Variability in the Deformability of Red Blood Cells: Application to Treating Premature Newborns with Blood Transfusion
Abstract
1. Introduction
- Preterm neonates, particularly with very low birth weight, have relatively large RBCs, leading to increased mechanical stress during their passage through the microcirculation, particularly the capillaries.
- Effective perfusion in newborns depends mainly on the lower viscosity of neonatal plasma and an increased capillary density.
- These aspects emphasize the crucial role of RBC deformability in maintaining sufficient oxygen delivery in neonatal critical care.
2. Results
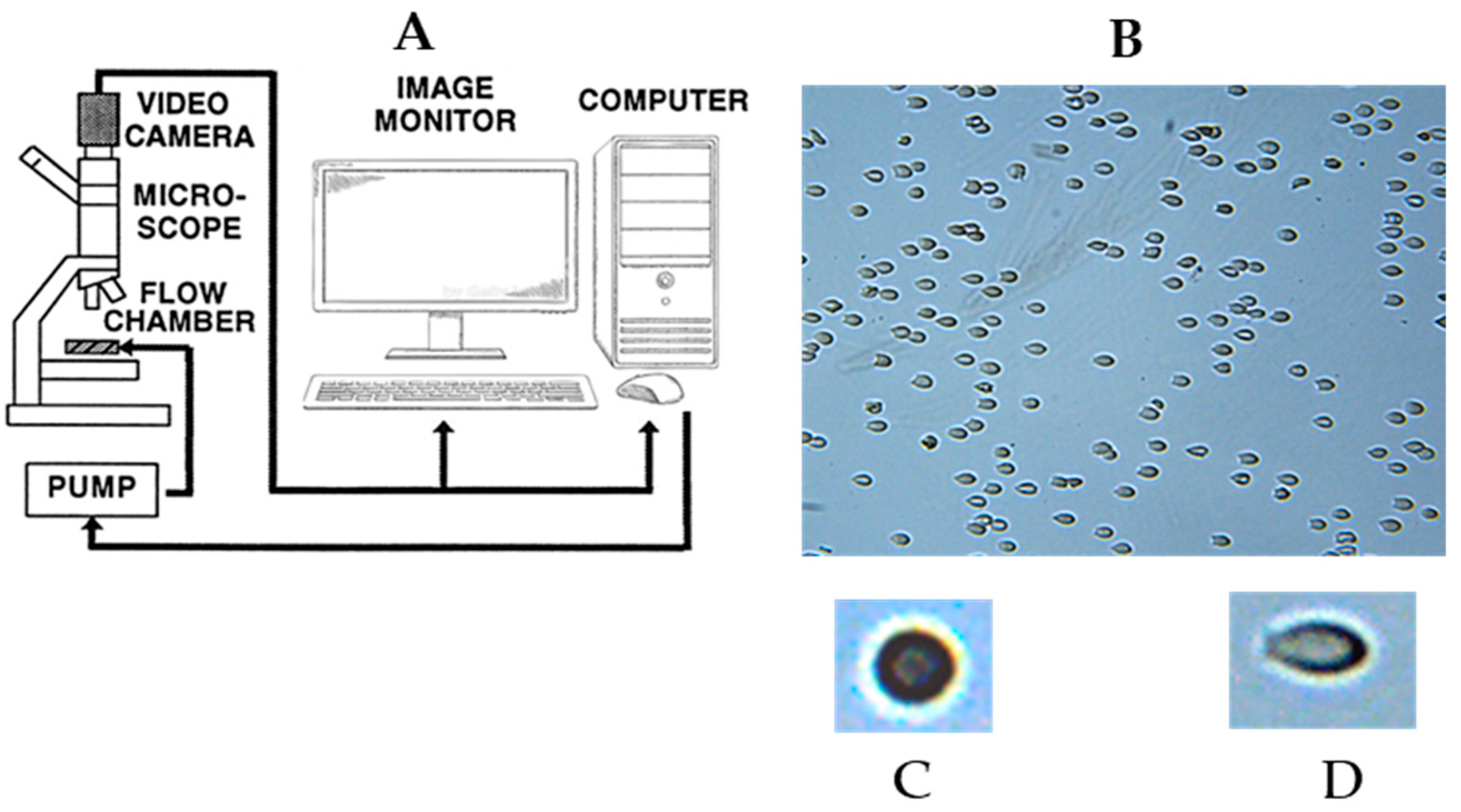
2.1. Deformability of Red Blood Cells from PN and PRBC Units
2.2. Determination of RBC Membrane Proteins for Assessing Cell Deformability
3. Discussion
3.1. Unique Characteristics of Microcirculation in Premature Neonates
3.2. Variability in Deformability of Donors’ and Recipients’ RBCs, and Its Implications for Blood Transfusion Outcomes
- The conventional supply of PRBC units according to their storage duration, even when shortly stored, is not sufficient for assuring that the hemodynamic functionality (expressed primarily by the deformability of the transfused RBCs) is better than that of the recipients’ RBCs; the RBC deformability should be specifically determined for each PRBC unit, independently of the storage duration.
- Cord blood RBCs exhibit, on average, higher deformability than PRBCs, but with considerable variability. Therefore, their use for transfusion to premature newborns does not necessarily secure the provision of RBCs with better deformability than that of the newborn recipients.
- Before transfusion, selection of PRBCs with deformability that is better than, or at least equal to, that of the recipients’ RBCs can be used for personalized, patient-specific blood transfusion to ensure safer and more efficient outcomes.
- The selection of PRBCs is particularly pertinent to transfusions in neonates, as their RBC deformability is generally superior to that of PRBCs collected from adult donors. As noted above, premature neonates, especially those with very low birth weight (VLBW; <1500 g), are prone to developing pathologies (e.g., NEC, IVH, BPD, ROP), which are linked to compromised microcirculation [50,61,62], in which RBC deformability plays a major role [63,64]. High RBC deformability allows smooth passage through the capillaries and effective oxygen delivery [65]. Conversely, less deformable/rigid RBCs can exacerbate hypoxia, increase vascular resistance, and impede perfusion, potentially worsening these conditions [66,67,68,69]. Accordingly, the transfusion of RBCs with low deformability can impair the transfusion outcome, as expressed by the transfusion-induced change in Hb increment and skin blood perfusion [24,25]. Therefore, the transfusion of PRBCs with proper deformability is essential for safe and efficient RBC transfusion, thereby reducing the potential for developing circulatory pathologies.
3.3. Future Perspectives
4. Materials and Methods
4.1. Materials
4.2. Preterm Newborn Population
4.3. Preparation of RBC Samples
4.3.1. Packed RBC Collection
4.3.2. Cord Blood Collection
4.3.3. RBC Sample Preparation
4.4. Determination of RBC Deformability
4.5. Determination of RBC Membrane Protein Composition
4.5.1. Preparation of RBC Membranes
4.5.2. Determination of Membrane-Bound Hb and Membrane Protein Content
4.6. Statistical Analysis
5. Conclusions
- The conventional FIFO criterion is not sufficient for ensuring an optimal transfusion outcome, and the hemodynamic functionality of PRBCs, as expressed by the flow-affecting properties of RBCs, should be precisely determined for each PRBC unit, independently of the storage duration.
- Cord blood RBCs exhibit, on average, better hemodynamic functionality than PRBCs, but this does not always guarantee the provision of RBCs with better deformability than that of the newborn recipients.
- Comparison of the PRBCs’ deformability to that of the intended blood recipient’s cells can be used for personalized, patient-specific transfusion, with more efficient outcomes. This is particularly pertinent to the transfusion to neonates, as their RBC deformability is generally superior to that of adult RBCs.
- The correlations between the molecular composition of the RBC membrane and the cell deformability (Table 3) may potentially be applied for safer and more efficient transfusion to PNs by selecting a PRBC unit with deformability that is better than, or at least equal to, that of the recipient.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RBC | Red blood cell |
| CRBC | RBC from cord blood |
| PRBC | Packed red blood cell |
| EGTA | Ethylene glycol tetraacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| PMSF | Phenylmethylsulphonyl fluoride |
| BSA | Bovine serum albumin |
| Hb | Hemoglobin |
| HBB | Hemoglobin β-subunits |
| FIFO | First-in first-out |
| SBF | Skin blood flow |
| PN | Premature newborn |
| ELBW | Extremely low birth weight |
| NEC | Necrotizing enterocolitis |
| ROP | Retinopathy of prematurity |
| BPD | Bronchopulmonary dysplasia |
| CFA | Cell flow analyzer |
| ER | Elongation ratio |
| MER | Median elongation ratio |
| LDFC | Low-deformable cell |
| UDFC | Undeformable cell |
| HDFC | High-deformable cell |
References
- Bancalari, E.; Jain, D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J. Management during the first 72 h of age of the periviable infant: An evidence-based review. Semin. Perinatol. 2014, 38, 17–24. [Google Scholar] [CrossRef]
- Fabie, N.A.V.; Pappas, K.B.; Feldman, G.L. The Current State of Newborn Screening in the United States. Pediatr. Clin. N. Am. 2019, 66, 369–386. [Google Scholar] [CrossRef]
- Tan, A.P.; Svrckova, P.; Cowan, F.; Chong, W.K.; Mankad, K. Intracranial hemorrhage in neonates: A review of etiologies, patterns and predicted clinical outcomes. Eur. J. Paediatr. Neurol. 2018, 22, 690–717. [Google Scholar] [CrossRef]
- Zangari, A.; Noviello, C.; Nobile, S.; Cobellis, G.; Gulia, C.; Piergentili, R.; Gigli, S.; Carnielli, V. Surgical management of Necrotizing Enterocolitis in an Incredibly Low Birth Weight infant and review of the Literature. Clin. Ter. 2017, 168, e297–e299. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Patel, R.M. Red Blood Cell Transfusion, Anemia, Feeding, and the Risk of Necrotizing Enterocolitis. Clin. Perinatol. 2023, 50, 669–681. [Google Scholar] [CrossRef]
- von Lindern, J.S.; Lopriore, E. Management and prevention of neonatal anemia: Current evidence and guidelines. Expert. Rev. Hematol. 2014, 7, 195–202. [Google Scholar] [CrossRef]
- Glaser, K.; Hartel, C.; Dammann, O.; Herting, E.; Andres, O.; Speer, C.P.; Gopel, W.; Stahl, A.; German Neonatal, N. Erythrocyte transfusions are associated with retinopathy of prematurity in extremely low gestational age newborns. Acta Paediatr. 2023, 112, 2507–2515. [Google Scholar] [CrossRef]
- Wang, X.; Rao, R.; Li, H.; Lei, X.; Dong, W. Red Blood Cell Transfusion for Incidence of Retinopathy of Prematurity: Prospective Multicenter Cohort Study. JMIR Pediatr. Parent. 2024, 7, e60330. [Google Scholar] [CrossRef]
- Bahr, T.M.; Snow, G.L.; Christensen, T.R.; Davenport, P.; Henry, E.; Tweddell, S.M.; Ilstrup, S.J.; Yoder, B.A.; Ohls, R.K.; Sola-Visner, M.C.; et al. Can Red Blood Cell and Platelet Transfusions Have a Pathogenic Role in Bronchopulmonary Dysplasia? J. Pediatr. 2024, 265, 113836. [Google Scholar] [CrossRef] [PubMed]
- McGrady, G.A.; Rettig, P.J.; Istre, G.R.; Jason, J.M.; Holman, R.C.; Evatt, B.L. An outbreak of necrotizing enterocolitis. Association with transfusions of packed red blood cells. Am. J. Epidemiol. 1987, 126, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Blau, J.; Calo, J.M.; Dozor, D.; Sutton, M.; Alpan, G.; La Gamma, E.F. Transfusion-related acute gut injury: Necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J. Pediatr. 2011, 158, 403–409. [Google Scholar] [CrossRef]
- Christensen, R.D.; Lambert, D.K.; Henry, E.; Wiedmeier, S.E.; Snow, G.L.; Baer, V.L.; Gerday, E.; Ilstrup, S.; Pysher, T.J. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion 2010, 50, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Janjindamai, W.; Prapruettrong, A.; Thatrimontrichai, A.; Dissaneevate, S.; Maneenil, G.; Geater, A. Risk of Necrotizing Enterocolitis Following Packed Red Blood Cell Transfusion in Very Low Birth Weight Infants. Indian J. Pediatr. 2019, 86, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Patel, R.M.; Christensen, R.D. Anemia, red blood cell transfusions, and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 47–51. [Google Scholar] [CrossRef]
- Nair, J.; Lakshminrusimha, S. Role of NO and other vascular mediators in the etiopathogenesis of necrotizing enterocolitis. Front. Biosci. 2019, 11, 9–28. [Google Scholar] [CrossRef]
- Sood, B.G.; Rambhatla, A.; Thomas, R.; Chen, X. Decreased hazard of necrotizing enterocolitis in preterm neonates receiving red cell transfusions. J. Matern. Fetal Neonatal Med. 2016, 29, 737–744. [Google Scholar] [CrossRef]
- Khashu, M.; Dame, C.; Lavoie, P.M.; De Plaen, I.G.; Garg, P.M.; Sampath, V.; Malhotra, A.; Caplan, M.D.; Kumar, P.; Agrawal, P.B.; et al. Current Understanding of Transfusion-associated Necrotizing Enterocolitis: Review of Clinical and Experimental Studies and a Call for More Definitive Evidence. Newborn 2022, 1, 201–208. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Lipowsky, H.H. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am. J. Physiol. 1999, 277, H2145–H2157. [Google Scholar] [CrossRef]
- Matot, I.; Katz, M.; Pappo, O.; Zelig, O.; Corchia, N.; Yedgar, S.; Barshtein, G.; Bennett-Guerrero, E.; Abramovitch, R. Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit. Care Med. 2013, 41, 842–849. [Google Scholar] [CrossRef]
- Sakr, Y.; Chierego, M.; Piagnerelli, M.; Verdant, C.; Dubois, M.J.; Koch, M.; Creteur, J.; Gullo, A.; Vincent, J.L.; De Backer, D. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit. Care Med. 2007, 35, 1639–1644. [Google Scholar] [CrossRef]
- McHedlishvili, G. Disturbed blood flow structuring as critical factor of hemorheological disorders in microcirculation. Clin. Hemorheol. Microcirc. 1998, 19, 315–325. [Google Scholar]
- Barshtein, G.; Goldschmidt, N.; Pries, A.R.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of transfused red blood cells is a potent effector of transfusion-induced hemoglobin increment: A study with beta-thalassemia major patients. Am. J. Hematol. 2017, 92, E559–E560. [Google Scholar] [CrossRef]
- Barshtein, G.; Pries, A.R.; Goldschmidt, N.; Zukerman, A.; Orbach, A.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation 2016, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Unit-to-unit variability in the deformability of red blood cells. Transfus. Apher. Sci. 2020, 59, 102876. [Google Scholar] [CrossRef]
- Koch, C.G.; Figueroa, P.I.; Li, L.; Sabik, J.F., 3rd; Mihaljevic, T.; Blackstone, E.H. Red blood cell storage: How long is too long? Ann. Thorac. Surg. 2013, 96, 1894–1899. [Google Scholar] [CrossRef] [PubMed]
- Bellach, L.; Eigenschink, M.; Hassanein, A.; Savran, D.; Salzer, U.; Mullner, E.W.; Repa, A.; Klebermass-Schrehof, K.; Wisgrill, L.; Giordano, V.; et al. Packed red blood cell transfusion in preterm infants. Lancet Haematol. 2022, 9, e615–e626. [Google Scholar] [CrossRef]
- Howarth, C.; Banerjee, J.; Aladangady, N. Red Blood Cell Transfusion in Preterm Infants: Current Evidence and Controversies. Neonatology 2018, 114, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Collard, K.J. Transfusion related morbidity in premature babies: Possible mechanisms and implications for practice. World J. Clin. Pediatr. 2014, 3, 19–29. [Google Scholar] [CrossRef]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 722896. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Arbell, D.; Barkan, R.; Livshits, L.; Pajic-Lijakovic, I.; Yedgar, S. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 12755. [Google Scholar] [CrossRef]
- Barshtein, G.; Livshits, L.; Gural, A.; Arbell, D.; Barkan, R.; Pajic-Lijakovic, I.; Yedgar, S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. Int. J. Mol. Sci. 2024, 25, 5814. [Google Scholar] [CrossRef]
- Pellegrino, C.; Stone, E.F.; Valentini, C.G.; Teofili, L. Fetal Red Blood Cells: A Comprehensive Review of Biological Properties and Implications for Neonatal Transfusion. Cells 2024, 13, 1843. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell Proteom. 2016, 15, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Mino, M. Vitamin E and preterm infants. Free Radic. Biol. Med. 2022, 180, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Colin, F.C.; Gallois, Y.; Rapin, D.; Meskar, A.; Chabaud, J.J.; Vicariot, M.; Menez, J.F. Impaired fetal erythrocytes’ filterability: Relationship with cell size, membrane fluidity, and membrane lipid composition. Blood 1992, 79, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.L.; Altaf, W.; Lall, R.; Wapnir, R.A. Cord blood red cell osmotic fragility: A comparison between preterm and full-term newborn infants. Early Hum. Dev. 2003, 72, 37–46. [Google Scholar] [CrossRef]
- Christensen, R.D.; Jopling, J.; Henry, E.; Wiedmeier, S.E. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J. Perinatol. 2008, 28, 24–28. [Google Scholar] [CrossRef]
- Sahoo, J.; Mahapatra, C.; Sahoo, B.B.; Sahoo, N. Changes in hematological and biochemical parameters and rate of hemolysis during the storage of packed RBC units: A prospective study. J. Lab. Physicians 2024, 17, 88–94. [Google Scholar] [CrossRef]
- Maitoza, L.A.; Neeman, E.; Funaro, M.; Pierce, R.W. Relevance of Microvascular Flow Assessments in Critically Ill Neonates and Children: A Systematic Review. Pediatr. Crit. Care Med. 2020, 21, 373–384. [Google Scholar] [CrossRef]
- Puchwein-Schwepcke, A.; Grzybowski, A.K.; Genzel-Boroviczeny, O.; Nussbaum, C. Effects of Prematurity on the Cutaneous Microcirculatory Network in the First Weeks of Life. Front. Pediatr. 2019, 7, 198. [Google Scholar] [CrossRef]
- Linderkamp, O.; Stadler, A.A.; Zilow, E.P. In vitro models of microcirculation in the human fetus, neonate and adult. Pediatr. Res. 1992, 32, 615. [Google Scholar] [CrossRef][Green Version]
- Piety, N.Z.; Stutz, J.; Yilmaz, N.; Xia, H.; Yoshida, T.; Shevkoplyas, S.S. Microfluidic capillary networks are more sensitive than ektacytometry to the decline of red blood cell deformability induced by storage. Sci. Rep. 2021, 11, 604. [Google Scholar] [CrossRef]
- Sosa, J.M.; Nielsen, N.D.; Vignes, S.M.; Chen, T.G.; Shevkoplyas, S.S. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin. Hemorheol. Microcirc. 2014, 57, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemodynamic Functionality of Transfused Red Blood Cells in the Microcirculation of Blood Recipients. Front. Physiol. 2018, 9, 41. [Google Scholar] [CrossRef]
- Arbell, D.; Bin-Nun, A.; Zugayar, D.; Eventov-Friedman, S.; Chepel, N.; Srebnik, N.; Hamerman, C.; Wexler, T.L.R.; Barshtein, G.; Yedgar, S. Deformability of cord blood vs. newborns’ red blood cells: Implication for blood transfusion. J. Matern. Fetal Neonatal Med. 2022, 35, 3270–3275. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Preparation of packed red blood cell units in the blood bank: Alteration in red blood cell deformability. Transfus. Apher. Sci. 2020, 59, 102738. [Google Scholar] [CrossRef]
- Dang, D.; Gu, X.; Jiang, S.; Li, W.; Zhou, W.; Cao, Y.; Lee, S.K.; Wu, H.; Zhou, J.; Chinese Neonatal, N. RBC transfusion and necrotizing enterocolitis in very preterm infants: A multicenter observational study. Sci. Rep. 2024, 14, 14345. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Besner, G.E. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin. Pediatr. Surg. 2013, 22, 83–87. [Google Scholar] [CrossRef]
- Yedgar, S.; Arbell, D.; Barshtein, G. Hemodynamic Functionality of Stored Red Blood Cells: An Important Metric of Blood Unit Quality. Ann. Thorac. Surg. 2019, 108, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Rasmusen, T.L.; Zelig, O.; Arbell, D.; Yedgar, S. Inter-donor variability in deformability of red blood cells in blood units. Transfus. Med. 2020, 30, 492–496. [Google Scholar] [CrossRef]
- Hess, J.R.; Biomedical Excellence for Safer Transfusion, C. Scientific problems in the regulation of red blood cell products. Transfusion 2012, 52, 1827–1835. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Papageorgiou, E.G.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion 2015, 55, 2659–2671. [Google Scholar] [CrossRef]
- Bosman, G.J.; Stappers, M.; Novotny, V.M. Changes in band 3 structure as determinants of erythrocyte integrity during storage and survival after transfusion. Blood Transfus. 2010, 8 (Suppl. 3), s48–s52. [Google Scholar] [CrossRef]
- Dern, R.J.; Gwinn, R.P.; Wiorkowski, J.J. Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. J. Lab. Clin. Med. 1966, 67, 955–965. [Google Scholar]
- Tarasev, M.; Alfano, K.; Chakraborty, S.; Zubair, A. Evaluation of Novel In-Vitro RBC Fragility Metrics as Age-Independent Measures of Stored RBC Quality. Transfusion 2011, 51, 79a. [Google Scholar]
- Bianchi, M.; Giannantonio, C.; Spartano, S.; Fioretti, M.; Landini, A.; Molisso, A.; Tesfagabir, G.M.; Tornesello, A.; Barbagallo, O.; Valentini, C.G.; et al. Allogeneic umbilical cord blood red cell concentrates: An innovative blood product for transfusion therapy of preterm infants. Neonatology 2015, 107, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Papacci, P.; Valentini, C.G.; Barbagallo, O.; Vento, G.; Teofili, L. Umbilical cord blood as a source for red-blood-cell transfusion in neonatology: A systematic review. Vox Sang. 2018, 113, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.G.; Casanova, M.A.; Samarkanova, D.; Aldecoa-Bilbao, V.; Teresa-Palacio, M.; Busquets, E.F.; Figueras-Aloy, J.; Salvia-Roiges, M.; Querol, S. Feasibility of umbilical cord blood as a source of red blood cell transfusion in preterm infants. Blood Transfus 2021, 19, 510–517. [Google Scholar] [CrossRef]
- Kluckow, M. The Pathophysiology of Low Systemic Blood Flow in the Preterm Infant. Front. Pediatr. 2018, 6, 29. [Google Scholar] [CrossRef]
- Park, Y.S. Perspectives: Understanding the Pathophysiology of Intraventricular Hemorrhage in Preterm Infants and Considering of the Future Direction for Treatment. J. Korean Neurosurg. Soc. 2023, 66, 298–307. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Bagchi, P. A computational study of red blood cell deformability effect on hemodynamic alteration in capillary vessel networks. Sci. Rep. 2022, 12, 4304. [Google Scholar] [CrossRef]
- Hurd, T.C.; Dasmahapatra, K.S.; Rush, B.F., Jr.; Machiedo, G.W. Red blood cell deformability in human and experimental sepsis. Arch. Surg. 1988, 123, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Z.; Liu, H.; Bian, X.; Tang, W. Erythrocytes enhance oxygen-carrying capacity through self-regulation. Front. Physiol. 2025, 16, 1592176. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Shamoun, M.; Seu, K.G.; Tanski, T.; Kalfa, T.A.; Eniola-Adefeso, O. Characterizing bulk rigidity of rigid red blood cell populations in sickle-cell disease patients. Sci. Rep. 2021, 11, 7909. [Google Scholar] [CrossRef]
- Raat, N.J.; Verhoeven, A.J.; Mik, E.G.; Gouwerok, C.W.; Verhaar, R.; Goedhart, P.T.; de Korte, D.; Ince, C. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit. Care Med. 2005, 33, 39–45, discussion 238–239. [Google Scholar] [CrossRef]
- Tsai, A.G.; Hofmann, A.; Cabrales, P.; Intaglietta, M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: The experimental evidence. Transfus. Apher. Sci. 2010, 43, 69–78. [Google Scholar] [CrossRef]
- Rashidi, Y.; Simionato, G.; Zhou, Q.; John, T.; Kihm, A.; Bendaoud, M.; Kruger, T.; Bernabeu, M.O.; Kaestner, L.; Laschke, M.W.; et al. Red blood cell lingering modulates hematocrit distribution in the microcirculation. Biophys. J. 2023, 122, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Kriebardis, A.G.; Papassideri, I.S. Aging and death signalling in mature red cells: From basic science to transfusion practice. Blood Transfus. 2010, 8 (Suppl. S3), s39–s47. [Google Scholar] [CrossRef]
- Asaro, R.J.; Zhu, Q.; Cabrales, P. Erythrocyte Aging, Protection via Vesiculation: An Analysis Methodology via Oscillatory Flow. Front. Physiol. 2018, 9, 1607. [Google Scholar] [CrossRef]
- Ma, S.R.; Xia, H.F.; Gong, P.; Yu, Z.L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines 2023, 11, 2798. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, X.; Pivkin, I.V.; Dao, M.; Karniadakis, G.E.; Suresh, S. Lipid bilayer and cytoskeletal interactions in a red blood cell. Proc. Natl. Acad. Sci. USA 2013, 110, 13356–13361. [Google Scholar] [CrossRef]
- Livshits, L.; Peretz, S.; Bogdanova, A.; Zoabi, H.; Eitam, H.; Barshtein, G.; Galindo, C.; Feldman, Y.; Pajic-Lijakovic, I.; Koren, A.; et al. The Impact of Ca(2+) on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells 2023, 12, 2280. [Google Scholar] [CrossRef]
- Freitas Leal, J.K.; Lasonder, E.; Sharma, V.; Schiller, J.; Fanelli, G.; Rinalducci, S.; Brock, R.; Bosman, G. Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes 2020, 8, 6. [Google Scholar] [CrossRef]
- Gautier, E.F.; Ducamp, S.; Leduc, M.; Salnot, V.; Guillonneau, F.; Dussiot, M.; Hale, J.; Giarratana, M.C.; Raimbault, A.; Douay, L.; et al. Comprehensive Proteomic Analysis of Human Erythropoiesis. Cell Rep. 2016, 16, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Mean ± SD | p Value | PRBC | CRBC | |||
|---|---|---|---|---|---|---|---|
| PRBC | CRBC | Max | Min | Max | Min | ||
| MER | 1.52 ± 0.11 | 1.61 ± 0.07 | 1.34 × 10−12 | 1.77 | 1.24 | 1.75 | 1.41 |
| %HDFC | 4.22 ± 3.38 | 8.51 ± 3.64 | 1.60 × 10−14 | 17.77 | 0.30 | 20.8 | 1.90 |
| %LDFC | 20.70 ± 13.9 | 14.10 ± 5.52 | 2.8 × 10−6 | 66.00 | 2.0 | 31.5 | 6.3 |
| %UDFC | 3.18 ± 3.83 | 2.21 ± 1.06 | 0.019 | 21.00 | 0.12 | 5.50 | 0.35 |
| Parameters | U120 | U128 | U139 | U160 | U172 | CRBC |
|---|---|---|---|---|---|---|
| MER | 1.52 | 1.60 | 1.49 | 1.63 | 1.43 | 1.55 |
| %HDFC | 4.74 | 7.41 | 4.08 | 13.82 | 2.09 | 6.14 |
| % LDFC | 16.40 | 9.73 | 21.33 | 15.25 | 30.53 | 14.48 |
| %UDFC | 1.83 | 1.21 | 2.87 | 2.54 | 3.94 | 1.12 |
| Protein | R | p-Value |
|---|---|---|
| HBB | 0.618 | 0.019 |
| Ezrin | 0.626 | 0.013 |
| Stomatin | 0.510 | 0.053 |
| Band 4.1 | 0.552 | 0.035 |
| Flotillin 2 | 0.571 | 0.032 |
| Flotillin 1 | 0.632 | 0.015 |
| HBB and Ezrin | 0.702 | 0.017 |
| HBB and Stomatin | 0.706 | 0.016 |
| HBB and Band 4.1 | 0.73 | 0.01 |
| HBB and Flotillin 2 | 0.76 | 0.009 |
| HBB and Flotillin 1 | 0.77 | 0.007 |
| Parameter | Value, Av ± S.D |
|---|---|
| Gestation age, weeks | 25.47 ± 1.31 |
| Birth weight, g | 752 ± 157 |
| Gender, F/M | 38/40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbell, D.; Gural, A.; Barshtein, G.; Abu-Leil, S.; Luques, L.; Gazer, B.; Yedgar, S. Variability in the Deformability of Red Blood Cells: Application to Treating Premature Newborns with Blood Transfusion. Int. J. Mol. Sci. 2025, 26, 8144. https://doi.org/10.3390/ijms26178144
Arbell D, Gural A, Barshtein G, Abu-Leil S, Luques L, Gazer B, Yedgar S. Variability in the Deformability of Red Blood Cells: Application to Treating Premature Newborns with Blood Transfusion. International Journal of Molecular Sciences. 2025; 26(17):8144. https://doi.org/10.3390/ijms26178144
Chicago/Turabian StyleArbell, Dan, Alexander Gural, Gregory Barshtein, Sinan Abu-Leil, Lisandro Luques, Benny Gazer, and Saul Yedgar. 2025. "Variability in the Deformability of Red Blood Cells: Application to Treating Premature Newborns with Blood Transfusion" International Journal of Molecular Sciences 26, no. 17: 8144. https://doi.org/10.3390/ijms26178144
APA StyleArbell, D., Gural, A., Barshtein, G., Abu-Leil, S., Luques, L., Gazer, B., & Yedgar, S. (2025). Variability in the Deformability of Red Blood Cells: Application to Treating Premature Newborns with Blood Transfusion. International Journal of Molecular Sciences, 26(17), 8144. https://doi.org/10.3390/ijms26178144







