Impact of Photobiomodulation on the Pro-Osteogenic Activity of Dental Pulp Mesenchymal Stem/Stromal Cells
Abstract
1. Introduction
2. Results
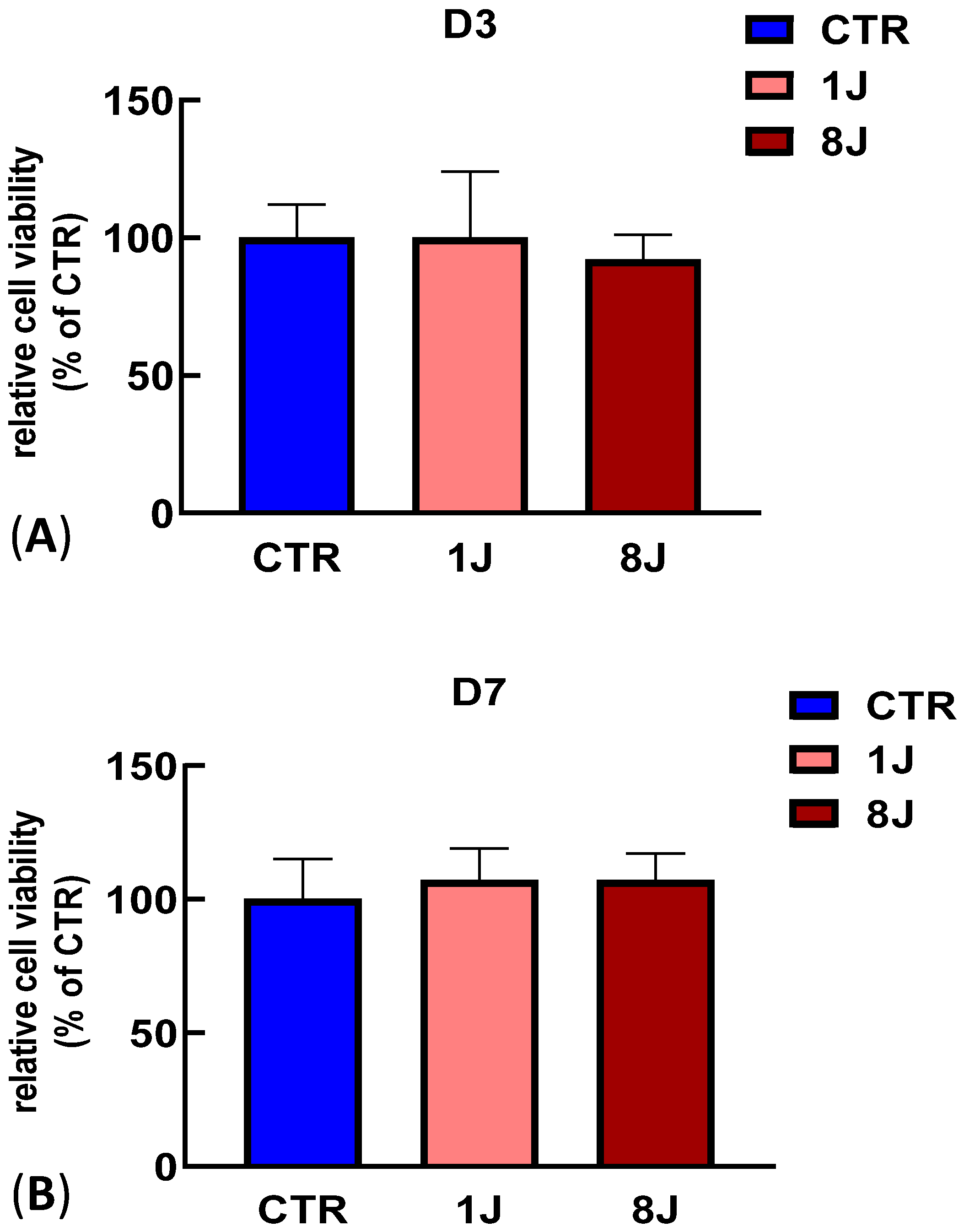
2.1. Cell Viability Assay Demonstrated a Lack of Photodamage in Irradiated DP-MSCs
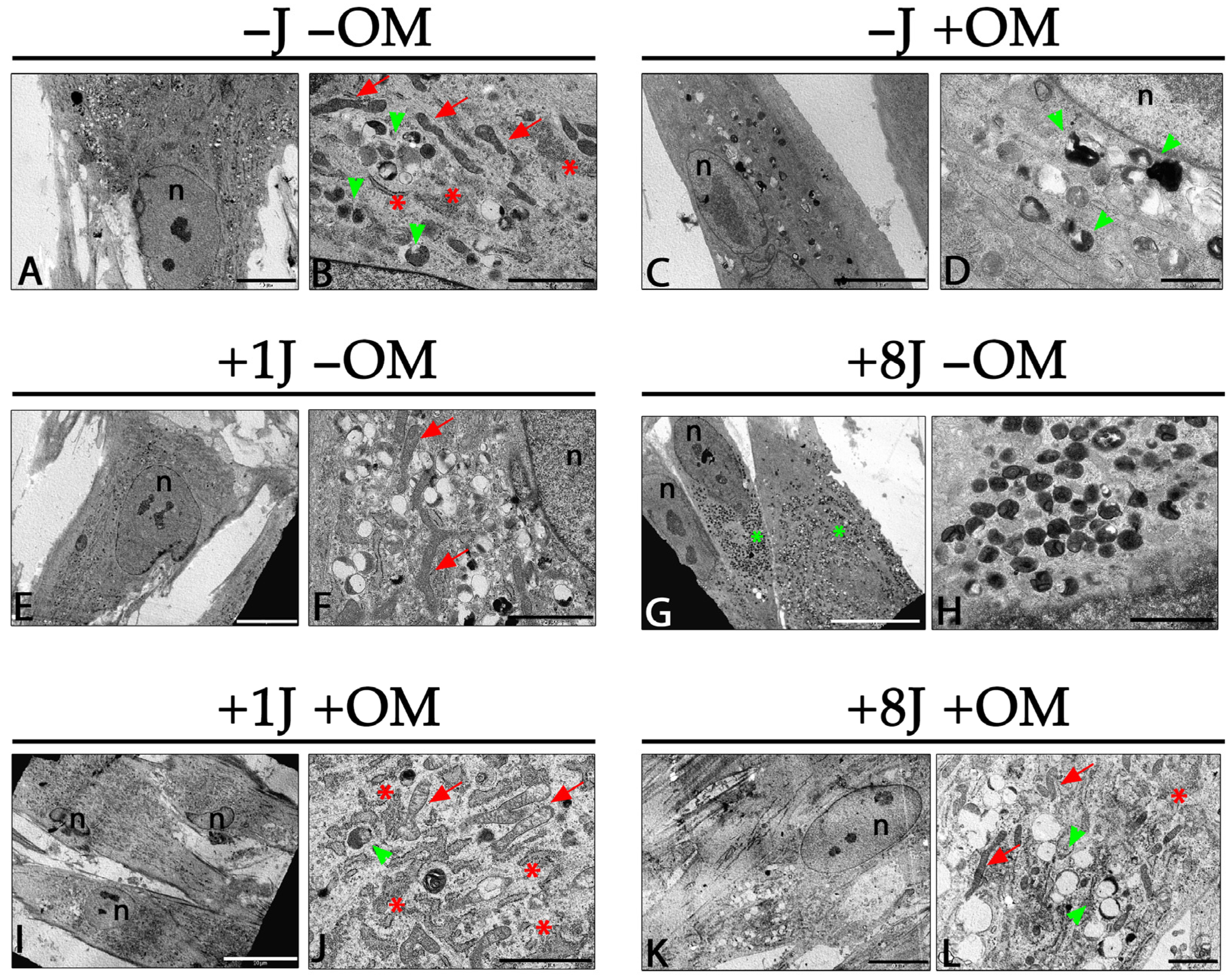
2.2. Ultrastructural Analysis Demonstrated a Strong Induction of Autophagy by PBM, Which Was Mitigated by Inducing Osteogenic Differentiation
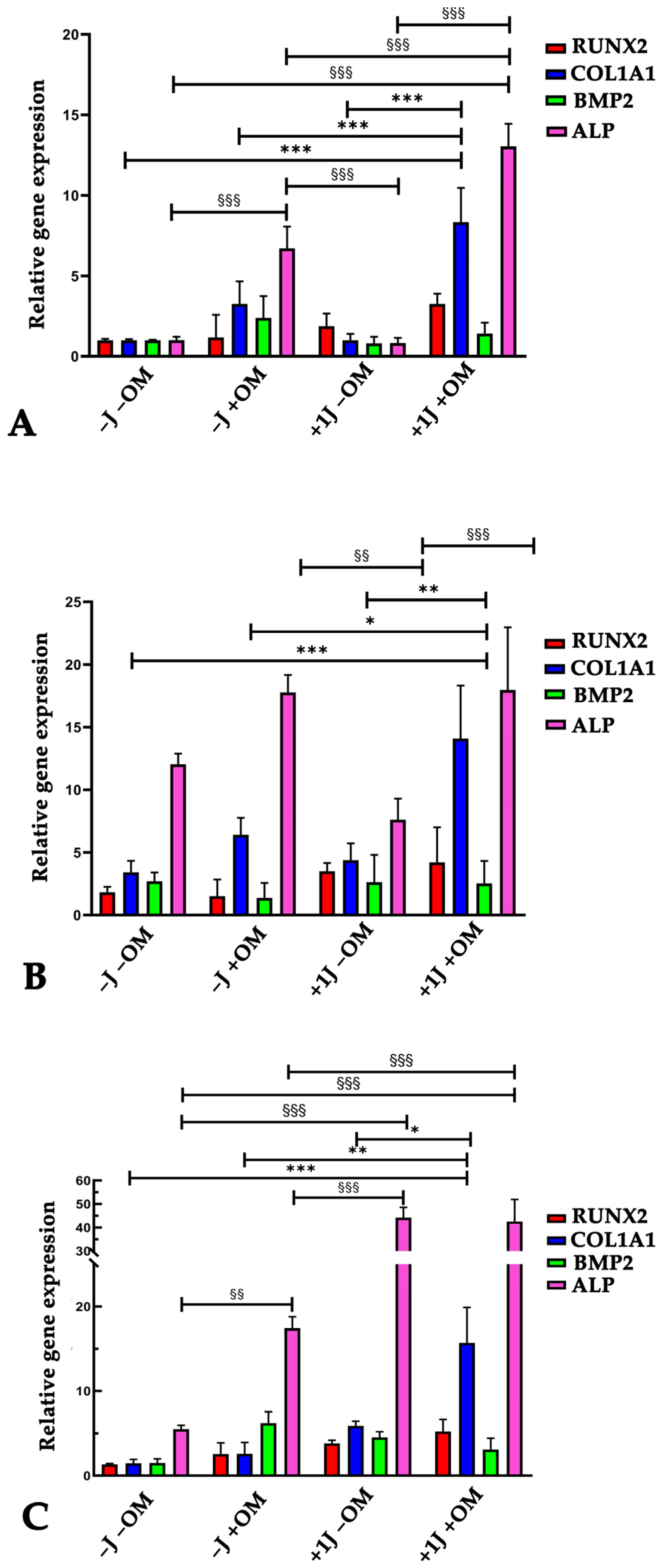
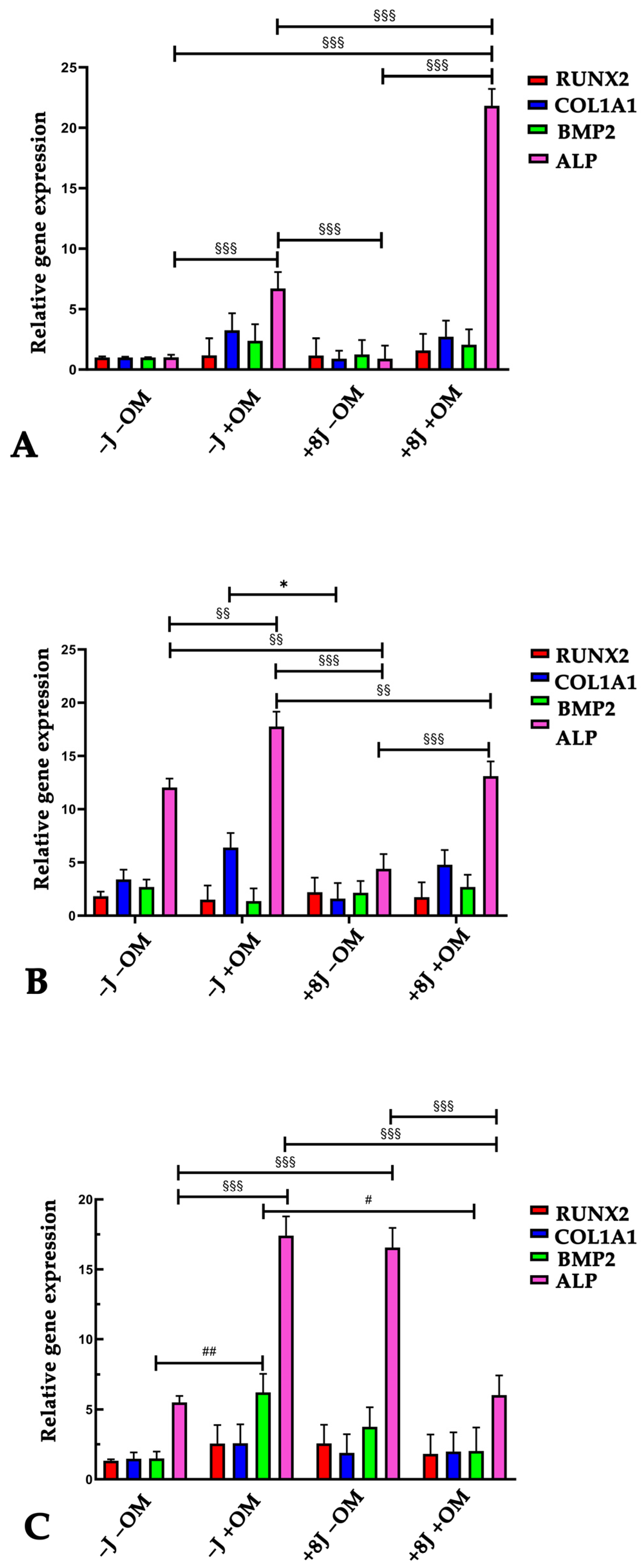
2.3. Laser Irradiation Enhanced the Ability of DP-MSCs to Differentiate Toward an Osteogenic Phenotype
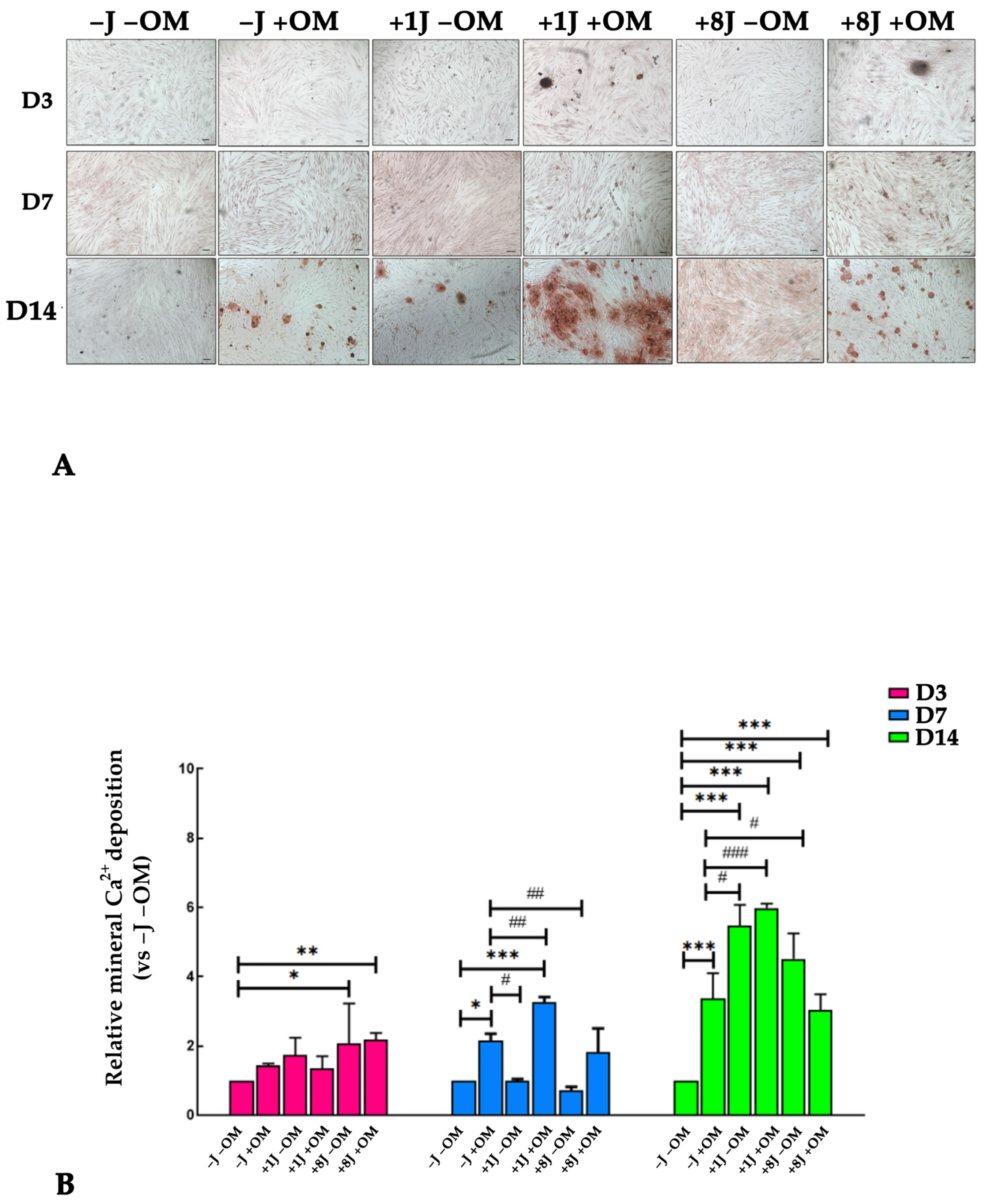
2.4. Laser Irradiation Enhanced the Ability of DP-MSCs to Deposit Mineral Crystals
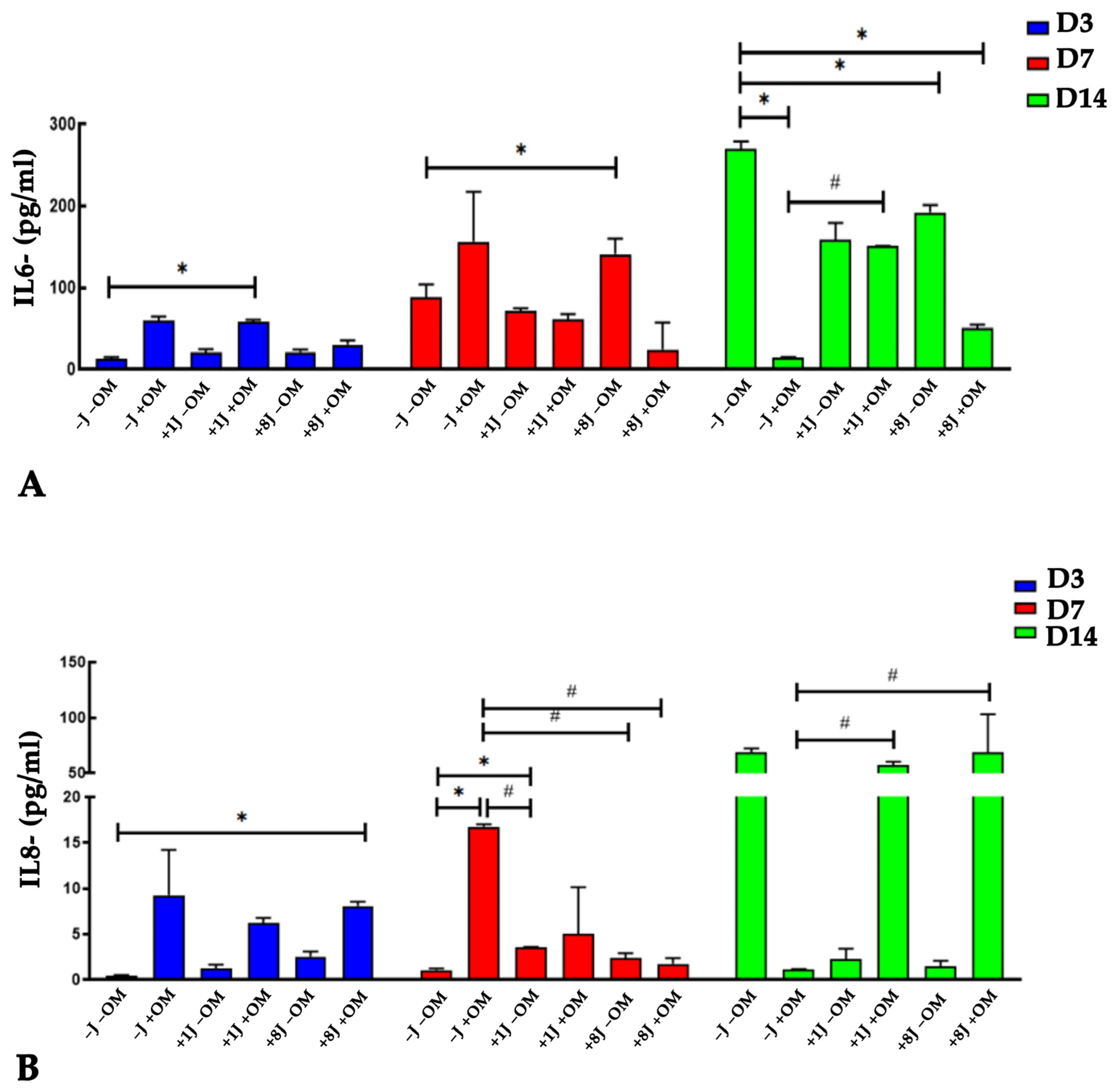
2.5. Laser Irradiation Regulated the Release of IL-6 and IL-8 Cytokines During Osteogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Human Dental Pulp Mesenchymal Stem/Stromal Cells (DP-MSCs)
4.2. PBM Irradiation Protocol and Sample Preparation
4.3. Cell Viability Assay
4.4. Osteogenic Differentiation
4.5. Transmission Electron Microscopy (TEM)
4.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Alizarin Red
4.8. IL-6 and IL-8 Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E. Photobiomodulation: A review of the molecular evidence for low level light therapy. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Pasternak-Mnich, K.; Ziemba, B.; Szwed, A.; Kopacz, K.; Synder, M.; Bryszewska, M.; Kujawa, J. Effect of Photobiomodulation Therapy on the Increase of Viability and Proliferation of Human Mesenchymal Stem Cells. Lasers Surg. Med. 2019, 51, 824–833. [Google Scholar] [CrossRef]
- Firoozi, P.; Amiri, M.A.; Soghli, N.; Farshidfar, N.; Hakimiha, N.; Fekrazad, R. The Role of Photobiomodulation on Dental-Derived Stem Cells in Regenerative Dentistry: A Comprehensive Systematic Review. Curr. Stem Cell Res. Ther. 2024, 19, 559–586. [Google Scholar] [CrossRef]
- Etemadi, A.; Sadatmansouri, S.; Sodeif, F.; Jalalishirazi, F.; Chiniforush, N. Photobiomodulation Effect of Different Diode Wavelengths on the Proliferation of Human Gingival Fibroblast Cells. Photochem. Photobiol. 2021, 97, 1123–1128. [Google Scholar] [CrossRef]
- AlShahrani, I.; Togoo, R.A.; Hosmani, J.; Alhaizaey, A. Photobiomodulation in acceleration of orthodontic tooth movement: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102220–102229. [Google Scholar] [CrossRef]
- Fernandes, M.R.U.; Suzuki, S.S.; Suzuki, H.; Martinez, E.F.; Garcez, A.S. Photobiomodulation increases intrusion tooth movement and modulates IL-6, IL-8 and IL-1β expression during orthodontically bone remodeling. J. Biophotonics 2019, 12, 201800311. [Google Scholar] [CrossRef]
- Martins, B.G.; de Moura, V.S.; Fujii, D.N.; Garcez, A.S.; Suzuki, S.S. Photobiomodulation stimulates surrounding bone formation and increases stability of titanium alloy miniscrews in ovariectomized rats. Lasers Med. Sci. 2022, 37, 2917–2924. [Google Scholar] [CrossRef]
- Kuffler, D.P. Photobiomodulation in promoting wound healing: A review. Regen. Med. 2016, 11, 107–122. [Google Scholar] [CrossRef]
- González-Muñoz, A.; Cuevas-Cervera, M.; Pérez-Montilla, J.J.; Aguilar-Núñez, D.; Hamed-Hamed, D.; Aguilar-García, M.; Pruimboom, L.; Navarro-Ledesma, S. Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare 2023, 11, 938. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation Dose Parameters in Dentistry: A Systematic Review and Meta-Analysis. Dent. J. 2020, 8, 114. [Google Scholar] [CrossRef]
- Engel, K.W.; Khan, I.; Arany, P.R. Cell lineage responses to photobiomodulation therapy. J. Biophotonics 2016, 9, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.; Fischer, B.V.; Vitali, F.C.; Santos, P.S.; Teixeira, C.D.S.; Queiroz, Í.O.A.; Sivieri-Araujo, G.; Dos Santos, P.H.; Garcia, L.D.F.R. Advances in laser-assisted regenerative endodontic procedures: A scoping review. J. Dent. 2025, 158, 105783–105792. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Chinigò, G.; Genova, T.; Munaron, L.; Mussano, F. Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines 2021, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells. 2019, 11, 347–374. [Google Scholar] [CrossRef]
- Alanazi, A.; Munir, H.; Alassiri, M.; Ward, L.S.C.; McGettrick, H.M.; Nash, G.B. Comparative adhesive and migratory properties of mesenchymal stem cells from different tissues. Biorheology 2019, 56, 15–30. [Google Scholar] [CrossRef]
- Zou, D.; Vigen, M.; Putnam, A.J.; Cao, C.; Tarlé, S.A.; Guinn, T.; Kaigler, D. Phenotypic, trophic, and regenerative properties of mesenchymal stem cells from different osseous tissues. Cell Tissue Res. 2022, 388, 75–88. [Google Scholar] [CrossRef]
- Poblano-Pérez, L.I.; Castro-Manrreza, M.E.; González-Alva, P.; Fajardo-Orduña, G.R.; Montesinos, J.J. Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential. Int. J. Mol. Sci. 2024, 25, 1986. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived from Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624–717644. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef]
- Gholami, L.; Khorsandi, K.; Nooshabadi, V.T.; Shahabi, S.; Jazaeri, M.; Esfahani, H.; Faradonbeh, D.R.; Malekshahi, Z.V.; Afsartala, Z.; Mostafa, N. Effect of Photobiomodulation on Structure and Function of Extracellular Vesicle Secreted from Mesenchymal Stem Cells. Photochem. Photobiol. 2022, 98, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int. 2015, 2015, 974864. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.H.C.; Mesquita-Ferrari, R.A.; Rodrigues, M.F.S.D.; da Silva, D.F.T.; Ribeiro, B.G.; Alves, A.N.; Garcia, M.P.; Nunes, F.D.; da Silva Junior, E.M.; França, C.M.; et al. Photobiomodulation and different macrophages phenotypes during muscle tissue repair. J. Cell Mol. Med. 2018, 22, 4922–4934. [Google Scholar] [CrossRef]
- Mineroff, J.; Maghfour, J.; Ozog, D.M.; Lim, H.W.; Kohli, I.; Jagdeo, J. Photobiomodulation CME part II: Clinical applications in dermatology. J. Am. Acad. Dermatol. 2024, 91, 805–815. [Google Scholar] [CrossRef]
- Fernandes, M.R.U.; Martinez, E.F.; Teti, G.; Suzuki, S.S.; Aranha, A.C.C. Effect of Simvastatin Combined with Photobiomodulation Therapy on Orthodontic Tooth Movement and the Expression of Metalloproteinases and Inhibitors—An Experimental Study. Lasers Med. Sci. 2025, 40, 191. [Google Scholar] [CrossRef]
- Aggarwal, I.; Lio, P.A. Photobiomodulation therapy and low-level light therapy in wound healing. Lasers Med. Sci. 2023, 38, 239. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.P.; de Jesus Guirro, R.R.; Orellana, M.D.; de Santis, G.C.; Farina Junior, J.A.; de Oliveira Guirro, E.C. Photobiomodulation with laser and led on mesenchymal stem cells viability and wound closure in vitro. Lasers Med. Sci. 2024, 39, 205. [Google Scholar] [CrossRef]
- Fekrazad, R.; Asefi, S.; Allahdadi, M.; Kalhori, K.A. Effect of Photobiomodulation on Mesenchymal Stem Cells. Photomed. Laser Surg. 2016, 34, 533–542. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Benedicenti, S.; Marchegiani, A.; Sabbieti, M.G.; Agas, D. Steering the multipotent mesenchymal cells towards an anti-inflammatory and osteogenic bias via photobiomodulation therapy: How to kill two birds with one stone. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef]
- Marques, M.M.; Diniz, I.M.; de Cara, S.P.; Pedroni, A.C.; Abe, G.L.; D’Almeida-Couto, R.S.; Lima, P.L.; Tedesco, T.K.; Moreira, M.S. Photobiomodulation of Dental Derived Mesenchymal Stem Cells: A Systematic Review. Photomed. Laser Surg. 2016, 34, 500–508. [Google Scholar] [CrossRef]
- Bayat, M.; Asgari, M.; Abdollahifar, M.A.; Moradi, A.; Zare, F.; Kouhkheil, R.; Gazor, R.; Ebrahiminia, A.; Karbasaraea, Z.S.; Chien, S. Photobiomodulation and mesenchymal stem cell-conditioned medium for the repair of experimental critical-size defects. Lasers Med. Sci. 2024, 39, 158. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, E.A.; Alvarez, M.M.P.; Pelosine, A.M.; Carrilho, M.R.O.; Tersariol, I.L.S.; Nascimento, F.D. Laser Photobiomodulation 808 nm: Effects on Gene Expression in Inflammatory and Osteogenic Biomarkers in Human Dental Pulp Stem Cells. Front. Pharmacol. 2022, 12, 782095. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmar, A.A.; Abuarqoub, D.; Ababneh, N.; Jafar, H.; Zalloum, S.; Ismail, M.; Arany, P.; Awidi, A. Potential Effects of Photobiomodulation Therapy on Human Dental Pulp Stem Cells. Appl. Sci. 2024, 14, 124. [Google Scholar] [CrossRef]
- Etemadi, A.; Aghaie, M.; Sayar, F.; Chiniforush, N. Effect of photobiomodulation therapy with 660 and 980 nm diode lasers on differentiation of periodontal ligament mesenchymal stem cells. Sci. Rep. 2024, 14, 20587. [Google Scholar] [CrossRef]
- Barboza, C.A.; Ginani, F.; Soares, D.M.; Henriques, A.C.; Freitas Rde, A. Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein 2014, 12, 75–81. [Google Scholar] [CrossRef]
- Rahmati, A.; Abbasi, R.; Najafi, R.; Rezaei-Soufi, L.; Karkehabadi, H. Effect of diode low level laser and red light emitting diode irradiation on cell proliferation and osteogenic/odontogenic differentiation of stem cells from the apical papilla. BMC Oral Health 2022, 22, 543. [Google Scholar] [CrossRef]
- Teti, G.; Cavallo, C.; Grigolo, B.; Giannini, S.; Facchini, A.; Mazzotti, A.; Falconi, M. Ultrastructural analysis of human bone marrow mesenchymal stem cells during in vitro osteogenesis and chondrogenesis. Microsc. Res. Tech. 2012, 75, 596–604. [Google Scholar] [CrossRef]
- Moreno-Blas, D.; Adell, T.; González-Estévez, C. Autophagy in Tissue Repair and Regeneration. Cells 2025, 14, 282. [Google Scholar] [CrossRef]
- Rossin, D.; Perrelli, M.G.; Lo Iacono, M.; Rastaldo, R.; Giachino, C. Dynamic Interplay Between Autophagy and Oxidative Stress in Stem. Cells: Implications for Regenerative Medicine. Antioxidants 2025, 14, 691. [Google Scholar] [CrossRef]
- Hong, H.H.; Hong, A.; Wang, C.C.; Huang, E.W.; Chiang, C.C.; Yen, T.H.; Huang, Y.F. Calcitriol exerts a mineralization-inductive effect comparable to that of vitamin C in cultured human periodontium cells. Am. J. Transl. Res. 2019, 11, 2304–2316. [Google Scholar] [PubMed]
- Abdelgawad, L.M.; Abdelaziz, A.M.; Sabry, D.; Abdelgwad, M. Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression. Biomol. Concepts. 2020, 11, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ye, Y.; Shi, X.; Li, N.; Chen, Y.; Shi, X.; Chen, H. Photobiomodulation promotes osteogenic differentiation of mesenchymal stem cells and increases P-Akt levels in vitro. Sci. Rep. 2025, 15, 17844. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.P.; Hertel, F.C.; Fernandes, E.; Oliveira, H.F.; Arany, P.; Beloti, M.M.; Marques, M.M.; Ferraz, E.P. Enhancing osteoblast differentiation and bone repair: The priming effect of photobiomodulation on adipose stromal cells. J. Photochem. Photobiol. B Biol. 2024, 260, 113040. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, F.; Chen, X.; Luo, M.; Hu, L.; Lu, Y. The Role of Mitochondria-Derived Reactive Oxygen Species in Hyperthermia-Induced Platelet Apoptosis. PLoS ONE 2013, 8, e75044. [Google Scholar] [CrossRef]
- Turrioni, A.P.; Basso, F.G.; Montoro, L.A.; Almeida Lde, F.; Costa, C.A.; Hebling, J. Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J. Dent. 2014, 42, 1292–1299. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Milward, M.R.; Hadis, M.A.; Kuehne, S.A.; Cooper, P.R. Photobiomodulation of mineralisation in mesenchymal stem cells. Photochem. Photobiol. Sci. 2021, 20, 699–714. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Gerosa, L.; Banfi, G.; Lombardi, G. The bone-muscle unit: From mechanical coupling to soluble factors-mediated signaling. Mol. Aspects Med. 2025, 103, 101367. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Danz, J.C.; Degen, M. Selective modulation of the bone remodeling regulatory system through orthodontic tooth movement—A review. Front. Oral Health 2025, 6, 1472711. [Google Scholar] [CrossRef]
| (A) | ||
|---|---|---|
| Group | 1J | OM |
| 1 | − | − |
| 2 | − | + |
| 3 | + | − |
| 4 | + | + |
| (B) | ||
| Group | 8J | OM |
| 1 | − | − |
| 2 | − | + |
| 3 | + | − |
| 4 | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.R.U.; Teti, G.; Gatta, V.; Longhin, A.; Aranha, A.C.C.; Falconi, M. Impact of Photobiomodulation on the Pro-Osteogenic Activity of Dental Pulp Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2025, 26, 8174. https://doi.org/10.3390/ijms26178174
Fernandes MRU, Teti G, Gatta V, Longhin A, Aranha ACC, Falconi M. Impact of Photobiomodulation on the Pro-Osteogenic Activity of Dental Pulp Mesenchymal Stem/Stromal Cells. International Journal of Molecular Sciences. 2025; 26(17):8174. https://doi.org/10.3390/ijms26178174
Chicago/Turabian StyleFernandes, Marcella Rodrigues Ueda, Gabriella Teti, Valentina Gatta, Aurora Longhin, Ana Cecilia Corrêa Aranha, and Mirella Falconi. 2025. "Impact of Photobiomodulation on the Pro-Osteogenic Activity of Dental Pulp Mesenchymal Stem/Stromal Cells" International Journal of Molecular Sciences 26, no. 17: 8174. https://doi.org/10.3390/ijms26178174
APA StyleFernandes, M. R. U., Teti, G., Gatta, V., Longhin, A., Aranha, A. C. C., & Falconi, M. (2025). Impact of Photobiomodulation on the Pro-Osteogenic Activity of Dental Pulp Mesenchymal Stem/Stromal Cells. International Journal of Molecular Sciences, 26(17), 8174. https://doi.org/10.3390/ijms26178174






