Deoxyshikonin Inhibits Influenza A Virus Infection at an Early Stage
Abstract
1. Introduction
2. Results
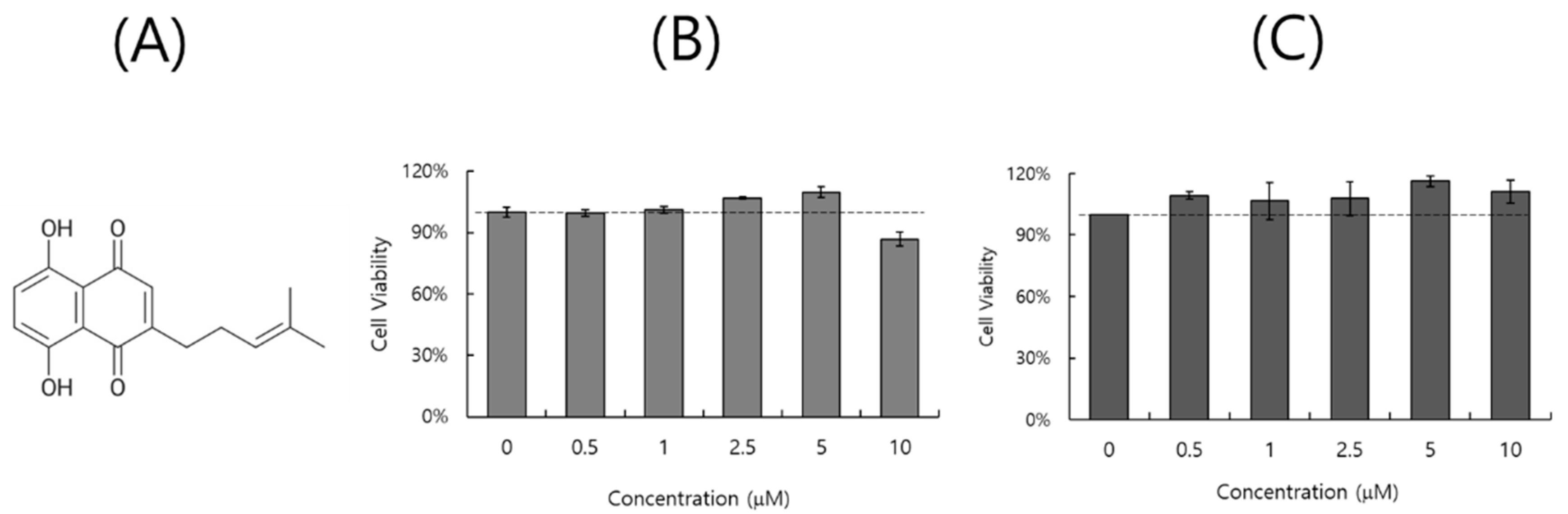
2.1. Effect of DS on Cell Viability
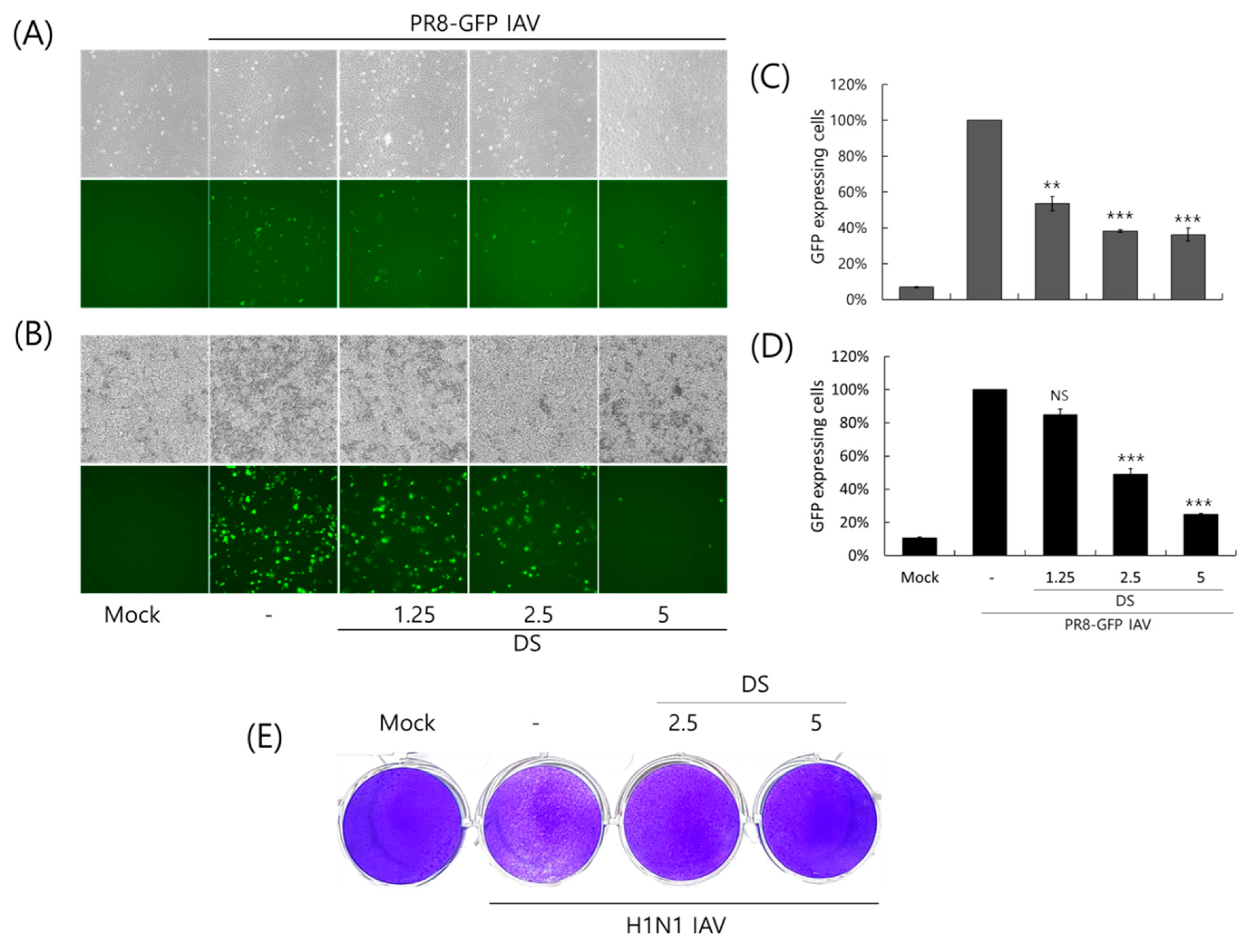
2.2. Anti-Influenza Viral Effect of DS
2.3. Repressive Effect of DS on IAV Protein Expression
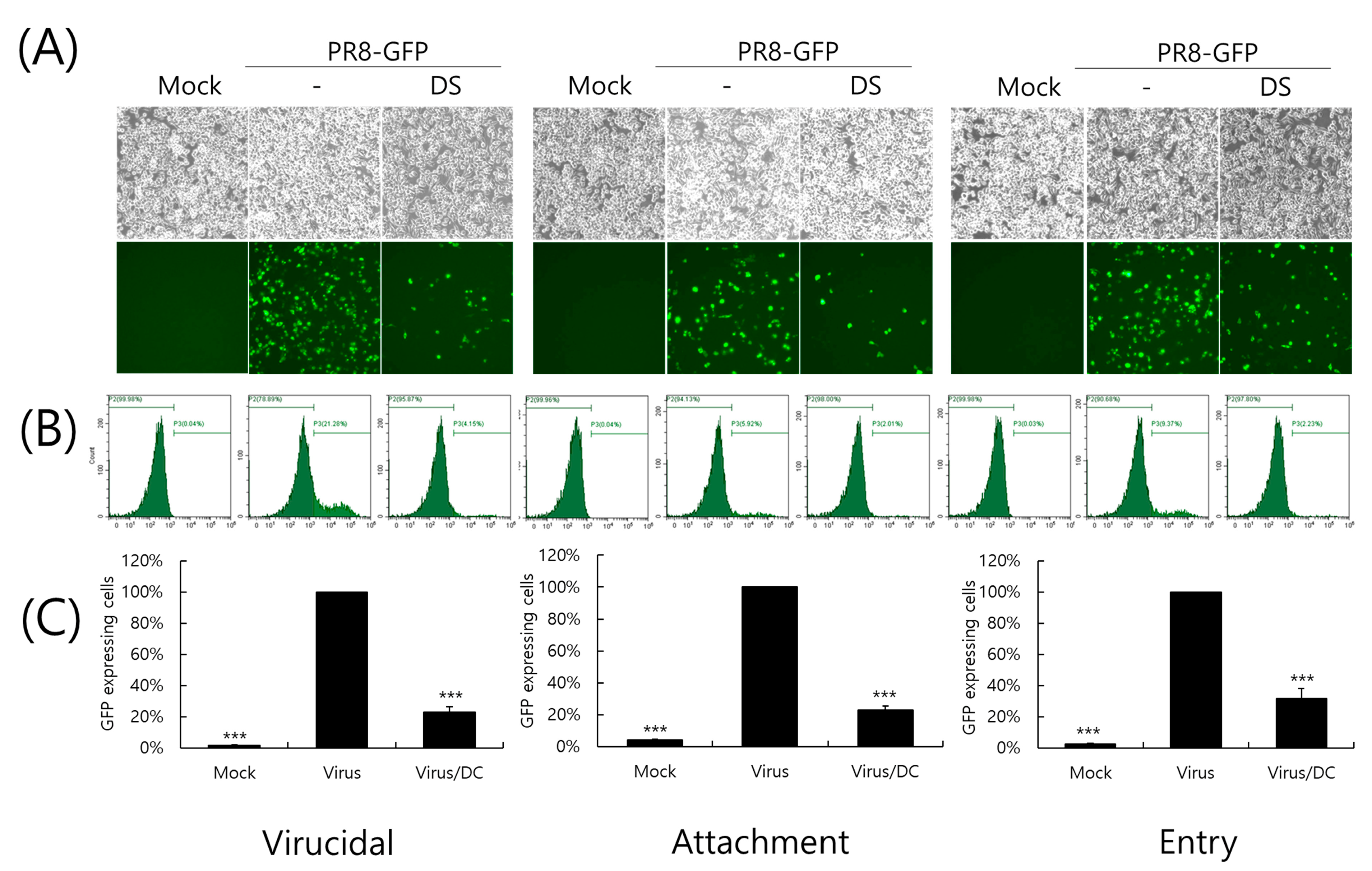
2.4. Inhibitory Effect of DS on IAV Infection at an Early Stage
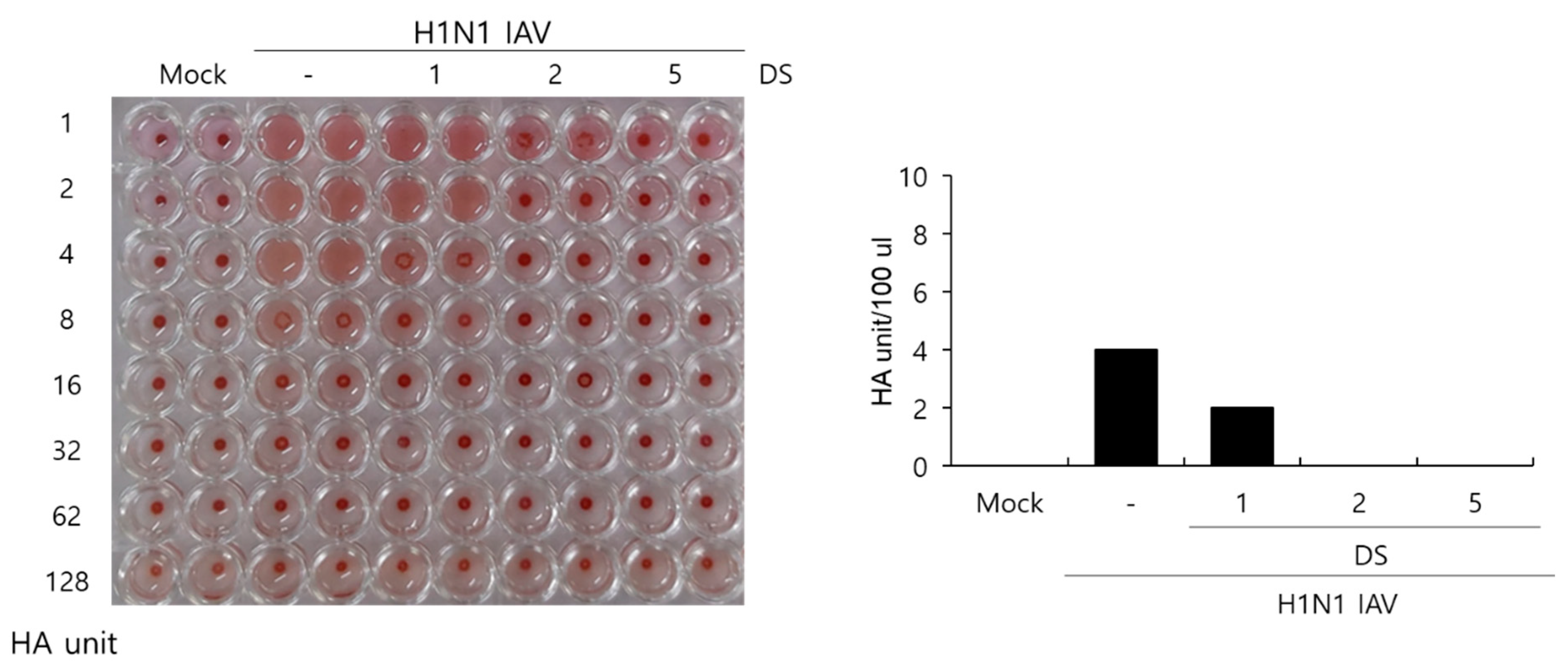
2.5. Effect of DS on Hemagglutination by IAV Infection
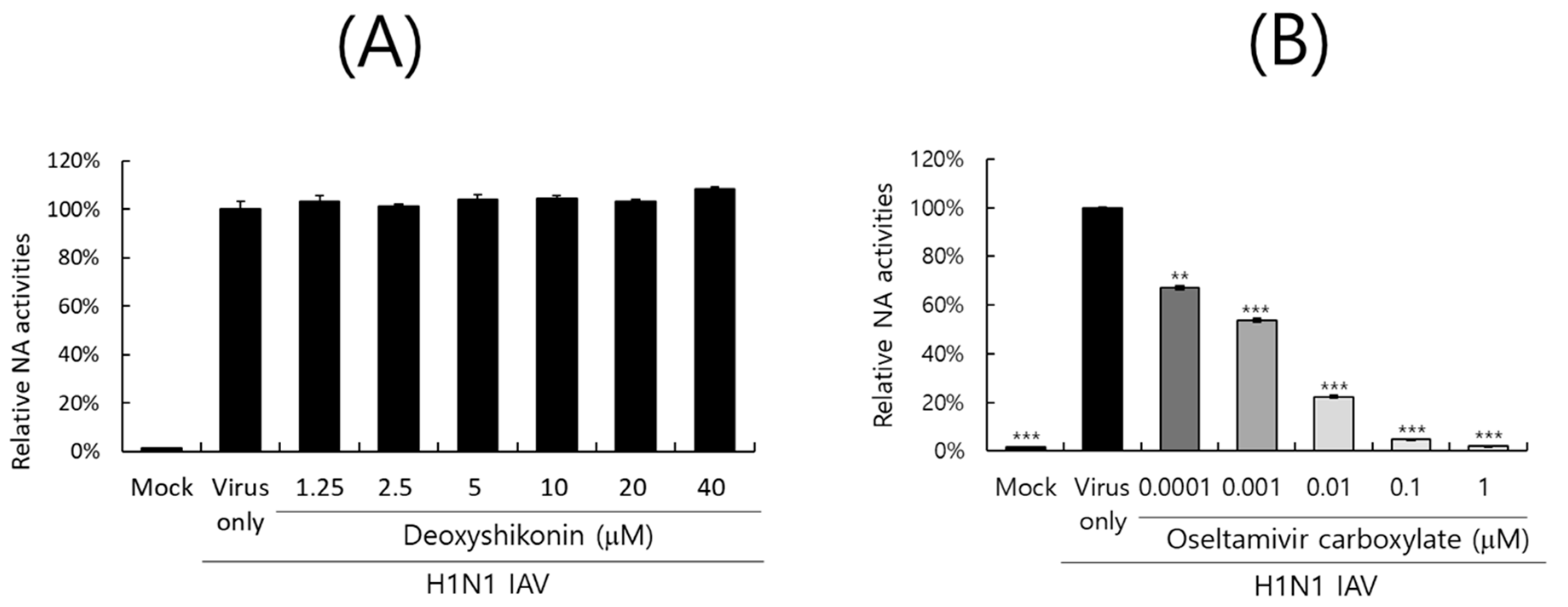
2.6. Effect of DS on IAV Neuraminidase Activity
3. Discussion
4. Materials and Methods
4.1. Reagents, Cell Culture, and Viruses
4.2. Cytotoxicity Determination
4.3. Antiviral Assay Against Influenza Virus Infection
4.4. Viral Attachment, Entry, and Virucidal Assay
4.5. Immunofluorescence Analysis Against IAV Proteins
4.6. Hemagglutination Inhibition Assay Using RBC
4.7. Neuraminidase Inhibition Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Abed, Y.; Pizzorno, A.; Bouhy, X.; Boivin, G. Role of permissive neuraminidase mutations in influenza A/Brisbane/59/2007-like (H1N1) viruses. PLoS Pathog. 2011, 7, e1002431. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D.; Gong, L.I.; Baltimore, D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 2010, 328, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Long, J.K.; Mossad, S.B.; Goldman, M.P. Antiviral agents for treating influenza. Cleve Clin. J. Med. 2000, 67, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, S.M.; Nahata, M.C. Rimantadine: A clinical perspective. Ann. Pharmacother. 1995, 29, 299–310. [Google Scholar] [CrossRef]
- Samson, M.; Pizzorno, A.; Abed, Y.; Boivin, G. Influenza virus resistance to neuraminidase inhibitors. Antivir. Res. 2013, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sharma, S.D.; Kumar, A.; Ende, Z.; Mishina, M.; Wang, Y.; Falls, Z.; Samudrala, R.; Pohl, J.; Knight, P.R.; et al. Antiviral Approaches against Influenza Virus. Clin. Microbiol. Rev. 2023, 36, e0004022. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, P.N.; Kao, S.H.; Wu, H.H.; Hsiao, Y.H.; Huang, T.Y.; Wang, P.H.; Yang, S.F. Deoxyshikonin triggers apoptosis in cervical cancer cells through p38 MAPK-mediated caspase activation. Environ. Toxicol. 2024, 39, 4308–4317. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Chen, L. Deoxyshikonin Inhibits Viability and Glycolysis by Suppressing the Akt/mTOR Pathway in Acute Myeloid Leukemia Cells. Front. Oncol. 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y. Deoxyshikonin inhibits cisplatin resistance of non-small-cell lung cancer cells by repressing Akt-mediated ABCB1 expression and function. J. Biochem. Mol. Toxicol. 2020, 34, e22560. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, Y.; Long, X.; Zhu, Z.; Zhou, Y.; Ye, H.; Zeng, X.; Zheng, X. Deoxyshikonin isolated from Arnebia euchroma inhibits colorectal cancer by down-regulating the PI3K/Akt/mTOR pathway. Pharm. Biol. 2019, 57, 412–423. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, M.S.; Hwang, G.S.; Yamabe, N.; Yoo, J.E.; Kang, K.S.; Kim, J.C.; Lee, J.G.; Ham, J.; Lee, H.L. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2018, 19, 3660. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Popovic, S.L.; Zaric, M.M.; Baskic, D.D.; Krstic, G.B.; Tesevic, V.V.; Kacaniova, M.M. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI J. 2017, 16, 73–88. [Google Scholar] [CrossRef]
- Sasaki, K.; Abe, H.; Yoshizaki, F. In vitro antifungal activity of naphthoquinone derivatives. Biol. Pharm. Bull. 2002, 25, 669–670. [Google Scholar] [CrossRef]
- Li, C.; Fukushi, Y.; Kawabata, J.; Tahara, S.; Mizutani, J.; Uyeda, I. Antiviral and antifungal activities of some naphthoquinones isolated from the roots of Lithospermum erythrorhizon. J. Pestic. Sci. 1988, 23, 54–57. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; He, B.; Pu, R.; Cui, Y.; Zhou, G. Deoxyshikonin inhibited rotavirus replication by regulating autophagy and oxidative stress through SIRT1/FoxO1/Rab7 axis. Microb. Pathog. 2023, 178, 106065. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S. Influenza Virus Entry inhibitors. Adv. Exp. Med. Biol. 2022, 1366, 123–135. [Google Scholar] [CrossRef]
- Mair, C.E.; Grienke, U.; Wilhelm, A.; Urban, E.; Zehl, M.; Schmidtke, M.; Rollinger, J.M. Anti-Influenza Triterpene Saponins from the Bark of Burkea africana. J. Nat. Prod. 2018, 81, 515–523. [Google Scholar] [CrossRef]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yeh, C.Y.; Cheng, J.C.; Huang, Y.Q.; Hsu, K.C.; Lin, Y.F.; Lu, C.H. Potent sialic acid inhibitors that target influenza A virus hemagglutinin. Sci. Rep. 2021, 11, 8637. [Google Scholar] [CrossRef]
- Basu, A.; Antanasijevic, A.; Wang, M.; Li, B.; Mills, D.M.; Ames, J.A.; Nash, P.J.; Williams, J.D.; Peet, N.P.; Moir, D.T.; et al. New small molecule entry inhibitors targeting hemagglutinin-mediated influenza a virus fusion. J. Virol. 2014, 88, 1447–1460. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Wang, J. Inhibitors targeting the influenza virus hemagglutinin. Curr. Med. Chem. 2015, 22, 1361–1382. [Google Scholar] [CrossRef]
- Lin, D.; Luo, Y.; Yang, G.; Li, F.; Xie, X.; Chen, D.; He, L.; Wang, J.; Ye, C.; Lu, S.; et al. Potent influenza A virus entry inhibitors targeting a conserved region of hemagglutinin. Biochem. Pharmacol. 2017, 144, 35–51. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Shen, X.; Liu, S. Influenza A virus entry inhibitors targeting the hemagglutinin. Viruses 2013, 5, 352–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, Q.; Caffrey, M.; Rong, L.; Du, R. Small Molecule Inhibitors of Influenza Virus Entry. Pharmaceuticals 2021, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Badani, H.; Garry, R.F.; Wimley, W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta 2014, 1838, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, G.; Chai, N.; Park, S.; Chiang, N.; Lin, Z.; Chiu, H.; Fong, R.; Yan, D.; Kim, J.; Zhang, J.; et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 2013, 14, 93–103. [Google Scholar] [CrossRef]
- Belser, J.A.; Lu, X.; Szretter, K.J.; Jin, X.; Aschenbrenner, L.M.; Lee, A.; Hawley, S.; Kim, D.H.; Malakhov, M.P.; Yu, M.; et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J. Infect. Dis. 2007, 196, 1493–1499. [Google Scholar] [CrossRef]
- Triana-Baltzer, G.B.; Gubareva, L.V.; Nicholls, J.M.; Pearce, M.B.; Mishin, V.P.; Belser, J.A.; Chen, L.M.; Chan, R.W.; Chan, M.C.; Hedlund, M.; et al. Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS ONE 2009, 4, e7788. [Google Scholar] [CrossRef]
- Sacramento, C.Q.; Marttorelli, A.; Fintelman-Rodrigues, N.; de Freitas, C.S.; de Melo, G.R.; Rocha, M.E.; Kaiser, C.R.; Rodrigues, K.F.; da Costa, G.L.; Alves, C.M.; et al. Aureonitol, a Fungi-Derived Tetrahydrofuran, Inhibits Influenza Replication by Targeting Its Surface Glycoprotein Hemagglutinin. PLoS ONE 2015, 10, e0139236. [Google Scholar] [CrossRef]
- Cho, W.K.; Choi, H.J.; Ahmad, S.S.; Choi, I.; Ma, J.Y. Antiviral Effect of Amentoflavone Against Influenza Viruses. Int. J. Mol. Sci. 2024, 25, 12426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, H.; Zhang, Z.; Ou, W.; Xu, F.; Luo, L.; Liu, Y.; Chen, W.; Chen, J. Antiviral Effect of Ginsenosides rk1 against Influenza a Virus Infection by Targeting the Hemagglutinin 1-Mediated Virus Attachment. Int. J. Mol. Sci. 2023, 24, 4967. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, M.M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.-K.; Ma, J.Y. Deoxyshikonin Inhibits Influenza A Virus Infection at an Early Stage. Int. J. Mol. Sci. 2025, 26, 8158. https://doi.org/10.3390/ijms26178158
Cho W-K, Ma JY. Deoxyshikonin Inhibits Influenza A Virus Infection at an Early Stage. International Journal of Molecular Sciences. 2025; 26(17):8158. https://doi.org/10.3390/ijms26178158
Chicago/Turabian StyleCho, Won-Kyung, and Jin Yeul Ma. 2025. "Deoxyshikonin Inhibits Influenza A Virus Infection at an Early Stage" International Journal of Molecular Sciences 26, no. 17: 8158. https://doi.org/10.3390/ijms26178158
APA StyleCho, W.-K., & Ma, J. Y. (2025). Deoxyshikonin Inhibits Influenza A Virus Infection at an Early Stage. International Journal of Molecular Sciences, 26(17), 8158. https://doi.org/10.3390/ijms26178158





