Disrupted Biotensegrity in the Fiber Cellular Fascial Network and Neuroma Microenvironment: A Conceptual Framework for “Phantom Limb Pain”
Abstract
1. Introduction
2. Current Theories of Phantom Limb Pain
3. Methods
4. A Theoretical Biophysical Model to Help Explain PLP Pathogenesis
4.1. Building Blocks for a Model of Biophysical Tensegrity
- i.
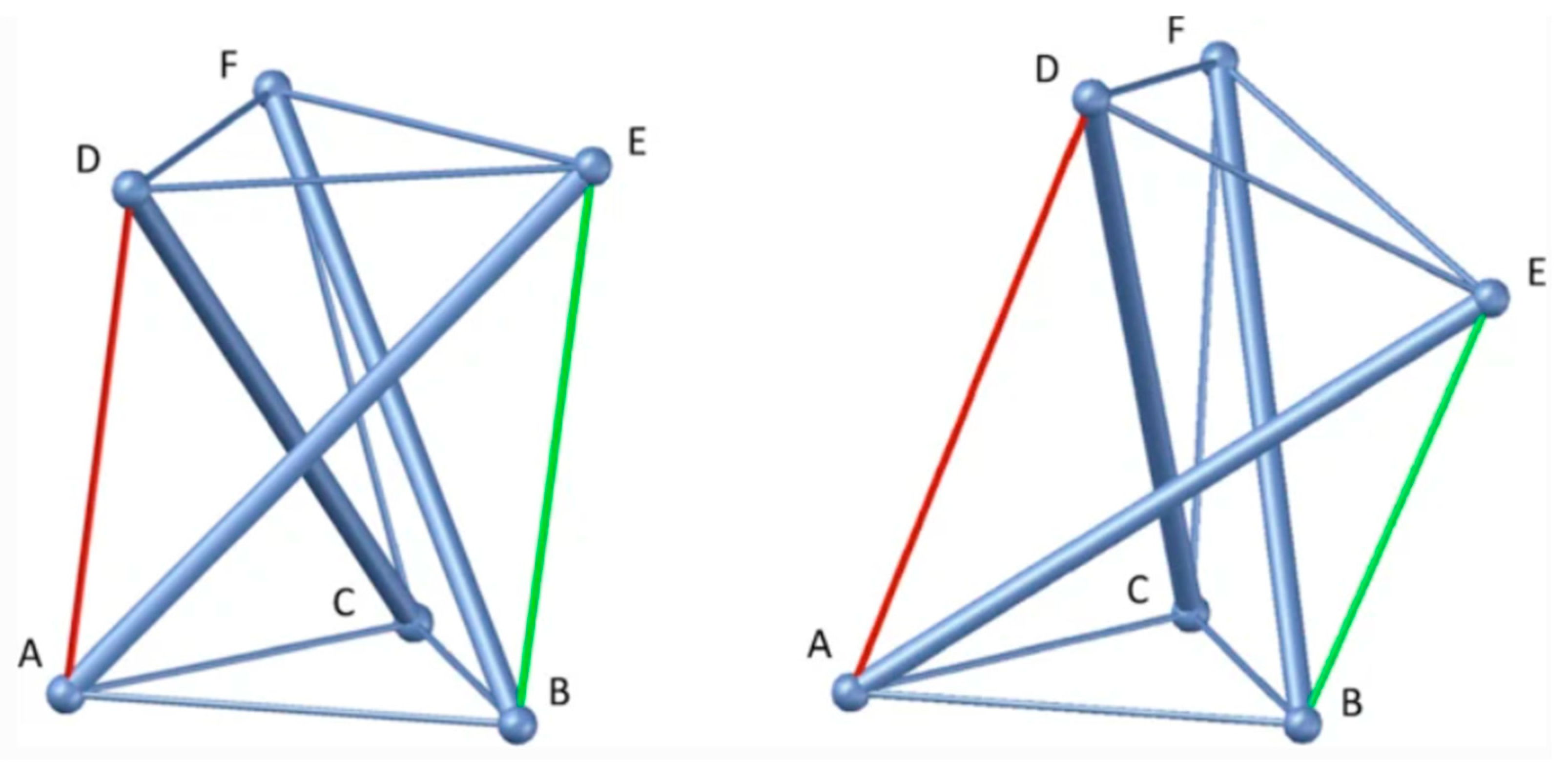
- Tensegrity: The extracellular matrix and fascial tissue make up a complex dynamic multifunctional three-dimensional interconnected network of connective tissue that extends throughout the human body, surrounding, permeating, and connecting muscles, epimysia, perimysia, tendons, ligaments, retinacula, septa, aponeuroses, blood vessels, epineuria, periostea, and connective tissue sheaths at various depths and layers, while undergoing constant remodeling and exhibiting tensegrity-type properties [7,27,28]. ‘Tensegrity’ is an architectural term basically referring to the interplay of compressive and tensional element forces that enable the dynamic behavior and stabilization of one connected structure [27,29]. The concept of ‘biotensegrity’ integrates complex biological aspects of living systems into a biophysical model where each “separate part” of the system is valued with relation to the whole [30]. Biotensegrity provides a more practical and synergistic view of the human body as a functional system requiring both movement and stability. It upgrades the century’s-old over-simplified concept that the skeleton is the frame upon which soft tissue is “draped,” and instead, implements more complex biomechanical concepts, more fitting of real living vertebrates [28,30]. Figure 1 below shows a simple tensegrity structure as an illustration. The displayed structure is stabilized by compression and tension force elements (e.g., bones and muscular-tendinous-fascial tissue in our context). Alterations in one part of the system affect other force elements and the overall state of the entire system as well. Box 1 summarizes the molecular and cellular composition of fascia.
- Fibroblast cells: fibroblast are a diverse family of cells that are crucial for synthesizing and regulating the ECM. Myofibroblasts, a phenotype that has smooth muscle cell-like behavior, can also be found.
- Adipocytes: fat cells can be found within fascial layers, particularly in the superficial fascia.
- Various immune cells, such as macrophages, might be present, especially in inflammatory conditions.
- Mast cells: these immune cells are found in connective tissue and can release inflammatory mediators.
- Vascular cells: endothelial cells line the blood vessels that course through the fascia.
- Nerve cells: sensory nerve endings, nociceptors, mechanoreceptors, proprioceptors, and sympathetic nerve fibers are embedded within the fascial network. While technically neural tissue, they are inextricably linked to the surrounding ECM and interstitium.
- ii.
- Soft tissue kinetic chains are load-bearing myofascial pathways: The (fascio)musculoskeletal system is capable of transmitting mechanical forces to a distance by means of myofascial chains [31]. Myofascial chains are anatomical mechanical links that exist in the human body and allow for force transmission to nearby and distant body regions via continuity of muscular-tendinous-fascial tissue. In this way, for instance, force in the lower limb (e.g., hamstrings) can cross joints and be transmitted to the trunk and affect the lumbar musculature (e.g., via sacrotuberous ligament and thoracolumbar fascia) [32].
- iii.
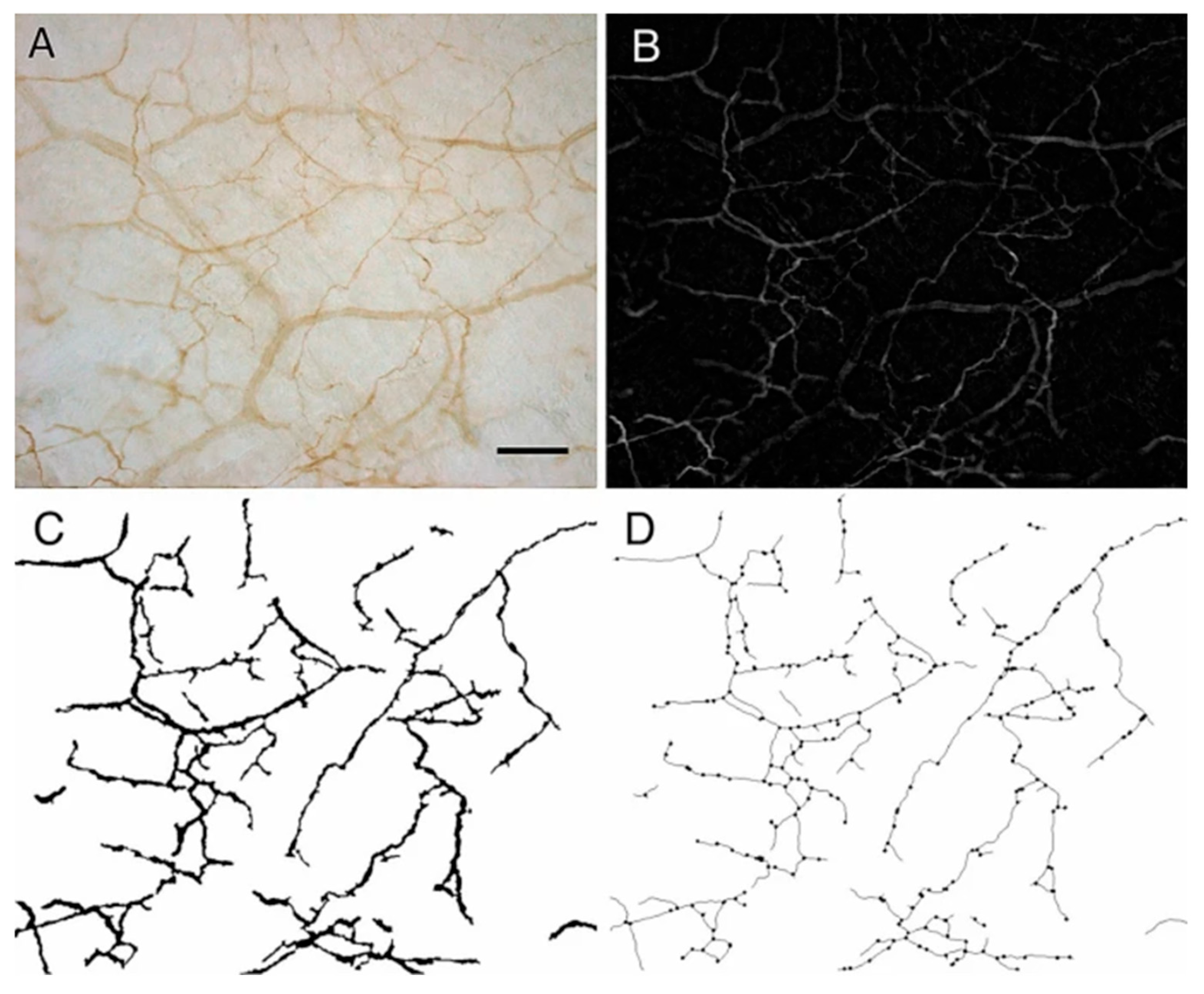
- Mechanical and chemical alterations (densification, fibrosis, pro-inflammatory substances, shear strain, etc.) within the myofascial system can lead to the development of pain [33,34,35], and may help explain myofascial pain syndrome [28,33,36] and fibromyalgia syndrome [7]. Fascia contains a dense network of sensory nerve endings and nociceptors that play a part in the perception of pain [35,37]. In Figure 2A–D below a magnified sample of the innervated fascia is seen, with an impressive network of sympathetic nerve fibers as was demonstrated by Fede et al. (2021) [38]. Myofascial tissue (superficial fascia, perimysium, endomysium, etc.) is richly innervated and contains mechanoreceptors, proprioceptors, and nociceptors, thus playing a role in the generation of pain [28,39]. Abnormal mechanical forces and nociceptive inflammatory mediators secreted by myofibroblasts and local cells (e.g., tumor necrosis factor-alpha, interleukin 1-beta, substance P and neuropeptide Y) may trigger pain via activation of local peripheral sensory receptors. When peripheral nociception is activated, neuronal signaling is then relayed to the nervous system through spinal nociceptors that project to the thalamus and then onward to cortical and subcortical brain network areas that are responsible for pain. Also, extracellular matrix (ECM) stiffness seems to be a crucial factor in the behavior and function of nerve cells [40]. Researchers have investigated the effect of substrate matrix rigidity on neuronal cells in vitro, and found a marked difference in growth dynamics, synaptic density and electrophysiological activity of cortical neuronal networks when comparing cultures grown in substrates with 100-fold differences in Young’s modulus [41]. Matrix stiffness is a significant parameter that modulates Schwann cell function and behavior [42]. Box 2 describes biomolecular empirics that are at the basis of the matrix–neuron interactions in this model.
- iv.
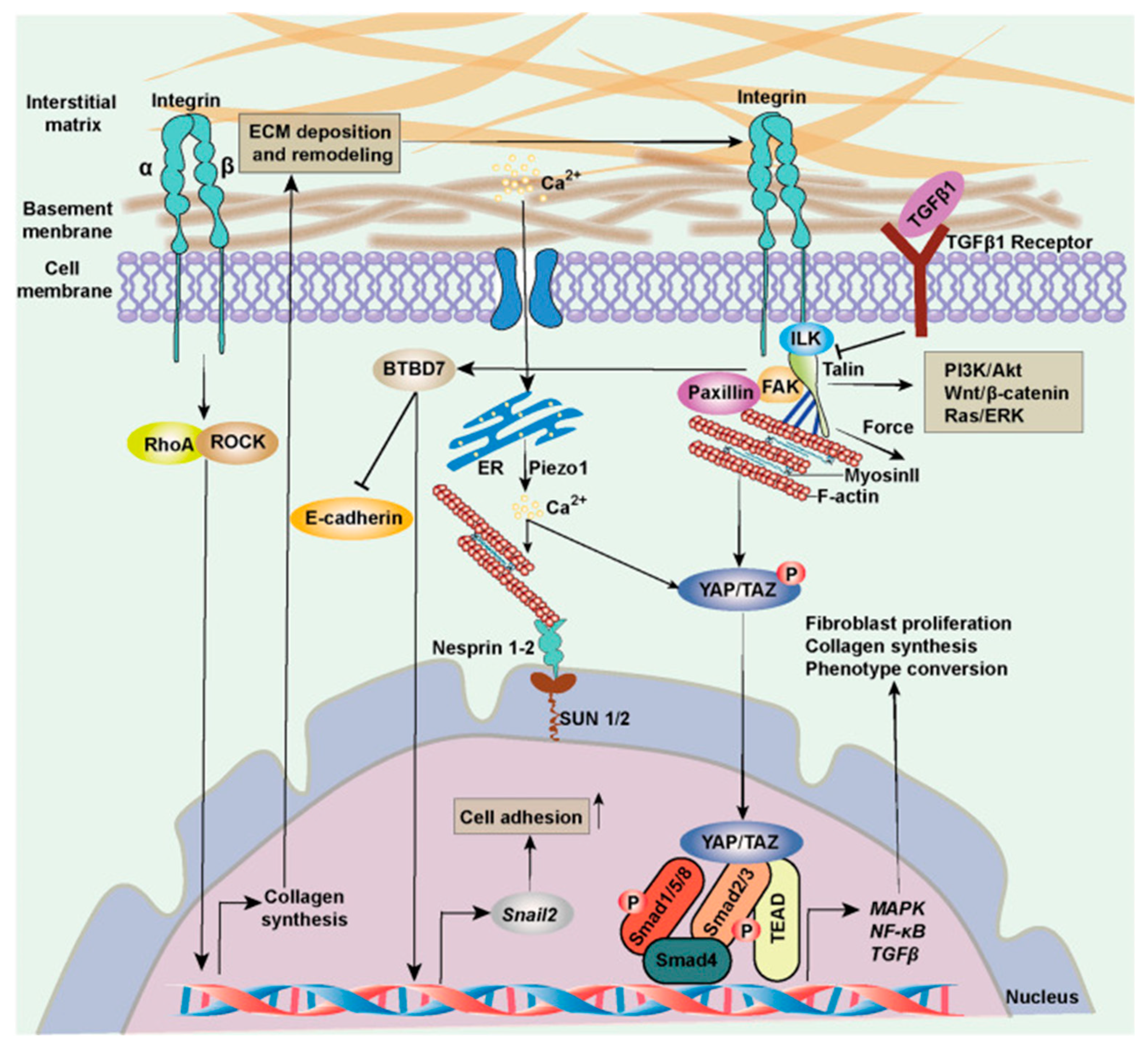
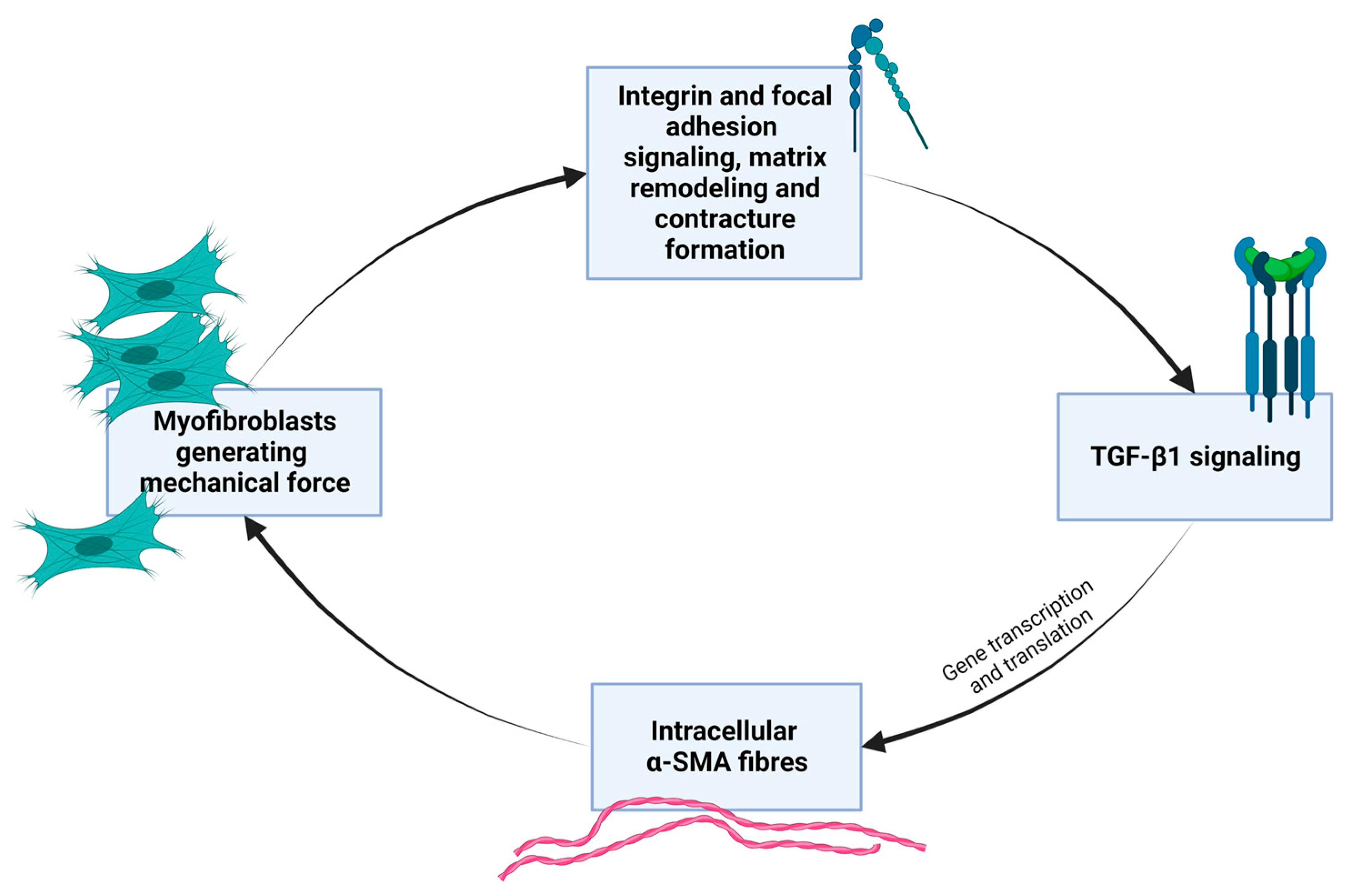
- Myofibroblasts express a smooth muscle cell-like behavior and are induced by mechanical strain and biochemical cues that can stimulate the process of fibroblast-to-myofibroblast trans-differentiation and proliferation [43]. The natural mechanobiology of myofibroblasts is relevant in times of scarring and wound healing in granulation tissue, but they are also found in other tissues. Myofibroblasts are cells normally found in fascia and maintain basal mechanical tissue tone [28,36]. By synthesizing contractile protein machinery and actively remodeling the surrounding matrix material and as they are sensitive to mechanical stimuli and operate with mechano-transducing signaling pathways, myofibroblasts can generate tissue contracture in a positive-feedback loop [43]. Box 3 gives further molecular elaboration on this mechanobiological festivity. Figure 3 outlines the basic self-perpetuating loop of myofibroblast contractile activity that transpires as transforming growth factor beta both enhances mechanical contractile activity of myofibroblasts while its levels are also sustained by mechanical stress. Figure 4 shows, in general, the cellular signaling pathway concerned here, which is activated in fibroblasts in response to increased ECM stiffness and mechanical and biochemical cues.
- v.
- In addition to matrix remodeling and generating pre-stress in tissue, myofibroblasts can electrically couple to nearby cells by use of gap junctions, and contract in collaboration [36,43]. Fibroblasts form a widespread reticular network of cells in soft tissue with potentially major physiological importance. Langevin et al. [51] have shown, using confocal microscopy, histochemistry, immunohistochemistry and electron microscopy, that cultured fibroblasts of mouse subcutaneous tissue as well as cultured human fibroblasts form abundant cell processes and many points of cell-to-cell contact with each other. About 30% of such processes could be followed continuously from one cell to another using confocal microscopy. Other investigators have reported data consistent with this when investigating human fibroblasts and in vivo samples [52,53]. When fibroblasts experience mechanical stimuli they initiate cellular responses ranging from cytosolic intracellular calcium concentration and adenosine triphosphate release, to activation of intracellular signaling pathway, actin polymerization, and gene expression. It is possible that oscillations of calcium waves are a main facilitator of intercellular communication of fibroblasts by fluctuations in the levels of cytosolic calcium and its effect on downstream cell signaling pathways [51]. The nature of these oscillations likely depends, among several different factors, on substrate rigidity [54].
4.2. Examples of Post Intervention Complications and Pain: Exploring a Biophysical Framework of Tensegrity
- Plantar fasciitis—A study involving 37 patients with plantar fasciitis treated with corticosteroid injections at the calcaneal origin reported that 30% experienced a sudden tearing sensation in the heel, while others exhibited gradual symptom progression [57]. While the injections alleviated initial heel pain, new complications soon emerged, including metatarsal pain, midfoot discomfort (dorsal and lateral), foot weakness, swelling, and metatarsal fractures. All cases showed evidence of plantar fascia rupture [57]. The new symptoms eventually resolved in most patients within a period of one year but for others the symptoms persisted.
- New onset Boutonniere deformity develops after treating Dupuytren’s disease [60].
- Trigger finger is more likely to occur following carpal tunnel release [61].
- Treatment for lateral epicondylitis requires “unrelated” arthroscopic decompression of the shoulder joint [62].
- Compartment syndrome of the foot occurred following spine surgery [63].
5. Amputation in a Framework of Osteomyofascial Tensegrity
5.1. Considering the Relevance of Biotensegrity for PLP
5.2. Neuroma as a Focal Sensor of the Biotensegrity System and a Source of Sensations and Pain
5.3. Flexion Contracture and Its Relevance to a Tensegrity Framework
5.4. Summary of the Neuro-Mechanobiological Model for Rethinking PLP
- The increased tension and stiffness caused by myofibroblast activity can directly stimulate mechanosensitive nociceptors or polymodal fibers within the myofascial tissue. These nociceptors respond to mechanical deformation and pressure, firing signals that are interpreted as pain. Mechanosensitive ion channels (e.g., Piezo1/2, TRP channels, stretch-activated channels) mediate the conversion of mechanical stimuli into electrochemical signals. Also, in certain instances, the altered mechanical environment can lower their threshold for activation, making them more easily triggered.
- Under conditions of increased ECM stiffness, long-term transcriptional and epigenetic level adaptations in nerve cells would lead to changes in their structure and function, including intracellular cytoskeleton architecture, which can affect neuronal electrophysiology. Researchers have investigated the effect of substrate matrix rigidity on neuronal cells in vitro, and found a marked difference in growth dynamics, synaptic density and electrophysiological activity of cortical neuronal networks when comparing cultures grown in substrates with 100-fold differences in Young’s modulus [41]. The pre-synaptic density was two times higher on stiff substrates and consistently the number of action potentials and miniature synaptic currents was enhanced on stiff substrates [41].
- Excessive collagen deposition and tissue remodeling associated with myofibroblast activity can potentially lead to the entrapment or compression of small nerve fibers within the deep or superficial fascia. Involvement of the neurovascular bundle can also add to the clinical presentation.
- The effects of higher substrate rigidity on Schwann cells [42] add another neuropathic component to the mechanism.
- Beyond mechanical forces, myofibroblasts can release various pro-inflammatory mediators and growth factors that can sensitize nociceptors in the surrounding tissue, lowering their threshold for activation by mechanical or chemical stimuli. This can lead to hyperalgesia and allodynia.
- Local hypoxic conditions in muscle caused by epimysial and perimysial compression can lead to the release of algogenic substances from the affected tissue, and to the activation of chemoreceptors on nociceptors, and low-grade inflammation.
- Myofibroblasts can communicate with neighboring cells, including other fibroblasts, myocytes, and potentially nerve cells, via gap junctions. This allows for the direct transfer of electrical and chemical signals, potentially contributing to the propagation of pain signals.
- Changes in the mechanical properties of fascia due to myofibroblasts can affect proprioception and kinaesthesia. This altered sensory feedback might lead to compensatory movements, muscle imbalances, and increased strain on other tissues, which can indirectly contribute to pain.
- Compression of the dorsal root ganglion (and conceivably also sympathetic chains) may lower thresholds for electrophysiological activity and can even cause them to fire signals spontaneously. Increased rigidity and compressive forces in the ECM microenvironment may constitute a sufficient endogenous stimulus to independently initiate this phenomenon, in the absence of external stimuli.
- A similar effect as for the previous point (point 9), with regard to a stump neuroma.
6. Discussion
6.1. Biotensegrity as a Useful Framework to Help Explain Osteomyofascia Phenomena and Anomalies
6.2. Therapeutic Implications
6.3. Means to Test the Theoretical Model
- One straightforward non-invasive approach would be to use shear wave elastography, or magnetic resonance elastography for more accuracy, which can help measure the stiffness of myofascial tissue and to compare patients and healthy controls. Compared to control subjects, fibromyalgia/PLP patients should have increased values as measured with shear wave/magnetic resonance elastography, not only at the specific clinical tender spots, but diffusely. Pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage (according to the definition of the International Association for the Study of Pain). We would not necessarily expect the area with pain to be strictly correlated only with measured tissue stiffness because pain is the sum of multiple factors, starting from the nerve itself, its microenvironment, the density of innervation, tethering or entrapment, inhibitory pathways, and all the way up to the brain and consciousness. We would, however, expect to find some correlation between imbalance in the overall biotensegrity structure and pain, and to find a correlation between improvement in the overall measured stiffness/imbalance and improvement in clinical complaints, focusing on cases where other known pathologies have been excluded. While the clinical picture may draw attention to a certain anatomical location or tender spot or to a limb, it does not mean the overall pathology is necessarily reflected in this way. Pain, perhaps not intuitive at first, might in certain cases reflect a weak “node” in the tensegrity “dome” being influenced by tension/taut bands/contractures from elsewhere. Each patient can have a different steady state of the biotensegrity system (resulting in a different clinical presentation). We would also expect to find a close causal association between substrate stiffness of ECM and neuroma activity, or between myofibroblast aberrant mechano-activity and neuroma neuroactivity.
- Biopsies can be taken (e.g., subcutaneous tissue, perimysium, perineurium, periosteum) to investigate whether there is an increase in myofibroblast density, or if cells express higher levels of smooth muscle actin or other proto/myofibroblast markers. In muscles, numbers of-SMA–positive cells per 100 myofibers should be higher when compared to control subjects. Due to tensegrity dynamics, a taut band or any area with myofibroblasts might be stress shielded thus causing pain in a more distant region that seems innocent upon focal inspection. But since myofibroblast can de-differentiate and leave behind a remodeled dysfunctional fascia, testing only by this method might actually be deceptive. Beyond cell numbers, immunohistochemical analysis can characterize the deposition and organization of key ECM macromolecules such as collagen I, collagen III, fibronectin, and proteoglycans, assessing changes in fiber alignment and cross-linking that contribute to increased rigidity. cryo-electron tomography can be used to visualize ECM.
- Overall, the secretory profile of myofibroblasts should be altered correspondently, reflecting an overactive state under higher substrate stiffness. Classic systemic markers are not easy to make out because the mechanism is not endocrine or blood-mediated in the main, and there is no leukocyte-driven overt inflammation. In addition, this model has an inherent variability in terms of which anatomical structures are involved. Transcriptomic and proteomic analyses could reveal altered gene and protein expression profiles indicative of an overactive mechanotransduction cascade, involving pathways like YAP/TAZ, RhoA/ROCK, and TGF-β signaling, reflecting higher substrate stiffness.
- Above a certain threshold of substrate stiffness, mechanosignaling would disrupt the intracellular balance between pro-survival/proliferative and apoptotic signals. This is expected to be reflected by gene expression and compensatory mechanisms of myofascial fibroblasts.
- Invasive interventions are expected to affect PLP disease course, especially if operating on the path of the myofascial chains that are most relevant. A treatment that respects principles of tensegrity might be effective in treating PLP, though this depends on whether and in which manner a neuroma is involved. Neuromas complicate the sensory experience. The focus of this paper is less with amputations of traumatic etiology where it is suggested [78] that acute nerve damage (destroyed nerve plexuses due to traction or pulling forces) play a larger role in the postamputation pain state. Nevertheless, neuroma pain and myofascial pain can co-occur.
- Measuring muscle damping should reflect increased muscle tension rather than spasticity. Pendulousness of the limbs of patients compared to age, sex, and body-mass index matched controls could be a simple clinical test to start with.
- Measuring intramuscular pressure via a pressure gauge has been used in studying fibromyalgia [8]. Studying multiple muscles of persons ranked high on the Fibromyalgia Impact Questionnaire score might be insightful; however, the use of invasive measurement tools would alter the measured result and not be ideal for patient safety reasons. Needling multiple sites without respecting tensegrity principles is expected to alter the tensegrity structure and exacerbate the abnormality.
- Traction force microscopy can be used to quantify cellular forces exerted on substrates.
- Biophysical tests—rheometry, strain elastography, stress-relaxation tests, biaxial testing, compression tests, atomic force microscopy, optical coherence elastography, dynamic mechanical analysis, etc., of fascial/myofascial tissue might be insightful, although these would have to take into account the complexity of the model and possible confounding factors, and control for hypermobility syndrome. Age, sex, pH, temperature, hydration, hyaluronic acid composition, adipocytes, cell phenotype and density, are all variables that may affect the properties of fascia in vivo.
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha-smooth muscle actin |
| CNS | Central nervous system |
| CRT | Cortical remapping theory |
| DRG | Dorsal root ganglion |
| ECM | Extracellular matrix |
| PLP | Phantom limb pain |
| TGF-β1 | Transforming growth factor beta 1 |
| YAP | Yes-associated protein |
References
- Flor, H. Phantom-limb pain: Characteristics, causes, and treatment. Lancet Neurol. 2002, 1, 182–189. [Google Scholar] [CrossRef]
- Ephraim, P.L.; Wegener, S.T.; MacKenzie, E.J.; Dillingham, T.R.; Pezzin, L.E. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch. Phys. Med. Rehabil. 2005, 86, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Limakatso, K.; Bedwell, G.J.; Madden, V.J.; Parker, R. The prevalence and risk factors for phantom limb pain in people with amputations: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0240431. [Google Scholar] [CrossRef] [PubMed]
- Marbach, J.J.; Raphael, K.G. Phantom tooth pain: A new look at an old dilemma. Pain Med. 2000, 1, 68–77. [Google Scholar] [CrossRef]
- Jung, B.F.; Ahrendt, G.M.; Oaklander, A.L.; Dworkin, R.H. Neuropathic pain following breast cancer surgery: Proposed classification and research update. Pain 2003, 104, 1–13. [Google Scholar] [CrossRef]
- Collins, K.L.; Russell, H.G.; Schumacher, P.J.; Robinson-Freeman, K.E.; O’Conor, E.C.; Gibney, K.D.; Yambem, O.; Dykes, R.W.; Waters, R.S.; Tsao, J.W. A review of current theories and treatments for phantom limb pain. J. Clin. Investig. 2018, 128, 2168–2176. [Google Scholar] [CrossRef]
- Plaut, S. Scoping review and interpretation of myofascial pain/fibromyalgia syndrome: An attempt to assemble a medical puzzle. PLoS ONE 2022, 17, e0263087. [Google Scholar] [CrossRef]
- Katz, R.S.; Leavitt, F.; Small, A.K.; Small, B.J. Erratum in: Intramuscular Pressure Is Almost Three Times Higher in Fibromyalgia Patients: A Possible Mechanism for Understanding the Muscle Pain and Tenderness. J. Rheumatol. 2021, 48, 598–602. [Google Scholar] [CrossRef]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. 2021, 131, e144201. [Google Scholar] [CrossRef]
- Adkisson, C.D.; Yip, L.; Armstrong, M.J.; Stang, M.T.; Carty, S.E.; McCoy, K.L. Fibromyalgia symptoms and medication requirements respond to parathyroidectomy. Surgery 2014, 156, 1614–1620, discussion 1620–1621. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.A.; Boros, M.J.; Mancl, T.; Elgamal, M.H.; Song, S.; Wisadrattanapong, T. The effect of laparoscopic Roux-en-Y gastric bypass on fibromyalgia. Obes. Surg. 2008, 18, 652–655. [Google Scholar] [CrossRef]
- Li, Z.; Wei, H.; Piirainen, S.; Chen, Z.; Kalso, E.; Pertovaara, A.; Tian, L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016, 58, 107–117. [Google Scholar] [CrossRef]
- Flor, H.; Nikolajsen, L.; Staehelin Jensen, T. Phantom limb pain: A case of maladaptive CNS plasticity? Nat. Rev. Neurosci. 2006, 7, 873–881. [Google Scholar] [CrossRef]
- Brazenor, G.A.; Malham, G.M.; Teddy, P.J. Can Central Sensitization After Injury Persist as an Autonomous Pain Generator? A Comprehensive Search for Evidence. Pain Med. 2022, 23, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Velasco, E.; Flores-Cortés, M.; Guerra-Armas, J.; Flix-Díez, L.; Gurdiel-Álvarez, F.; Donado-Bermejo, A.; van den Broeke, E.N.; Pérez-Cervera, L.; Delicado-Miralles, M. Is chronic pain caused by central sensitization? A review and critical point of view. Neurosci. Biobehav. Rev. 2024, 167, 105886. [Google Scholar] [CrossRef]
- Makin, T.R.; Scholz, J.; Henderson Slater, D.; Johansen-Berg, H.; Tracey, I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain 2015, 138, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.S.; Howard, C.; Buxbaum, L.J. Aberrant activity in an intact residual muscle is associated with phantom limb pain in above-knee amputees. J. Neurophysiol. 2021, 125, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.E.; Lotterie, J.A.; Cassol, E.; Lazorthes, Y.; Sol, J.C.; Berry, I. Cortical areas involved in virtual movement of phantom limbs: Comparison with normal subjects. Neurosurgery 2003, 53, 1342–1352, discussion 1352–1353. [Google Scholar] [CrossRef]
- Makin, T.R.; Scholz, J.; Filippini, N.; Henderson Slater, D.; Tracey, I.; Johansen-Berg, H. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 2013, 4, 1570. [Google Scholar] [CrossRef]
- Jensen, T.S.; Nikolajsen, L. Phantom pain and other phenomena after amputation. In Textbook of Pain, 4th ed.; Wall, P.D., Melzack, R.A., Eds.; Churchill Livingstone: Edinburgh, UK, 1999; pp. 799–814. [Google Scholar]
- Waxman, S.G.; Hains, B.C. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006, 29, 207–215. [Google Scholar] [CrossRef]
- Li, C.X.; Chappell, T.D.; Ramshur, J.T.; Waters, R.S. Forelimb amputation-induced reorganization in the ventral posterior lateral nucleus (VPL) provides a substrate for large-scale cortical reorganization in rat forepaw barrel subfield (FBS). Brain Res. 2014, 1583, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Melzack, R. Pain ‘memories’ in phantom limbs: Review and clinical observations. Pain 1990, 43, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Tung, M.L.; Murphy, I.C.; Griffin, S.C.; Alphonso, A.L.; Hussey-Anderson, L.; Hughes, K.E.; Weeks, S.R.; Merritt, V.; Yetto, J.M.; Pasquina, P.F.; et al. Observation of limb movements reduces phantom limb pain in bilateral amputees. Ann. Clin. Transl. Neurol. 2014, 1, 633–638. [Google Scholar] [CrossRef]
- Sherman, R.A.; Sherman, C.J.; Bruno, G.M. Psychological factors influencing chronic phantom limb pain: An analysis of the literature. Pain 1987, 28, 285–295. [Google Scholar] [CrossRef]
- Sherman, R.A.; Sherman, C.J.; Parker, L. Chronic phantom and stump pain among American veterans: Results of a survey. Pain 1984, 18, 83–95. [Google Scholar] [CrossRef]
- Tadeo, I.; Berbegall, A.P.; Escudero, L.M.; Alvaro, T.; Noguera, R. Biotensegrity of the extracellular matrix: Physiology, dynamic mechanical balance, and implications in oncology and mechanotherapy. Front. Oncol. 2014, 4, 39. [Google Scholar] [CrossRef]
- Chaitow, L.; Schleip, R.; Huijing, P.; Findley, T.W. (Eds.) Fascia: The Tensional Network of the Human Body—The Science and Clinical Applications in Manual and Movement Therapy, 2nd ed.; Churchill Livingstone/Elsevier: London, UK, 2012; pp. 121–142, 156–177. [Google Scholar]
- Micheletti, A.; Podio-Guidugli, P. Seventy years of tensegrities (and counting). Arch. Appl. Mech. 2022, 92, 2525–2548. [Google Scholar] [CrossRef]
- Scarr, G. Biotensegrity: What is the big deal? J. Bodyw. Mov. Ther. 2020, 24, 134–137. [Google Scholar] [CrossRef]
- Wilke, J.; Krause, F.; Vogt, L.; Banzer, W. What Is Evidence-Based About Myofascial Chains: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 454–461. [Google Scholar] [CrossRef]
- Krause, F.; Wilke, J.; Vogt, L.; Banzer, W. Intermuscular force transmission along myofascial chains: A systematic review. J. Anat. 2016, 228, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial components of the myofascial pain syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Pirri, C.; Stecco, C. Fascial entrapment neuropathy. Clin. Anat. 2019, 32, 883–890. [Google Scholar] [CrossRef]
- Kondrup, F.; Gaudreault, N.; Venne, G. The deep fascia and its role in chronic pain and pathological conditions: A review. Clin. Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411. [Google Scholar] [CrossRef]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; De Caro, R.; Stecco, C. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a new hidden neural network into deep fasciae. Sci. Rep. 2021, 11, 12623. [Google Scholar] [CrossRef]
- Suarez-Rodriguez, V.; Fede, C.; Pirri, C.; Petrelli, L.; Loro-Ferrer, J.F.; Rodriguez-Ruiz, D.; De Caro, R.; Stecco, C. Fascial Innervation: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5674. [Google Scholar] [CrossRef]
- Kayal, C.; Moeendarbary, E.; Shipley, R.J.; Phillips, J.B. Mechanical Response of Neural Cells to Physiologically Relevant Stiffness Gradients. Adv. Healthc. Mater. 2020, 9, e1901036. [Google Scholar] [CrossRef]
- Lantoine, J.; Grevesse, T.; Villers, A.; Delhaye, G.; Mestdagh, C.; Versaevel, M.; Mohammed, D.; Bruyère, C.; Alaimo, L.; Lacour, S.P.; et al. Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials 2016, 89, 14–24. [Google Scholar] [CrossRef]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, J.; Ding, B.; Xue, K.; Sun, X.; Qian, S. Mechanical Strain Regulates Myofibroblast Differentiation of Human Scleral Fibroblasts by YAP. Front. Physiol. 2021, 12, 712509. [Google Scholar] [CrossRef]
- Martin, K.; Pritchett, J.; Llewellyn, J.; Mullan, A.F.; Athwal, V.S.; Dobie, R.; Harvey, E.; Zeef, L.; Farrow, S.; Streuli, C.; et al. PAK Proteins and YAP-1 Signalling Downstream of Integrin Beta-1 in Myofibroblasts Promote Liver Fibrosis. Nat. Commun. 2016, 7, 12502. [Google Scholar] [CrossRef]
- Tarbit, E.; Singh, I.; Peart, J.N.; Bivol, S.; Rose’Meyer, R.B. Increased release of serotonin from rat primary isolated adult cardiac myofibroblasts. Sci. Rep. 2021, 11, 20376. [Google Scholar] [CrossRef]
- Majno, G.; Gabbiani, G.; Hirschel, B.J.; Ryan, G.B.; Statkov, P.R. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science 1971, 173, 548–550. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Y.; Deng, N.; Huang, G.; Taghavifar, F.; Geng, Y.; Liu, N.; Kulur, V.; Yao, C.; Chen, P.; et al. Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell Rep. 2018, 22, 3625–3640. [Google Scholar] [CrossRef]
- Sun, K.H.; Chang, Y.; Reed, N.I.; Sheppard, D. α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L824–L836. [Google Scholar] [CrossRef]
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts form a body-wide cellular network. Histochem. Cell Biol. 2004, 122, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Novotny, G.E.; Gnoth, C. Variability of fibroblast morphology in vivo: A silver impregnation study on human digital dermis and subcutis. J. Anat. 1991, 177, 195–207. [Google Scholar] [PubMed]
- Ko, K.; Arora, P.; Lee, W.; McCulloch, C. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am. J. Physiol. Cell Physiol. 2000, 279, C147–C157. [Google Scholar] [CrossRef]
- Lembong, J.; Sabass, B.; Sun, B.; Rogers, M.E.; Stone, H.A. Mechanics regulates ATP-stimulated collective calcium response in fibroblast cells. J. R. Soc. Interface 2015, 12, 20150140. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Stecco, C.; Giordani, F.; Fan, C.; Biz, C.; Pirri, C.; Frigo, A.C.; Fede, C.; Macchi, V.; Masiero, S.; De Caro, R. Role of fasciae around the median nerve in pathogenesis of carpal tunnel syndrome: Microscopic and ultrasound study. J. Anat. 2020, 236, 660–667. [Google Scholar] [CrossRef]
- Sellman, J.R. Plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1994, 15, 376–381. [Google Scholar] [CrossRef]
- Acevedo, J.I.; Beskin, J.L. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hettrich, C.M.; DiCarlo, E.F.; Faryniarz, D.; Vadasdi, K.B.; Williams, R.; Hannafin, J.A. The effect of myofibroblasts and corticosteroid injections in adhesive capsulitis. J. Shoulder Elbow Surg. 2016, 25, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan-Cerveró, R.; Carrera-Hueso, F.J.; Vazquez-Ferreiro, P.; Gomez-Herrero, D. Adverse Effects of Collagenase in the Treatment of Dupuytren Disease: A Systematic Review. BioDrugs 2017, 31, 105–115. [Google Scholar] [CrossRef]
- Lin, F.Y.; Manrique, O.J.; Lin, C.L.; Cheng, H.T. Incidence of trigger digits following carpal tunnel release: A nationwide, population-based retrospective cohort study. Medicine 2017, 96, e7355. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Skrepnik, N.V.; Edwards, S.G.; Jones, G.L.; Sampson, S.; Vermillion, D.A.; Ramsey, M.L.; Karli, D.C.; Rettig, A.C. Efficacy of platelet-rich plasma for chronic tennis elbow: A double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am. J. Sports Med. 2014, 42, 463–471. [Google Scholar] [CrossRef]
- Stotts, A.K.; Carroll, K.L.; Schafer, P.G.; Santora, S.D.; Branigan, T.D. Medial compartment syndrome of the foot: An unusual complication of spine surgery. Spine 2003, 28, E118–E120. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Ceccherelli, F.; Labeeb, A.A.; Biella, G.E. Phantom limb pain relief by contralateral myofascial injection with local anaesthetic in a placebo-controlled study: Preliminary results. J. Rehabil. Med. 2009, 41, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Di, Z.; Tian, H.F.; Zhang, Q.A. Contralateral Acupuncture for the Treatment of Phantom Limb Pain and Phantom Limb Sensation in Oncologic Lower Limb Amputee: A Case Report. Front. Neurosci. 2021, 15, 713548. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Vogt, L.; Niederer, D.; Banzer, W. Is remote stretching based on myofascial chains as effective as local exercise? A randomised-controlled trial. J. Sports Sci. 2017, 35, 2021–2027. [Google Scholar] [CrossRef]
- Nordez, A.; Gross, R.; Andrade, R.; Le Sant, G.; Freitas, S.; Ellis, R.; McNair, P.J.; Hug, F. Non-Muscular Structures Can Limit the Maximal Joint Range of Motion during Stretching. Sports Med. 2017, 47, 1925–1929. [Google Scholar] [CrossRef]
- Behm, D.G.; Cavanaugh, T.; Quigley, P.; Reid, J.C.; Nardi, P.S.; Marchetti, P.H. Acute bouts of upper and lower body static and dynamic stretching increase non-local joint range of motion. Eur. J. Appl. Physiol. 2016, 116, 241–249. [Google Scholar] [CrossRef]
- Chen, B.; Cui, S.; Xu, M.; Zhang, Z.; Liu, C. Effects of Isometric Plantar-Flexion on the Lower Limb Muscle and Lumbar Tissue Stiffness. Front. Bioeng. Biotechnol. 2022, 9, 810250. [Google Scholar] [CrossRef]
- Prabu Raja, G.; Shyamasunder Bhat, N.; Marie Cruz, A.; Prabhu, A.; Fernandes, S.; Naaz, N. The anatomical myofascial continuum between the neck and eyes. Clin. Anat. 2022, 35, 340–346. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Badalamente, M.A.; Hurst, L.C.; Ellstein, J.; McDevitt, C.A. The pathobiology of human neuromas: An electron microscopic and biochemical study. J. Hand Surg. Br. 1985, 10, 49–53. [Google Scholar] [CrossRef]
- Akbar, M.; McLean, M.; Garcia-Melchor, E.; Crowe, L.A.; McMillan, P.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; McInnes, I.B.; et al. Fibroblast activation and inflammation in frozen shoulder. PLoS ONE 2019, 14, e0215301. [Google Scholar] [CrossRef]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, È.; Wang, F.; Bories, C.; Joly-Beauparlant, C.; et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J. Exp. Med. 2020, 217, e20191430. [Google Scholar] [CrossRef]
- Manjavachi, M.N.; Motta, E.M.; Marotta, D.M.; Leite, D.F.P.; Calixto, J.B. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain 2010, 151, 345–355. [Google Scholar] [CrossRef]
- Sugawara, O.; Atsuta, Y.; Iwahara, T.; Muramoto, T.; Watakabe, M.; Takemitsu, Y. The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia. An analysis of ectopic firing using an in vitro model. Spine 1996, 21, 2089–2094. [Google Scholar] [CrossRef]
- Economides, J.M.; DeFazio, M.V.; Attinger, C.E.; Barbour, J.R. Prevention of Painful Neuroma and Phantom Limb Pain After Transfemoral Amputations Through Concomitant Nerve Coaptation and Collagen Nerve Wrapping. Neurosurgery 2016, 79, 508–513. [Google Scholar] [CrossRef]
- Reiestad, F.; Kulkarni, J. Role of myofascial trigger points in post-amputation pain: Causation and management. Prosthet. Orthot. Int. 2013, 37, 120–123. [Google Scholar] [CrossRef]
- Farmer, S.E.; James, M. Contractures in orthopaedic and neurological conditions: A review of causes and treatment. Disabil. Rehabil. 2001, 23, 549–558. [Google Scholar]
- Jaegers, S.M.; Arendzen, J.H.; de Jongh, H.J. Changes in hip muscles after above-knee amputation. Clin. Orthop. Relat. Res. 1995, 312, 76–284. [Google Scholar] [CrossRef]
- Poonsiri, J.; Dijkstra, P.U.; Geertzen, J.H.B. Fitting transtibial and transfemoral prostheses in persons with a severe flexion contracture: Problems and solutions—A systematic review. Disabil. Rehabil. 2022, 44, 3749–3759. [Google Scholar] [CrossRef]
- Su, E.P. Fixed flexion deformity and total knee arthroplasty. J. Bone Jt. Surg. Br. 2012, 94, 112–115. [Google Scholar] [CrossRef]
- Sasabe, R.; Sakamoto, J.; Goto, K.; Honda, Y.; Kataoka, H.; Nakano, J.; Origuchi, T.; Endo, D.; Koji, T.; Okita, M. Effects of joint immobilization on changes in myofibroblasts and collagen in the rat knee contracture model. J. Orthop. Res. 2017, 35, 1998–2006. [Google Scholar] [CrossRef]
- Chamay, A.; Gabbiani, G. Digital contracture deformity after implantation of a silicone prosthesis: Light and electron microscopic study. J. Hand Surg. Am. 1978, 3, 266–270. [Google Scholar] [CrossRef]
- Schleip, R.; Klingler, W. Active contractile properties of fascia. Clin. Anat. 2019, 32, 891–895. [Google Scholar] [CrossRef]
- Goldman, B. Chronic pain and the search for alternative treatments. CMAJ Can. Med. Assoc. J. 1991, 145, 508–509, 512–513. [Google Scholar]
- Milton, L.M.; Chessick, R.D. Psychosomatic aspects of peptic ulcer. Q. Bull. Northwest Univ. Med. Sch. 1962, 36, 70–73. [Google Scholar]
- Sherman, R.A.; Griffin, V.D.; Evans, C.B.; Grana, A.S. Temporal relationships between changes in phantom limb pain intensity and changes in surface electromyogram of the residual limb. Int. J. Psychophysiol. 1992, 13, 71–77. [Google Scholar] [CrossRef]
- Khoury, A.L.; Keane, H.; Varghese, F.; Hosseini, A.; Mukhtar, R.; Eder, S.E.; Weinstein, P.R.; Esserman, L.J. Trigger point injection for post-mastectomy pain: A simple intervention with high rate of long-term relief. NPJ Breast Cancer 2021, 7, 123. [Google Scholar] [CrossRef]
- Wilk, I.; Kurpas, D.; Mroczek, B.; Andrzejewski, W.; Okręglicka-Forysiak, E.; Krawiecka-Jaworska, E.; Kassolik, K. Application of Tensegrity Massage to Relive Complications After Mastectomy—Case Report. Rehabil. Nurs. 2015, 40, 294–304. [Google Scholar] [CrossRef]
- Kassolik, K.; Andrzejewski, W.; Brzozowski, M.; Wilk, I.; Górecka-Midura, L.; Ostrowska, B.; Krzyżanowski, D.; Kurpas, D. Comparison of massage based on the tensegrity principle and classic massage in treating chronic shoulder pain. J. Manip. Physiol. Ther. 2013, 36, 418–427. [Google Scholar] [CrossRef]
- Kassolik, K.; Jaskólska, A.; Kisiel-Sajewicz, K.; Marusiak, J.; Kawczyński, A.; Jaskólski, A. Tensegrity principle in massage demonstrated by electro- and mechanomyography. J. Bodyw. Mov. Ther. 2009, 13, 164–170. [Google Scholar] [CrossRef]
- Chiu, P.E.; Fu, Z.; Sun, J.; Jian, G.W.; Li, T.M.; Chou, L.W. Efficacy of Fu’s Subcutaneous Needling in Treating Soft Tissue Pain of Knee Osteoarthritis: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 7184. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.M.; Zhang, K.X.; Wang, S.; Wang, N.; Wang, N.; Li, X.; Huang, L.P. Effectiveness of Mirror Therapy for Phantom Limb Pain: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Schone, H.R.; Baker, C.I.; Katz, J.; Nikolajsen, L.; Limakatso, K.; Flor, H.; Makin, T.R. Making sense of phantom limb pain. J. Neurol. Neurosurg. Psychiatry 2022, 93, 833–843. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaut, S. Disrupted Biotensegrity in the Fiber Cellular Fascial Network and Neuroma Microenvironment: A Conceptual Framework for “Phantom Limb Pain”. Int. J. Mol. Sci. 2025, 26, 8161. https://doi.org/10.3390/ijms26178161
Plaut S. Disrupted Biotensegrity in the Fiber Cellular Fascial Network and Neuroma Microenvironment: A Conceptual Framework for “Phantom Limb Pain”. International Journal of Molecular Sciences. 2025; 26(17):8161. https://doi.org/10.3390/ijms26178161
Chicago/Turabian StylePlaut, Shiloh. 2025. "Disrupted Biotensegrity in the Fiber Cellular Fascial Network and Neuroma Microenvironment: A Conceptual Framework for “Phantom Limb Pain”" International Journal of Molecular Sciences 26, no. 17: 8161. https://doi.org/10.3390/ijms26178161
APA StylePlaut, S. (2025). Disrupted Biotensegrity in the Fiber Cellular Fascial Network and Neuroma Microenvironment: A Conceptual Framework for “Phantom Limb Pain”. International Journal of Molecular Sciences, 26(17), 8161. https://doi.org/10.3390/ijms26178161





