Transcriptomic Analysis of the Rainbow Trout Response to Single and Co-Infections with Myxobolus cerebralis and Tetracapsuloides bryosalmonae at Sites of Parasite Entry
Abstract
1. Introduction
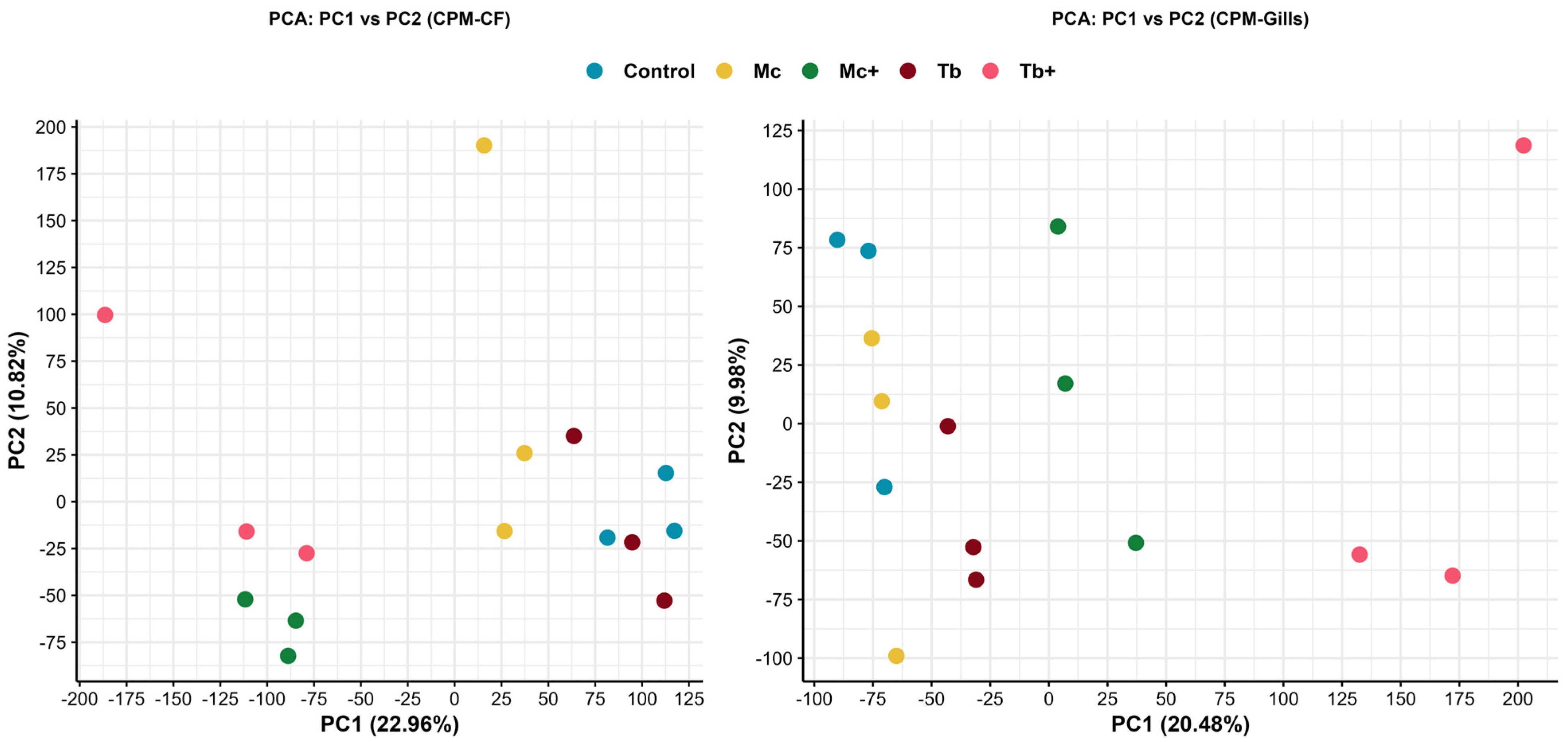
2. Results
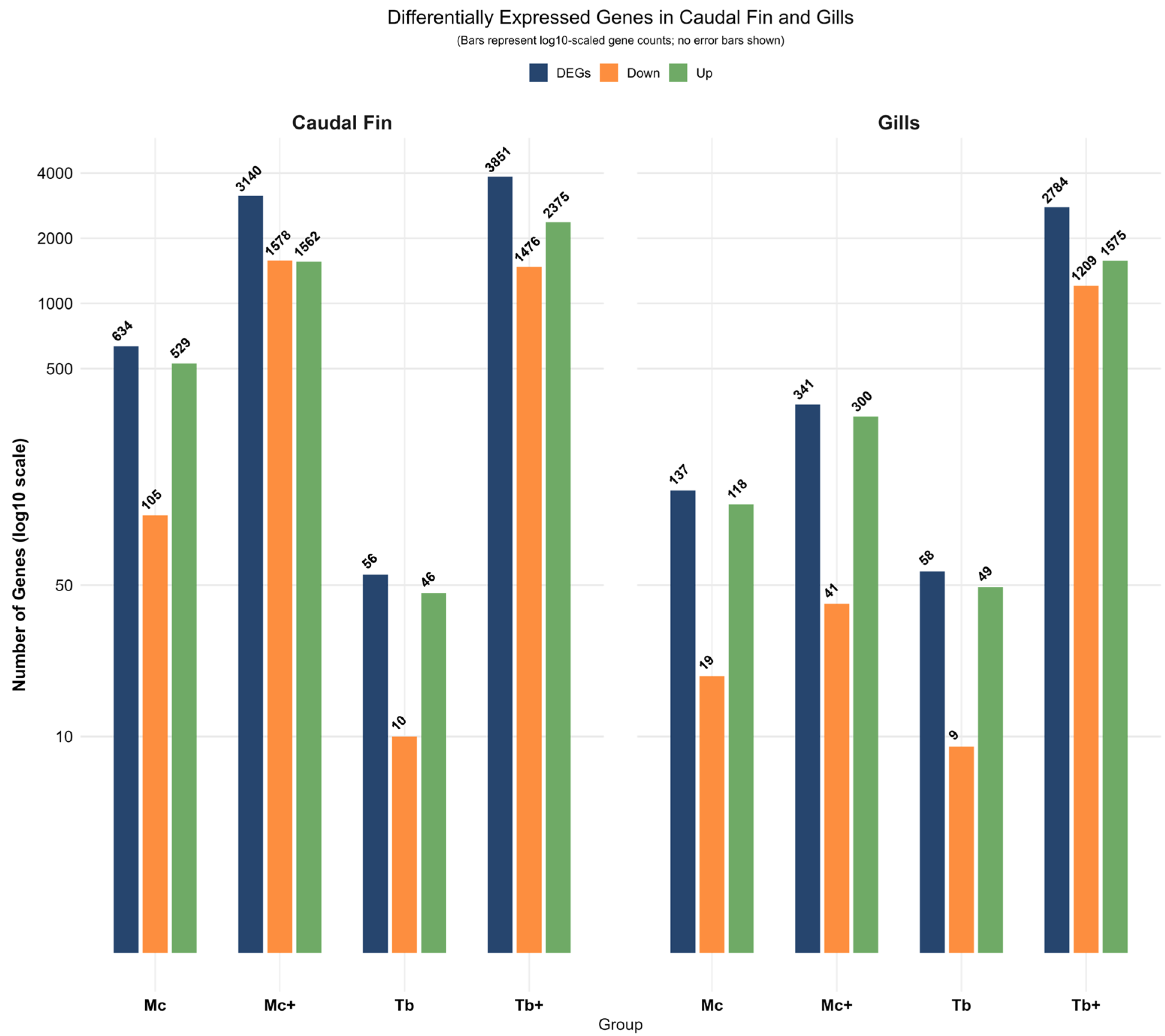
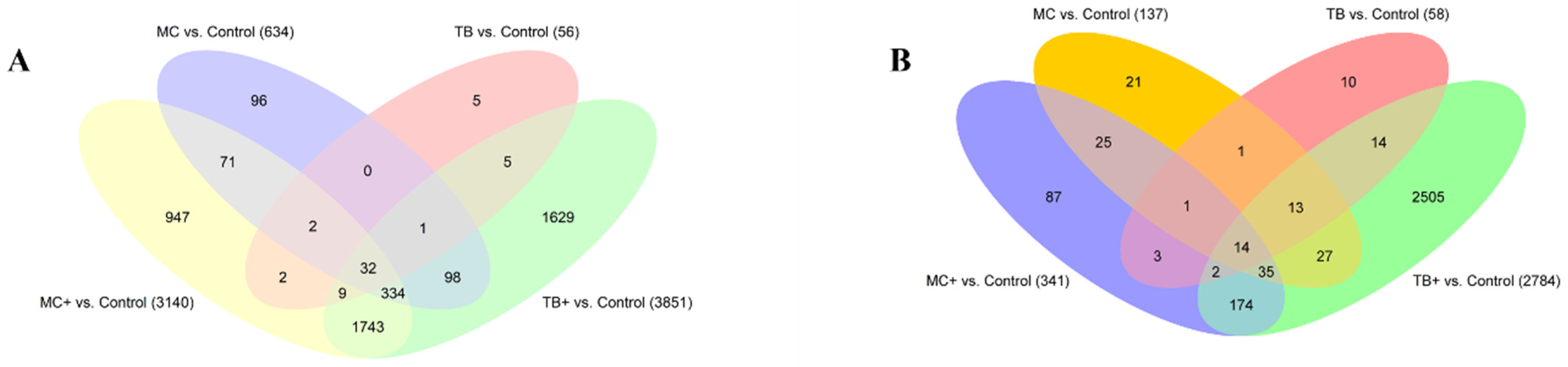
2.1. Differential Expression Analysis in Caudal Fins
2.2. Differential Expression Analysis in Gills
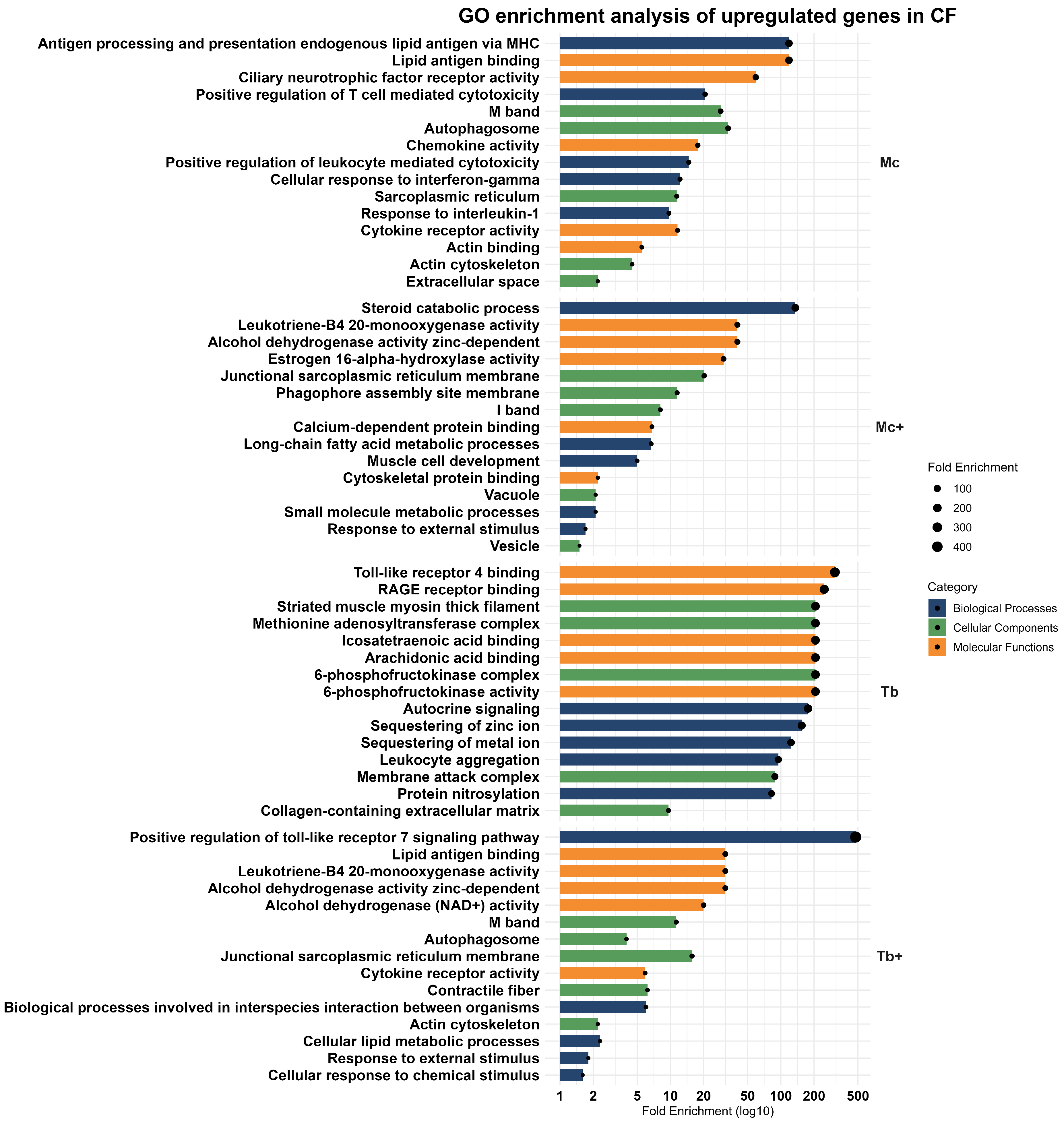
2.3. Upregulated Genes in the Caudal Fins (CF)
2.3.1. Upregulated Genes After M. cerebralis Infection (Mc)
2.3.2. Upregulated Genes After Co-Infection with T. bryosalmonae (Mc+)
2.3.3. Upregulated Genes After Infection with T. bryosalmonae (Tb)
2.3.4. Upregulated Genes After Co-Infection with M. cerebralis (Tb+)
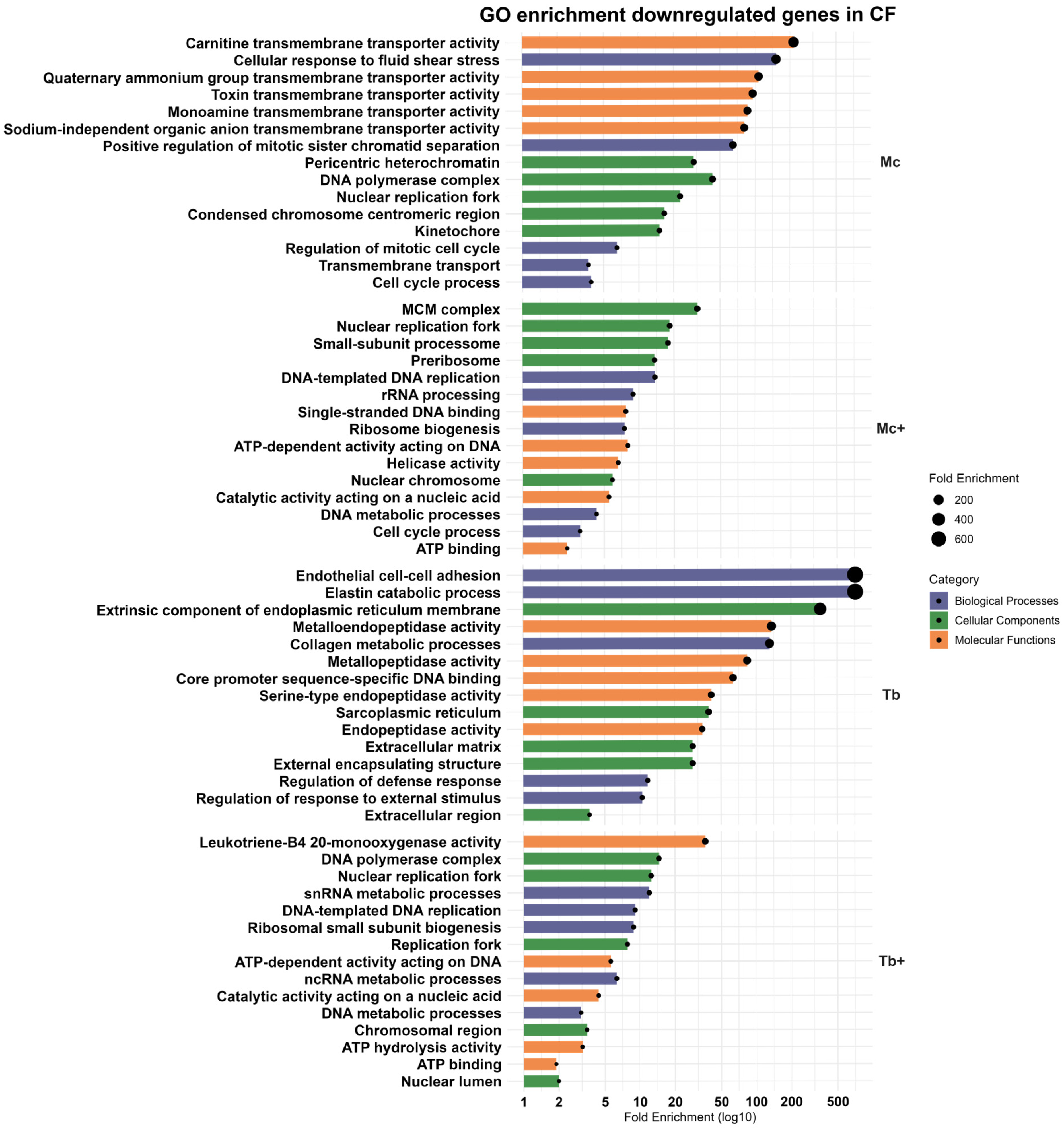
2.4. Downregulated Genes in the Caudal Fins (CF)
2.4.1. Downregulated Genes in M. cerebralis-Infected Group (Mc)
2.4.2. Downregulated Genes After Co-Infection with T. bryosalmonae (Mc+)
2.4.3. Downregulated Genes in T. bryosalmonae-Infected Group (Tb)
2.4.4. Downregulated Genes After Co-Infection with M. cerebralis (Tb+)
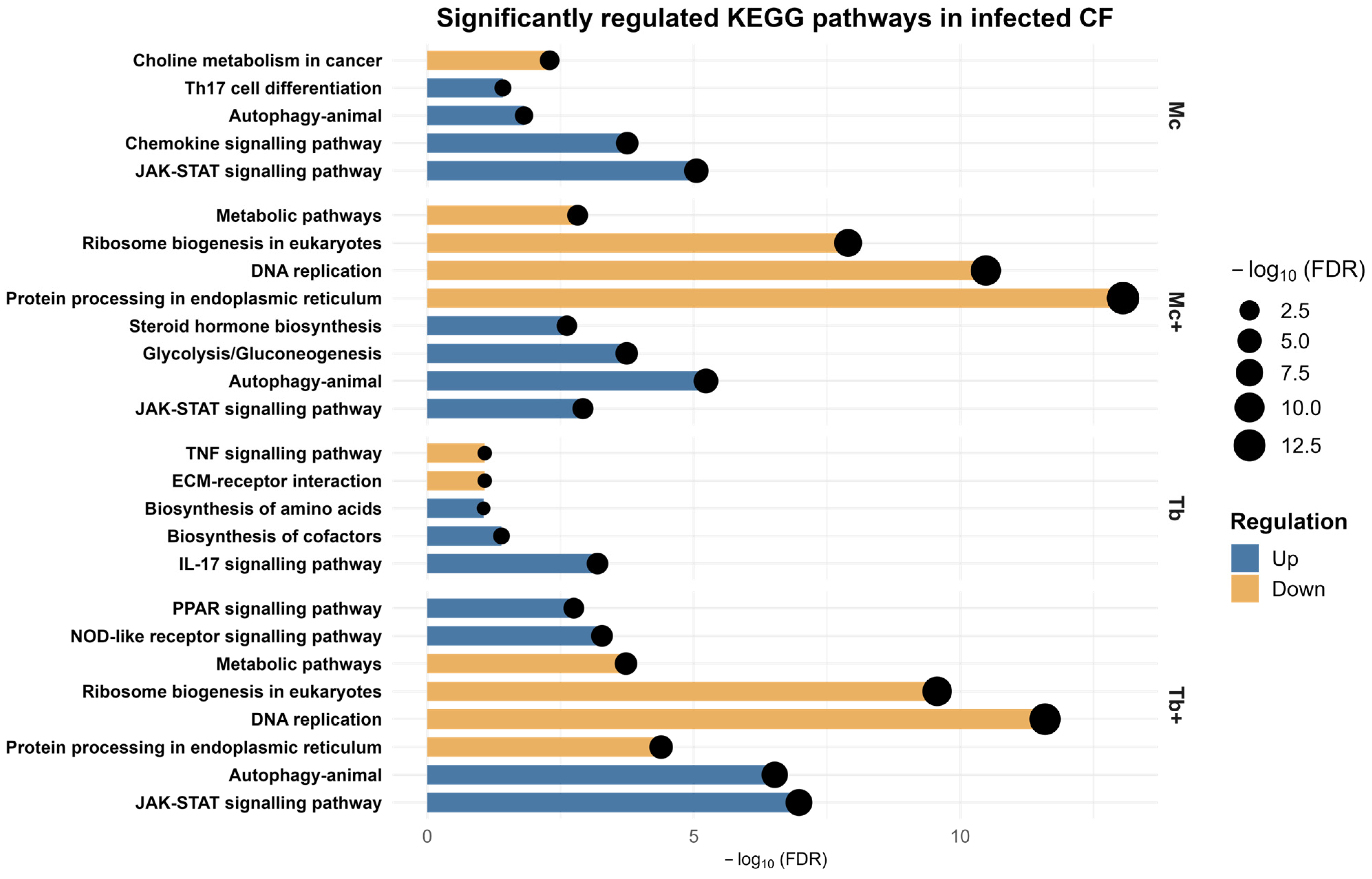
2.5. KEGG Pathway Analysis of Caudal Fin DEGs
2.6. Upregulated Genes in Gills
2.6.1. Upregulated Genes in the Gills After a Single Infection with M. cerebralis (Mc)
2.6.2. Upregulated Genes in the Gills After Co-Infection with T. bryosalmonae (Mc+)
2.6.3. Upregulated Genes in the Gills After Single Infection with T. bryosalmonae (Tb)
2.6.4. Upregulated Genes in the Gills After Co-Infection with M. cerebralis (Tb+)
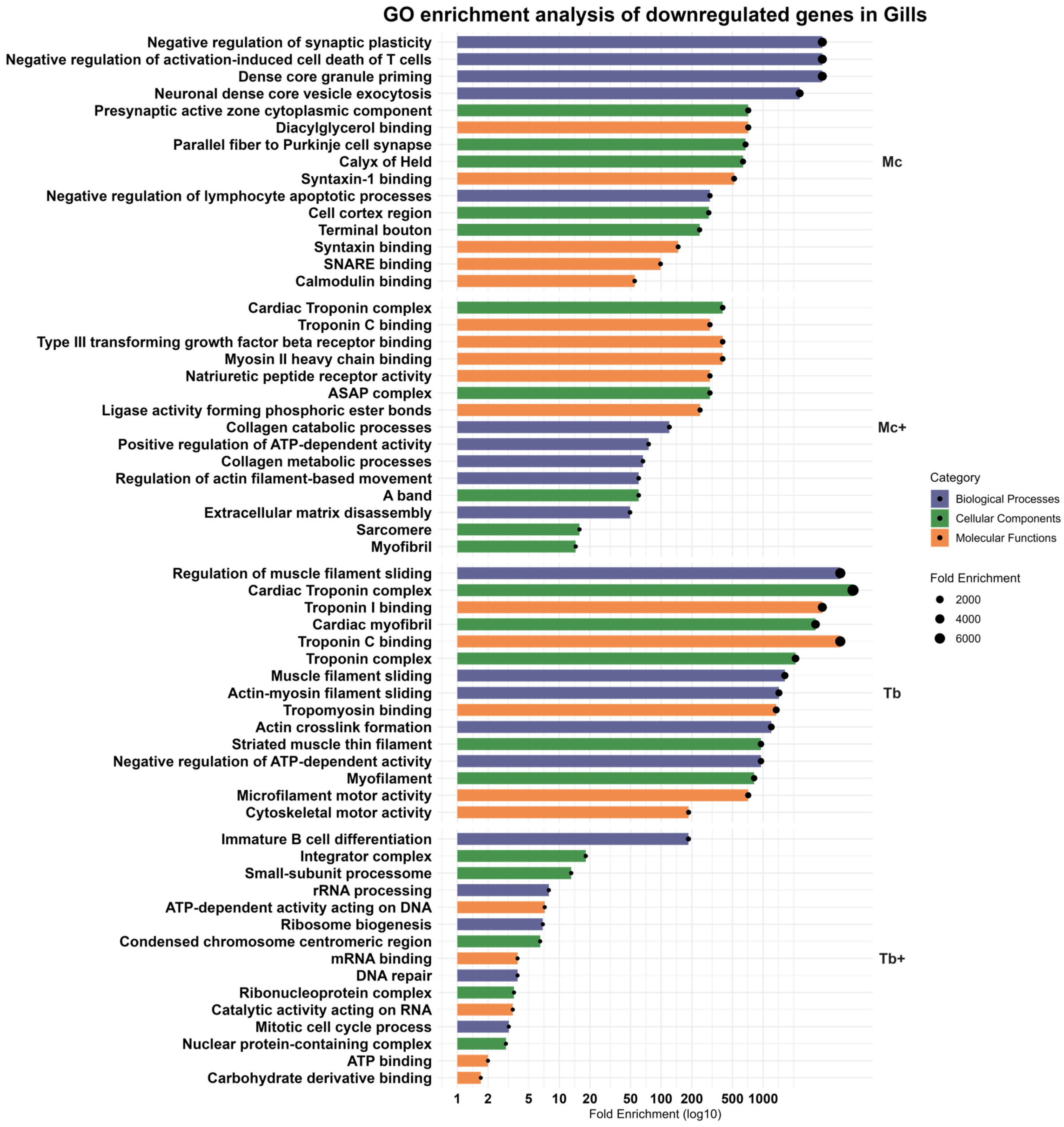
2.7. Downregulated Genes in the Gills
2.7.1. Downregulated Genes in the Gills After M. cerebralis Infection (Mc)
2.7.2. Downregulated Genes in the Gills After Co-Infection with T. bryosalmonae (Mc+)
2.7.3. Downregulated Genes in the Gills Following Single Infection with T. bryosalmonae (Tb)
2.7.4. Downregulated Genes in the Gills Following Co-Infection with M. cerebralis (Tb+)
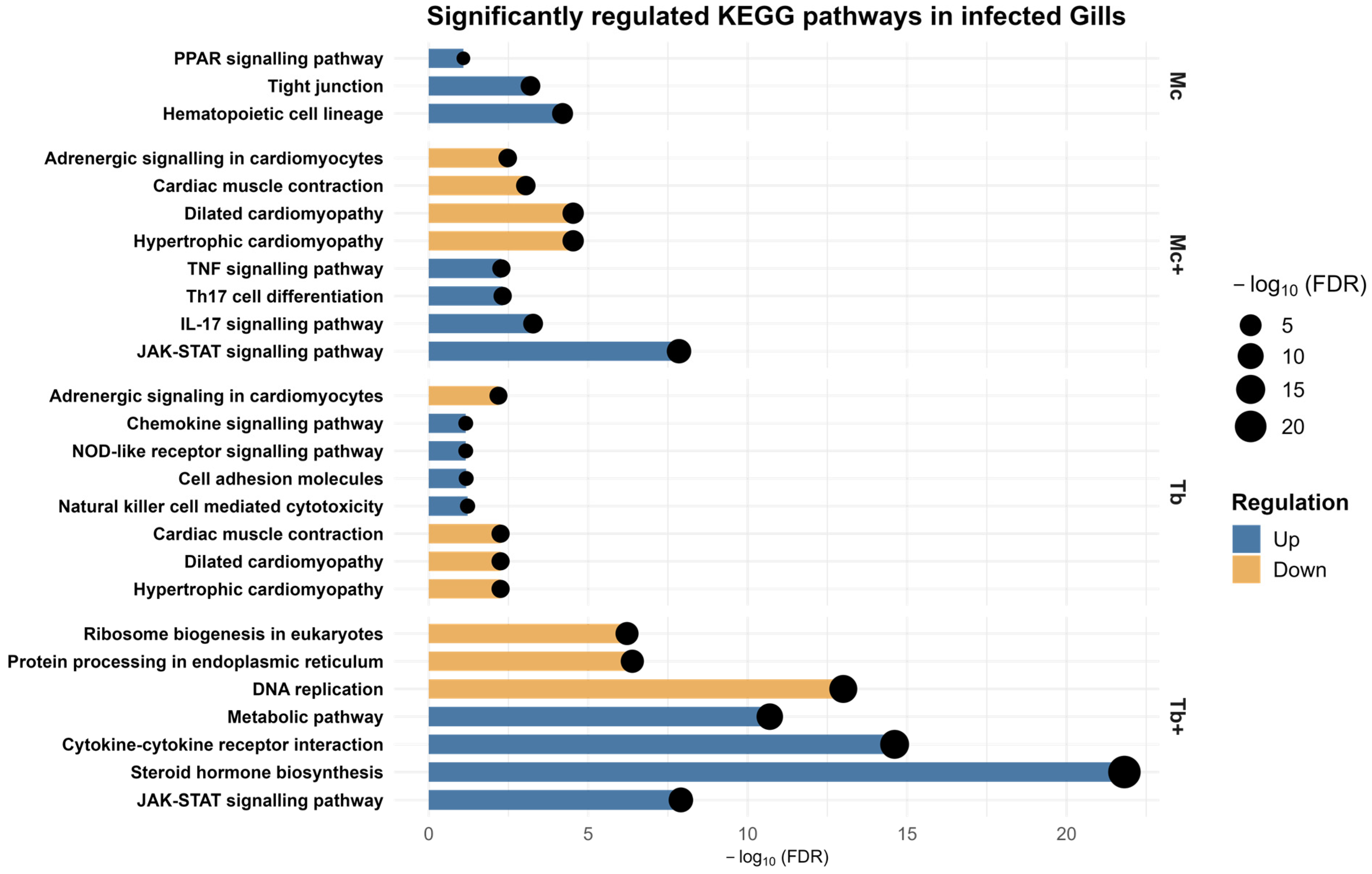
2.8. KEGG Pathway Analysis of Gills DEGs
3. Discussion
3.1. Transcriptomic Response to M. cerebralis Single Infection (Mc)
3.2. Transcriptomic Response to Co-Infection with T. bryosalmonae (Mc+)
3.3. Transcriptomic Response to T. bryosalmonae Single Infection (Tb)
3.4. Transcriptomic Response to Co-Infection with M. cerebralis (Tb+)
3.5. KEGG Pathway Analysis at the Portals of Entry
3.6. Integration with Previous Proteomic Findings
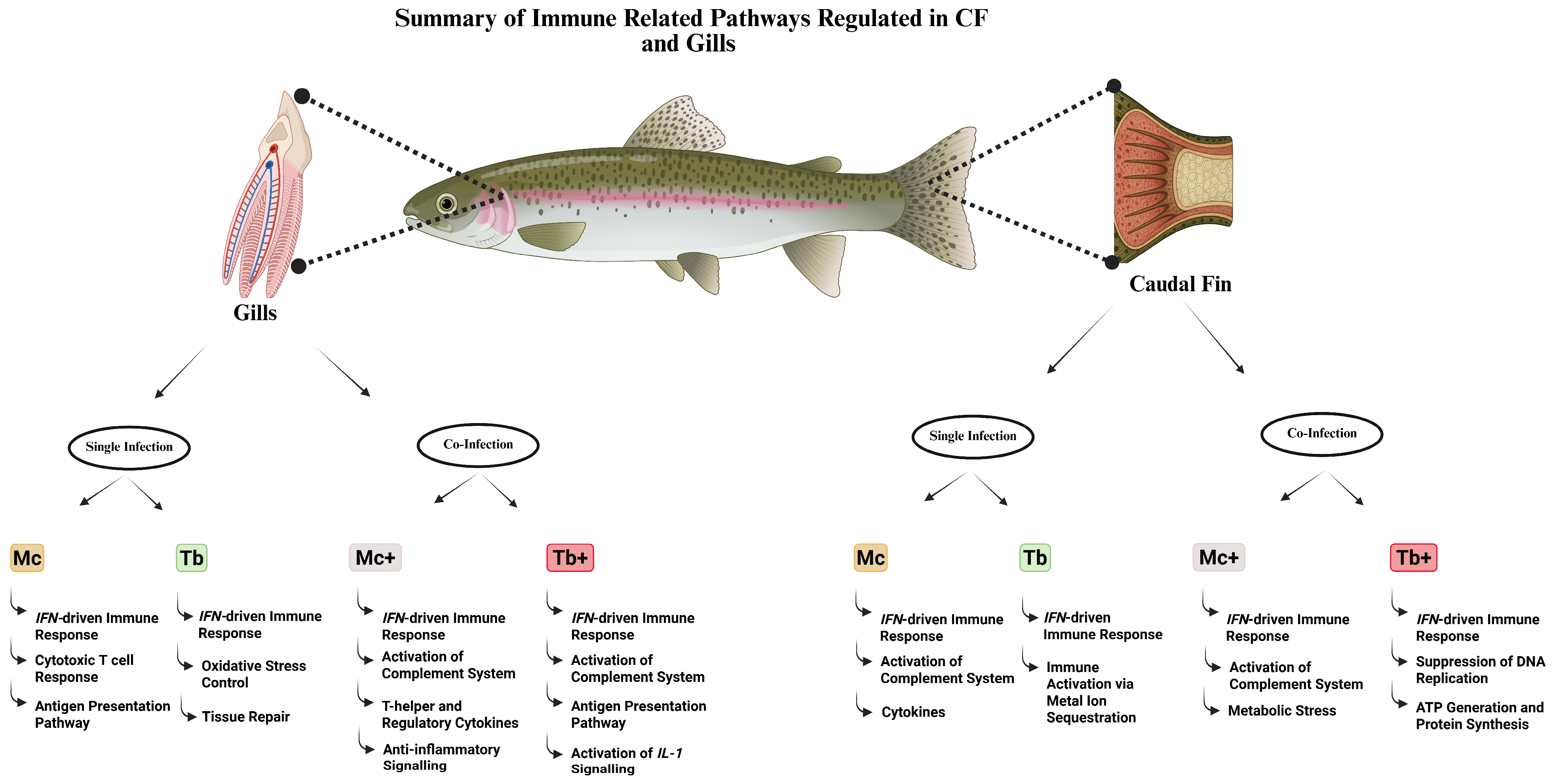
3.7. Overview of Immune Response
4. Materials and Methods
4.1. Experimental Design and Fish Sampling
4.2. RNA Extraction, Library Preparation, and Sequencing
4.3. Mapping and Differential Expression Analysis of Host Genes
4.4. Comparative Analysis with Proteomic Study
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WD | Whirling Disease |
| PKD | Proliferative Kidney Disease |
| TAMs | Triactinomyxon Spores |
| SOCS | Suppressor of Cytokine Signaling |
| STAT | Signal Transducer and Activator of Transcription |
| CNTF | Ciliary Neurotrophic Factor Receptor Activity |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopaedia of Genes and Genomes |
| CF | Caudal Fins |
| HSP | Heat Shock Protein |
| MCP | Monocyte Chemotactic Protein |
| PAS | Phagophore Assembly Site |
| TNF | Tumor Necrosis Factor |
| IFI44 | Interferon Induced Protein 44 |
| RSAD2 | Radical S-adenosyl Methionine Domain Containing 2 |
| ISG15 | ISG15 Ubiquitin-Like Modifier |
| TLR7 | Toll-Like Receptor 7 |
References
- Jones, S.R.M.; Bartholomew, J.L.; Zhang, J.Y. Mitigating Myxozoan Disease Impacts on Wild Fish Populations. In Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015; pp. 397–413. [Google Scholar]
- Allison, E.H.; Perry, A.L.; Badjeck, M.; Neil Adger, W.; Brown, K.; Conway, D.; Halls, A.S.; Pilling, G.M.; Reynolds, J.D.; Andrew, N.L.; et al. Vulnerability of National Economies to the Impacts of Climate Change on Fisheries. Fish Fish. 2009, 10, 173–196. [Google Scholar] [CrossRef]
- Henderson, M.; Okamura, B. The Phylogeography of Salmonid Proliferative Kidney Disease in Europe and North America. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 1729–1736. [Google Scholar] [CrossRef]
- Gorgoglione, B.; Kotob, M.; Unfer, G.; El-Matbouli, M. First Proliferative Kidney Disease Outbreak in Austria, Linking to the Aetiology of Black Trout Syndrome Threatening Autochthonous Trout Populations. Dis. Aquat. Org. 2016, 119, 117–128. [Google Scholar] [CrossRef]
- Kotob, M.H.; Gorgoglione, B.; Kumar, G.; Abdelzaher, M.; Saleh, M.; El-Matbouli, M. The Impact of Tetracapsuloides Bryosalmonae and Myxobolus Cerebralis Co-Infections on Pathology in Rainbow Trout. Parasit. Vectors 2017, 10, 442. [Google Scholar] [CrossRef]
- Hedrick, R.; McDowell, T.; Gay, M.; Marty, G.; Georgiadis, M.; MacConnell, E. Comparative Susceptibility of Rainbow Trout Oncorhynchus mykiss and Brown Trout Salmo Trutta to Myxobolus Cerebralis, the Cause of Salmonid Whirling Disease. Dis. Aquat. Org. 1999, 37, 173–183. [Google Scholar] [CrossRef]
- Sarker, S.; Kallert, D.; Hedrick, R.; El-Matbouli, M. Whirling Disease Revisited: Pathogenesis, Parasite Biology and Disease Intervention. Dis. Aquat. Org. 2015, 114, 155–175. [Google Scholar] [CrossRef]
- EL–Matbouli, M.; Hoffmann, R.W.; Mandok, C. Light and Electron Microscopic Observations on the Route of the Triactinomyxon-sporoplasm of Myxobolus cerebralis from Epidermis into Rainbow Trout Cartilage. J. Fish Biol. 1995, 46, 919–935. [Google Scholar] [CrossRef]
- Kotob, M.H.; Kumar, G.; Saleh, M.; Gorgoglione, B.; Abdelzaher, M.; El-Matbouli, M. Differential Modulation of Host Immune Genes in the Kidney and Cranium of the Rainbow Trout (Oncorhynchus mykiss) in Response to Tetracapsuloides Bryosalmonae and Myxobolus Cerebralis Co-Infections. Parasit. Vectors 2018, 11, 326. [Google Scholar] [CrossRef]
- Okamura, B.; Hartikainen, H.; Schmidt-Posthaus, H.; Wahli, T. Life Cycle Complexity, Environmental Change and the Emerging Status of Salmonid Proliferative Kidney Disease. Freshw. Biol. 2011, 56, 735–753. [Google Scholar] [CrossRef]
- Morris, D.J.; Adams, A.; Richards, R.H. In Situ Hybridisation Identifies the Gill as a Portal of Entry for PKX (Phylum Myxozoa), the Causative Agent of Proliferative Kidney Disease in Salmonids. Parasitol. Res. 2000, 86, 950–956. [Google Scholar] [CrossRef]
- Longshaw, M.; Le Deuff, R.; Harris, A.F.; Feist, S.W. Development of Proliferative Kidney Disease in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Following Short-term Exposure to Tetracapsula bryosalmonae Infected Bryozoans. J. Fish Dis. 2002, 25, 443–449. [Google Scholar] [CrossRef]
- Grabner, D.; El-Matbouli, M. Transmission of Tetracapsuloides Bryosalmonae (Myxozoa: Malacosporea) to Fredericella Sultana (Bryozoa: Phylactolaemata) by Various Fish Species. Dis. Aquat. Org. 2008, 79, 133–139. [Google Scholar] [CrossRef]
- COX, F.E.G. Concomitant Infections, Parasites and Immune Responses. Parasitology 2001, 122, S23–S38. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The Impact of Co-Infections on Fish: A Review. Vet. Res. 2016, 47, 98. [Google Scholar] [CrossRef]
- BRADLEY, J.E.; JACKSON, J.A. Measuring Immune System Variation to Help Understand Host-Pathogen Community Dynamics. Parasitology 2008, 135, 807–823. [Google Scholar] [CrossRef]
- Alarcón, M.; Thoen, E.; Poppe, T.T.; Bornø, G.; Mohammad, S.N.; Hansen, H. Co-infection of Nucleospora cyclopteri (Microsporidia) and Kudoa islandica (Myxozoa) in Farmed Lumpfish, Cyclopterus lumpus L., in Norway: A Case Report. J. Fish Dis. 2016, 39, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.S.; Sommerville, C.; Wootten, R. Molecular Studies on the Seasonal Occurrence and Development of Five Myxozoans in Farmed Salmo trutta L. Parasitology 2005, 132, 193. [Google Scholar] [CrossRef] [PubMed]
- Peeler, E.J.; Feist, S.W.; Longshaw, M.; Thrush, M.A.; St-Hilaire, S. An Assessment of the Variation in the Prevalence of Renal Myxosporidiosis and Hepatitis in Wild Brown Trout, Salmo trutta L., within and between Rivers in South-West England. J. Fish Dis. 2008, 31, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Hummel, K.; Schlosser, S.; Razzazi-Fazeli, E.; Bartholomew, J.L.; Holzer, A.; Secombes, C.J.; El-Matbouli, M. The Myxozoans Myxobolus Cerebralis and Tetracapsuloides Bryosalmonae Modulate Rainbow Trout Immune Responses: Quantitative Shotgun Proteomics at the Portals of Entry after Single and Co-Infections. Front. Cell. Infect. Microbiol. 2024, 14, 1369615. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; El-Matbouli, M.; Saleh, M. The Immune Response to the Myxozoan Parasite Myxobolus Cerebralis in Salmonids: A Review on Whirling Disease. Int. J. Mol. Sci. 2023, 24, 17392. [Google Scholar] [CrossRef]
- Vaumourin, E.; Vourc’h, G.; Gasqui, P.; Vayssier-Taussat, M. The Importance of Multiparasitism: Examining the Consequences of Co-Infections for Human and Animal Health. Parasit. Vectors 2015, 8, 545. [Google Scholar] [CrossRef]
- Shivam, S.; El-Matbouli, M.; Kumar, G. Development of Fish Parasite Vaccines in the OMICs Era: Progress and Opportunities. Vaccines 2021, 9, 179. [Google Scholar] [CrossRef]
- Sudhagar, A.; Ertl, R.; Kumar, G.; El-Matbouli, M. Transcriptome Profiling of Posterior Kidney of Brown Trout, Salmo Trutta, during Proliferative Kidney Disease. Parasit. Vectors 2019, 12, 569. [Google Scholar] [CrossRef]
- Maor-Landaw, K.; Smirnov, M.; Brekhman, V.; Ofek-Lalzar, M.; Yahav, T.; Lotan, T. Infection by the Parasite Myxobolus Bejeranoi (Cnidaria: Myxozoa) Suppresses the Immune System of Hybrid Tilapia. Microorganisms 2022, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Shivam, S.; Ertl, R.; Sexl, V.; El-Matbouli, M.; Kumar, G. Differentially Expressed Transcripts of Tetracapsuloides Bryosalmonae (Cnidaria) between Carrier and Dead-End Hosts Involved in Key Biological Processes: Novel Insights from a Coupled Approach of FACS and RNA Sequencing. Vet. Res. 2023, 54, 51. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, B.; Wang, T.; Secombes, C.J.; Holland, J.W. Immune Gene Expression Profiling of Proliferative Kidney Disease in Rainbow Trout Oncorhynchus mykiss Reveals a Dominance of Anti-Inflammatory, Antibody and T Helper Cell-like Activities. Vet. Res. 2013, 44, 55. [Google Scholar] [CrossRef] [PubMed]
- Boshra, H.; Gelman, A.E.; Sunyer, J.O. Structural and Functional Characterization of Complement C4 and C1s-Like Molecules in Teleost Fish: Insights into the Evolution of Classical and Alternative Pathways. J. Immunol. 2004, 173, 349–359. [Google Scholar] [CrossRef]
- Harris, P.D.; Soleng, A.; Bakke, T.A. Killing of Gyrodactylus salaris (Platyhelminthes, Monogenea) Mediated by Host Complement. Parasitology 1998, 117, 137–143. [Google Scholar] [CrossRef]
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. The Parasitic Ciliate Ichthyophthirius multifiliis Induces Expression of Immune Relevant Genes in Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2004, 27, 409–417. [Google Scholar] [CrossRef]
- Liu, H.; Peatman, E.; Wang, W.; Abernathy, J.; Liu, S.; Kucuktas, H.; Terhune, J.; Xu, D.-H.; Klesius, P.; Liu, Z. Molecular Responses of Ceruloplasmin to Edwardsiella ictaluri Infection and Iron Overload in Channel Catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2011, 30, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Zapryanova, D.; Urku, C.; Nikolov, G.; Atanasoff, A. The Significance of Ceruloplasmin and C-Reactive Protein as Possible Markers for the Diagnosis of Myxobolous Infection Caused by Myxobolus Cerebralis in Rainbow Trout (Oncorhynchus mykiss). Bemepuнapcкu Жypнaл Penyблuкe Cpncкe 2023, 23, 32–44. (In Russian) [Google Scholar] [CrossRef]
- Cao, J.; Tan, X. Comparative Analysis of the Tetraspanin Gene Family in Six Teleost Fishes. Fish Shellfish Immunol. 2018, 82, 432–441. [Google Scholar] [CrossRef]
- Perdiguero, P.; Jiménez-Barrios, P.; Morel, E.; Abós, B.; Tafalla, C. Single-Cell Atlas of Rainbow Trout Peripheral Blood Leukocytes and Profiling of Their Early Response to Infectious Pancreatic Necrosis Virus. Front. Immunol. 2024, 15, 1404209. [Google Scholar] [CrossRef]
- Castro, R.; Abós, B.; González, L.; Aquilino, C.; Pignatelli, J.; Tafalla, C. Molecular Characterization of CD9 and CD63, Two Tetraspanin Family Members Expressed in Trout B Lymphocytes. Dev. Comp. Immunol. 2015, 51, 116–125. [Google Scholar] [CrossRef] [PubMed]
- May, A.P.; Whiteheart, S.W.; Weis, W.I. Unraveling the Mechanism of the Vesicle Transport ATPase NSF, the N-Ethylmaleimide-Sensitive Factor. J. Biol. Chem. 2001, 276, 21991–21994. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Montero, R.; Kumar, G.; Sudhagar, A.; Friedl, A.; Köllner, B.; El-Matbouli, M. Kinetics of Local and Systemic Immune Cell Responses in Whirling Disease Infection and Resistance in Rainbow Trout. Parasit. Vectors 2019, 12, 249. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef]
- Lindenstrøm, T.; Secombes, C.J.; Buchmann, K. Expression of Immune Response Genes in Rainbow Trout Skin Induced by Gyrodactylus Derjavini Infections. Vet. Immunol. Immunopathol. 2004, 97, 137–148. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Hu, Y.; Li, A.; Xu, Y.; Jiang, B.; Lu, G.; Luo, X. Transcriptomic Variation of Locally-Infected Skin of Epinephelus Coioides Reveals the Mucosal Immune Mechanism against Cryptocaryon Irritans. Fish Shellfish Immunol. 2017, 66, 398–410. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Jafaar, R.M.; Dirks, R.P.; Buchmann, K. Transcriptomic Analysis of Immunity in Rainbow Trout (Oncorhynchus mykiss) Gills Infected by Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2019, 86, 486–496. [Google Scholar] [CrossRef]
- Gao, H.; Li, K.; Ai, K.; Geng, M.; Cao, Y.; Wang, D.; Yang, J.; Wei, X. Interleukin-12 Induces IFN-γ Secretion and STAT Signaling Implying Its Potential Regulation of Th1 Cell Response in Nile Tilapia. Fish Shellfish Immunol. 2023, 140, 108974. [Google Scholar] [CrossRef]
- Baerwald, M.R. Temporal Expression Patterns of Rainbow Trout Immune-Related Genes in Response to Myxobolus Cerebralis Exposure. Fish Shellfish Immunol. 2013, 35, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, M.W.; Anderson, K.D.; Lambert, P.D.; Yancopoulos, G.D.; Wiegand, S.J. The Ciliary Neurotrophic Factor and Its Receptor, CNTFRα. Pharm. Acta Helv. 2000, 74, 265–272. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. Identification and Expression Analysis of Two Fish-Specific IL-6 Cytokine Family Members, the Ciliary Neurotrophic Factor (CNTF)-like and M17 Genes, in Rainbow Trout Oncorhynchus mykiss. Mol. Immunol. 2009, 46, 2290–2298. [Google Scholar] [CrossRef]
- Oates, A.C.; Pratt, S.J.; Vail, B.; Yan, Y.; Ho, R.K.; Johnson, S.L.; Postlethwait, J.H.; Zon, L.I. The Zebrafish Klf Gene Family. Blood 2001, 98, 1792–1801. [Google Scholar] [CrossRef]
- Gómez-Abellán, V.; Sepulcre, M.P. The Role of Prostaglandins in the Regulation of Fish Immunity. Mol. Immunol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Mihaljevic, I.; Popovic, M.; Zaja, R.; Smital, T. Phylogenetic, Syntenic, and Tissue Expression Analysis of Slc22 Genes in Zebrafish (Danio rerio). BMC Genom. 2016, 17, 626. [Google Scholar] [CrossRef]
- Wetsaphan, N.; Rimphanitchayakit, V.; Tassanakajon, A.; Somboonwiwat, K. PmSERPIN3 from Black Tiger Shrimp Penaeus Monodon Is Capable of Controlling the ProPO System. Dev. Comp. Immunol. 2013, 41, 110–119. [Google Scholar] [CrossRef]
- Yuan, M.; Ning, M.; Wei, P.; Hao, W.; Jing, Y.; Gu, W.; Wang, W.; Meng, Q. The Function of Serpin-2 from Eriocheir sinensis in Spiroplasma eriocheiris Infection. Fish Shellfish Immunol. 2018, 76, 21–26. [Google Scholar] [CrossRef]
- Apitanyasai, K.; Chang, C.-C.; Ng, T.H.; Ng, Y.S.; Liou, J.-H.; Lo, C.-F.; Lin, S.-S.; Wang, H.-C. Penaeus Vannamei Serine Proteinase Inhibitor 7 (LvSerpin7) Acts as an Immune Brake by Regulating the ProPO System in AHPND-Affected Shrimp. Dev. Comp. Immunol. 2020, 106, 103600. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Qin, Z.; Yao, J.; Jiang, C.; Song, L.; Dunham, R.; Liu, Z. The Serpin Superfamily in Channel Catfish: Identification, Phylogenetic Analysis and Expression Profiling in Mucosal Tissues after Bacterial Infections. Dev. Comp. Immunol. 2015, 49, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Klesius, P.H. Apolipoprotein A1 in Channel Catfish: Transcriptional Analysis, Antimicrobial Activity, and Efficacy as Plasmid DNA Immunostimulant against Aeromonas hydrophila Infection. Fish Shellfish Immunol. 2013, 35, 1129–1137. [Google Scholar] [CrossRef]
- Villarroel, F.; Bastías, A.; Casado, A.; Amthauer, R.; Concha, M.I. Apolipoprotein A-I, an Antimicrobial Protein in Oncorhynchus mykiss: Evaluation of Its Expression in Primary Defence Barriers and Plasma Levels in Sick and Healthy Fish. Fish Shellfish Immunol. 2007, 23, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sukeda, M.; Shiota, K.; Kondo, M.; Nagasawa, T.; Nakao, M.; Somamoto, T. Innate Cell-Mediated Cytotoxicity of CD8+ T Cells against the Protozoan Parasite Ichthyophthirius multifiliis in the Ginbuna Crucian Carp, Carassius auratus langsdorfii. Dev. Comp. Immunol. 2021, 115, 103886. [Google Scholar] [CrossRef]
- Kordon, A.O.; Pinchuk, L.; Karsi, A. Adaptive Immune System in Fish. Turk. J. Fish. Aquat. Sci. 2021, 22, TRJFAS20235. [Google Scholar] [CrossRef]
- Fischer, U.; Utke, K.; Somamoto, T.; Köllner, B.; Ototake, M.; Nakanishi, T. Cytotoxic Activities of Fish Leucocytes. Fish Shellfish Immunol. 2006, 20, 209–226. [Google Scholar] [CrossRef]
- Heutschi, D. Genetic Analysis of Genes Linked to Lumen Formation in the Zebrafish Vasculature. Ph.D. Thesis, University of Basel, Basel, Switzerland, 14 December 2021. [Google Scholar]
- Tacchi, L.; Bron, J.E.; Taggart, J.B.; Secombes, C.J.; Bickerdike, R.; Adler, M.A.; Takle, H.; Martin, S.A.M. Multiple Tissue Transcriptomic Responses to Piscirickettsia salmonis in Atlantic Salmon (Salmo salar). Physiol. Genom. 2011, 43, 1241–1254. [Google Scholar] [CrossRef]
- Shao, R.; Liu, J.; Lan, Y.; Liao, X.; Zhang, J.; Xu, W.; Mai, K.; Ai, Q.; Wan, M. Vitamin D Impacts on the Intestinal Health, Immune Status and Metabolism in Turbot (Scophthalmus maximus L.). Br. J. Nutr. 2022, 128, 2083–2096. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Evenhuis, J.P. Molecular Characterization of Atrogin-1/F-Box Protein-32 (FBXO32) and F-Box Protein-25 (FBXO25) in Rainbow Trout (Oncorhynchus mykiss): Expression across Tissues in Response to Feed Deprivation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 248–257. [Google Scholar] [CrossRef]
- Sugasawa, T.; Komine, R.; Manevich, L.; Tamai, S.; Takekoshi, K.; Kanki, Y. Gene Expression Profile Provides Novel Insights of Fasting-Refeeding Response in Zebrafish Skeletal Muscle. Nutrients 2022, 14, 2239. [Google Scholar] [CrossRef]
- Kent, M.L.; Hedrick, R.P. Effects of Cortisol Implants on the PKX Myxosporean Causing Proliferative Kidney Disease in Rainbow Trout, Salmo Gairdneri. J. Parasitol. 1987, 73, 455–461. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Mahdy, O.A.; El-Saied, M.A.; Mohammed, F.F.; Salem, M.A. Novel Insights into Immune Stress Markers Associated with Myxosporeans Gill Infection in Nile Tilapia (Molecular and Immunohistochemical Studies). PLoS ONE 2024, 19, e0303702. [Google Scholar] [CrossRef]
- Aedo, J.E.; Zuloaga, R.; Boltaña, S.; Molina, A.; Valdés, J.A. Membrane-Initiated Cortisol Action Modulates Early Pyruvate Dehydrogenase Kinase 2 (Pdk2) Expression in Fish Skeletal Muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 233, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Bik, D.P.; Ruzicka, T.; Merk, H.F.; Bickers, D.R. Cytochrome P-450-Dependent Omega-Oxidation of Leukotriene B4 in Rodent and Human Epidermis. J. Investig. Dermatol. 1989, 93, 231–235. [Google Scholar] [CrossRef]
- Zhou, Z.; He, Y.; Wang, S.; Wang, Y.; Shan, P.; Li, P. Autophagy Regulation in Teleost Fish: A Double-Edged Sword. Aquaculture 2022, 558, 738369. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Y.; Liu, J.; Ren, G.; Li, Z.; Tian, Y. Transcriptome Analysis Reveals the DNA Replication Genes Response to Vibrio anguillarum and NNV Infection in Jinhu Grouper (Epinephelus fuscoguttatus♀ × Epinephelus tukulal♂). Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 54, 101421. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Mizuno, S. Synergistic Effects of Infectious Haematopoietic Necrosis Virus and Flavobacterium psychrophilum Co-infection on the Mortality and Pathophysiology of Masu Salmon Parr Oncorhynchus masou. J. Fish Biol. 2025. [Google Scholar] [CrossRef]
- Yao, H.H.-C.; Whoriskey, W.; Capel, B. Desert Hedgehog/Patched 1 Signaling Specifies Fetal Leydig Cell Fate in Testis Organogenesis. Genes Dev. 2002, 16, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Dong, Z.; Zhang, N.; Wang, L.; Shao, C.; Xu, W. Cloning, Expression and Functional Analysis of the Desert Hedgehog (Dhh) Gene in Chinese Tongue Sole (Cynoglossus semilaevis). Gene Expr. Patterns 2021, 39, 119163. [Google Scholar] [CrossRef]
- Król, E.; Noguera, P.; Shaw, S.; Costelloe, E.; Gajardo, K.; Valdenegro, V.; Bickerdike, R.; Douglas, A.; Martin, S.A.M. Integration of Transcriptome, Gross Morphology and Histopathology in the Gill of Sea Farmed Atlantic Salmon (Salmo salar): Lessons From Multi-Site Sampling. Front. Genet. 2020, 11, 610. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Bailey, C.; Holland, J.W.; Secombes, C.J.; Tafalla, C. A Portrait of the Immune Response to Proliferative Kidney Disease (PKD) in Rainbow Trout. Parasite Immunol. 2020, 42, e12730. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T Cells in Fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef]
- Brent, S. McKenzie. Understanding the IL-23–IL-17 Immune Pathway. Trends Immunol. 2006, 27, 17–23. [Google Scholar]
- Saleh, M.; Friedl, A.; Srivastava, M.; Soliman, H.; Secombes, C.J.; El-Matbouli, M. STAT3/SOCS3 Axis Contributes to the Outcome of Salmonid Whirling Disease. PLoS ONE 2020, 15, e0234479. [Google Scholar] [CrossRef]
- Hsiao, C.; Tsai, W.; Horng, L.; Tsai, H. Molecular Structure and Developmental Expression of Three Muscle-type Troponin T Genes in Zebrafish. Dev. Dyn. 2003, 227, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.E.; Schiavi, J.; Ulerich, A.D.; Weaver, F.E.; Coughlin, D.J. Myosin Regulatory Light Chain Expression in Trout Muscle. J. Exp. Zool. A Ecol. Genet. Physiol. 2008, 309A, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Garcia de la serrana, D.; Wreggelsworth, K.; Johnston, I.A. Duplication of a Single Myhz1.1 Gene Facilitated the Ability of Goldfish (Carassius auratus) to Alter Fast Muscle Contractile Properties With Seasonal Temperature Change. Front. Physiol. 2018, 9, 1724. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Lin, T.; Feng, G.; Wang, X.; Zhang, D. Muscle Fiber Plasticity, Stress Physiology, and Muscle Transcriptome Determine the Inter-Individual Difference of Swimming Performance in the Large Yellow Croaker (Larimichthys crocea). Aquaculture 2023, 567, 739247. [Google Scholar] [CrossRef]
- Johnson, K.R.; Olson, K.R. Responses of the Trout Cardiac Natriuretic Peptide System to Manipulation of Salt and Water Balance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1170–R1179. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sato, K.; Kunisaki, N.; Kimura, S. Characterization of a Rainbow Trout Matrix Metalloproteinase Capable of Degrading Type I Collagen. Eur. J. Biochem. 2000, 267, 6943–6950. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.-A.; Geng, R.; Niu, S.; Zuo, H.; Weng, S.; He, J.; Xu, X. A Kelch Motif-Containing Protein KLHDC2 Regulates Immune Responses against Vibrio Parahaemolyticus and White Spot Syndrome Virus in Penaeus vannamei. Fish Shellfish Immunol. 2022, 127, 187–194. [Google Scholar] [CrossRef]
- Vestal, D.J. Review: The Guanylate-Binding Proteins (GBPs): Proinflammatory Cytokine-Induced Members of the Dynamin Superfamily with Unique GTPase Activity. J. Interferon Cytokine Res. 2005, 25, 435–443. [Google Scholar] [CrossRef]
- Robertsen, B.; Zou, J.; Secombes, C.; Leong, J.-A. Molecular and Expression Analysis of an Interferon-Gamma-Inducible Guanylate-Binding Protein from Rainbow Trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2006, 30, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Immune Cell Regulation by Autocrine Purinergic Signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Trautmann, A. Extracellular ATP in the Immune System: More than Just a “Danger Signal”. Sci. Signal. 2009, 2, pe6. [Google Scholar] [CrossRef]
- Bailey, C.; Strepparava, N.; Wahli, T.; Segner, H. Exploring the Immune Response, Tolerance and Resistance in Proliferative Kidney Disease of Salmonids. Dev. Comp. Immunol. 2019, 90, 165–175. [Google Scholar] [CrossRef]
- Diaz-Ochoa, V.E.; Jellbauer, S.; Klaus, S.; Raffatellu, M. Transition Metal Ions at the Crossroads of Mucosal Immunity and Microbial Pathogenesis. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Brunet, F.G.; Fraser, F.W.; Binder, M.J.; Smith, A.D.; Kintakas, C.; Dancevic, C.M.; Ward, A.C.; McCulloch, D.R. The Evolutionary Conservation of the A Disintegrin-like and Metalloproteinase Domain with Thrombospondin-1 Motif Metzincins across Vertebrate Species and Their Expression in Teleost Zebrafish. BMC Evol. Biol. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Chourasia, D.; Ng, K.W.; Khel, N.B.; Parhar, I.S. Cloning and Localization of Immediate Early Response 2 (Ier2) Gene in the Brain of Medaka. J. Chem. Neuroanat. 2016, 77, 24–29. [Google Scholar] [CrossRef]
- Xu, P.; Huang, M. Small Peptides as Modulators of Serine Proteases. Curr. Med. Chem. 2020, 27, 3686–3705. [Google Scholar] [CrossRef]
- Di Cera, E. Serine Proteases. IUBMB Life 2009, 61, 510–515. [Google Scholar] [CrossRef]
- Schonfeld, M.; Villar, M.T.; Artigues, A.; Weinman, S.A.; Tikhanovich, I. Arginine Methylation of Integrin Alpha-4 Prevents Fibrosis Development in Alcohol-Associated Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, L.; Li, J.; Chen, Y.; Yao, Y.; Zeng, R.; Shushakova, N.; Haller, H.; Xu, G.; Rong, S. C-X3-C Motif Chemokine Ligand 1/Receptor 1 Regulates the M1 Polarization and Chemotaxis of Macrophages after Hypoxia/Reoxygenation Injury. Chronic Dis. Transl. Med. 2021, 7, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wang, X.; Liu, B.; Chen, M.; Ning, J.; Liu, H.; Liu, G.; Xu, X.; Zhang, X.; Yu, K.; et al. Potential Roles of IFI44 Genes in High Resistance to Vibrio in Hybrids of Argopecten scallops. Fish Shellfish Immunol. 2023, 135, 108702. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, M.R.; Petersen, J.L.; Hedrick, R.P.; Schisler, G.J.; May, B. A Major Effect Quantitative Trait Locus for Whirling Disease Resistance Identified in Rainbow Trout (Oncorhynchus mykiss). Heredity 2011, 106, 920–926. [Google Scholar] [CrossRef]
- Baerwald, M.R.; Welsh, A.B.; Hedrick, R.P.; May, B. Discovery of Genes Implicated in Whirling Disease Infection and Resistance in Rainbow Trout Using Genome-Wide Expression Profiling. BMC Genom. 2008, 9, 37. [Google Scholar] [CrossRef]
- Saleh, M.; Friedl, A.; Srivastava, M.; Secombes, C.J.; El-Matbouli, M. Modulation of Local and Systemic Immune Responses in Brown Trout (Salmo trutta) Following Exposure to Myxobolus cerebralis. Fish Shellfish Immunol. 2020, 106, 844–851. [Google Scholar] [CrossRef]
- Chaumont, L.; Jouneau, L.; Huetz, F.; van Muilekom, D.R.; Peruzzi, M.; Raffy, C.; Le Hir, J.; Minke, J.; Boudinot, P.; Collet, B. Unexpected Regulatory Functions of Cyprinid Viperin on Inflammation and Metabolism. BMC Genom. 2024, 25, 650. [Google Scholar] [CrossRef]
- He, Y.; Shen, Y.; Zhao, J.; Chen, X. The Peritoneum of Fish Expresses a Specific Gene Pattern. Aquac. Fish. 2024, 9, 573–580. [Google Scholar] [CrossRef]
- Mazelet, L.; Dietrich, J.; Rolland, J.L. New RT-QPCR Assay for Viral Nervous Necrosis Virus Detection in Sea Bass, Dicentrarchus labrax (L.): Application and Limits for Hatcheries Sanitary Control. Fish Shellfish Immunol. 2011, 30, 27–32. [Google Scholar] [CrossRef]
- Xue, X.; Feng, C.Y.; Hixson, S.M.; Johnstone, K.; Anderson, D.M.; Parrish, C.C.; Rise, M.L. Characterization of the Fatty Acyl Elongase (Elovl) Gene Family, and Hepatic Elovl and Delta-6 Fatty Acyl Desaturase Transcript Expression and Fatty Acid Responses to Diets Containing Camelina Oil in Atlantic Cod (Gadus morhua). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 175, 9–22. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Wang, L.; Ma, T.; Xu, W.; Chen, Z.; Zhou, Q.; Zheng, G.; Chen, S. Molecular Characterization and Expression Analysis of SKIV Infection of Interferon-Induced Protein with Tetratricopeptide Repeats 1 (IFIT1) in Epinephelus Lanceolatus. J. Ocean Univ. China 2021, 20, 383–392. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Modulation of Fish Growth Hormone Levels by Salinity, Temperature, Pollutants and Aquaculture Related Stress: A Review. Rev. Fish Biol. Fish. 2009, 19, 97–120. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, G.; Jin, Z.; Zhang, C.; Huang, Y. The Transcription Factor C-Fos Plays a Negative Role in Prawn Immunity against WSSV Infection. Aquaculture 2022, 553, 738080. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent Advances on the Complement System of Teleost Fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-Reactive Protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef]
- Aragão, C.; Corte-Real, J.; Costas, B.; Dinis, M.T.; Conceição, L.E.C. Stress Response and Changes in Amino Acid Requirements in Senegalese Sole (Solea Senegalensis Kaup 1858). Amino Acids 2008, 34, 143–148. [Google Scholar] [CrossRef]
- Costas, B.; Aragão, C.; Mancera, J.M.; Dinis, M.T.; Conceição, L.E.C. High Stocking Density Induces Crowding Stress and Affects Amino Acid Metabolism in Senegalese Sole Solea Senegalensis (Kaup 1858) Juveniles. Aquac. Res. 2007, 39, 1–9. [Google Scholar] [CrossRef]
- Tejpal, C.S.; Pal, A.K.; Sahu, N.P.; Ashish Kumar, J.; Muthappa, N.A.; Vidya, S.; Rajan, M.G. Dietary Supplementation of L-Tryptophan Mitigates Crowding Stress and Augments the Growth in Cirrhinus mrigala Fingerlings. Aquaculture 2009, 293, 272–277. [Google Scholar] [CrossRef]
- Metcalf, V.J.; Brennan, S.O.; George, P.M. The Antarctic Toothfish (Dissostichus mawsoni) Lacks Plasma Albumin and Utilises High Density Lipoprotein as Its Major Palmitate Binding Protein. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 124, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Noël, E.S.; dos Reis, M.; Arain, Z.; Ober, E.A. Analysis of the Albumin/α-Fetoprotein/Afamin/Group Specific Component Gene Family in the Context of Zebrafish Liver Differentiation. Gene Expr. Patterns 2010, 10, 237–243. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Huising, M.O.; van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.J.; Verburg-van Kemenade, B.M.L. Neuroendocrine–Immune Interactions in Fish: A Role for Interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. The Interleukins of Fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic Characterization of Larval Zebrafish (Danio rerio) with Partial Knockdown of the Cacna1a Gene. Mol. Neurobiol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- Zapata, A.G. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology 2022, 11, 747. [Google Scholar] [CrossRef]
- Petrie-Hanson, L.; Hohn, C.; Hanson, L. Characterization of Rag1 Mutant Zebrafish Leukocytes. BMC Immunol. 2009, 10, 8. [Google Scholar] [CrossRef]
- Gaffen, S.L. Biology of Recently Discovered Cytokines: Interleukin-17—A Unique Inflammatory Cytokine with Roles in Bone Biology and Arthritis. Arthritis Res. Ther. 2004, 6, 240. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Wang, A.; Husain, M.; Xu, Q.; Secombes, C.J. Identification of the Salmonid IL-17A/F1a/b, IL-17A/F2b, IL-17A/F3 and IL-17N Genes and Analysis of Their Expression Following in Vitro Stimulation and Infection. Immunogenetics 2015, 67, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Seiliez, I.; Gabillard, J.-C.; Riflade, M.; Sadoul, B.; Dias, K.; Avérous, J.; Tesseraud, S.; Skiba, S.; Panserat, S. Amino Acids Downregulate the Expression of Several Autophagy-Related Genes in Rainbow Trout Myoblasts. Autophagy 2012, 8, 364–375. [Google Scholar] [CrossRef] [PubMed]
- MORRIS, D.J.; ADAMS, A. Sporogony of Tetracapsuloides bryosalmonae in the Brown Trout Salmo trutta and the Role of the Tertiary Cell during the Vertebrate Phase of Myxozoan Life Cycles. Parasitology 2008, 135, 1075–1092. [Google Scholar] [CrossRef]
- Osborn, D.P.S.; Emrahi, L.; Clayton, J.; Tabrizi, M.T.; Wan, A.Y.B.; Maroofian, R.; Yazdchi, M.; Garcia, M.L.E.; Galehdari, H.; Hesse, C.; et al. Autosomal Recessive Cardiomyopathy and Sudden Cardiac Death Associated with Variants in MYL3. Genet. Med. 2021, 23, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Fu, N.; Guo, S.; Yan, Z.; Xu, Y.; Hu, H.; Menzel, C.; Chen, W.; Li, Y.; Zeng, R.; et al. Estimating Accuracy of RNA-Seq and Microarrays with Proteomics. BMC Genom. 2009, 10, 161. [Google Scholar] [CrossRef]
- Garbis, S.; Lubec, G.; Fountoulakis, M. Limitations of Current Proteomics Technologies. J. Chromatogr. A 2005, 1077, 1–18. [Google Scholar] [CrossRef]
- Nie, L.; Wu, G.; Culley, D.E.; Scholten, J.C.M.; Zhang, W. Integrative Analysis of Transcriptomic and Proteomic Data: Challenges, Solutions and Applications. Crit. Rev. Biotechnol. 2007, 27, 63–75. [Google Scholar] [CrossRef]
- El-Matbouli, M.; Mattes, M.; Soliman, H. Susceptibility of Whirling Disease (WD) Resistance and WD Susceptible Strains of Rainbow Trout Oncorhynchus mykiss to Tetracapsuloides bryosalmonae, Yersinia ruckeri and Viral Haemorrhagic Septicaemia Virus. Aquaculture 2009, 288, 299–304. [Google Scholar] [CrossRef]
- Rucker, U.; El-Matbouli, M. Sequence Analysis of OmNramp α and Quantitative Expression of Nramp Homologues in Different Trout Strains after Infection with Myxobolus Cerebralis. Dis. Aquat. Org. 2007, 76, 223–230. [Google Scholar] [CrossRef]
- WOAH. Diseases of Fish: General Information. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2021; pp. 223–233. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.00_INTRO_FISH.pdf (accessed on 31 December 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G: Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]

| Pathways | Regulation | Group | Pathway ID | Enrichment FDR | Pathway Genes | Fold Enrichment | nGenes | Genes |
|---|---|---|---|---|---|---|---|---|
| Antigen processing and presentation endogenous lipid antigen via MHC | Upregulated Pathways | Mc | GO:0048006 | 2.25 × 10−8 | 5 | 118.6 | 5 | CD1D, CD1A, CD1C, CD1B, CD1E |
| Lipid antigen binding | Mc | GO:0030882 | 5.50 × 10−9 | 5 | 118.6 | 5 | CD1D, CD1A, CD1C, CD1B, CD1E | |
| Steroid catabolic process | Mc+ | GO:0006706 | 4.27 × 10−3 | 26 | 135.4 | 2 | CYP24A1, CYP1A2 | |
| Leukotriene-B4 20-monooxygenase activity | Mc+ | GO:0050051 | 7.35 × 10−7 | 5 | 40.4 | 5 | CYP4F11, CYP4F2, CYP4F12, CYP4F3, CYP4A11 | |
| Toll-like receptor 4 binding | Tb | GO:0035662 | 3.40 × 10−4 | 4 | 309.2 | 2 | S100A8, S100A9 | |
| RAGE receptor binding | Tb | GO:0050786 | 6.27 × 10−8 | 10 | 247.4 | 4 | S100A4, S100A8, S100A7, S100A9 | |
| Positive reg. of toll-like receptor 7 signaling pathway | Tb+ | GO:0034157 | 3.96 × 10−2 | 6 | 476.7 | 1 | RSAD2 | |
| Lipid antigen binding | Tb+ | GO:0030882 | 3.441 × 10−6 | 5 | 31.4 | 5 | CD1D, CD1A, CD1C, CD1B, CD1E | |
| Carnitine transmembrane transporter activity | Downregulated Pathways | Mc | GO:0015226 | 1.06 × 10−3 | 4 | 215.9 | 2 | SLC22A5, SLC22A4 |
| Cellular response to fluid shear stress | Mc | GO:0071498 | 4.50 × 10−3 | 20 | 152.5 | 2 | PTGS2, KLF4 | |
| MCM complex | Mc+ | GO:0042555 | 1.76 × 10−8 | 11 | 31.8 | 7 | MCM6, MCM9, MCM3, MCM7, MMS22L, TONSL, MCMBP | |
| Nuclear replication fork | Mc+ | GO:0043596 | 5.02 × 10−13 | 38 | 18.4 | 14 | POLA2, MCM10, POLA1, POLD2, TIMELESS, RPA2, MMS22L, PRIM1, WDHD1, PRPF19, PCNA, SMARCA5, TONSL, MCM3 | |
| Elastin catabolic proc. | Tb | GO:0060309 | 3.98 × 10−2 | 4 | 715.0 | 1 | MMP12 | |
| Endothelial cell–cell adhesion | Tb | GO:0071603 | 3.98 × 10−2 | 4 | 715.0 | 1 | THBS4 | |
| Leukotriene-B4 20-monooxygenase activity | Tb+ | GO:0050051 | 9.46 × 10−7 | 5 | 36.5 | 5 | CYP4F11, CYP4F2, CYP4F12, CYP4F3, CYP4A11 | |
| DNA polymerase complex | Tb+ | GO:0042575 | 1.115 × 10−6 | 20 | 14.6 | 8 | POLA2, POLE2, POLA1, POLD2, PRIM1, DNA2, POLG, MCM3 |
| Sr No. | Pathways | Group | Fold Enrichment | Pathway ID | No. of Identified Genes | Genes |
|---|---|---|---|---|---|---|
| Upregulated Pathways | ||||||
| 1 | JAK-STAT signaling pathway | Mc | 8.049990405 | hsa04630 | 11 | IL23R, GHR, IL2RG, IL3RA, IL6R, IL11RA, IL12RB1, IL13RA1, IL17D, CRLF2, OSMR |
| 2 | Chemokine signaling pathway | Mc | 6.207036866 | hsa04062 | 10 | CCL26, CCL1, CCL2, CCL7, CCL8, CCL11, CCL13, CCL22, CCL24, CX3CL1 |
| 3 | Th17 cell differentiation | Mc | 5.488629822 | hsa04659 | 5 | IL23R, TBX21, IL2RG, IL6R, IL12RB1 |
| 4 | Autophagy-animal | Mc+ | 4.865435851 | hsa04140 | 17 | GABARAPL2, CTSL, ATG14, ATG4B, ATG9B, PIK3R4, SH3GLB1, PDPK1, ZFYVE1, PRKAA2, TP53INP2, RAB1A, DEPTOR, RAB7A, ATG9A, IRS4, ULK2 |
| 5 | JAK-STAT signaling pathway | Mc+ | 3.487425697 | hsa04630 | 14 | IL23R, GHR, IL2RG, IL3RA, IL6R, IL11RA, IL12RB1, IL13RA1, IL13RA2, CRLF2, STAT5A, STAT5B, SOCS2, OSMR |
| 6 | IL-17 signaling pathway | Tb | 26.59808195 | hsa04657 | 4 | S100A7A, S100A7, S100A8, S100A9 |
| 7 | JAK-STAT signaling pathway | Tb+ | 4.656288156 | hsa04630 | 24 | SOCS4, IL23R, GHR, IFNAR1, IFNAR2, IFNGR1, IL2RG, IL3RA, IL6R, IL10RB, IL11RA, IL12RB1, IL13RA1, JAK1, JAK2, PDGFRB, IL17D, CRLF2, STAT1, STAT2, STAT5A, STAT5B, SOCS2, OSMR |
| Downregulated Pathways | ||||||
| 8 | Protein processing in endoplasmic reticulum | Mc+ | 6.457418086 | hsa04141 | 30 | PDIA6, SEC23B, CKAP4, DAD1, EIF2S1, STT3B, SEC31A, DNAJC5G, SEC61A1, HSPA2, HSPA5, HSP90AA1, STT3A, LMAN1, P4HB, DNAJC10, UGGT2, DNAJC3, MAN1C1, RPN1, RPN2, RRBP1, DNAJC1, SSR4, HSP90B1, VCP, CALR, TXNDC5, CAPN2, PLAA |
| 9 | DNA replication | Mc+ | 14.14652888 | hsa03030 | 14 | DNA2, FEN1, POLA2, LIG1, MCM3, MCM6, MCM7, PCNA, POLA1, POLD2, POLE2, PRIM1, RFC2, RPA2 |
| 10 | Ribosome biogenesis in eukaryotes | Mc+ | 7.558813206 | hsa03008 | 16 | EMG1, UTP14A, POP1, WDR36, DKC1, WDR43, REXO2, EIF6, SNU13, GAR1, HEATR1, NAT10, UTP6, PWP2, NOL6, UTP14C |
| 11 | ECM-receptor interaction | Tb | 32.50142045 | hsa04512 | 1 | THBS4 |
| 12 | TNF signaling pathway | Tb | 25.53683036 | hsa04668 | 1 | MMP3 |
| 13 | DNA replication | Tb+ | 15.2053429 | hsa03030 | 15 | DNA2, FEN1, POLA2, LIG1, MCM3, MCM6, MCM7, PCNA, POLA1, POLD2, POLE2, PRIM1, RFC2, RFC4, RPA2 |
| 14 | Protein processing in endoplasmic reticulum | Tb+ | 3.886809547 | hsa04141 | 18 | SEC23B, DAD1, EIF2S1, STT3B, SEC31A, SEC61G, SEC61A1, HSPA2, HSP90AA1, LMAN1, MAN1C1, RPN1, RPN2, RRBP1, DNAJC1, SSR4, CALR, TXNDC5 |
| Pathways | Regulation | Group | Pathway ID | Enrichment FDR | Pathway Genes | Fold Enrichment | nGenes | Genes |
|---|---|---|---|---|---|---|---|---|
| Antigen processing and presentation endogenous lipid antigen via MHC | Upregulated Pathways | Mc | GO:0048006 | 3.98 × 10−12 | 5 | 762.7 | 5 | CD1D, CD1A, CD1C, CD1B, CD1E |
| Exogenous lipid antigen binding | Mc | GO:0030884 | 1.86 × 10−13 | 5 | 762.7 | 5 | CD1D, CD1A, CD1C, CD1B, CD1E | |
| Collagen type IX trimer | Mc+ | GO:0005594 | 2.04 × 10−3 | 3 | 111.3 | 2 | COL9A3, COL9A2 | |
| CCR3 chemokine receptor binding | Mc+ | GO:0031728 | 4.36 × 10−5 | 5 | 100.2 | 5 | CCL26, CCL24, CCL11 | |
| Integrin alpha4-beta1 complex | Tb | GO:0034668 | 4.40 × 10−2 | 3 | 401.4 | 1 | ITGA4 | |
| Interleukin-8 receptor binding | Tb | GO:0005153 | 3.76 × 10−2 | 3 | 401.4 | 1 | CX3CL1 | |
| Alcohol dehydrogenase activity zinc-dependent | Tb+ | GO:0004024 | 2.09 × 10−11 | 7 | 63.0 | 7 | ADH6, ADH1A, ADH7, ADH1B, ADH5, ADH4, ADH1C | |
| Humoral immune response mediated by circulating immunoglobulin | Tb+ | GO:0002455 | 1.03 × 10−3 | 67 | 60.3 | 3 | CRP, APCS, C3 | |
| Negative reg. of synaptic plasticity | Downregulated Pathways | Mc | GO:0031914 | 7.65 × 10−3 | 3 | 3813.5 | 1 | UNC13C |
| Dense core granule priming | Mc | GO:0061789 | 7.65 × 10−3 | 3 | 3813.5 | 1 | UNC13C | |
| Cardiac Troponin complex | Mc+ | GO:1990584 | 3.06 × 10−2 | 3 | 401.4 | 1 | TNNT2 | |
| Myosin II heavy chain binding | Mc+ | GO:0032038 | 22.99 × 10−2 | 3 | 401.4 | 1 | MYL3 | |
| Cardiac Troponin complex | Tb | GO:1990584 | 1.70 × 10−3 | 3 | 7627.0 | 1 | TNNT2 | |
| Regulation of muscle filament sliding | Tb | GO:0032971 | 1.59 × 10−2 | 4 | 5720.3 | 1 | TNNT2 | |
| Immature B cell differentiation | Tb+ | GO:0002327 | 1.37 × 10−2 | 13 | 185.3 | 2 | RAG1, RAG2 | |
| Integrator complex | Tb+ | GO:0032039 | 3.05 × 10−11 | 30 | 18.2 | 12 | INTS6L, SAGE1, CT45A5, CT45A1, CT45A3, CT45A10, CT45A9, CT45A2, CT45A7, CT45A8, CT45A6, INTS14 |
| Sr No. | Pathways | Group | Fold Enrichment | Pathway ID | No. of Identified Genes | Genes |
|---|---|---|---|---|---|---|
| Upregulated Pathways | ||||||
| 1 | JAK-STAT signaling pathway | Mc+ | 12.37 | hsa04630 | 12 | SOCS4, CSF3R, IL23R, GHR, IL2RG, IL3RA, IL6R, IL11RA, IL12RB1, IL13RA1, CRLF2, OSMR |
| 2 | IL-17 signaling pathway | Mc+ | 10.77 | hsa04657 | 6 | IL17RA, IL1B, PTGS2, CCL2, CCL7, CCL11 |
| 3 | Th17 cell differentiation | Mc+ | 7.73 | hsa04659 | 5 | IL23R, IL1B, IL2RG, IL6R, IL12RB1 |
| 4 | TNF signaling pathway | Mc+ | 7.45 | hsa04668 | 5 | IL1B, PTGS2, BCL3, CCL2, CX3CL1 |
| 5 | Natural killer-cell-mediated cytotoxicity | Tb | 18.52 | hsa04650 | 2 | ITGB2, PRF1 |
| 6 | JAK-STAT signaling pathway | Tb+ | 5.23 | hsa04630 | 21 | CISH, SOCS4, CSF3R, IL23R, EGFR, GHR, IL2RB, IL2RG, IL3RA, IL6R, IL10RB, IL11RA, IL12RB1, IL13RA1, JAK1, CRLF2, STAT1, STAT2, STAT3, SOCS2, OSMR |
| 7 | Cytokine–cytokine receptor interaction | Tb+ | 5.21 | hsa04060 | 38 | CCL26, IL31RA, CSF1R, CSF3R, IL23R, IL17RA, IL36RN, GHR, IL36B, IL37, IL36A, FAS, IL1B, IL1RN, IL2RB, IL2RG, IL3RA, IL6R, IL10RB, IL11RA, IL12RB1, IL13RA1, IL36G, CCL1, CCL2, CCL7, CCL8, CCL11, CCL13, CCL22, CCL24, CX3CL1, CRLF2, CXCR4, IL1F10, TNFSF14, TNFSF10, OSMR |
| Downregulated Pathways | ||||||
| 8 | Hypertrophic cardiomyopathy | Tb | 254.23 | hsa05410 | 1 | TNNT2 |
| 9 | Dilated cardiomyopathy | Tb | 238.34 | hsa05414 | 1 | TNNT2 |
| 10 | Hypertrophic cardiomyopathy | Mc+ | 53.52 | hsa05410 | 4 | MYL3, ATP2A1, TGFB3, TNNT2 |
| 11 | Dilated cardiomyopathy | Mc+ | 50.17 | hsa05414 | 4 | MYL3, ATP2A1, TGFB3, TNNT2 |
| 12 | Cardiac muscle contraction | Mc+ | 41.52 | hsa04260 | 3 | MYL3, ATP2A1, TNNT2 |
| 13 | DNA replication | Tb+ | 18.91 | hsa03030 | 15 | DNA2, FEN1, POLA2, LIG1, MCM3, MCM6, MCM7, PCNA, POLA1, POLD2, POLE2, PRIM1, RFC2, RFC4, RPA2 |
| 14 | Protein processing in the endoplasmic reticulum | Tb+ | 5.104008171 | hsa04141 | 19 | CKAP4, DAD1, EIF2S1, STT3B, SEC31A, SEC61A1, HSP90AA1, STT3A, LMAN1, UGGT2, RPN1, RPN2, RRBP1, DNAJC1, SSR4, VCP, CALR, TXNDC5, PLAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, N.; Ertl, R.; Ghanei-Motlagh, R.; Secombes, C.J.; El-Matbouli, M.; Holzer, A.S.; Saleh, M. Transcriptomic Analysis of the Rainbow Trout Response to Single and Co-Infections with Myxobolus cerebralis and Tetracapsuloides bryosalmonae at Sites of Parasite Entry. Int. J. Mol. Sci. 2025, 26, 8148. https://doi.org/10.3390/ijms26178148
Akram N, Ertl R, Ghanei-Motlagh R, Secombes CJ, El-Matbouli M, Holzer AS, Saleh M. Transcriptomic Analysis of the Rainbow Trout Response to Single and Co-Infections with Myxobolus cerebralis and Tetracapsuloides bryosalmonae at Sites of Parasite Entry. International Journal of Molecular Sciences. 2025; 26(17):8148. https://doi.org/10.3390/ijms26178148
Chicago/Turabian StyleAkram, Naveed, Reinhard Ertl, Reza Ghanei-Motlagh, Christopher J. Secombes, Mansour El-Matbouli, Astrid S. Holzer, and Mona Saleh. 2025. "Transcriptomic Analysis of the Rainbow Trout Response to Single and Co-Infections with Myxobolus cerebralis and Tetracapsuloides bryosalmonae at Sites of Parasite Entry" International Journal of Molecular Sciences 26, no. 17: 8148. https://doi.org/10.3390/ijms26178148
APA StyleAkram, N., Ertl, R., Ghanei-Motlagh, R., Secombes, C. J., El-Matbouli, M., Holzer, A. S., & Saleh, M. (2025). Transcriptomic Analysis of the Rainbow Trout Response to Single and Co-Infections with Myxobolus cerebralis and Tetracapsuloides bryosalmonae at Sites of Parasite Entry. International Journal of Molecular Sciences, 26(17), 8148. https://doi.org/10.3390/ijms26178148







