Dynamic Intercellular Networks in the CNS: Mechanisms of Crosstalk from Homeostasis to Neurodegeneration
Abstract
1. Introduction
2. The Intercellular Communication Landscape in the CNS: Modes and Mechanisms
2.1. Ligand–Receptor Signaling: The Canonical Signaling Backbone
2.2. Gap Junctions and Metabolic Coupling
2.3. Ionic and Metabolic Exchanges
2.4. Extracellular Vesicles (EVs)
2.5. Contact-Dependent Signaling
2.6. Neurotransmitter and Gliotransmitter Crosstalk
3. Technologies for Mapping Intercellular Communication in the CNS
3.1. Single-Cell and Single-Nucleus RNA Sequencing: Resolving Cellular Identity and Communication Potential
3.2. Spatial Transcriptomics: Embedding Communication into Anatomical Context
3.3. Integrative Frameworks: Linking Cell Identity, Space, and Function
4. Cell–Cell Communication in a Healthy CNS: A Hierarchical Network of Homeostatic Signaling
- Multicellular tissue homeostasis;
- Synaptic refinement;
- Developmental lineage coordination;
- Long-range functional integration.
4.1. Multicellular Crosstalk Supporting Tissue-Level Homeostasis
4.2. Localized Glial Control of Synaptic Refinement
4.3. Cross-Compartment Coordination of Lineage Specification
4.4. System-Level Integration in Long-Term Memory Encoding
4.5. Integrative Summary: Multiscale Communication in the Healthy CNS
5. Age-Associated Remodeling of Intercellular Communication Networks
5.1. Chronic Inflammation and Glial Crosstalk in Aging
5.2. Neurovascular Unit (NVU) Decline and BBB Dysfunction
5.3. Synaptic Vulnerability and Disrupted Neuron–Glia Communication
5.4. OPCs Dysfunction and Myelin Integrity Loss
5.5. Integrative Single-Cell Atlas Highlights Glial-Centered Aging Signatures
5.6. Microglia-Driven Myelin and Cognitive Decline
5.7. Microglia Maintain Intercellular Balance to Prevent Degeneration
5.8. Immune–Neural Crosstalk Limits Regeneration in Aging Peripheral Nervous System
5.9. Genetic Studies Highlight Intercellular Communication Pathways in Brain Aging
5.10. Integrative Summary: Aging-Associated Remodeling of CNS Networks
6. Dysregulated Intercellular Communication in CNS Diseases
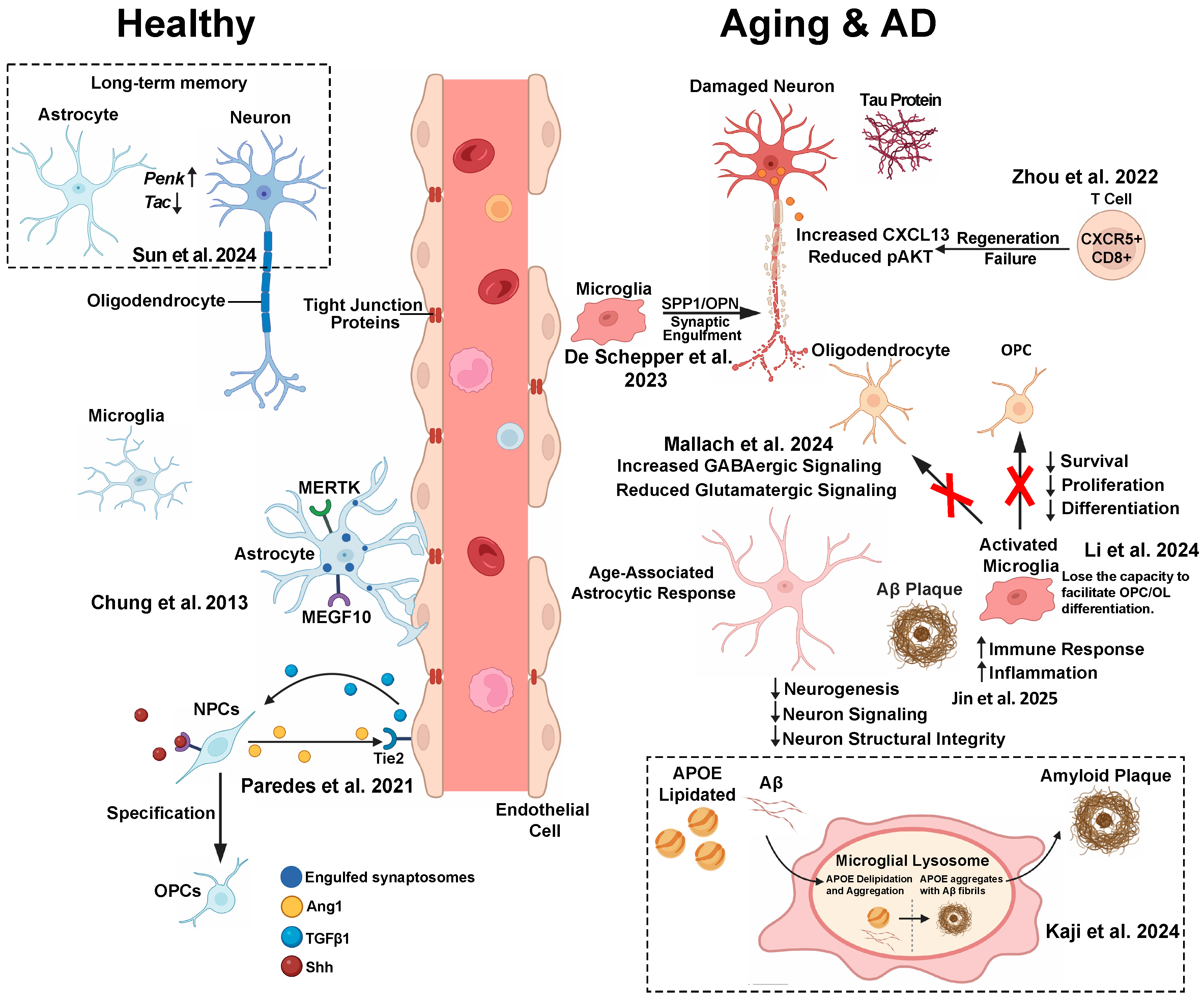
6.1. Alzheimer’s Disease: Glial–Neuronal Crosstalk in Neurodegeneration (Figure 1)
6.1.1. Microglia–Astrocyte Interactions and Amyloid Plaque Microenvironment
6.1.2. Astrocyte–Neuron Lipid Trafficking Dysfunction
6.1.3. Microglia–Neuron Lipid Crosstalk and APOE4 Toxicity
6.1.4. Microglia–Astrocyte Axis in APOE Aggregation and Aβ Plaque Formation
6.1.5. APOE4 Alters Microglia–Neuron Communication Through Calcium Signaling
6.1.6. Perivascular Cell–Microglial Communication
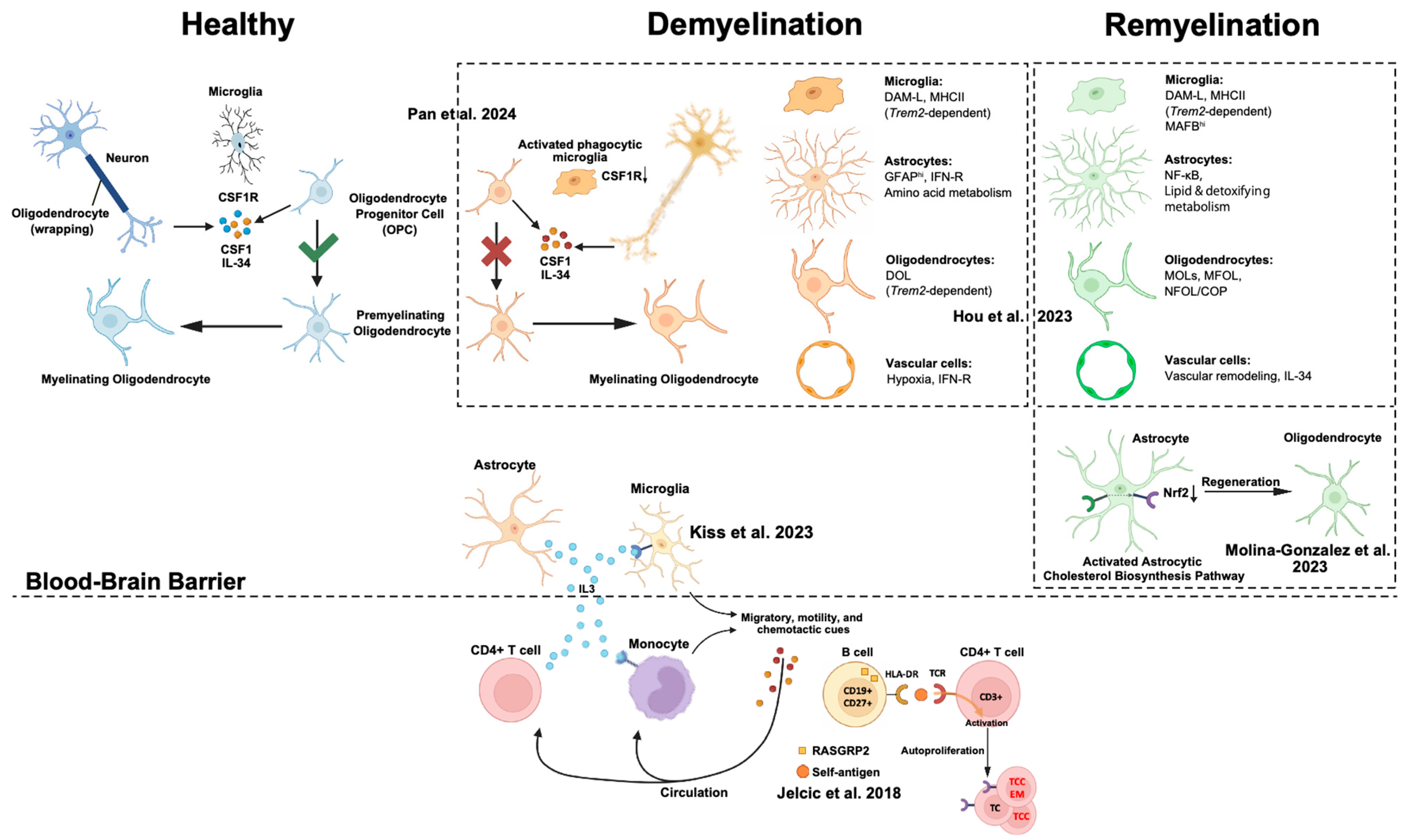
6.2. Demyelinating Disease: Rewiring Communication in Myelin Injury and Repair
6.2.1. Microglia–Oligodendrocyte Lineage Crosstalk
6.2.2. Astrocyte-Derived Cholesterol Supports Repair
6.2.3. Astrocyte–Microglia Immune Modulation
6.2.4. Glial–Immune Cytokine Signaling
6.2.5. Adaptive Immune Cell Crosstalk in MS
6.2.6. Communication Failure in Leukodystrophy
6.3. Summary
7. Therapeutic Implications of Dysregulated Intercellular Communication in the CNS
- Progressive imbalance between homeostatic and pathological signaling
- 2.
- Context-dependent disease phenotypes shaped by local microenvironments
- 3.
- Disruption of spatial organization in intercellular signaling
- Enhancing protective glia–neuron signaling, including promotion of lipid metabolism and mitigation of excitotoxicity;
- Reprogramming glial states to favor anti-inflammatory and pro-regenerative phenotypes;
- Stabilizing neurovascular interactions, such as reinforcing blood–brain barrier integrity and perivascular signaling;
- Spatially targeted delivery of therapeutic agents to lesion cores and vulnerable tissue niches.
8. Future Directions and Challenges
9. Conclusions
- Multiscale Mapping of CNS Communication: Future studies should integrate spatial transcriptomics, proteomics, metabolomics, and functional imaging to build high-resolution atlases that capture CNS signaling across different scales—from molecular gradients to whole-brain networks.
- Functional Interrogation of Crosstalk Pathways: Genetic and optogenetic perturbation platforms, including CRISPRa/i, DREADDs, and intersectional viral tools, can be leveraged to selectively activate or inhibit specific communication axes in defined cell populations and time windows.
- Translational Application and Biomarker Discovery: By identifying conserved or disease-specific communication motifs, especially those shared across species or patient cohorts, we can prioritize signaling hubs as therapeutic targets or diagnostic biomarkers. The emergence of spatially resolved, patient-derived data will be crucial in this endeavor.
| Conditions | Pathways | Citation |
|---|---|---|
| Health | CX3CL1-CX3CR1 pathway | Zhao et al. [59] |
| MEGF10/MERTK phagocytic pathways | Chung et al. [48] | |
| Ang1-Tie2 pathway | Paredes et al. [49] | |
| BDNF/MAPK/CREB/ubiquitination/Penk/Tac pathways | Sun et al. [50] | |
| Aging | VEGF/Angiopoietin-Tie/PDGF pathways | Linnerbauer et al. [38] |
| Zhang et al. [74] | ||
| JAM2-JAM3/TNF-NOTCH1 pathways | Li et al. [52] | |
| CXCL13/CXCR5/NFkB/pAKT/pS6 pathways | Rhinn et al. [84] | |
| AD | GABAergic pathway | Mallach et al. [42] |
| JAK/STAT pathways | Kaji et al. [54] | |
| SPP1 phagocytic pathway | De Schepper et al. [55] | |
| Demyelination | TREM2/IL-33/ST2 pathways | Hou et al. [90] |
| PD-L1/PD-1 pathway | Linnerbauer et al. [93] | |
| IL-3/IL-3RA pathway | Kiss et al. [91] | |
| HLA-DR15/TCR pathways | Jelcic et al. [92] | |
| CSF1R/CSF1/IL-34 pathway | Pan et al. [9] | |
| Nrf2/Cholesterol biosynthesis pathways | Molina-Gonzalez et al. [23] |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Gomez, M.; Alberini, C.M.; Deneen, B.; Drummond, G.T.; Manninen, T.; Sur, M.; Vicentic, A. Neuron–Glial Interactions: Implications for Plasticity, Behavior, and Cognition. J. Neurosci. 2024, 44, e1231242024. [Google Scholar] [CrossRef] [PubMed]
- Grosche, J.; Matyash, V.; Möller, T.; Verkhratsky, A.; Reichenbach, A.; Kettenmann, H. Microdomains for neuron–glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 1999, 2, 139–143. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef]
- Janesick, A.; Shelansky, R.; Gottscho, A.D.; Wagner, F.; Williams, S.R.; Rouault, M.; Beliakoff, G.; Morrison, C.A.; Oliveira, M.F.; Sicherman, J.T.; et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun. 2023, 14, 8353. [Google Scholar] [CrossRef]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
- Pan, J.; Fores-Martos, J.; Delpirou Nouh, C.; Jensen, T.D.; Vallejo, K.; Cayrol, R.; Ahmadian, S.; Ashley, E.A.; Greicius, M.D.; Cobos, I. Deciphering glial contributions to CSF1R-related disorder via single-nuclear transcriptomic profiling: A case study. Acta Neuropathol. Commun. 2024, 12, 139. [Google Scholar] [CrossRef]
- Merritt, C.R.; Ong, G.T.; Church, S.E.; Barker, K.; Danaher, P.; Geiss, G.; Hoang, M.; Jung, J.; Liang, Y.; McKay-Fleisch, J.; et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020, 38, 586–599. [Google Scholar] [CrossRef]
- Chen, K.H.; Boettiger, A.N.; Moffitt, J.R.; Wang, S.; Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 2015, 348, aaa6090. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, L.; Dong, H.; Yang, M.; Ding, W.; Zhou, X.; Lu, T.; Liu, Z.; Zhou, X.; Wang, M.; et al. Decoding the spatiotemporal regulation of transcription factors during human spinal cord development. Cell Res. 2024, 34, 193–213. [Google Scholar] [CrossRef]
- Chang, J.; Lu, J.; Liu, Q.; Xiang, T.; Zhang, S.; Yi, Y.; Li, D.; Liu, T.; Liu, Z.; Chen, X.; et al. Single-cell multi-stage spatial evolutional map of esophageal carcinogenesis. Cancer Cell 2025, 43, 380–397.e7. [Google Scholar] [CrossRef]
- Benjamin, K.; Bhandari, A.; Kepple, J.D.; Qi, R.; Shang, Z.; Xing, Y.; An, Y.; Zhang, N.; Hou, Y.; Crockford, T.L.; et al. Multiscale topology classifies cells in subcellular spatial transcriptomics. Nature 2024, 630, 943–949. [Google Scholar] [CrossRef]
- Efremova, M.; Vento-Tormo, M.; Teichmann, S.A.; Vento-Tormo, R. CellPhoneDB: Inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 2020, 15, 1484–1506. [Google Scholar] [CrossRef] [PubMed]
- Browaeys, R.; Saelens, W.; Saeys, Y. NicheNet: Modeling intercellular communication by linking ligands to target genes. Nat. Methods 2020, 17, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Plikus, M.V.; Nie, Q. CellChat for systematic analysis of cell–cell communication from single-cell transcriptomics. Nat. Protoc. 2025, 20, 180–219. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Chang, W.Y.; Malewska, A.; Napolitano, F.; Gahan, J.C.; Unni, N.; Zhao, M.; Yuan, R.; Wu, F.; et al. Mapping cellular interactions from spatially resolved transcriptomics data. Nat. Methods 2024, 21, 1830–1842. [Google Scholar] [CrossRef]
- Gunner, G.; Cheadle, L.; Johnson, K.M.; Ayata, P.; Badimon, A.; Mondo, E.; Nagy, M.A.; Liu, L.; Bemiller, S.M.; Kim, K.-W.; et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 2019, 22, 1075–1088. [Google Scholar] [CrossRef]
- Diniz, L.P.; Tortelli, V.; Matias, I.; Morgado, J.; Bérgamo Araujo, A.P.; Melo, H.M.; Seixas da Silva, G.S.; Alves-Leon, S.V.; de Souza, J.M.; Ferreira, S.T.; et al. Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer’s Disease Model. J. Neurosci. 2017, 37, 6797–6809. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigão, P.; Stipursky, J.; Kahn, S.A.; Romão, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-induced Synaptogenesis Is Mediated by Transforming Growth Factor β Signaling through Modulation of d-Serine Levels in Cerebral Cortex Neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]
- Droguerre, M.; Tsurugizawa, T.; Duchêne, A.; Portal, B.; Guiard, B.P.; Déglon, N.; Rouach, N.; Hamon, M.; Mouthon, F.; Ciobanu, L.; et al. A New Tool for In Vivo Study of Astrocyte Connexin 43 in Brain. Sci. Rep. 2019, 9, 18292. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Holloway, R.K.; Jiwaji, Z.; Dando, O.; Kent, S.A.; Emelianova, K.; Lloyd, A.F.; Forbes, L.H.; Mahmood, A.; Skripuletz, T.; et al. Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration. Nat. Commun. 2023, 14, 3372. [Google Scholar] [CrossRef] [PubMed]
- Bellot-Saez, A.; Kékesi, O.; Morley, J.W.; Buskila, Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci. Biobehav. Rev. 2017, 77, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.S.; Frostig, R.D. Astrocyte-neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion. Sci. Rep. 2023, 13, 12799. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Torrini, F.; Gil-Garcia, M.; Cardellini, J.; Frigerio, R.; Basso, M.; Gori, A.; Arosio, P. Monitoring neurodegeneration through brain-derived extracellular vesicles in biofluids. Trends Pharmacol. Sci. 2025, 46, 468–479. [Google Scholar] [CrossRef]
- Arvanitaki, E.S.; Goulielmaki, E.; Gkirtzimanaki, K.; Niotis, G.; Tsakani, E.; Nenedaki, E.; Rouska, I.; Kefalogianni, M.; Xydias, D.; Kalafatakis, I.; et al. Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. USA 2024, 121, e2317402121. [Google Scholar] [CrossRef]
- Lyons, A.; Downer, E.J.; Crotty, S.; Nolan, Y.M.; Mills, K.H.; Lynch, M.A. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. J. Neurosci. 2007, 27, 8309–8313. [Google Scholar] [CrossRef]
- Rabaneda-Lombarte, N.; Vidal-Taboada, J.M.; Valente, T.; Ezquerra, M.; Fernández-Santiago, R.; Martí, M.J.; Compta, Y.; Saura, J.; Solà, C. Altered expression of the immunoregulatory ligand-receptor pair CD200-CD200R1 in the brain of Parkinson’s disease patients. npj Park. Dis. 2022, 8, 27. [Google Scholar] [CrossRef]
- Guo, R.; Han, D.; Song, X.; Gao, Y.; Li, Z.; Li, X.; Yang, Z.; Xu, Z. Context-dependent regulation of Notch signaling in glial development and tumorigenesis. Sci. Adv. 2023, 9, eadi2167. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Han, L.; Liu, Z.; Jing, Z.; Liu, Y.; Peng, Y.; Chang, H.; Lei, J.; Wang, K.; Xu, Y.; Liu, W.; et al. Single-cell spatial transcriptomic atlas of the whole mouse brain. Neuron 2025, 113, 2141–2160.e9. [Google Scholar] [CrossRef]
- Otero-Garcia, M.; Mahajani, S.U.; Wakhloo, D.; Tang, W.; Xue, Y.-Q.; Morabito, S.; Pan, J.; Oberhauser, J.; Madira, A.E.; Shakouri, T.; et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 2022, 110, 2929–2948.e8. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Spitzer, S.O.; Sitnikov, S.; Kamen, Y.; Evans, K.A.; Kronenberg-Versteeg, D.; Dietmann, S.; de Faria, O., Jr.; Agathou, S.; Káradóttir, R.T. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 2019, 101, 459–471.e5. [Google Scholar] [CrossRef] [PubMed]
- Piwecka, M.; Rajewsky, N.; Rybak-Wolf, A. Single-cell and spatial transcriptomics: Deciphering brain complexity in health and disease. Nat. Rev. Neurol. 2023, 19, 346–362. [Google Scholar] [CrossRef]
- Qian, X.; Coleman, K.; Jiang, S.; Kriz, A.J.; Marciano, J.H.; Luo, C.; Cai, C.; Manam, M.D.; Caglayan, E.; Lai, A.; et al. Spatial transcriptomics reveals human cortical layer and area specification. Nature 2025, 644, 153–163. [Google Scholar] [CrossRef]
- Mallach, A.; Zielonka, M.; van Lieshout, V.; An, Y.; Khoo, J.H.; Vanheusden, M.; Chen, W.-T.; Moechars, D.; Arancibia-Carcamo, I.L.; Fiers, M.; et al. Microglia-astrocyte crosstalk in the amyloid plaque niche of an Alzheimer’s disease mouse model, as revealed by spatial transcriptomics. Cell Rep. 2024, 43, 114216. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, S.; Äijö, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivová, Ž.; Saarenpää, S.; Saiz-Castro, G.; et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef]
- Alsema, A.M.; Wijering, M.H.C.; Miedema, A.; Kotah, J.M.; Koster, M.; Rijnsburger, M.; van Weering, H.R.J.; de Vries, H.E.; Baron, W.; Kooistra, S.M.; et al. Spatially resolved gene signatures of white matter lesion progression in multiple sclerosis. Nat. Neurosci. 2024, 27, 2341–2353. [Google Scholar] [CrossRef]
- Horan, K.; Williams, A.C. Mapping out multiple sclerosis with spatial transcriptomics. Nat. Neurosci. 2024, 27, 2270–2272. [Google Scholar] [CrossRef]
- Lerma-Martin, C.; Badia-i-Mompel, P.; Ramirez Flores, R.O.; Sekol, P.; Schäfer, P.S.L.; Riedl, C.J.; Hofmann, A.; Thäwel, T.; Wünnemann, F.; Ibarra-Arellano, M.A.; et al. Cell type mapping reveals tissue niches and interactions in subcortical multiple sclerosis lesions. Nat. Neurosci. 2024, 27, 2354–2365. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, M.; Lee, J.; Sziraki, A.; Anderson, S.; Zhang, Z.; Xu, Z.; Jiang, W.; Ge, S.; Nelson, P.T.; et al. Tracking cell-type-specific temporal dynamics in human and mouse brains. Cell 2023, 186, 4345–4364.e24. [Google Scholar] [CrossRef]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Paredes, I.; Vieira, J.R.; Shah, B.; Ramunno, C.F.; Dyckow, J.; Adler, H.; Richter, M.; Schermann, G.; Giannakouri, E.; Schirmer, L.; et al. Oligodendrocyte precursor cell specification is regulated by bidirectional neural progenitor–endothelial cell crosstalk. Nat. Neurosci. 2021, 24, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, Z.; Jiang, X.; Chen, M.B.; Dong, H.; Liu, J.; Südhof, T.C.; Quake, S.R. Spatial transcriptomics reveal neuron–astrocyte synergy in long-term memory. Nature 2024, 627, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Yao, Z.; van Velthoven, C.T.J.; Kaplan, E.S.; Glattfelder, K.; Barlow, S.T.; Boyer, G.; Carey, D.; Casper, T.; Chakka, A.B.; et al. Brain-wide cell-type-specific transcriptomic signatures of healthy ageing in mice. Nature 2025, 638, 182–196. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Publisher Correction: Transcriptional and epigenetic decoding of the microglial aging process. Nat. Aging 2024, 4, 276. [Google Scholar] [CrossRef]
- Zhou, L.; Kong, G.; Palmisano, I.; Cencioni, M.T.; Danzi, M.; De Virgiliis, F.; Chadwick, J.S.; Crawford, G.; Yu, Z.; De Winter, F.; et al. Reversible CD8 T cell–neuron cross-talk causes aging-dependent neuronal regenerative decline. Science 2022, 376, eabd5926. [Google Scholar] [CrossRef]
- Kaji, S.; Berghoff, S.A.; Spieth, L.; Schlaphoff, L.; Sasmita, A.O.; Vitale, S.; Büschgens, L.; Kedia, S.; Zirngibl, M.; Nazarenko, T.; et al. Apolipoprotein E aggregation in microglia initiates Alzheimer’s disease pathology by seeding β-amyloidosis. Immunity 2024, 57, 2651–2668.e12. [Google Scholar] [CrossRef]
- De Schepper, S.; Ge, J.Z.; Crowley, G.; Ferreira, L.S.S.; Garceau, D.; Toomey, C.E.; Sokolova, D.; Rueda-Carrasco, J.; Shin, S.-H.; Kim, J.-S.; et al. Perivascular cells induce microglial phagocytic states andsynaptic engulfment via SPP1 in mouse models of Alzheimer’sdisease. Nat. Neurosci. 2023, 26, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Tani, H.; Dulla, C.G.; Farzampour, Z.; Taylor-Weiner, A.; Huguenard, J.R.; Reimer, R.J. A Local Glutamate-Glutamine Cycle Sustains Synaptic Excitatory Transmitter Release. Neuron 2014, 81, 888–900. [Google Scholar] [CrossRef]
- Lines, J.; Martin, E.D.; Kofuji, P.; Aguilar, J.; Araque, A. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun. 2020, 11, 3689. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Ouyang, X.; Wang, F.; Li, Q.; Xu, Z.; Ji, D.; Wu, Q.; Zhang, J.; Lu, C.; et al. The effect of CX3CL1/CX3CR1 signal axis on microglia in central nervous system diseases. J. Neurorestoratol. 2023, 11, 100042. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Mironova, Y.A.; Jouroukhin, Y.; Chew, J.; Vidensky, S.; Farah, M.H.; Pletnikov, M.V.; Bergles, D.E.; Morrison, B.M.; Rothstein, J.D. MCT1 Deletion in Oligodendrocyte Lineage Cells Causes Late-Onset Hypomyelination and Axonal Degeneration. Cell Rep. 2021, 34, 108610. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Škandík, M.; Friess, L.; Vázquez-Cabrera, G.; Keane, L.; Grabert, K.; Cruz De los Santos, M.; Posada-Pérez, M.; Baleviciute, A.; Cheray, M.; Joseph, B. Age-associated microglial transcriptome leads to diminished immunogenicity and dysregulation of MCT4 and P2RY12/P2RY13 related functions. Cell Death Discov. 2025, 11, 16. [Google Scholar] [CrossRef]
- Javanmehr, N.; Saleki, K.; Alijanizadeh, P.; Rezaei, N. Microglia dynamics in aging-related neurobehavioral and neuroinflammatory diseases. J. Neuroinflamm. 2022, 19, 273. [Google Scholar] [CrossRef]
- Pan, J.; Ma, N.; Yu, B.; Zhang, W.; Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflamm. 2020, 17, 97. [Google Scholar] [CrossRef]
- Labarta-Bajo, L.; Allen, N.J. Astrocytes in aging. Neuron 2025, 113, 109–126. [Google Scholar] [CrossRef]
- Pan, J.; Zhong, J.; Geng, J.; Oberhauser, J.; Shi, S.; Wan, J. Microglial Lyzl4 Facilitates β-Amyloid Clearance in Alzheimer’s Disease. Adv. Sci. 2024, 12, e2412184. [Google Scholar] [CrossRef]
- Olah, M.; Patrick, E.; Villani, A.-C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, N.; Zhong, J.; Yu, B.; Wan, J.; Zhang, W. Age-associated changes in microglia and astrocytes ameliorate blood-brain barrier dysfunction. Mol. Ther.-Nucleic Acids 2021, 26, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Nyúl-Tóth, Á.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; DelFavero, J.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wiley, G.; et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. GeroScience 2020, 42, 429–444. [Google Scholar] [CrossRef]
- Tyurikova, O.; Kopach, O.; Zheng, K.; Rathore, D.; Codadu, N.; Wu, S.-Y.; Shen, Y.; Campbell, R.E.; Wykes, R.C.; Volynski, K.; et al. Astrocyte Kir4.1 expression level territorially controls excitatory transmission in the brain. Cell Rep. 2025, 44, 115299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kontos, C.D.; Annex, B.H.; Popel, A.S. Angiopoietin-Tie Signaling Pathway in Endothelial Cells: A Computational Model. iScience 2019, 20, 497–511. [Google Scholar] [CrossRef]
- Smith, L.K.; He, Y.; Park, J.-S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Mahoney, M.; Huang, Y.; Korte, N.; Yvanka de Soysa, T.; Wilton, D.K.; Knorr, E.; Mastro, K.; Chang, A.; Zhang, A.; et al. Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain. Cell 2024, 187, 4193–4212.e24. [Google Scholar] [CrossRef]
- Dejanovic, B.; Wu, T.; Tsai, M.-C.; Graykowski, D.; Gandham, V.D.; Rose, C.M.; Bakalarski, C.E.; Ngu, H.; Wang, Y.; Pandey, S.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2022, 2, 837–850. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef]
- Heo, D.; Kim, A.A.; Neumann, B.; Doze, V.N.; Xu, Y.K.T.; Mironova, Y.A.; Slosberg, J.; Goff, L.A.; Franklin, R.J.M.; Bergles, D.E. Transcriptional profiles of mouse oligodendrocyte precursor cells across the lifespan. Nat. Aging 2025, 5, 675–690. [Google Scholar] [CrossRef]
- Zou, P.; Wu, C.; Liu, T.C.-Y.; Duan, R.; Yang, L. Oligodendrocyte progenitor cells in Alzheimer’s disease: From physiology to pathology. Transl. Neurodegener. 2023, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.N.; Fields, R.D. Regulation of myelination by microglia. Sci. Adv. 2021, 7, eabk1131. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.T.; Carver, C.M.; Rodriguez, S.L.; Wang, L.; Zhang, X.; Schafer, M.J. Aging and senescent fates of oligodendrocyte precursor cells in the mouse brain. npj Aging 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Munro, D.A.D.; Bestard-Cuche, N.; McQuaid, C.; Chagnot, A.; Shabestari, S.K.; Chadarevian, J.P.; Maheshwari, U.; Szymkowiak, S.; Morris, K.; Mohammad, M.; et al. Microglia protect against age-associated brain pathologies. Neuron 2024, 112, 2732–2748.e8. [Google Scholar] [CrossRef]
- Rhinn, H.; Abeliovich, A. Differential Aging Analysis in Human Cerebral Cortex Identifies Variants in TMEM106B and GRN that Regulate Aging Phenotypes. Cell Syst. 2017, 4, 404–415.e5. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572. [Google Scholar] [CrossRef]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.B.; Leary, N.; Luna, X.; Meharena, H.S.; Scannail, A.N.; Bozzelli, P.L.; Samaan, G.; Murdock, M.H.; von Maydell, D.; Effenberger, A.H.; et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 2022, 29, 1197–1212.e8. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, Y.; Cai, Z.; Terekhova, M.; Swain, A.; Andhey, P.S.; Guimaraes, R.M.; Ulezko Antonova, A.; Qiu, T.; Sviben, S.; et al. Transcriptomic atlas and interaction networks of brain cells in mouse CNS demyelination and remyelination. Cell Rep. 2023, 42, 112293. [Google Scholar] [CrossRef]
- Kiss, M.G.; Mindur, J.E.; Yates, A.G.; Lee, D.; Fullard, J.F.; Anzai, A.; Poller, W.C.; Christie, K.A.; Iwamoto, Y.; Roudko, V.; et al. Interleukin-3 coordinates glial-peripheral immune crosstalk to incite multiple sclerosis. Immunity 2023, 56, 1502–1514.e8. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, I.; Al Nimer, F.; Wang, J.; Lentsch, V.; Planas, R.; Jelcic, I.; Madjovski, A.; Ruhrmann, S.; Faigle, W.; Frauenknecht, K.; et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4+ T Cells in Multiple Sclerosis. Cell 2018, 175, 85–100.e23. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Beyer, T.; Nirschl, L.; Farrenkopf, D.; Lößlein, L.; Vandrey, O.; Peter, A.; Tsaktanis, T.; Kebir, H.; Laplaud, D.; et al. PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Nat. Commun. 2023, 14, 5555. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Huang, R.; Pan, J. Dynamic Intercellular Networks in the CNS: Mechanisms of Crosstalk from Homeostasis to Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 8155. https://doi.org/10.3390/ijms26178155
Zheng Y, Huang R, Pan J. Dynamic Intercellular Networks in the CNS: Mechanisms of Crosstalk from Homeostasis to Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(17):8155. https://doi.org/10.3390/ijms26178155
Chicago/Turabian StyleZheng, Yutian, Rui Huang, and Jie Pan. 2025. "Dynamic Intercellular Networks in the CNS: Mechanisms of Crosstalk from Homeostasis to Neurodegeneration" International Journal of Molecular Sciences 26, no. 17: 8155. https://doi.org/10.3390/ijms26178155
APA StyleZheng, Y., Huang, R., & Pan, J. (2025). Dynamic Intercellular Networks in the CNS: Mechanisms of Crosstalk from Homeostasis to Neurodegeneration. International Journal of Molecular Sciences, 26(17), 8155. https://doi.org/10.3390/ijms26178155