | Inflammatory response | [108,109] |
| Target | Degraded Protein | Mechanism | Potential Use in OTM | References |
|---|---|---|---|---|
| NF-κB | p65/p50 subunits | Ubiquitin–proteasome degradation | Reduces RANKL induction | [110,111,112] |
| PROTAC uptake | Folate/HER2-coupled | Targeted intracellular delivery | Improves selectivity | [111,114] |
| Application Area | Validated AI Tools | Experimental/Research-Phase AI Models |
|---|---|---|
| Cephalometric analysis | CephX® (Orca Dental AI Ltd., Tel-Aviv, Israel), Vatech EzOrtho AI (Vatech Co., Ltd. Hwaseong-si, Republic of Korea, WebCeph™ (AssembleCircle Corp., Seongnam-si, Republic of Korea [137,138] | Generative adversarial networks (GANs) for automated landmark detection [139] |
| Treatment planning | 3Shape OrthoAnalyzer™ AI (3Shape A/S, Copenhagen, Denmark),(aligner sequencing), Dolphin Imaging AI (Patterson Dental (Dolphin Imaging & Management Solutions) Chatsworth, CA, USA [140] | Reinforcement learning for force system optimization [141] |
| Tooth segmentation | Deep learning CNNs for automated dental arch segmentation (FDA-cleared) [142] | Multimodal segmentation using fused intraoral scans and CBCT [143] |
| Outcome prediction | Aligner tracking algorithms based on validated movement thresholds [144] | Predictive AI models for treatment duration and risk of OEARR using EMR + radiomics [145] |
| Application | Function | Clinical Benefit | References |
|---|---|---|---|
| Virtual Twin Modeling | Simulates jaw/teeth movement | Precise appliance design | [148,150] |
| Predictive Treatment AI | Estimates treatment response based on data | Shorter duration, fewer side effects | [151,152] |
| Diagnostic AI Imaging | Detects malocclusion/pathology in radiographs | Enhanced diagnostic sensitivity | [152,153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinberg, T.; Jung, B.; Husari, A.; Bai, S.; Tomakidi, P. Shaping Orthodontics of the Future: Concepts and Implications from a Cellular and Molecular Perspective. Int. J. Mol. Sci. 2025, 26, 8203. https://doi.org/10.3390/ijms26178203
Steinberg T, Jung B, Husari A, Bai S, Tomakidi P. Shaping Orthodontics of the Future: Concepts and Implications from a Cellular and Molecular Perspective. International Journal of Molecular Sciences. 2025; 26(17):8203. https://doi.org/10.3390/ijms26178203
Chicago/Turabian StyleSteinberg, Thorsten, Britta Jung, Ayman Husari, Shuoqiu Bai, and Pascal Tomakidi. 2025. "Shaping Orthodontics of the Future: Concepts and Implications from a Cellular and Molecular Perspective" International Journal of Molecular Sciences 26, no. 17: 8203. https://doi.org/10.3390/ijms26178203
APA StyleSteinberg, T., Jung, B., Husari, A., Bai, S., & Tomakidi, P. (2025). Shaping Orthodontics of the Future: Concepts and Implications from a Cellular and Molecular Perspective. International Journal of Molecular Sciences, 26(17), 8203. https://doi.org/10.3390/ijms26178203






