The Role of Translation-Associated Proteins in p53 Modulation: Mechanisms and Implications
Abstract
1. Introduction: Translation and miRNA
2. Ribosome Biogenesis and Its Role in Cellular Function
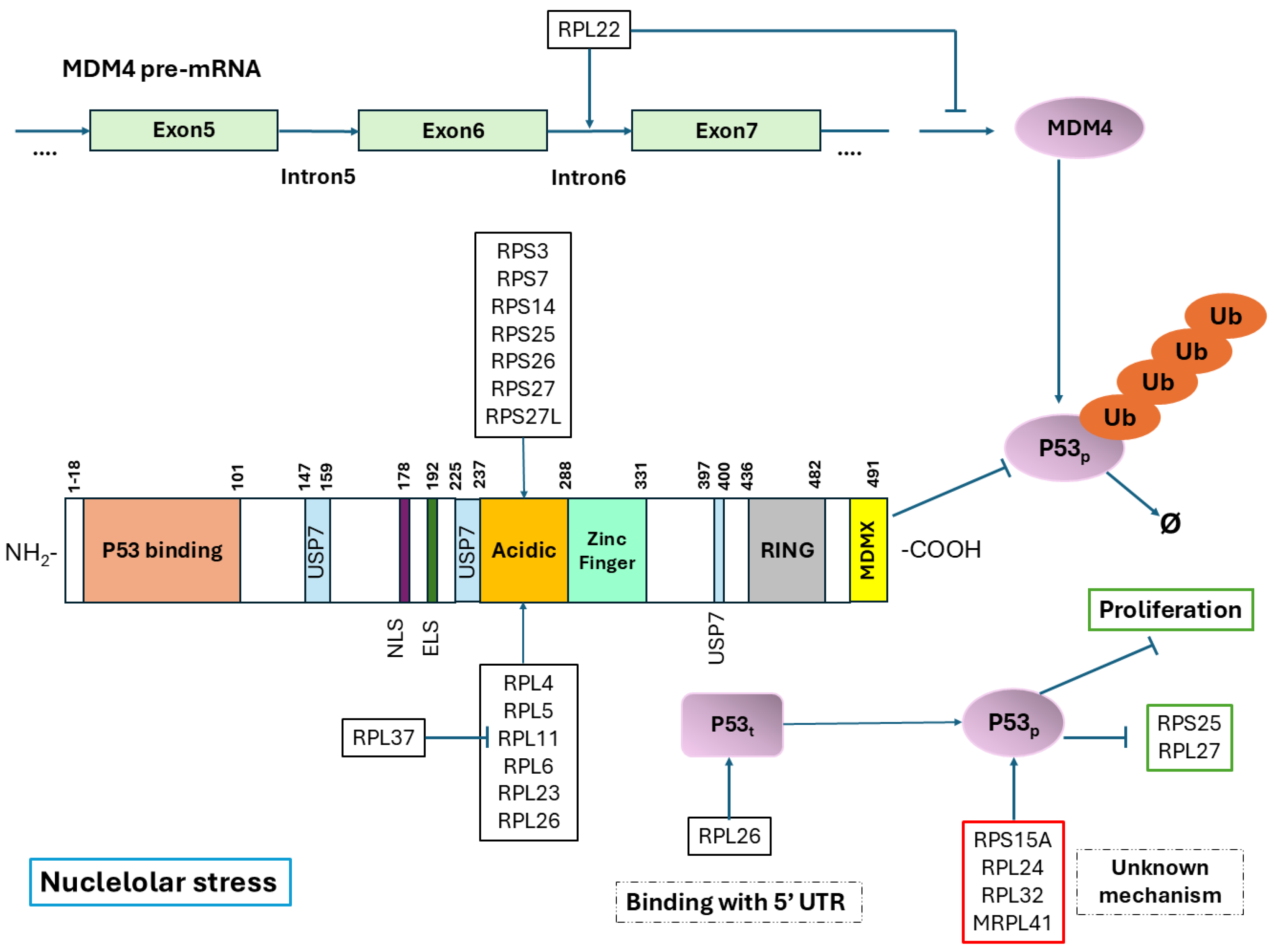
3. Ribosomal Proteins and p53 Regulation
4. Regulation of p53 with Ribosomal Proteins
4.1. Small Ribosomal Subunit Proteins
4.2. Large Ribosomal Subunit Proteins
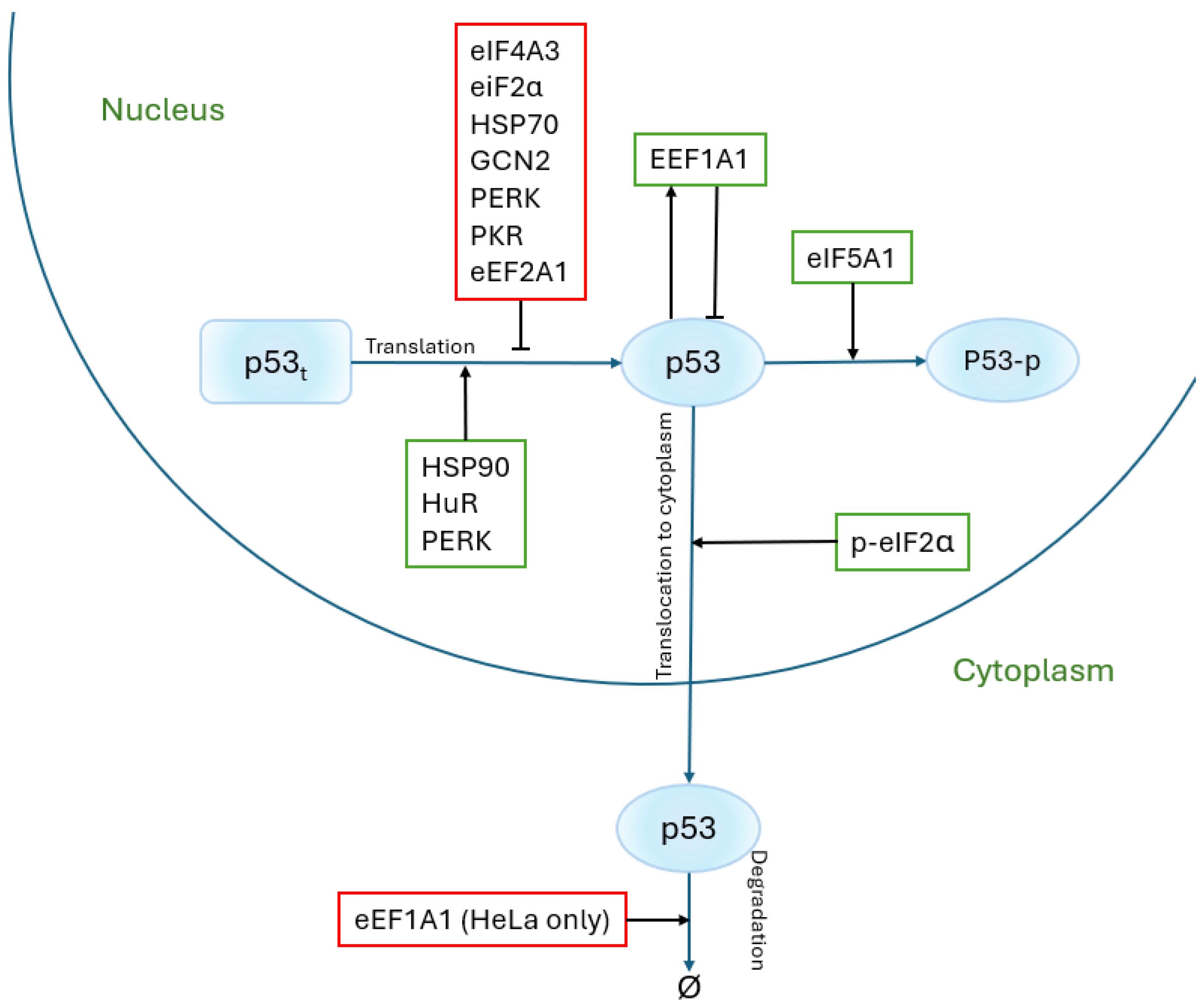
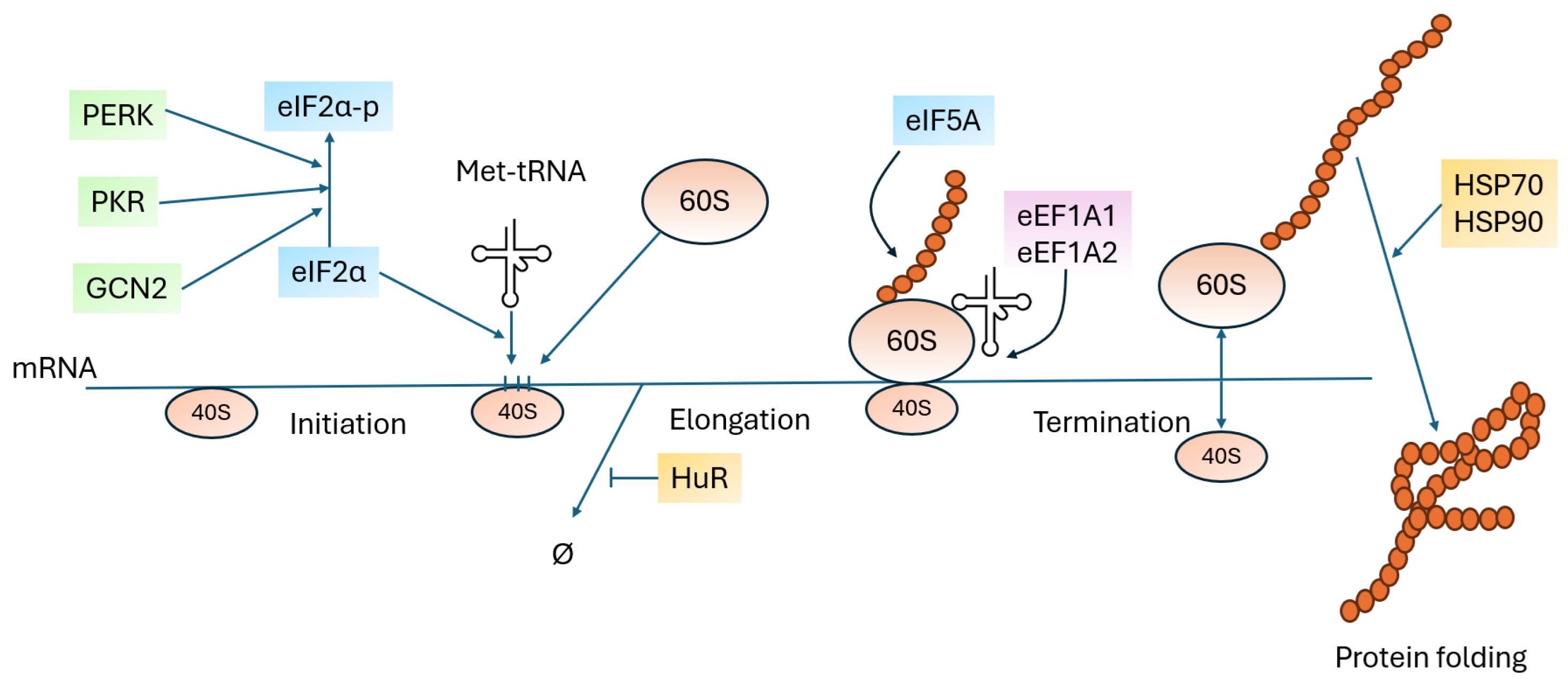
5. Other Proteins Involved in Translation
6. Conclusions
Funding
Conflicts of Interest
References
- Fonseca, B.D.; Smith, E.M.; Yelle, N.; Alain, T.; Bushell, M.; Pause, A. The ever-evolving role of mTOR in translation. Semin. Cell Dev. Biol. 2014, 36, 102–112. [Google Scholar] [CrossRef]
- Russo, A.; Russo, G. Ribosomal proteins control or bypass p53 during nucleolar stress. Int. J. Mol. Sci. 2017, 18, 140. [Google Scholar] [CrossRef]
- Pelletier, J.; Thomas, G.; Volarević, S. Ribosome biogenesis in cancer: New players and therapeutic avenues. Nat. Rev. Cancer 2018, 18, 51–63. [Google Scholar] [CrossRef]
- Stults, D.M.; Killen, M.W.; Pierce, H.H.; Pierce, A.J. Genomic architecture and inheritance of human ribosomal RNA gene clusters. Genome Res. 2008, 18, 13–18. [Google Scholar] [CrossRef]
- Castillo Duque de Estrada, N.M.; Thoms, M.; Flemming, D.; Hammaren, H.M.; Buschauer, R.; Ameismeier, M.; Baßler, J.; Beck, M.; Beckmann, R.; Hurt, E. Structure of nascent 5S RNPs at the crossroad between ribosome assembly and MDM2–p53 pathways. Nat. Struct. Mol. Biol. 2023, 30, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.; Ben-Shem, A.; De Loubresse, N.G.; Jenner, L.; Yusupova, G.; Yusupov, M. One core, two shells: Bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 2012, 19, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, D.L. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat. Struct. Mol. Biol. 2015, 22, 11–19. [Google Scholar] [CrossRef]
- Nicolas, E.; Parisot, P.; Pinto-Monteiro, C.; De Walque, R.; De Vleeschouwer, C.; Lafontaine, D.L. Involvement of human ribosomal proteins in nucleolar structure and p53-dependent nucleolar stress. Nat. Commun. 2016, 7, 11390. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Montanaro, L.; Derenzini, M. Ribosome biogenesis and control of cell proliferation: p53 is not alone. Cancer Res. 2012, 72, 1602–1607. [Google Scholar] [CrossRef]
- Golomb, L.; Volarevic, S.; Oren, M. p53 and ribosome biogenesis stress: The essentials. FEBS Lett. 2014, 588, 2571–2579. [Google Scholar] [CrossRef]
- Deisenroth, C.; Zhang, Y. Ribosome biogenesis surveillance: Probing the ribosomal protein-Mdm2-p53 pathway. Oncogene 2010, 29, 4253–4260. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H. Signaling to p53: Ribosomal proteins find their way. Cancer Cell 2009, 16, 369–377. [Google Scholar] [CrossRef]
- Lindström, M.S.; Jin, A.; Deisenroth, C.; White Wolf, G.; Zhang, Y. Cancer-associated mutations in the MDM2 zinc finger domain disrupt ribosomal protein interaction and attenuate MDM2-induced p53 degradation. Mol. Cell. Biol. 2007, 27, 1056–1068. [Google Scholar] [CrossRef]
- Liu, Y.; Deisenroth, C.; Zhang, Y. RP–MDM2–p53 pathway: Linking ribosomal biogenesis and tumor surveillance. Trends Cancer 2016, 2, 191–204. [Google Scholar] [CrossRef]
- Hannan, K.M.; Soo, P.; Wong, M.S.; Lee, J.K.; Hein, N.; Poh, P.; Wysoke, K.D.; Williams, T.D.; Montellese, C.; Smith, L.K.; et al. Nuclear stabilization of p53 requires a functional nucleolar surveillance pathway. Cell Rep. 2022, 41, 111571. [Google Scholar] [CrossRef]
- Sloan, K.E.; Bohnsack, M.T.; Watkins, N.J. The 5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar stress. Cell Rep. 2013, 5, 237–247. [Google Scholar] [CrossRef]
- Donati, G.; Peddigari, S.; Mercer, C.A.; Thomas, G. 5S ribosomal RNA is an essential component of a nascent ribosomal precursor complex that regulates the Hdm2-p53 checkpoint. Cell Rep. 2013, 4, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Pelava, A.; Schneider, C.; Watkins, N.J. The importance of ribosome production, and the 5S RNP–MDM2 pathway, in health and disease. Biochem. Soc. Trans. 2016, 44, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Ivanenkov, V.V.; Teng, T.; Thomas, G. Suprainduction of p53 by disruption of 40S and 60S ribosome biogenesis leads to the activation of a novel G2/M checkpoint. Genes Dev. 2012, 26, 1028–1040. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.; Shin, S.C.; Kim, J.H.; Kim, E.E.; Song, E.J. Ribosomal protein S2 interplays with MDM2 to induce p53. Biochem. Biophys. Res. Commun. 2020, 523, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Yadavilli, S.; Mayo, L.D.; Higgins, M.; Lain, S.; Hegde, V.; Deutsch, W.A. Ribosomal protein S3: A multi-functional protein that interacts with both p53 and MDM2 through its KH domain. DNA Repair 2009, 8, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Alam, E.; Maaliki, L.; Nasr, Z. Ribosomal protein S3 selectively affects colon cancer growth by modulating the levels of p53 and lactate dehydrogenase. Mol. Biol. Rep. 2020, 47, 6083–6090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Poyurovsky, M.V.; Li, Y.; Biderman, L.; Stahl, J.; Jacq, X.; Prives, C. Ribosomal protein S7 is both a regulator and a substrate of MDM2. Mol. Cell 2009, 35, 316–326. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Z.; Li, M.; Wang, W.; Li, Y.; Rayburn, E.; Hill, D.; Wang, H.; Zhang, R. Ribosomal protein S7 as a novel modulator of p53–MDM2 interaction: Binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene 2007, 26, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Q.; Liao, J.m.; Zhang, Q.; Lu, H. Ribosomal protein S14 unties the MDM2–p53 loop upon ribosomal stress. Oncogene 2013, 32, 388–396. [Google Scholar] [CrossRef]
- Hu, S.; Cai, J.; Fang, H.; Chen, Z.; Zhang, J.; Cai, R. RPS14 promotes the development and progression of glioma via p53 signaling pathway. Exp. Cell Res. 2023, 423, 113451. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Feng, Q.; Ren, L.; He, G.; Chang, W.; Zhu, D.; Yi, T.; Lin, Q.; Tang, W.; et al. Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int. J. Oncol. 2016, 48, 1628–1638. [Google Scholar] [CrossRef]
- Han, L.; Huo, Y.; Huang, L.; Zheng, Y.; Yu, X.; Zhang, N.; Yang, M. Genome-wide functional integration identified MAZ-controlled RPS14 dysregulation in hepatocellular carcinoma. Arch. Toxicol. 2024, 98, 985–997. [Google Scholar] [CrossRef]
- Li, X.; Di, S.; Nie, F.; Meng, W.; Huang, S. RPS15a knockdown impedes the progression of B-ALL by inducing p53-mediated nucleolar stress. Biochem. Biophys. Res. Commun. 2025, 763, 151768. [Google Scholar] [CrossRef]
- Zhang, H.M.; Li, H.N.; Qi, E.; Yang, Y.; Ma, H.; Ma, J.; Xiong, X.; Wang, J.; Yang, Z.F.; Liao, X.H. GGCT Inhibits Ferroptosis in PTC Cells by Upregulating p53 Through RPS15A. Cancer Sci. 2025, 116, 1592–1603. [Google Scholar] [CrossRef]
- Adilakshmi, T.; Laine, R.O. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J. Biol. Chem. 2002, 277, 4147–4151. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, H.; Wang, M.; Xu, W.; Zhang, R. Identification of ribosomal protein S25 (RPS25)–MDM2–p53 regulatory feedback loop. Oncogene 2013, 32, 2782–2791. [Google Scholar] [CrossRef]
- Xu, D.; Pan, J.; Fang, Y.; Zhao, L.; Su, Y. RpS25 is required for sperm elongation and individualization during Drosophila spermatogenesis. Biochem. Biophys. Res. Commun. 2024, 702, 149633. [Google Scholar] [CrossRef]
- Cui, D.; Li, L.; Lou, H.; Sun, H.; Ngai, S.; Shao, G.; Tang, J. The ribosomal protein S26 regulates p53 activity in response to DNA damage. Oncogene 2014, 33, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, Y. Ribosomal protein S27L is a direct p53 target that regulates apoptosis. Oncogene 2007, 26, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, Y.; He, H.; Sun, Y. Ribosomal protein S27-like and S27 interplay with p53-MDM2 axis as a target, a substrate and a regulator. Oncogene 2011, 30, 1798–1811. [Google Scholar] [CrossRef]
- Li, J.; Tan, J.; Zhuang, L.; Banerjee, B.; Yang, X.; Chau, J.F.L.; Lee, P.L.; Hande, M.P.; Li, B.; Yu, Q. Ribosomal protein S27-like, a p53-inducible modulator of cell fate in response to genotoxic stress. Cancer Res. 2007, 67, 11317–11326. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, Y.; Tang, F.; Wei, D.; Thomas, D.; Wang, X.; Liu, Y.; Zheng, P.; Sun, Y. Ribosomal protein S27-like is a physiological regulator of p53 that suppresses genomic instability and tumorigenesis. eLife 2014, 3, e02236. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, M.; Liu, X.; Xiong, X.; Sun, Y. Inactivation of ribosomal protein S27-like confers radiosensitivity via the Mdm2-p53 and Mdm2–MRN–ATM axes. Cell Death Dis. 2018, 9, 145. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Dai, M.S.; Sun, X.X. Ribosomal protein L4 is a novel regulator of the MDM2-p53 loop. Oncotarget 2016, 7, 16217. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Xu, K.; Zhang, C.; Wang, F.; Zhang, R.; Zhu, P. PRC1 promotes ovarian cancer progression by binding to RPL4 and increasing MDM2-mediated p53 ubiquitination. Exp. Cell Res. 2025, 447, 114509. [Google Scholar] [CrossRef]
- Teng, Y.; Lin, H.; Lin, Z.; Li, X.; Ruan, Y.; Pan, B.; Ge, J.; Zhu, Y.; Lin, D.; Ying, Q.; et al. CCT8 drives colorectal cancer progression via the RPL4-MDM2-p53 axis and immune modulation. BMC Med. Genom. 2025, 18, 77. [Google Scholar] [CrossRef]
- Bai, D.; Zhang, J.; Xiao, W.; Zheng, X. Regulation of the HDM2-p53 pathway by ribosomal protein L6 in response to ribosomal stress. Nucleic Acids Res. 2014, 42, 1799–1811. [Google Scholar] [CrossRef]
- Lei, Z.; Luo, Y.; Fu, Q.; Lu, J.; Wang, C.; Zhang, L.; Zhang, Z. Ribosomal protein L6 suppresses hepatocellular carcinoma by modulating FBXO22-mediated p53 degradation. Cell. Signal. 2025, 127, 111612. [Google Scholar] [CrossRef]
- Jansen, J.; Bohnsack, K.E.; Böhlken-Fascher, S.; Bohnsack, M.T.; Dobbelstein, M. The ribosomal protein L22 binds the MDM4 pre-mRNA and promotes exon skipping to activate p53 upon nucleolar stress. Cell Rep. 2024, 43, 114610. [Google Scholar] [CrossRef]
- Jin, A.; Itahana, K.; O’Keefe, K.; Zhang, Y. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol. Cell. Biol. 2004, 24, 7669–7680. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zheng, L. Differential genes and pathogenesis of sepsis-induced lung injury data analysis. Meds Clin. Med. 2023, 4, 128–131. [Google Scholar]
- Meng, X.; Tackmann, N.R.; Liu, S.; Yang, J.; Dong, J.; Wu, C.; Cox, A.D.; Zhang, Y. RPL23 links oncogenic RAS signaling to p53-mediated tumor suppression. Cancer Res. 2016, 76, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Ming, C.; Bai, X.; Zhao, L.; Yu, D.; Wang, X.; Wu, Y. RPL24 as a potential prognostic biomarker for cervical cancer treated by Cisplatin and concurrent chemoradiotherapy. Front. Oncol. 2023, 13, 1131803. [Google Scholar] [CrossRef]
- Ofir-Rosenfeld, Y.; Boggs, K.; Michael, D.; Kastan, M.B.; Oren, M. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol. Cell 2008, 32, 180–189. [Google Scholar] [CrossRef]
- Chen, J.; Guo, K.; Kastan, M.B. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 2012, 287, 16467–16476. [Google Scholar] [CrossRef]
- Takagi, M.; Absalon, M.J.; McLure, K.G.; Kastan, M.B. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 2005, 123, 49–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Yuan, Y.; Zhang, W.; Guan, W.; Wu, Z.; Jin, C.; Chen, H.; Zhang, L.; Yang, X.; et al. Negative regulation of HDM2 to attenuate p53 degradation by ribosomal protein L26. Nucleic Acids Res. 2010, 38, 6544–6554. [Google Scholar] [CrossRef]
- Reinhardt, L.S.; Groen, K.; Newton, C.; Avery-Kiejda, K.A. The role of truncated p53 isoforms in the DNA damage response. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1878, 188882. [Google Scholar]
- Xie, J.; Zhang, W.; Liang, X.; Shuai, C.; Zhou, Y.; Pan, H.; Yang, Y.; Han, W. RPL32 promotes lung cancer progression by facilitating p53 degradation. Mol.-Ther.-Nucleic Acids 2020, 21, 75–85. [Google Scholar] [CrossRef]
- Llanos, S.; Serrano, M. Depletion of ribosomal protein L37 occurs in response to DNA damage and activates p53 through the L11/MDM2 pathway. Cell Cycle 2010, 9, 4005–4012. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.A.; Kim, M.J.; Park, J.K.; Chung, Y.M.; Lee, J.H.; Chi, S.G.; Kim, J.S.; Do Yoo, Y. Mitochondrial ribosomal protein L41 suppresses cell growth in association with p53 and p27Kip1. Mol. Cell. Biol. 2005, 25, 6603–6616, Erratum in Mol. Cell. Biol. 2005, 25, 2072. [Google Scholar] [CrossRef]
- Guo, H.; Dong, Y.; Luo, D.; Gong, M.; Sun, J.; Wu, Z.; Liu, Z.; Zhong, L.; Jin, S. MRPL41, as a target for acupuncture, promotes neuron apoptosis in models of ischemic stroke via activating p53 pathway. Neurochem. Int. 2024, 180, 105881. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Anderson, D.E.; Barnitz, R.A.; Snow, A.; Bidere, N.; Zheng, L.; Hegde, V.; Lam, L.T.; Staudt, L.M.; Levens, D.; et al. Ribosomal protein S3: A KH domain subunit in NF-κB complexes that mediates selective gene regulation. Cell 2007, 131, 927–939. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Malygin, A.A.; Karpova, G.G. Human ribosomal protein S26 suppresses the splicing of its pre-mRNA. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2005, 1727, 134–140. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Zheng, D.; Liu, Y.J.; Fang, G.; Frankish, A.; Carriero, N.; Robilotto, R.; Cayting, P.; Gerstein, M. Comparative analysis of processed ribosomal protein pseudogenes in four mammalian genomes. Genome Biol. 2009, 10, R2. [Google Scholar] [CrossRef]
- Sun, X.X.; DeVine, T.; Challagundla, K.B.; Dai, M.S. Interplay between ribosomal protein S27a and MDM2 protein in p53 activation in response to ribosomal stress. J. Biol. Chem. 2011, 286, 22730–22741. [Google Scholar] [CrossRef]
- Dai, M.S.; Zeng, S.X.; Jin, Y.; Sun, X.X.; David, L.; Lu, H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell. Biol. 2004, 24, 7654–7668. [Google Scholar] [CrossRef]
- Dai, M.S.; Shi, D.; Jin, Y.; Sun, X.X.; Zhang, Y.; Grossman, S.R.; Lu, H. Regulation of the MDM2-p53 pathway by ribosomal protein L11 involves a post-ubiquitination mechanism. J. Biol. Chem. 2006, 281, 24304–24313. [Google Scholar] [CrossRef]
- Chen, J.; Kastan, M.B. 5′–3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes Dev. 2010, 24, 2146–2156. [Google Scholar] [CrossRef]
- Kimball, S.R. Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 1999, 31, 25–29. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, Y.S. Phosphorylation of eIF2α attenuates statin-induced apoptosis by inhibiting the stabilization and translocation of p53 to the mitochondria. Int. J. Oncol. 2013, 42, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, D.C.; Espinoza, J.A.; Zisi, A.; Sakkas, E.; Bartkova, J.; Katsori, A.M.; Boström, J.; Dyrskjøt, L.; Broholm, H.; Altun, M.; et al. The exon-junction complex helicase eIF4A3 controls cell fate via coordinated regulation of ribosome biogenesis and translational output. Sci. Adv. 2021, 7, eabf7561. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.P.; Wu, C.C.C.; Dever, T.E.; Buskirk, A.R.; Green, R. eIF5A functions globally in translation elongation and termination. Mol. Cell 2017, 66, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Li, H.Y.; Jin, B.F.; Ye, Q.N.; Zhou, T.; Yu, X.D.; Pan, X.; Man, J.H.; He, K.; Yu, M.; et al. A novel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis. J. Biol. Chem. 2004, 279, 49251–49258. [Google Scholar] [CrossRef]
- Jiang, X.; Baig, A.H.; Palazzo, G.; Del Pizzo, R.; Bortecen, T.; Groessl, S.; Zaal, E.A.; Amaya Ramirez, C.C.; Kowar, A.; Aviles-Huerta, D.; et al. P53-dependent hypusination of eIF5A affects mitochondrial translation and senescence immune surveillance. Nat. Commun. 2024, 15, 7458. [Google Scholar] [CrossRef]
- Martella, M.; Catalanotto, C.; Talora, C.; La Teana, A.; Londei, P.; Benelli, D. Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination suppress p53 translation and alters the association of eIF5A to the ribosomes. Int. J. Mol. Sci. 2020, 21, 4583. [Google Scholar] [CrossRef]
- Blanch, A.; Robinson, F.; Watson, I.R.; Cheng, L.S.; Irwin, M.S. Eukaryotic translation elongation factor 1-alpha 1 inhibits p53 and p73 dependent apoptosis and chemotherapy sensitivity. PLoS ONE 2013, 8, e66436. [Google Scholar] [CrossRef]
- Pellegrino, R.; Calvisi, D.F.; Neumann, O.; Kolluru, V.; Wesely, J.; Chen, X.; Wang, C.; Wuestefeld, T.; Ladu, S.; Elgohary, N.; et al. EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent stabilization of MDM4 in hepatocellular carcinoma. Hepatology 2014, 59, 1886–1899. [Google Scholar] [CrossRef]
- Kato, M.V.; Sato, H.; Nagayoshi, M.; Ikawa, Y. Upregulation of the elongation factor-1α gene by p53 in association with death of an erythroleukemic cell line. Blood J. Am. Soc. Hematol. 1997, 90, 1373–1378. [Google Scholar]
- Walters, R.W.; Parker, R. Coupling of ribostasis and proteostasis: Hsp70 proteins in mRNA metabolism. Trends Biochem. Sci. 2015, 40, 552–559. [Google Scholar] [CrossRef]
- Boysen, M.; Kityk, R.; Mayer, M.P. Hsp70-and Hsp90-mediated regulation of the conformation of p53 DNA binding domain and p53 cancer variants. Mol. Cell 2019, 74, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, N.; Guo, J.; Xia, J.; Ruan, Y. Dysregulation of TTP and HuR plays an important role in cancers. Tumor Biol. 2016, 37, 14451–14461. [Google Scholar] [CrossRef]
- Ouhara, K.; Munenaga, S.; Kajiya, M.; Takeda, K.; Matsuda, S.; Sato, Y.; Hamamoto, Y.; Iwata, T.; Yamasaki, S.; Akutagawa, K.; et al. The induced RNA-binding protein, HuR, targets 3′-UTR region of IL-6 mRNA and enhances its stabilization in periodontitis. Clin. Exp. Immunol. 2018, 192, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Mazan-Mamczarz, K.; Galbán, S.; de Silanes, I.L.; Martindale, J.L.; Atasoy, U.; Keene, J.D.; Gorospe, M. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. USA 2003, 100, 8354–8359. [Google Scholar] [CrossRef]
- Nakamura, H.; Kawagishi, H.; Watanabe, A.; Sugimoto, K.; Maruyama, M.; Sugimoto, M. Cooperative role of the RNA-binding proteins Hzf and HuR in p53 activation. Mol. Cell. Biol. 2011, 31, 1997–2009. [Google Scholar] [CrossRef]
- Nakamura, A.; Kimura, H. A new role of GCN2 in the nucleolus. Biochem. Biophys. Res. Commun. 2017, 485, 484–491. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, H.; Hou, Y.; Lei, T.; Wei, D.; Zhao, Y. Multiple roles of the stress sensor GCN2 in immune cells. Int. J. Mol. Sci. 2023, 24, 4285. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Chen, T.; Ming, J.; Sun, H.; Cao, P.; Fusco, D.N.; Chung, R.T.; Chorev, M.; Jin, Q.; Aktas, B.H. Dual Activators of Protein Kinase R (PKR) and Protein Kinase R-Like Kinase (PERK) Identify Common and Divergent Catalytic Targets. ChemBioChem 2013, 14, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Pluquet, O.; Papadakis, A.I.; Kazemi, S.; Qu, L.K.; Koromilas, A.E. The eIF2α kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J. Biol. Chem. 2007, 282, 31675–31687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałużyńska, D. The Role of Translation-Associated Proteins in p53 Modulation: Mechanisms and Implications. Int. J. Mol. Sci. 2025, 26, 8164. https://doi.org/10.3390/ijms26178164
Kałużyńska D. The Role of Translation-Associated Proteins in p53 Modulation: Mechanisms and Implications. International Journal of Molecular Sciences. 2025; 26(17):8164. https://doi.org/10.3390/ijms26178164
Chicago/Turabian StyleKałużyńska, Daria. 2025. "The Role of Translation-Associated Proteins in p53 Modulation: Mechanisms and Implications" International Journal of Molecular Sciences 26, no. 17: 8164. https://doi.org/10.3390/ijms26178164
APA StyleKałużyńska, D. (2025). The Role of Translation-Associated Proteins in p53 Modulation: Mechanisms and Implications. International Journal of Molecular Sciences, 26(17), 8164. https://doi.org/10.3390/ijms26178164





