Augmentation of the Benzyl Isothiocyanate-Induced Antiproliferation by NBDHEX in the HCT-116 Human Colorectal Cancer Cell Line
Abstract
1. Introduction
2. Results
2.1. Enhancing Effects of NBDHEX on BITC-Induced Antiproliferation
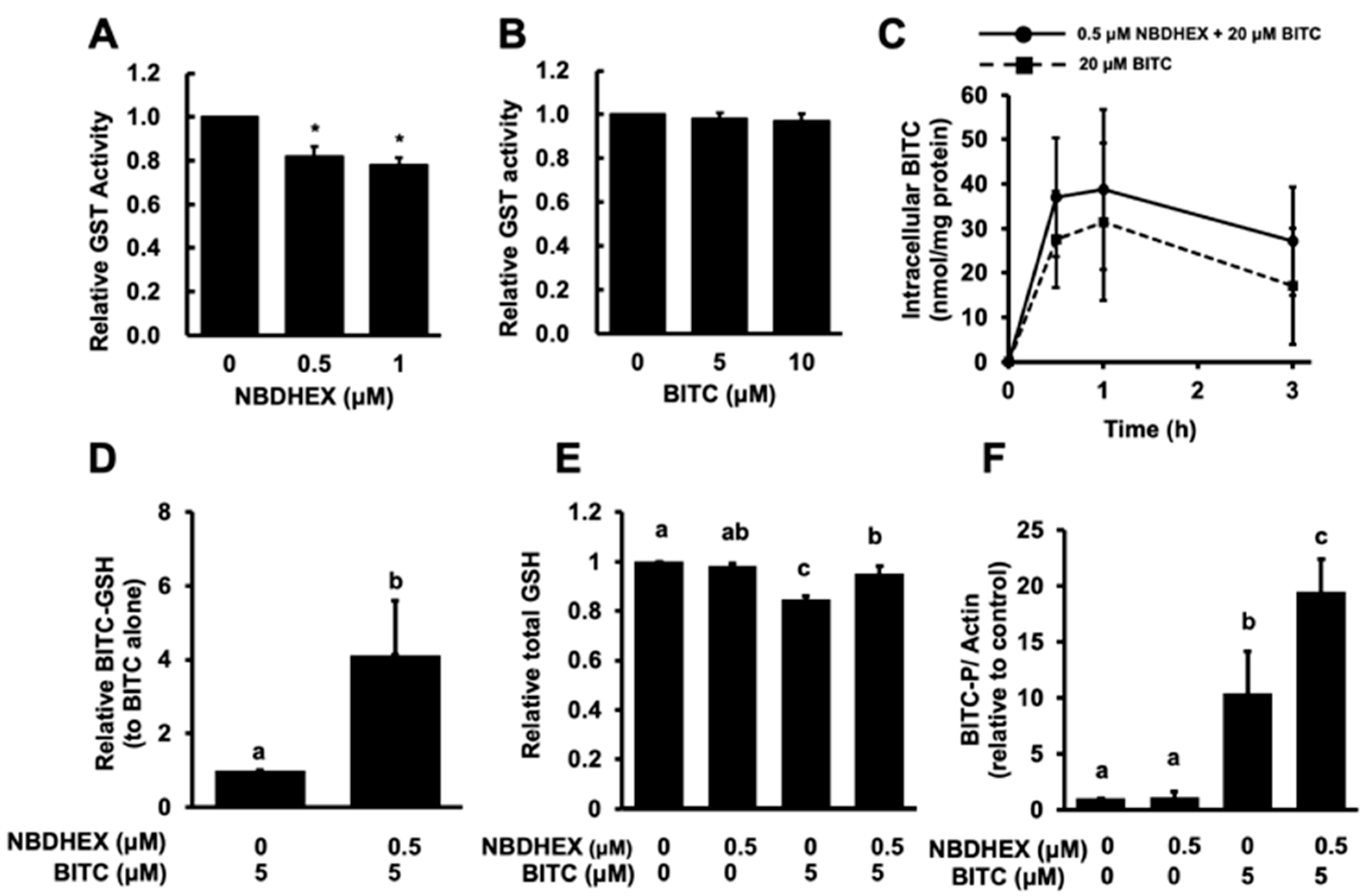
2.2. Modulatory Effect of NBDHEX on the Intracellular Metabolism of BITC
2.3. Enhancing Effects of NBDHEX on BITC-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture, Treatment, and Cell Viability Determination
4.3. Glutathione S-Transferase (GST) Activity Assay
4.4. Measurement of Intracellular BITC Accumulation
4.5. Measurement of Intracellular BITC–GSH Accumulation
4.6. Measurement of Intracellular GSH Level
4.7. Western Blot Analysis
4.8. Apoptosis Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BITC | benzyl isothiocyanate |
| ITC | isothiocyanate |
| MAPK | mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| MDR | multidrug resistance |
| GST | glutathione S-transferase |
| GSH | the reduced form of glutathione |
| MRP | multidrug resistance-associated protein |
| NBDHEX | 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol |
| CDNB | 1-chloro-2,4-dinitrobenzene |
| DTNB | 5,5′-dithiobis(2-nitrobenzoic acid) |
| GR | glutathione reductase |
References
- Nakamura, T.; Murata, Y.; Nakamura, Y. Characterization of benzyl isothiocyanate extracted from mashed green papaya by distillation. Food Chem. 2019, 299, 125118. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyoshi, N. Electrophiles in foods: The current status of isothiocyanates and their chemical biology. Biosci. Biotechnol. Biochem. 2010, 74, 242–255. [Google Scholar] [CrossRef]
- Dinh, T.N.; Parat, M.O.; Ong, Y.S.; Khaw, K.Y. Anticancer activities of dietary benzyl isothiocyanate: A comprehensive review. Pharmacol. Res. 2021, 169, 105666. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Nakamura, Y. Inhibition of drug resistance mechanisms improves the benzyl isothiocyanate-induced anti-proliferation in Human colorectal cancer cells. Curr. Pharmacol. Rep. 2020, 6, 306–314. [Google Scholar] [CrossRef]
- Huang, Y.P.; Jiang, Y.W.; Chen, H.Y.; Hsiao, Y.T.; Peng, S.F.; Chou, Y.C.; Yang, J.L.; Hsia, T.C.; Chung, J.G. Benzyl Isothiocyanate Induces Apoptotic Cell Death Through Mitochondria-dependent Pathway in Gefitinib-resistant NCI-H460 Human Lung Cancer Cells In Vitro. Anticancer Res. 2018, 38, 5165–5176. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol. Cancer Ther. 2005, 4, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Chiang, N.N.; Lu, Y.H.; Huang, Y.S.; Yang, J.S.; Tsai, S.C.; Lu, C.C.; Chen, F.A. Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. Biomedicine 2018, 8, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, G.; Yang, J.; Zhu, C.; Zeng, W.; Li, X.; Yun, Y.; Wen, T.; Pang, X.; Mamat, N.; et al. Benzyl isothiocyanate triggers apoptosis by initiating autophagy through the generation of ROS and modulating the MAPK and PI3K-AKT pathway in cervical cancer cells. Int. Immunopharmacol. 2025, 163, 115208. [Google Scholar] [CrossRef]
- Xiao, D.; Powolny, A.A.; Singh, S.V. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 2008, 283, 30151–30163. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wang, W.; Kong, A.N.; Tan, T.H. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J. Biol. Chem. 1998, 273, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Zhang, R.; Batra, S.; Shi, Y.; Srivastava, S.K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis 2009, 30, 1744–1753. [Google Scholar] [CrossRef]
- Panda, M.; Biswal, B.K. Cell signaling and cancer: A mechanistic insight into drug resistance. Mol. Biol. Rep. 2019, 46, 5645–5659. [Google Scholar] [CrossRef]
- Shi, H.; Lu, D.; Shu, Y.; Shi, W.; Lu, S.; Wang, K. Expression of multidrug-resistance-related proteins P-glycoprotein, glutathione-S-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Cancer Investig. 2008, 26, 344–351. [Google Scholar] [CrossRef]
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.T.; Chen, F.; Kohli, M.; Rago, C.; Cummins, J.M.; Dang, L.H. Glutathione S-transferase pi1 promotes tumorigenicity in HCT116 human colon cancer cells. Cancer Res. 2005, 65, 9485–9494. [Google Scholar] [CrossRef]
- Li, S.Y.; An, P.; Cai, H.Y.; Bai, X.; Zhang, Y.N.; Yu, B.; Zuo, F.Y.; Chen, G. Proteomic analysis of differentially expressed proteins involving in liver metastasis of human colorectal carcinoma. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 149–153. [Google Scholar]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Cummings, J.; Boyd, G.; Macpherson, J.S.; Wolf, H.; Smith, G.; Smyth, J.F.; Jodrell, D.I. Factors influencing the cellular accumulation of SN-38 and camptothecin. Cancer Chemother. Pharmacol. 2002, 49, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Maeng, Y.H.; Chang, W.Y.; Moon, P.G.; et al. Epigenetic alterations are involved in the overexpression of glutathione S-transferase π-1 in human colorectal cancers. Int. J. Oncol. 2014, 45, 1275–1283. [Google Scholar] [CrossRef]
- Sha, H.H.; Wang, Z.; Dong, S.C.; Hu, T.M.; Liu, S.W.; Zhang, J.Y.; Wu, Y.; Ma, R.; Wu, J.Z.; Chen, D.; et al. 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol: A promising new anticancer compound. Biosci. Rep. 2018, 38, BSR20171440. [Google Scholar] [CrossRef]
- Bruggeman, I.M.; Temmink, J.H.; van Bladeren, P.J. Glutathione- and cysteine-mediated cytotoxicity of allyl and benzyl isothiocyanate. Toxicol. Appl. Pharmacol. 1986, 83, 349–359. [Google Scholar] [CrossRef]
- Yang, Q.; Nakamura, T.; Seto, M.; Miyagawa, M.; Xu, W.; Zhu, B.; Munemasa, S.; Murata, Y.; Nakamura, Y. A multidrug resistance-associated protein inhibitor is a potential enhancer of the benzyl isothiocyanate-induced apoptosis induction in human colorectal cancer cells. J. Biochem. Mol. Toxicol. 2021, 35, e22791. [Google Scholar] [CrossRef]
- Yang, Q.; Miyagawa, M.; Liu, X.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. Methyl-β-cyclodextrin potentiates the BITC-induced anti-cancer effect through modulation of the Akt phosphorylation in human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 2158–2167. [Google Scholar] [CrossRef]
- Liu, X.; Takano, C.; Shimizu, T.; Yokobe, S.; Abe-Kanoh, N.; Zhu, B.; Nakamura, T.; Munemasa, S.; Murata, Y.; Nakamura, Y. Inhibition of phosphatidylinositide 3-kinase ameliorates antiproliferation by benzyl isothiocyanate in human colon cancer cells. Biochem. Biophys. Res. Commun. 2017, 491, 209–216. [Google Scholar] [CrossRef]
- De Luca, A.; Pellizzari Tregno, F.; Sau, A.; Pastore, A.; Palumbo, C.; Alama, A.; Cicconi, R.; Federici, G.; Caccuri, A.M. Glutathione S-transferase P1-1 as a target for mesothelioma treatment. Cancer Sci. 2013, 104, 223–230. [Google Scholar] [CrossRef]
- Federici, L.; Lo Sterzo, C.; Pezzola, S.; Di Matteo, A.; Scaloni, F.; Federici, G.; Caccuri, A.M. Structural basis for the binding of the anticancer compound 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol to human glutathione s-transferases. Cancer Res. 2009, 69, 8025–8034. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Chung, F.L. Binding to protein by isothiocyanates: A potential mechanism for apoptosis induction in human non small lung cancer cells. Nutr. Cancer 2008, 60 (Suppl. 1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Xiao, Z.; Hood, B.L.; Dakshanamurthy, S.; Wang, X.; Govind, S.; Conrads, T.P.; Veenstra, T.D.; Chung, F.L. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008, 283, 22136–22146. [Google Scholar] [CrossRef]
- Ascione, A.; Cianfriglia, M.; Dupuism, M.L.; Mallano, A.; Sau, A.; Pellizzari Tregno, F.; Pezzola, S.; Caccuri, A.M. The glutathione S-transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol overcomes the MDR1-P-glycoprotein and MRP1-mediated multidrug resistance in acute myeloid leukemia cells. Cancer Chemother. Pharmacol. 2009, 64, 419–424. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- Adler, V.; Yin, Z.; Fuchs, S.Y.; Benezra, M.; Rosario, L.; Tew, K.D.; Pincus, M.R.; Sardana, M.; Henderson, C.J.; Wolf, C.R.; et al. Regulation of JNK signaling by GSTp. EMBO J. 1999, 18, 1321–1334. [Google Scholar] [CrossRef]
- De Luca, A.; Federici, L.; De Canio, M.; Stella, L.; Caccuri, A.M. New insights into the mechanism of JNK1 inhibition by glutathione transferase P1-1. Biochemistry 2012, 51, 7304–7312. [Google Scholar] [CrossRef]
- Tang, Y.; Naito, S.; Abe-Kanoh, N.; Ogawa, S.; Yamaguchi, S.; Zhu, B.; Murata, Y.; Nakamura, Y. Benzyl isothiocyanate attenuates the hydrogen peroxide-induced interleukin-13 expression through glutathione S-transferase P induction in T lymphocytic leukemia cells. J. Biochem. Mol. Toxicol. 2018, 32, e22054. [Google Scholar] [CrossRef]
- Palumbo, C.; De Luca, A.; Rosato, N.; Forgione, M.; Rotili, D.; Caccuri, A.M. c-Jun N-terminal kinase activation by nitrobenzoxadiazoles leads to late-stage autophagy inhibition. J. Transl. Med. 2016, 14, 37. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Alnasser, S.M. The role of glutathione S-transferases in human disease pathogenesis and their current inhibitors. Genes Dis. 2024, 12, 101482. [Google Scholar] [CrossRef]
- Nakamura, T.; Kitamoto, N.; Osawa, T.; Kato, Y. Immunochemical detection of food-derived isothiocyanate as a lysine conjugate. Biosci. Biotechnol. Biochem. 2010, 74, 536–540. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Yano, A.; Satoh, A.; Munemasa, S.; Murata, Y.; Nakamura, T.; Nakamura, Y. Augmentation of the Benzyl Isothiocyanate-Induced Antiproliferation by NBDHEX in the HCT-116 Human Colorectal Cancer Cell Line. Int. J. Mol. Sci. 2025, 26, 8145. https://doi.org/10.3390/ijms26178145
Sun R, Yano A, Satoh A, Munemasa S, Murata Y, Nakamura T, Nakamura Y. Augmentation of the Benzyl Isothiocyanate-Induced Antiproliferation by NBDHEX in the HCT-116 Human Colorectal Cancer Cell Line. International Journal of Molecular Sciences. 2025; 26(17):8145. https://doi.org/10.3390/ijms26178145
Chicago/Turabian StyleSun, Ruitong, Aina Yano, Ayano Satoh, Shintaro Munemasa, Yoshiyuki Murata, Toshiyuki Nakamura, and Yoshimasa Nakamura. 2025. "Augmentation of the Benzyl Isothiocyanate-Induced Antiproliferation by NBDHEX in the HCT-116 Human Colorectal Cancer Cell Line" International Journal of Molecular Sciences 26, no. 17: 8145. https://doi.org/10.3390/ijms26178145
APA StyleSun, R., Yano, A., Satoh, A., Munemasa, S., Murata, Y., Nakamura, T., & Nakamura, Y. (2025). Augmentation of the Benzyl Isothiocyanate-Induced Antiproliferation by NBDHEX in the HCT-116 Human Colorectal Cancer Cell Line. International Journal of Molecular Sciences, 26(17), 8145. https://doi.org/10.3390/ijms26178145





