Antiviral Activity of Berbamine Against Influenza A Virus Infection
Abstract
1. Introduction
2. Results
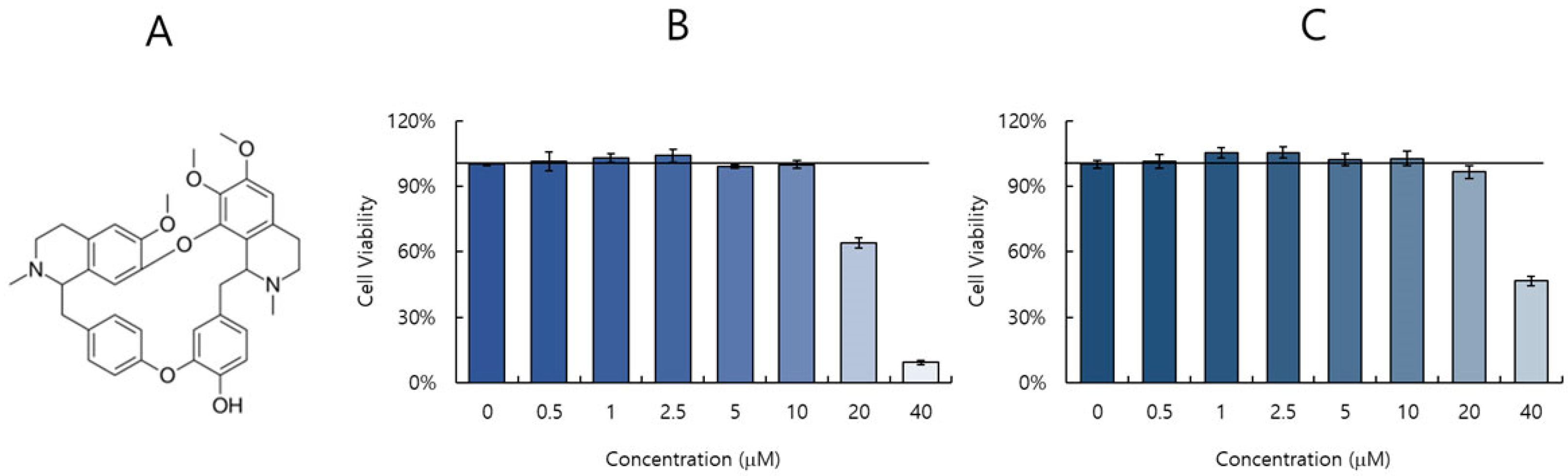
2.1. Cytotoxicity of Berbamine (BBM)
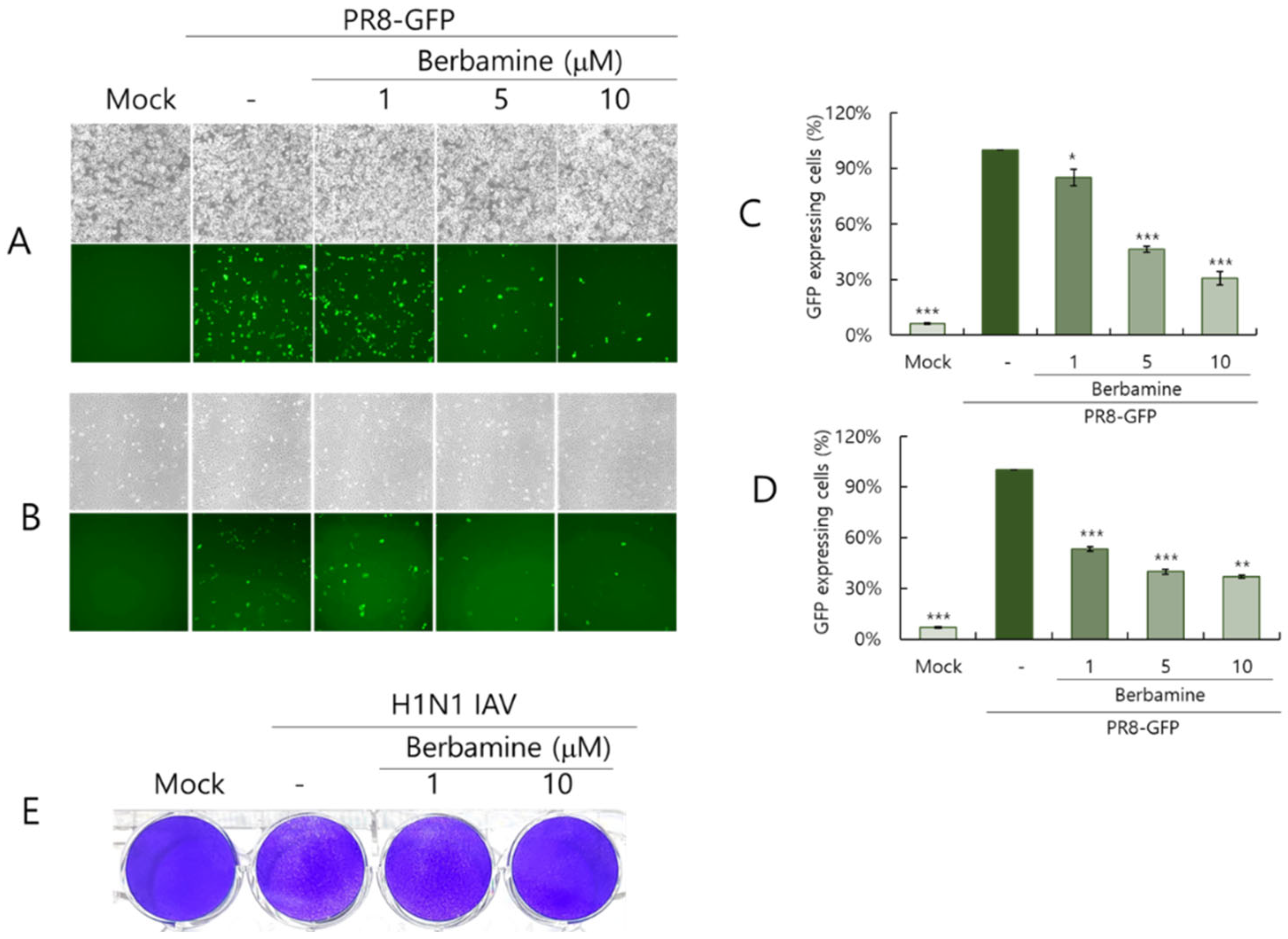
2.2. Berbamine Dose-Dependently Inhibits Influenza Virus Infection
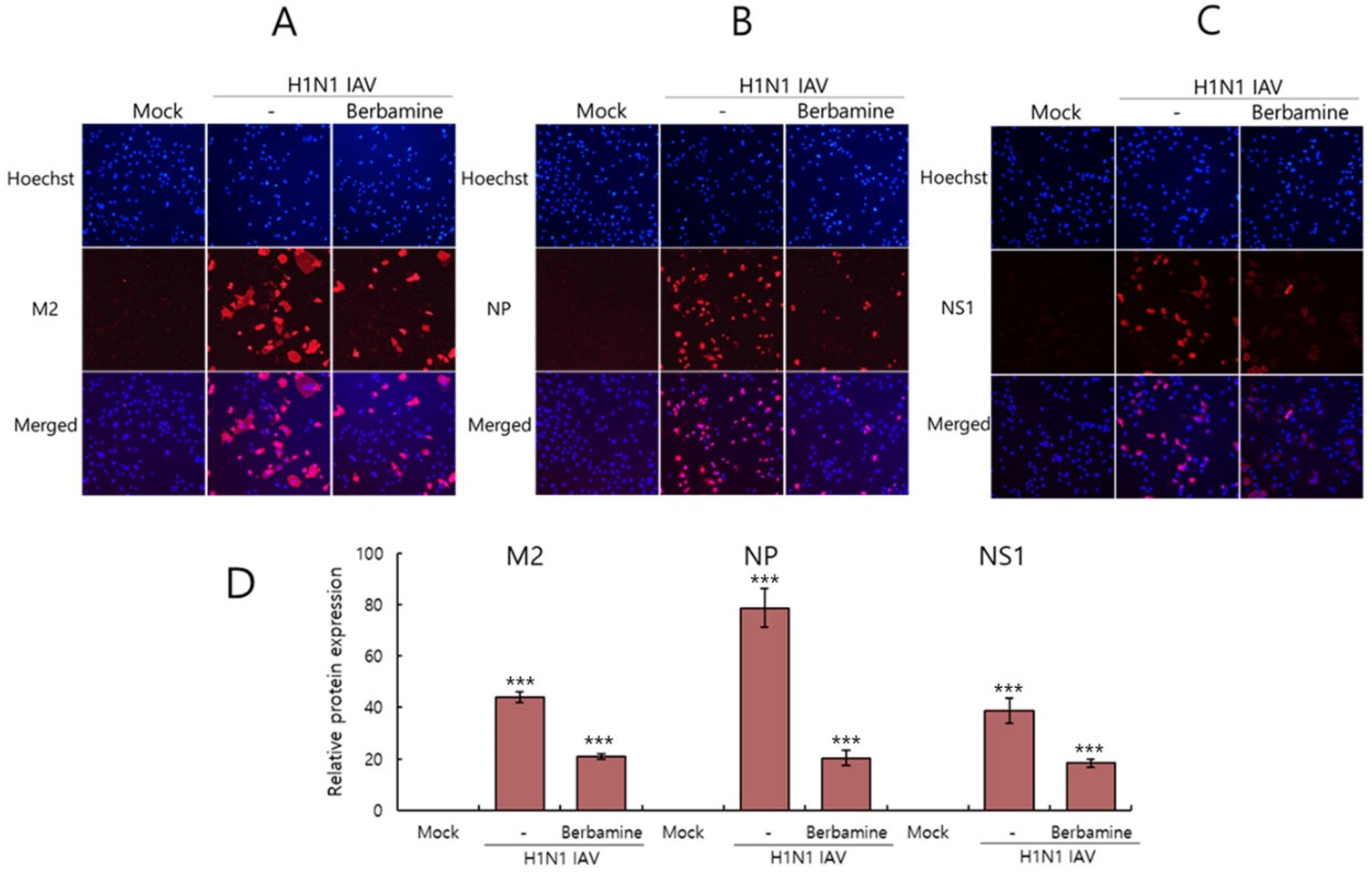
2.3. Immunofluorescence Results Confirmed the Anti-Influenza Viral Effect of Berbamine
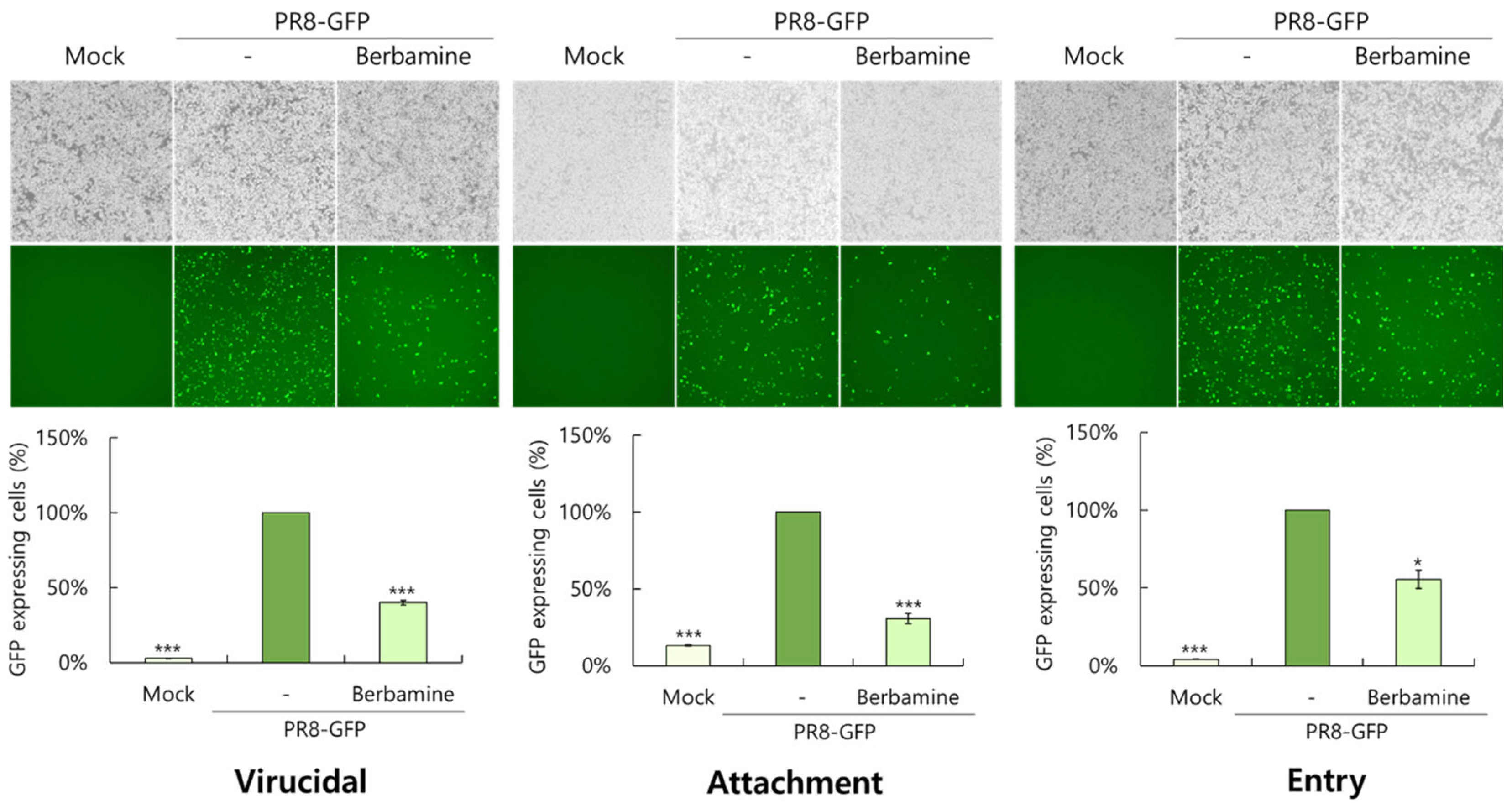
2.4. Berbamine Exerts a Virucidal Effect and the Blockage of Virus Binding and Entry on the Cells
2.5. Berbamine Dose-Dependently Suppresses Hemagglutination
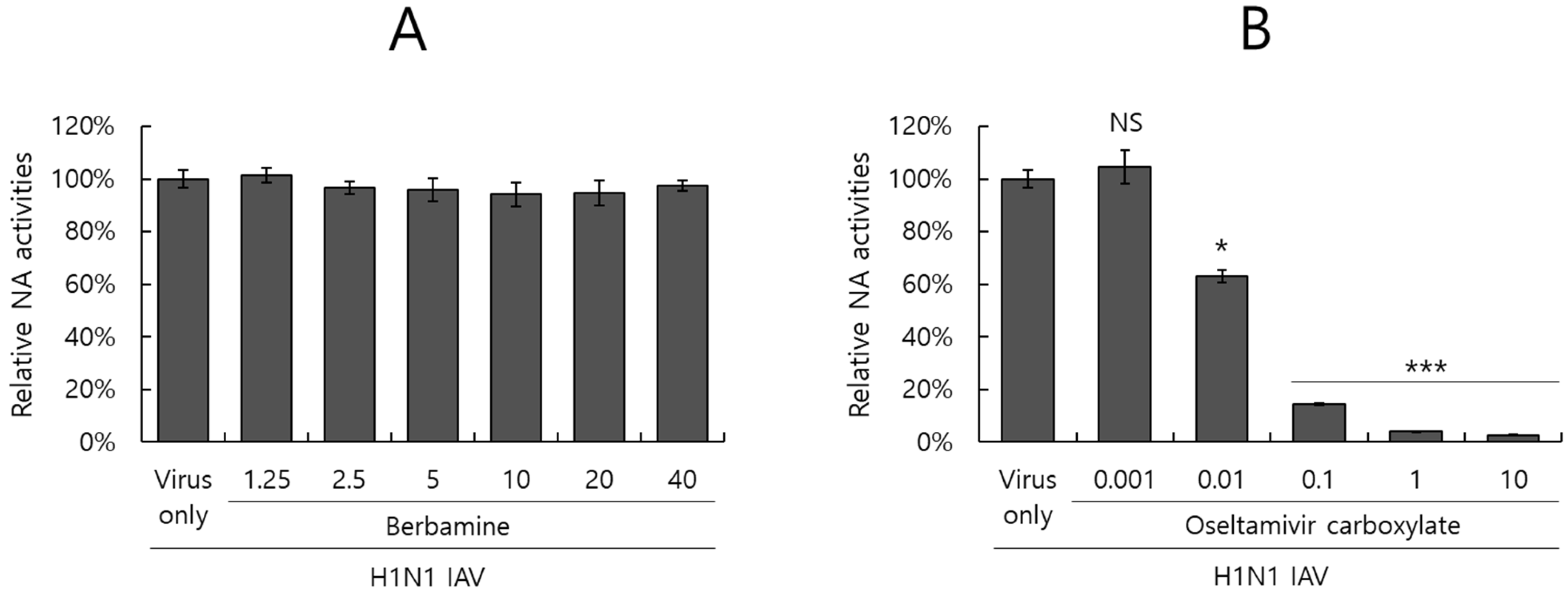
2.6. Berbamine Did Not Affect Neuraminidase Activity
3. Discussion
4. Materials and Methods
4.1. Materials, Cell Culture, and Viruses
4.2. Cell Viability Assay
4.3. Anti-Influenza Viral Assay
4.4. Time-of-Addition Assay
4.5. Immunofluorescence
4.6. Hemagglutination Inhibition Assay
4.7. Neuraminidase Inhibition Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macias, A.E.; McElhaney, J.E.; Chaves, S.S.; Nealon, J.; Nunes, M.C.; Samson, S.I.; Seet, B.T.; Weinke, T.; Yu, H. The disease burden of influenza beyond respiratory illness. Vaccine 2021, 39 (Suppl. S1), A6–A14. [Google Scholar] [CrossRef]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Nau, J.Y. Influenza virus of seasonal influenza, high increase of resistance to amantadine and rimantadine. Rev. Med. Suisse 2006, 2, 308. [Google Scholar]
- Abed, Y.; Pizzorno, A.; Bouhy, X.; Boivin, G. Role of permissive neuraminidase mutations in influenza A/Brisbane/59/2007-like (H1N1) viruses. PLoS Pathog. 2011, 7, e1002431. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D.; Gong, L.I.; Baltimore, D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 2010, 328, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bai, B.; Hong, Z.; Zhang, X.; Zhou, B. Berbamine (BBM), a Natural STAT3 Inhibitor, Synergistically Enhances the Antigrowth and Proapoptotic Effects of Sorafenib on Hepatocellular Carcinoma Cells. ACS Omega 2020, 5, 24838–24847. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, Y.; Jia, X.; Wang, X.; Guo, Y. Berbamine Inhibits the Biological Activities of Prostate Cancer Cells by Modulating the ROS/NF-kappaB Axis. Anti-Cancer Agents Med. Chem. 2023, 23, 1626–1633. [Google Scholar] [CrossRef]
- Liu, L.; Liang, D.; Zheng, Q.; Zhao, M.; Lv, R.; Tang, J.; Chen, N. Berbamine dihydrochloride suppresses the progression of colorectal cancer via RTKs/Akt axis. J. Ethnopharmacol. 2023, 303, 116025. [Google Scholar] [CrossRef]
- Yu, B.B.; Liu, L.L.; Yan, J.D.; Cao, J.B.; Cao, Y. Effect of berbamine on invasion and metastasis of human liver cancer SMMC-7721 cells and its possible mechanism. Anti-Cancer Drugs 2022, 33, e178–e185. [Google Scholar] [CrossRef]
- Liu, L.; Yan, J.; Cao, Y.; Yan, Y.; Shen, X.; Yu, B.; Tao, L.; Wang, S. Proliferation, migration and invasion of triple negative breast cancer cells are suppressed by berbamine via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol. Lett. 2021, 21, 70. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, G.N.; Du, G.M.; Pan, Y.; Song, W.Q.; Jiang, T.W.; Liu, H.L. Berbamine ameliorates ethanol-induced liver injury by inhibition of hepatic inflammation in mice. Chin. J. Nat. Med. 2020, 18, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kou, S.; He, K.; Han, Y.; Wang, Y.; Huang, T.; Zhou, X.; Xiao, Y.; Li, X.; Ye, X. Anti-hypercholesterolemic Effect of Berbamine Isolated from Rhizoma Coptidis in Hypercholesterolemic Zebrafish Induced by High-Cholesterol Diet. Iran. J. Pharm. Res. 2018, 17, 292–306. [Google Scholar]
- Zhang, C.; Zhong, Z.; Sang, W.; Ghorbani, F.; Ghalandari, B.; Mohamadali, M.; Irani, S.; Qian, Z.; Yi, C.; Yu, B. The Dibenzyl Isoquinoline Alkaloid Berbamine Ameliorates Osteoporosis by Inhibiting Bone Resorption. Front. Endocrinol. 2022, 13, 885507. [Google Scholar] [CrossRef]
- Tang, W.; Ma, J.; Chen, K.; Wang, K.; Chen, Z.; Chen, C.; Li, X.; Wang, Y.; Shu, Y.; Zhang, W.; et al. Berbamine ameliorates DSS-induced colitis by inhibiting peptidyl-arginine deiminase 4-dependent neutrophil extracellular traps formation. Eur. J. Pharmacol. 2024, 975, 176634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, L.; Gao, F.; Jian, W.; Chen, H.; Liao, M.; Qi, W. Berbamine Hydrochloride Inhibits African Swine Fever Virus Infection In Vitro. Molecules 2022, 28, 170. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Li, Q.; Wang, H.; Lv, K.; Ma, L.; Wang, Y.; Wang, J.; Zhang, Y.; Liu, M.; Li, X.; et al. Repurposing of berbamine hydrochloride to inhibit Ebola virus by targeting viral glycoprotein. Acta Pharm. Sin. B 2022, 12, 4378–4389. [Google Scholar] [CrossRef]
- Cloherty, A.P.M.; Rader, A.G.; Patel, K.S.; Perez-Vargas, J.; Thompson, C.A.H.; Ennis, S.; Niikura, M.; Wildenberg, M.E.; Muncan, V.; Schreurs, R.; et al. Berbamine suppresses intestinal SARS-CoV-2 infection via a BNIP3-dependent autophagy blockade. Emerg. Microbes Infect. 2023, 12, 2195020. [Google Scholar] [CrossRef]
- Li, Z.Y.; He, Y.X.; Ge, L.J.; Quan, R.; Chen, J.Z.; Hu, Y.; Sa, R.; Liu, J.H.; Ran, D.L.; Fu, Q.; et al. Berbamine, a bioactive alkaloid, suppresses equine herpesvirus type 1 in vitro and in vivo. Front. Vet. Sci. 2023, 10, 1163780. [Google Scholar] [CrossRef]
- Xiang, H.; Qiao, J.; Lin, H.; Li, J.; Li, Y.; Sun, H.; Wang, X.; Bi, R.; Zhang, Z.; Bo, Z.; et al. Berbamine inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2024, 298, 110244. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Zhang, L.; Zhang, J.; Wang, J.; Zou, Y.; Wang, J. Berbamine hydrochloride inhibits bovine viral diarrhea virus replication via interfering in late-stage autophagy. Virus Res. 2022, 321, 198905. [Google Scholar] [CrossRef]
- Huang, L.; Li, H.; Ye, Z.; Xu, Q.; Fu, Q.; Sun, W.; Qi, W.; Yue, J. Berbamine inhibits Japanese encephalitis virus (JEV) infection by compromising TPRMLs-mediated endolysosomal trafficking of low-density lipoprotein receptor (LDLR). Emerg. Microbes Infect. 2021, 10, 1257–1271. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S. Influenza Virus Entry inhibitors. Adv. Exp. Med. Biol. 2022, 1366, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Mair, C.E.; Grienke, U.; Wilhelm, A.; Urban, E.; Zehl, M.; Schmidtke, M.; Rollinger, J.M. Anti-Influenza Triterpene Saponins from the Bark of Burkea africana. J. Nat. Prod. 2018, 81, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yeh, C.Y.; Cheng, J.C.; Huang, Y.Q.; Hsu, K.C.; Lin, Y.F.; Lu, C.H. Potent sialic acid inhibitors that target influenza A virus hemagglutinin. Sci. Rep. 2021, 11, 8637. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef]
- Sacramento, C.Q.; Marttorelli, A.; Fintelman-Rodrigues, N.; de Freitas, C.S.; de Melo, G.R.; Rocha, M.E.; Kaiser, C.R.; Rodrigues, K.F.; da Costa, G.L.; Alves, C.M.; et al. Aureonitol, a Fungi-Derived Tetrahydrofuran, Inhibits Influenza Replication by Targeting Its Surface Glycoprotein Hemagglutinin. PLoS ONE 2015, 10, e0139236. [Google Scholar] [CrossRef]
- Cho, W.K.; Choi, H.J.; Ahmad, S.S.; Choi, I.; Ma, J.Y. Antiviral Effect of Amentoflavone Against Influenza Viruses. Int. J. Mol. Sci. 2024, 25, 12426. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, M.M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Zhang, Z.; Ou, W.; Xu, F.; Luo, L.; Liu, Y.; Chen, W.; Chen, J. Antiviral Effect of Ginsenosides rk1 against Influenza a Virus Infection by Targeting the Hemagglutinin 1-Mediated Virus Attachment. Int. J. Mol. Sci. 2023, 24, 4967. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.-K.; Choi, H.-J.; Ma, J.Y. Antiviral Activity of Berbamine Against Influenza A Virus Infection. Int. J. Mol. Sci. 2025, 26, 2819. https://doi.org/10.3390/ijms26062819
Cho W-K, Choi H-J, Ma JY. Antiviral Activity of Berbamine Against Influenza A Virus Infection. International Journal of Molecular Sciences. 2025; 26(6):2819. https://doi.org/10.3390/ijms26062819
Chicago/Turabian StyleCho, Won-Kyung, Hee-Jeong Choi, and Jin Yeul Ma. 2025. "Antiviral Activity of Berbamine Against Influenza A Virus Infection" International Journal of Molecular Sciences 26, no. 6: 2819. https://doi.org/10.3390/ijms26062819
APA StyleCho, W.-K., Choi, H.-J., & Ma, J. Y. (2025). Antiviral Activity of Berbamine Against Influenza A Virus Infection. International Journal of Molecular Sciences, 26(6), 2819. https://doi.org/10.3390/ijms26062819





