Placental Polycyclic Aromatic Hydrocarbon (PAH) Levels Are Associated with Spontaneous Preterm Birth
Abstract
1. Introduction
2. Results
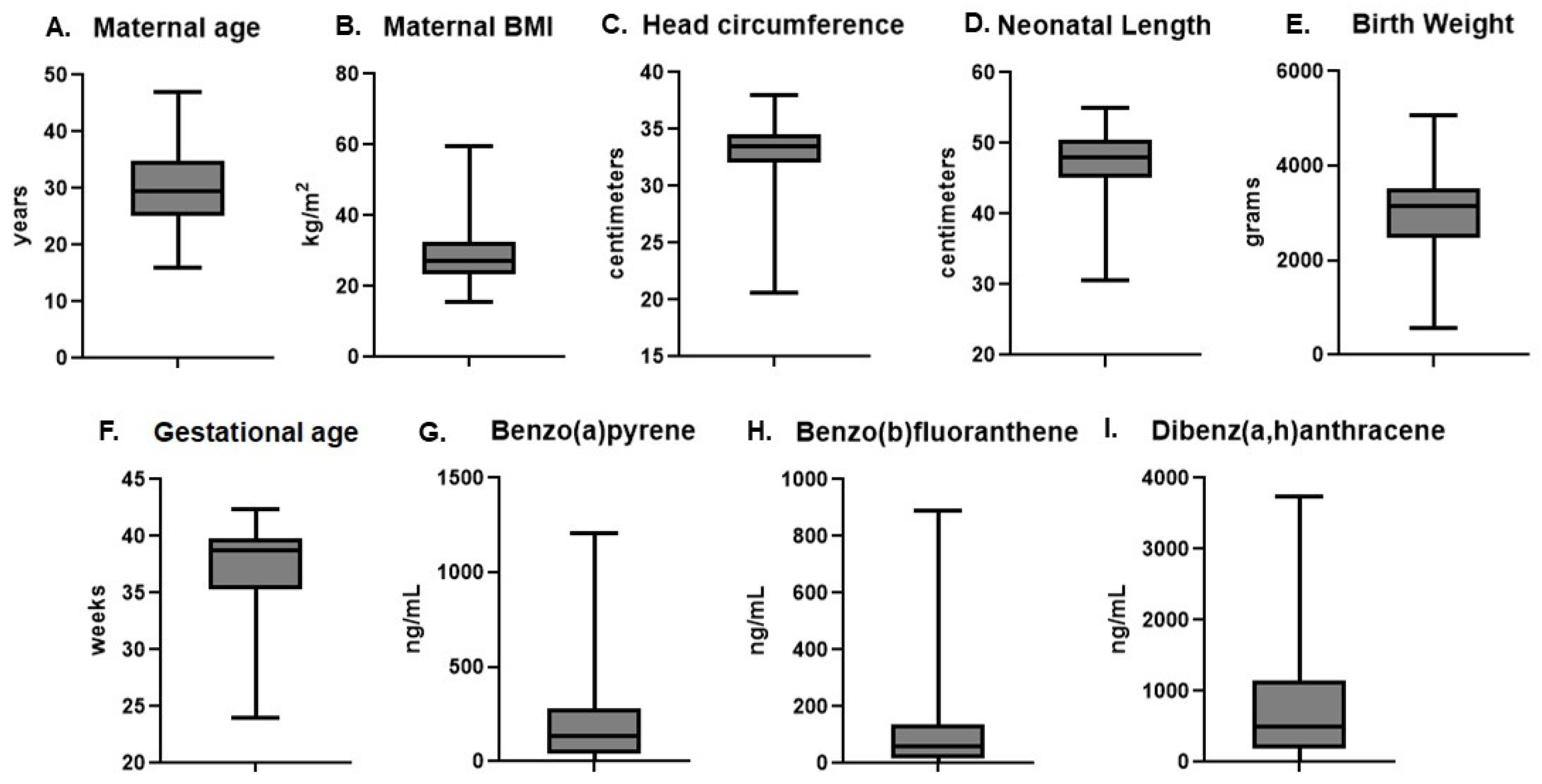
2.1. Subject Demographics and Placental PAH Levels
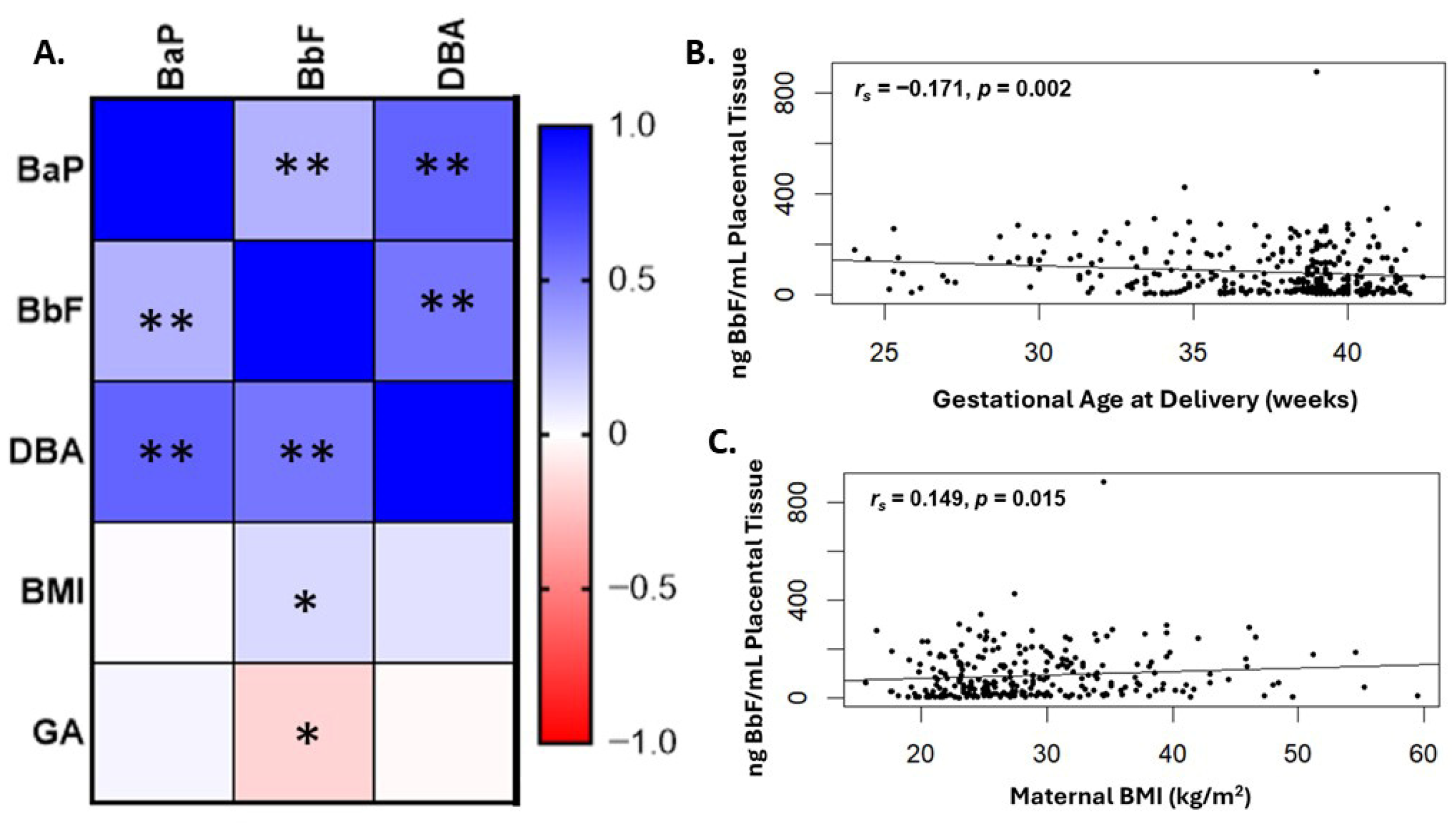
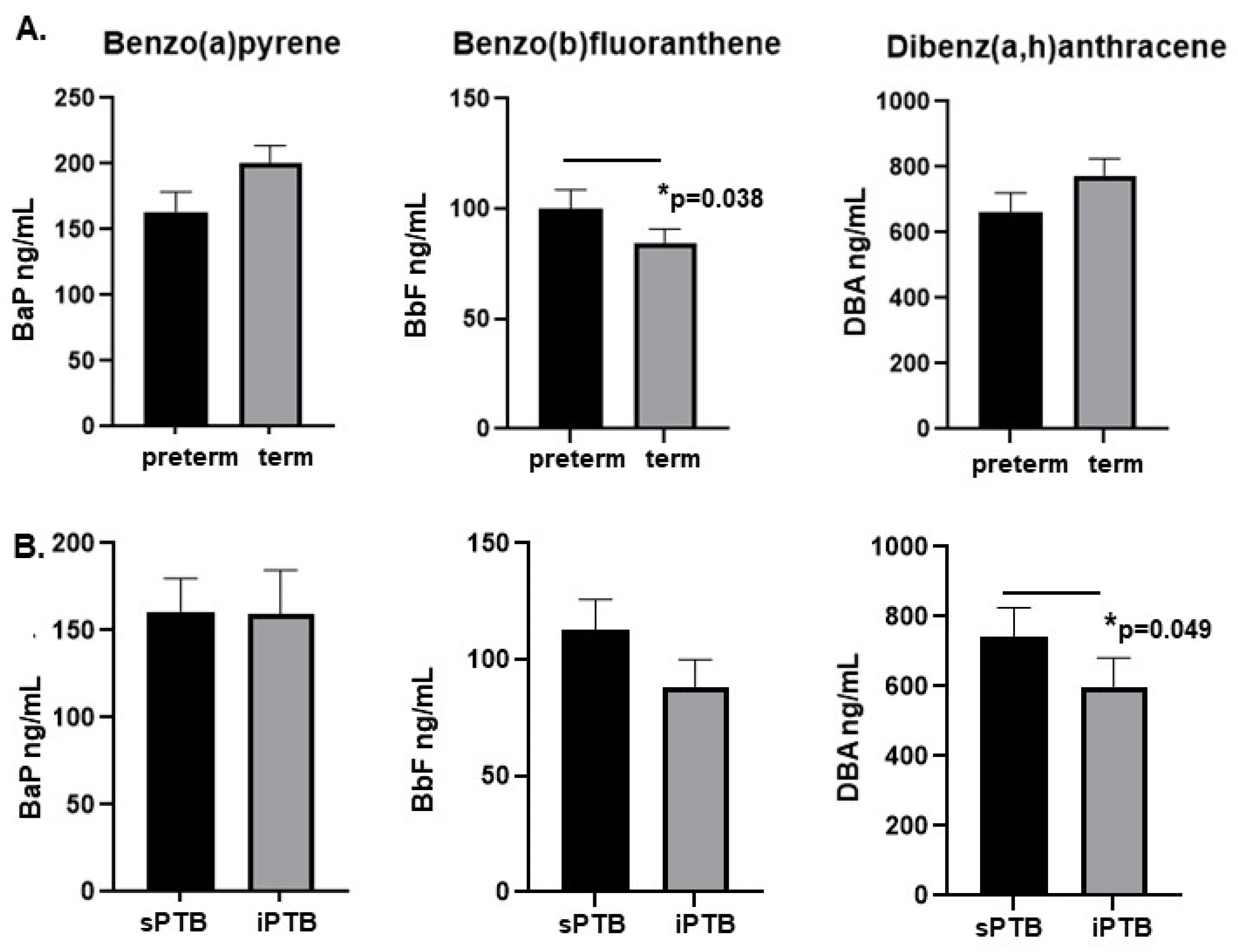
2.2. Levels of BbF Are Significantly Associated with Preterm Delivery
2.3. Levels of DBA Are Higher in Placentae from Spontaneous Preterm Deliveries Compared with Medically Indicated Preterm Delivery
2.4. Levels of BaP Are Higher in Placentae from Male Compared with Female Neonates
3. Discussion
4. Materials and Methods
4.1. Subjects and Tissue Samples
4.2. PAH Analysis of Placenta Tissue
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2021. Natl. Vital Stat. Rep. 2023, 72, 1–53. [Google Scholar]
- World Health Organization Fact Sheets- Preterm Birth. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 12 January 2025).
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- The Sustainable Development Goals Report 2023: Special Edition; United Nations: New York, NY, USA, 2023; Available online: https://unstats.un.org/sdgs/report/2023 (accessed on 12 January 2025).
- Howson, C.P.; Kinney, M.V.; McDougall, L.; Lawn, J.E. Born Too Soon Preterm Birth Action Group. Born too soon: Preterm birth matters. Reprod. Health 2013, 10, S1. [Google Scholar] [CrossRef]
- March of Dimes Report Card for the United States. 2024. Available online: https://www.marchofdimes.org/peristats/reports/united-states/report-card (accessed on 21 August 2025).
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm birth lifetime costs in the United States in 2016: An update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef]
- Ekwo, E.E.; Gosselink, C.A.; Moawad, A. Unfavorable outcome in penultimate pregnancy and premature rupture of membranes in successive pregnancy. Obstet. Gynecol. 1992, 80, 166–172. [Google Scholar]
- Andersen, H.F.; Nugent, C.E.; Wanty, S.D.; Hayashi, R.H. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am. J. Obstet. Gynecol. 1990, 163, 859–867. [Google Scholar] [CrossRef]
- Funaki, S.; Ogawa, K.; Ozawa, N.; Okamoto, A.; Morisaki, N.; Sago, H. Differences in pregnancy complications and outcomes by fetal gender among Japanese women: A multicenter cross-sectional study. Sci. Rep. 2020, 10, 18810. [Google Scholar] [CrossRef]
- Peelen, M.J.; Kazemier, B.M.; Ravelli, A.C.; De Groot, C.J.; Van Der Post, J.A.; Mol, B.W.; Hajenius, P.J.; Kok, M. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet. Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Gandhimadhi, D.; Mythili, R. Periodontal infection as a risk factor for preterm low birth weight. J. Indian Soc. Periodontol. 2010, 14, 114–120. [Google Scholar] [CrossRef]
- Walia, M.; Saini, N. Relationship between periodontal diseases and preterm birth: Recent epidemiological and biological data. Int. J. Appl. Basic Med. Res. 2015, 5, 2–6. [Google Scholar]
- Manuck, T.A. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin. Perinatol. 2017, 41, 511–518. [Google Scholar] [CrossRef]
- Dolatian, M.; Sharifi, N.; Mahmoodi, Z. Relationship of socioeconomic status, psychosocial factors, and food insecurity with preterm labor: A longitudinal study. Int. J. Reprod. Biomed. 2018, 16, 563–570. [Google Scholar] [CrossRef]
- Wadon, M.; Modi, N.; Wong, H.S.; Thapar, A.; O’Donovan, M.C. Recent advances in the genetics of preterm birth. Ann. Hum. Genet. 2020, 84, 205–213. [Google Scholar] [CrossRef]
- Chu, D.M.; Seferovic, M.; Pace, R.M.; Aagaard, K.M. The microbiome in preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 103–113. [Google Scholar] [CrossRef]
- Suter, M.A.; Aagaard, K.M.; Coarfa, C.; Robertson, M.; Zhou, G.; Jackson, B.P.; Thompson, D.; Putluri, V.; Putluri, N.; Hagan, J.; et al. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem. Biophys. Res. Commun. 2019, 516, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Singh, L.; Anand, M.; Taneja, A. Association Between Placental Polycyclic Aromatic Hydrocarbons (PAHS), Oxidative Stress, and Preterm Delivery: A Case-Control Study. Arch. Environ. Contam. Toxicol. 2018, 74, 218–227. [Google Scholar] [CrossRef]
- Padula, A.M.; Noth, E.M.; Hammond, S.K.; Lurmann, F.W.; Yang, W.; Tager, I.B.; Shaw, G.M. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ. Res. 2014, 135, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shang, L.; Wang, S.; Yang, W.; Huang, L.; Qi, C.; Gurcan, A.; Yang, Z.; Chung, M.C. The association between prenatal exposure to polycyclic aromatic hydrocarbons and birth weight: A meta-analysis. PLoS ONE 2020, 15, e0236708. [Google Scholar] [CrossRef]
- Choi, H.; Harrison, R.; Komulainen, H.; Delgado Saborit, J.M. Polycyclic aromatic hydrocarbons. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK138709 (accessed on 21 August 2025).
- Dong, X.; Wang, Q.; Peng, J.; Wu, M.; Pan, B.; Xing, B. Transfer of polycyclic aromatic hydrocarbons from mother to fetus in relation to pregnancy complications. Sci. Total. Environ. 2018, 15, 61–68. [Google Scholar] [CrossRef]
- Paquette, A.G.; Lapehn, S.; Freije, S.; MacDonald, J.; Bammler, T.; Day, D.B.; Loftus, C.T.; Kannan, K.; Alex, M.W.; Bush, N.R.; et al. Placental transcriptomic signatures of prenatal exposure to Hydroxy-Polycyclic aromatic hydrocarbons. Environ. Int. 2023, 172, 107763. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, L.; Pavek, P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr. Pharm. Biotechnol. 2011, 12, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Björvang, R.D.; Mamsen, L.S. Sexually Dimorphic Accumulation of Persistent Organic Pollutants in Fetuses. Front. Toxicol. 2022, 4, 909307. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.H.; Won, S.; Ku, J.L.; Moon, H.B.; Park, J.; Choi, G.; Kim, S.; Choi, K. Maternal exposures to persistent organic pollutants are associated with DNA methylation of thyroid hormone-related genes in placenta differently by infant sex. Environ. Int. 2019, 130, 104956. [Google Scholar] [CrossRef]
- Leonetti, C.; Butt, C.M.; Hoffman, K.; Hammel, S.C.; Miranda, M.L.; Stapleton, H.M. Brominated flame retardants in placental tissues: Associations with infant sex and thyroid hormone endpoints. Environ. Health 2016, 15, 113. [Google Scholar] [CrossRef]
- Mamsen, L.S.; Björvang, R.D.; Mucs, D.; Vinnars, M.T.; Papadogiannakis, N.; Lindh, C.H.; Andersen, C.Y.; Damdimopoulou, P. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ. Int. 2019, 124, 482–492. [Google Scholar] [CrossRef]
- ATSDR, Public Health Statement, Polycyclic Aromatic Hydrocarbons. 1990, US Department of Health and Human Services Atlanta, GA. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp69-c1-b.pdf (accessed on 21 August 2025).
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lutz, W.K.; Gaylor, D. Significance of DNA adducts at low dose: Shortening the time to spontaneous tumor occurrence. Regul Toxicol. Pharmacol. 1996, 23 Pt 1, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wild, R.; Feingold, K.R. Effect of Pregnancy on Lipid Metabolism and Lipoprotein Levels. Endotext [Internet], (2023). Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK498654/ (accessed on 7 August 2025).
- Brettell, R.; Yeh, P.S.; Impey, L.W. Examination of the association between male gender and preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Navarro, C.; Gregorio-Hernández, R.; Pérez-Pérez, A.; Rodríguez-Corrales, E.; Vigil-Vázquez, S.; Arriaga-Redondo, M.; Merino-Hernández, A.; Sánchez-Luna, M. Impact of placental pathology on the risk of bronchopulmonary dysplasia in preterm infants: The role of gestational age and sex. Eur. J. Pediatr. 2025, 184, 211. [Google Scholar] [CrossRef]
- Leek, C.; Cantu, A.; Sonti, S.; Gutierrez, M.C.; Eldredge, L.; Sajti, E.; Xu, H.N.; Lingappan, K. Role of sex as a biological variable in neonatal alveolar macrophages. Redox Biol. 2024, 75, 103296. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta 2013, 34, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.C.; Jansson, T.; Bale, T.L.; Powell, T.L. Maternal-fetal cross-talk via the placenta: Influence on offspring development and metabolism. Development 2023, 150, dev202088. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.; Abramovici, A.; Showalter, L.; Hu, M.; Shope, C.D.; Varner, M.; Aagaard-Tillery, K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 2010, 59, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez, C.; Sehgal, N.; Eick, S.M.; Barr, D.B.; Panuwet, P.; Yakimavets, V.; Chen, K.; Shankar, K.; Pearson, K.J.; Andres, A.; et al. Sex-specific effects of in utero exposure to per-and polyfluoroalkyl substances on placental development. Environ. Res. 2025, 270, 120868. [Google Scholar] [CrossRef]
- Antony, K.M.; Hemarajata, P.; Chen, J.; Morris, J.; Cook, C.; Masalas, D.; Gedminas, M.; Brown, A.; Versalovic, J.; Aagaard, K. Generation and validation of a universal perinatal database and biospecimen repository: PeriBank. J. Perinatol. 2016, 36, 921–929. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hummel, G.; Banerjee, S.; Putluri, V.; Malick, I.; Johnson, G.; Kamal, A.H.M.; Ambati, C.S.R.; Putluri, N.; Showalter, L.; Shope, C.D.; et al. Placental Polycyclic Aromatic Hydrocarbon (PAH) Levels Are Associated with Spontaneous Preterm Birth. Int. J. Mol. Sci. 2025, 26, 8179. https://doi.org/10.3390/ijms26178179
Hummel G, Banerjee S, Putluri V, Malick I, Johnson G, Kamal AHM, Ambati CSR, Putluri N, Showalter L, Shope CD, et al. Placental Polycyclic Aromatic Hydrocarbon (PAH) Levels Are Associated with Spontaneous Preterm Birth. International Journal of Molecular Sciences. 2025; 26(17):8179. https://doi.org/10.3390/ijms26178179
Chicago/Turabian StyleHummel, Gwendolynn, Sohini Banerjee, Vasanta Putluri, Inaara Malick, Grace Johnson, Abu Hena Mostafa Kamal, Chandra Shekar R. Ambati, Nagireddy Putluri, Lori Showalter, Cynthia D. Shope, and et al. 2025. "Placental Polycyclic Aromatic Hydrocarbon (PAH) Levels Are Associated with Spontaneous Preterm Birth" International Journal of Molecular Sciences 26, no. 17: 8179. https://doi.org/10.3390/ijms26178179
APA StyleHummel, G., Banerjee, S., Putluri, V., Malick, I., Johnson, G., Kamal, A. H. M., Ambati, C. S. R., Putluri, N., Showalter, L., Shope, C. D., Hagan, J., Aagaard, K. M., Moorthy, B., & Suter, M. A. (2025). Placental Polycyclic Aromatic Hydrocarbon (PAH) Levels Are Associated with Spontaneous Preterm Birth. International Journal of Molecular Sciences, 26(17), 8179. https://doi.org/10.3390/ijms26178179








