A Lack of Complete Linkage Disequilibrium Between c.1236G>A and c.1129-5923C>G HapB3 Variants of DPYD: A Call to Revise European Pharmacogenetic Guidelines
Abstract
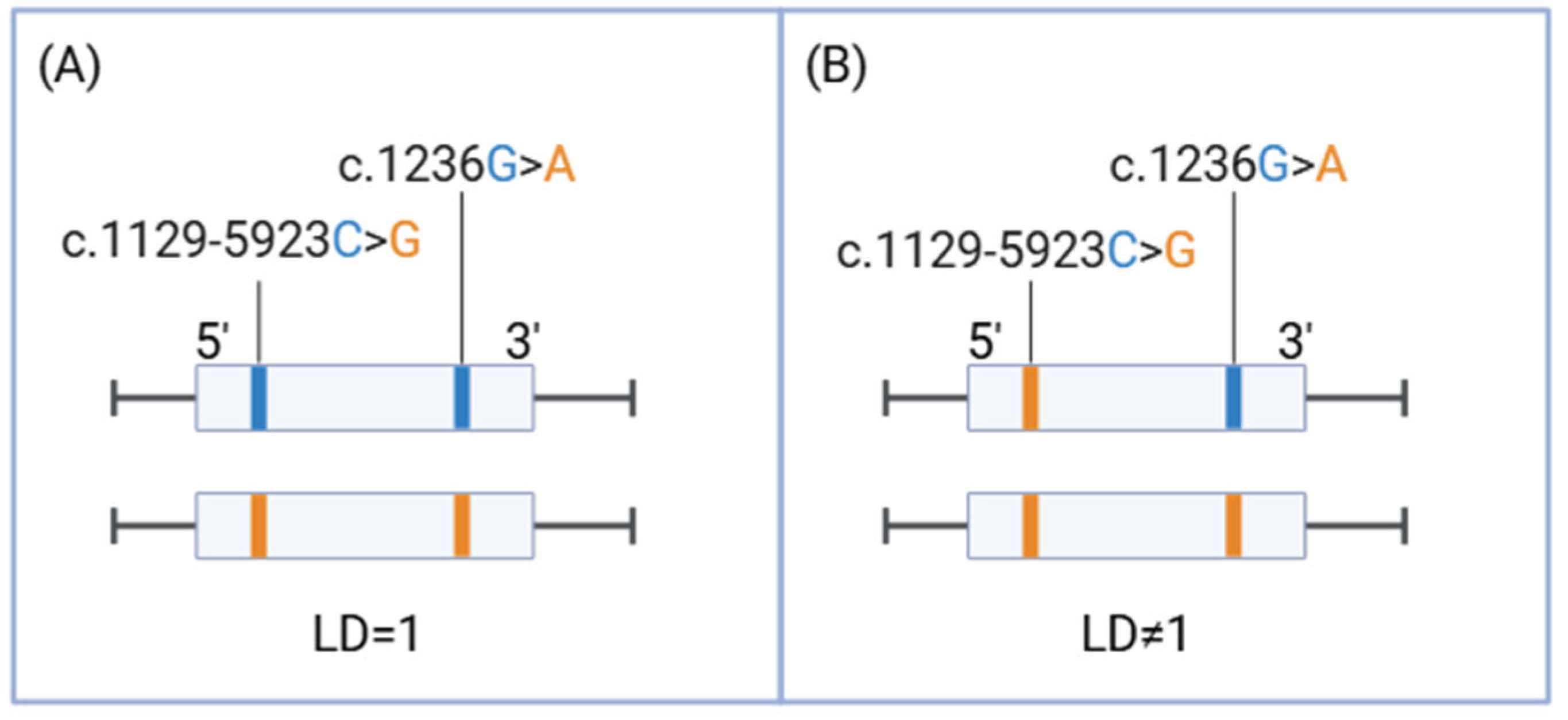
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Selection
4.2. Genotyping
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| DPD | Dihydropyrimidine dehydrogenase |
| SNPs | Single nucleotide polymorphisms |
| LD | Linkage disequilibrium |
| WES | Whole exome sequencing |
| PharmVar | Pharmacogene Variation Consortium |
| CPIC® | Clinical Pharmacogenetics Implementation Consortium |
| EMA | European Medicine Agency |
| EPAR | European Public Assessment Reports |
| AEMPS | Spanish Agency for Medicines and Health Products |
| Swissmedic | Swiss Agency for Therapeutic Products |
| FDA | Food and Drug Administration |
| DPWG | Dutch Pharmacogenetics Working Group |
| AIOM | Italian Association of Medical Oncology |
| SIF | Italian Society of Pharmacology |
| RNPGx | French National Network of Pharmacogenetics |
| SEFF/SEOM | Pharmacogenomics Society and the Spanish Society of Medical Oncology |
| FPGMX | Pharmacogenetic Unit of the Public Foundation of Genomic Medicine |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
References
- Simões, A.R.; Fernández-Rozadilla, C.; Maroñas, O.; Carracedo, Á. The Road so Far in Colorectal Cancer Pharmacogenomics: Are We Closer to Individualised Treatment? J. Pers. Med. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Henricks, L.M.; Opdam, F.L.; Beijnen, J.H.; Cats, A.; Schellens, J.H.M. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: Call for a drug label update. Ann. Oncol. 2017, 28, 2915–2922. [Google Scholar] [CrossRef]
- Toffoli, G.; Giodini, L.; Buonadonna, A.; Berretta, M.; De Paoli, A.; Scalone, S.; Miolo, G.; Mini, E.; Nobili, S.; Lonardi, S.; et al. Clinical validity of a DPYD-based pharmacogenetic test to predict severe toxicity to fluoropyrimidines. Int. J. Cancer 2015, 137, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, D.; Henricks, L.M.; Sonke, G.S.; Deenen, M.J.; Froehlich, T.K.; Amstutz, U.; Largiadèr, C.R.; Jennings, B.A.; Marinaki, A.M.; Sanderson, J.D.; et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 1639–1650. [Google Scholar] [CrossRef]
- Wei, X.; Elizondo, G.; Sapone, A.; McLeod, H.L.; Raunio, H.; Fernandez-Salguero, P.; Gonzalez, F.J. Characterization of the human dihydropyrimidine dehydrogenase gene. Genomics 1998, 51, 391–400. [Google Scholar] [CrossRef]
- Johnson, M.R.; Wang, K.; Tillmanns, S.; Albin, N.; Diasio, R.B. Structural organization of the human dihydropyrimidine dehydrogenase gene. Cancer Res. 1997, 57, 1660–1663. [Google Scholar]
- PharmGKB. Available online: https://www.pharmgkb.org/ (accessed on 12 January 2025).
- Amstutz, U.; Farese, S.; Aebi, S.; Largiadèr, C.R. Dihydropyrimidine dehydrogenase gene variation and severe 5-fluorouracil toxicity: A haplotype assessment. Pharmacogenomics 2009, 10, 931–944. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141, 456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Froehlich, T.K.; Amstutz, U.; Aebi, S.; Joerger, M.; Largiadèr, C.R. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early-onset fluoropyrimidine toxicity. Int. J. Cancer 2015, 136, 730–739. [Google Scholar] [CrossRef]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Deac, A.L.; Burz, C.C.; Bocşe, H.F.; Bocşan, I.C.; Buzoianu, A.D. A review on the importance of genotyping and phenotyping in fluoropyrimidine treatment. Med. Pharm. Rep. 2020, 93, 223–230. [Google Scholar] [CrossRef]
- Gil-Rodriguez, A.; Recarey-Rama, S.; Rodríguez-Viyuela, A.; Cruz, R.; Barros, F.; Carracedo, A.; Maroñas, O. Differences in DPYD Population Frequencies Observed in Galicians Compared to Europeans and Spanish from PhotoDPYD Study. Pharmaceuticals 2025, 18, 515. [Google Scholar] [CrossRef]
- PharmGKB. DPYD Frequency Table. Gene-Specific Information. Tables for DPYD. Available online: https://www.pharmgkb.org/page/dpydRefMaterials (accessed on 30 March 2025).
- Schwab, M.; Zanger, U.M.; Marx, C.; Schaeffeler, E.; Klein, K.; Dippon, J.; Kerb, R.; Blievernicht, J.; Fischer, J.; Hofmann, U.; et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: A prospective clinical trial by the German 5-FU Toxicity Study Group. J. Clin. Oncol. 2008, 26, 2131–2138. [Google Scholar] [CrossRef]
- Seck, K.; Riemer, S.; Kates, R.; Ullrich, T.; Lutz, V.; Harbeck, N.; Schmitt, M.; Kiechle, M.; Diasio, R.; Gross, E. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in a cohort of Caucasian individuals. Clin. Cancer Res. 2005, 11, 5886–5892. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Meijer, J.; Mul, A.N.P.M.; Meinsma, R.; Schmid, V.; Dobritzsch, D.; Hennekam, R.C.M.; Mannens, M.M.A.M.; Kiechle, M.; Etienne-Grimaldi, M.-C.; et al. Intragenic deletions and a deep intronic mutation affecting pre-mRNA splicing in the dihydropyrimidine dehydrogenase gene as novel mechanisms causing 5-fluorouracil toxicity. Hum. Genet. 2010, 128, 529–538. [Google Scholar] [CrossRef]
- Nie, Q.; Shrestha, S.; Tapper, E.E.; Trogstad-Isaacson, C.; Bouchonville, K.; Lee, A.; Wu, R.; Jerde, C.; Wang, Z.; Kubica, P.; et al. Quantitative Contribution of rs75017182 to Dihydropyrimidine Dehydrogenase mRNA Splicing and Enzyme Activity. Clin. Pharmacol. Ther. 2017, 102, 662–670. [Google Scholar] [CrossRef]
- Meulendijks, D.; Henricks, L.M.; van Kuilenburg, A.B.; Jacobs, B.A.W.; Aliev, A.; Rozeman, L.; Meijer, J.; Beijnen, J.H.; de Graaf, H.; Cats, A.; et al. Patients homozygous for DPYD c.1129-5923C>G/haplotype B3 have partial DPD deficiency and require a dose reduction when treated with fluoropyrimidines. Cancer Chemother. Pharmacol. 2016, 78, 875–880. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Ensembl. Linkage Disequilibrium Data-Ensembl Genome Browser 113. Available online: https://www.ensembl.org/Help/View?id=197 (accessed on 26 March 2025).
- NIH. LDlink-National Institutes of Health. Available online: https://ldlink.nih.gov/?tab=home (accessed on 27 January 2025).
- gnomAD. 1-97573863-C-T|gnomAD v4.1.0. Available online: https://gnomad.broadinstitute.org/variant/1-97573863-C-T?dataset=gnomad_r4 (accessed on 20 February 2025).
- gnomAD. 1-97579893-G-C|gnomAD v4.1.0. Available online: https://gnomad.broadinstitute.org/variant/1-97579893-G-C?dataset=gnomad_r4 (accessed on 20 February 2025).
- Turner, A.J.; Haidar, C.E.; Yang, W.; Boone, E.C.; Offer, S.M.; Empey, P.E.; Haddad, A.; Tahir, S.; Scharer, G.; Broeckel, U.; et al. Updated DPYD HapB3 haplotype structure and implications for pharmacogenomic testing. Clin. Transl. Sci. 2024, 17, e13699. [Google Scholar] [CrossRef]
- All of Us Research Program Genomics Investigators. Genomic data in the All of Us Research Program. Nature 2024, 627, 340–346. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). EMA Recommendations on DPD Testing Prior to Treatment with Fluorouracil, Capecitabine, Tegafur and Flucytosine. Available online: https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine (accessed on 11 January 2025).
- European Medicines Agency (EMA). Xeloda. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xeloda (accessed on 28 March 2025).
- Agencia Española de Medicamentos y Productos Sanitarios. Fluorouracilo, Capecitabina, Tegafur y Flucitosina en Pacientes Con Déficit de Dihidropirimidina Deshidrogenasa. Available online: https://www.aemps.gob.es/informa/fluorouracilo-capecitabina-tegafur-y-flucitosina-en-pacientes-con-deficit-de-dihidropirimidina-deshidrogenasa/ (accessed on 30 March 2025).
- Agencia Española de Medicamentos y Productos Sanitarios. Base de Datos de Biomarcadores Farmacogenómicos. 29 July 2024. Available online: https://www.aemps.gob.es/medicamentos-de-uso-humano/base-de-datos-de-biomarcadores-farmacogenomicos/ (accessed on 17 June 2025).
- Hamzic, S.; Aebi, S.; Joerger, M.; Montemurro, M.; Ansari, M.; Amstutz, U.; Largiadèr, C. Fluoropyrimidine chemotherapy: Recommendations for DPYD genotyping and therapeutic drug monitoring of the Swiss Group of Pharmacogenomics and Personalised Therapy. Swiss. Med. Wkly. 2020, 150, w20375. [Google Scholar] [CrossRef]
- PharmGKB. Annotation of Swissmedic Label for Fluorouracil and DPYD. Available online: https://www.pharmgkb.org/labelAnnotation/PA166184450 (accessed on 22 March 2025).
- U.S. Food and Drug Administration. FDA Approves Safety Labeling Changes Regarding DPD Deficiency for Fluorouracil Injection Products. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-safety-labeling-changes-regarding-dpd-deficiency-fluorouracil-injection-products (accessed on 9 May 2025).
- U.S. Food and Drug Administration. Safety Announcement: FDA Highlights Importance of DPD Deficiency Discussions with Patients Prior to Capecitabine or 5FU Treatment. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/safety-announcement-fda-highlights-importance-dpd-deficiency-discussions-patients-prior-capecitabine (accessed on 9 May 2025).
- U.S. Food and Drug Administration. Fluorouracil-Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/210123s008lbl.pdf (accessed on 11 January 2025).
- Lunenburg, C.A.T.C.; van der Wouden, C.H.; Nijenhuis, M.; Rhenen, M.H.C.-V.; de Boer-Veger, N.J.; Buunk, A.M.; Houwink, E.J.F.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.N.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. Eur. J. Hum. Genet. 2020, 28, 508–517. [Google Scholar] [CrossRef]
- PharmGKB. Annotation of AIOM Guideline for Capecitabine, Fluorouracil, Tegafur and DPYD. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166312801 (accessed on 28 March 2025).
- Association of Medical Oncology (AIOM) and the Italian Society of Pharmacology (SIF). Raccomandazioni Per Analisi Farmacogenetiche. Available online: https://www.aiom.it/wp-content/uploads/2024/11/2024_Racc-analisi-farmacogenetiche-update.pdf (accessed on 11 January 2025).
- Quaranta, S.; Dupouey, J.; Colle, R.; Verstuyft, C. Pharmacogenetics of antidepressant drugs: State of the art and clinical implementation—Recommendations from the French National Network of Pharmacogenetics. Therapie 2017, 72, 311–318. [Google Scholar] [CrossRef]
- CPIC®. Guideline for Fluoropyrimidines and DPYD. Available online: https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/ (accessed on 11 January 2025).
- SEFF—Sociedad Española de Farmacogenética y Farmacogenómica. Fluoropirimidinas-Recomendaciones Grupos de Trabajo de la SEFF. Available online: https://seff.es/fluoropirimidinas/ (accessed on 28 March 2025).
- Ministerio de Sanidad. Catálogo de Pruebas Genéticas y Genómicas. Available online: https://cgen.sanidad.gob.es/#/mas-informacion (accessed on 28 March 2025).
- Gaedigk, A.; Casey, S.T.; Whirl-Carrillo, M.; Miller, N.A.; Klein, T.E. Pharmacogene Variation Consortium: A Global Resource and Repository for Pharmacogene Variation. Clin. Pharmacol. Ther. 2021, 110, 542–545. [Google Scholar] [CrossRef]
- PharmVar. Pharmacogene Variation Consortium (PharmVar). DPYD. Available online: https://www.pharmvar.org/gene/DPYD (accessed on 27 January 2025).
- PharmVar. Pharmacogene Variation Consortium. Gene Information Document-DPYD. Available online: https://a.storyblok.com/f/70677/x/7508dd7406/gene-info_dpyd_v1-5.pdf (accessed on 27 January 2025).
- PharmGKB. Gene-Specific Information Tables for DPYD. Available online: https://www.pharmgkb.org/page/dpydRefMaterials (accessed on 11 January 2025).
- Henricks, L.M.; Lunenburg, C.A.; Meulendijks, D.; Gelderblom, H.; Cats, A.; Swen, J.J.; Schellens, J.H.; Guchelaar, H.-J. Translating DPYD genotype into DPD phenotype: Using the DPYD gene activity score. Pharmacogenomics 2015, 16, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Henricks, L.M.; Lunenburg, C.A.T.C.; de Man, F.M.; Meulendijks, D.; Frederix, G.W.; Kienhuis, E.; Creemers, G.-J.; Baars, A.; Dezentjé, V.O.; Imholz, A.L.; et al. A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur. J. Cancer 2019, 107, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Venook, A.P. Reducing Fluorouracil Doses in Patients With Partial Dihydropyrimidine Dehydrogenase Deficiency Is a Treatment Safety Strategy, Not a Panacea of Precision Dosing. JCO Precis. Oncol. 2025, 9, e2500440. [Google Scholar] [CrossRef]
- Fiebrich-Westra, H.B.; Haroun, C.; van der Galiën, R.; den Besten-Bertholee, D.; Deenen, M.J.; Moes, D.J.A.R.; Bet, P.M.; de Groot, J.W.B.; Brohet, R.M.; van Kuilenburg, A.B.; et al. Precision Treatment of Patients With GI Cancer Using Pre-emptive DPYD Genotyping/Phenotyping Plus Pharmacokinetic-Guided Dosing of 5-Fluorouracil. JCO Precis. Oncol. 2025, 9, e2500062. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, M.; Iengo, G.; Massa, N.; Carlomagno, C. Dihydropyrimidine dehydrogenase polymorphisms in patients with gastrointestinal malignancies and their impact on fluoropyrimidine tolerability: Experience from a single Italian institution. World J. Gastrointest. Oncol. 2025, 17, 96822. [Google Scholar] [CrossRef]
- Ardizzone, A.; Bulzomì, M.; De Luca, F.; Silvestris, N.; Esposito, E.; Capra, A.P. Dihydropyrimidine Dehydrogenase Polymorphism c.2194G>A Screening Is a Useful Tool for Decreasing Gastrointestinal and Hematological Adverse Drug Reaction Risk in Fluoropyrimidine-Treated Patients. Curr. Issues Mol. Biol. 2024, 46, 9831–9843. [Google Scholar] [CrossRef]
- Bianchino, G.; Perrone, A.; Sgambato, A.; Sarno, I.; Nozza, F.; Omer, L.C.; Ulivi, M.; Traficante, A.; Campisi, B.; Russi, S.; et al. Application of dihydropyrimidine dehydrogenase deficiency testing for the prevention of fluoropyrimidine toxicity: A real-world experience in a Southern Italy cancer center. J. Chemother. 2025, 17, 1–7. [Google Scholar] [CrossRef]
- Lau, D.K.; Fong, C.; Arouri, F.; Cortez, L.; Katifi, H.; Gonzalez-Exposito, R.; Razzaq, M.B.; Li, S.; Macklin-Doherty, A.; Hernandez, M.A.; et al. Impact of pharmacogenomic DPYD variant guided dosing on toxicity in patients receiving fluoropyrimidines for gastrointestinal cancers in a high-volume tertiary centre. BMC Cancer 2023, 23, 380. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, N.H.; Pfeiffer, P.; Ewertz, M.; Fruekilde, P.; Feddersen, S.; Holm, H.; Bergmann, T.; Qvortrup, C.; Damkier, P. Implementation and clinical benefit of DPYD genotyping in a Danish cancer population. ESMO Open 2023, 8, 100782. [Google Scholar] [CrossRef]
- Paulsen, N.H.; Qvortrup, C.; Vojdeman, F.J.; Plomgaard, P.; Andersen, S.E.; Ramlov, A.; Bertelsen, B.; Rossing, M.; Nielsen, C.G.; Hoffmann-Lücke, E.; et al. Dihydropyrimidine dehydrogenase (DPD) genotype and phenotype among Danish cancer patients: Prevalence and correlation between DPYD-genotype variants and P-uracil concentrations. Acta Oncol. 2022, 61, 1400–1405. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.; Recarey-Rama, S.; Rodríguez-Viyuela, A.; Barros, F.; Carracedo, A.; Maroñas, O. Balance of care activity after EMA recommendation for DPYD gene testing in Galicia. Front. Pharmacol. 2025, 16, 1523536. [Google Scholar] [CrossRef] [PubMed]
- Miarons, M.; Manzaneque Gordón, A.; Riera, P.; Gutiérrez Nicolás, F.; RedDPYD Research Group with the Spanish Society of Hospital Pharmacy (SEFH). Allelic Frequency of DPYD Genetic Variants in Patients With Cancer in Spain: The PhotoDPYD Study. Oncologist 2023, 28, e304–e308. [Google Scholar] [CrossRef] [PubMed]
- Ragia, G.; Maslarinou, A.; Atzemian, N.; Biziota, E.; Koukaki, T.; Ioannou, C.; Balgkouranidou, I.; Kolios, G.; Kakolyris, S.; Xenidis, N.; et al. Implementing pharmacogenetic testing in fluoropyrimidine-treated cancer patients: DPYD genotyping to guide chemotherapy dosing in Greece. Front. Pharmacol. 2023, 14, 1248898. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Hamdane, S.; Garinet, S.; Blons, H.; Zaanan, A.; Paillaud, E.; Taieb, J.; Laprevote, O.; Loriot, M.-A.; Narjoz, C. A comprehensive population-based study comparing the phenotype and genotype in a pretherapeutic screen of dihydropyrimidine dehydrogenase deficiency. Br. J. Cancer 2020, 123, 811–818. [Google Scholar] [CrossRef]
- de Moraes, F.C.A.; de Almeida Barbosa, A.B.; Sano, V.K.T.; Kelly, F.A.; Burbano, R.M.R. Pharmacogenetics of DPYD and treatment-related mortality on fluoropyrimidine chemotherapy for cancer patients: A meta-analysis and trial sequential analysis. BMC Cancer 2024, 24, 1210. [Google Scholar] [CrossRef]
- Le Teuff, G.; Cozic, N.; Boyer, J.C.; Boige, V.; Diasio, R.B.; Taieb, J.; Meulendijks, D.; Palles, C.; Schwab, M.; Deenen, M.; et al. Dihydropyrimidine dehydrogenase gene variants for predicting grade 4–5 fluoropyrimidine-induced toxicity: FUSAFE individual patient data meta-analysis. Br. J. Cancer 2024, 130, 808–818. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher SCIENTIFIC. Applied BiosystemsTM QuantStudioTM 12K Flex Real-Time PCR System OpenArray® Experiments. Available online: https://www.thermofisher.com/content/dam/LifeTech/migration/files/pcr/pdfs.par.36605.file.dat/4470935b.pdf (accessed on 9 April 2025).
- Integrated DNA Technologies. rhAmpTM SNP Genotyping System. Available online: https://eu.idtdna.com/pages/products/qpcr-and-pcr/genotyping/rhamp-snp-genotyping (accessed on 9 April 2025).

| Number of Patients | c.1236G>A Positive | c.1236G>A Negative |
|---|---|---|
| Sex (N, %) | ||
| Male | 29 (63.04%) | 265 (57.36%) |
| Female | 17 (36.96%) | 197 (42.64%) |
| Age (mean ± SD) | 68.78 ± 10.75 | ND |
| Genotyping results (N, %): | ||
| c.1129-5923C>G * | ||
| C/G | 45 (97.83%) | 462 (100%) |
| G/G | 1 (2.17%) * | --- |
| c.1236G>A | ||
| G/A | 46 (100%) | 462 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Rodriguez, A.; Recarey-Rama, S.; Fernández Montes, A.; Rodríguez-Viyuela, A.; Barros, F.; Carracedo, A.; Maroñas, O. A Lack of Complete Linkage Disequilibrium Between c.1236G>A and c.1129-5923C>G HapB3 Variants of DPYD: A Call to Revise European Pharmacogenetic Guidelines. Int. J. Mol. Sci. 2025, 26, 8136. https://doi.org/10.3390/ijms26178136
Gil-Rodriguez A, Recarey-Rama S, Fernández Montes A, Rodríguez-Viyuela A, Barros F, Carracedo A, Maroñas O. A Lack of Complete Linkage Disequilibrium Between c.1236G>A and c.1129-5923C>G HapB3 Variants of DPYD: A Call to Revise European Pharmacogenetic Guidelines. International Journal of Molecular Sciences. 2025; 26(17):8136. https://doi.org/10.3390/ijms26178136
Chicago/Turabian StyleGil-Rodriguez, Almudena, Sheila Recarey-Rama, Ana Fernández Montes, Ana Rodríguez-Viyuela, Francisco Barros, Angel Carracedo, and Olalla Maroñas. 2025. "A Lack of Complete Linkage Disequilibrium Between c.1236G>A and c.1129-5923C>G HapB3 Variants of DPYD: A Call to Revise European Pharmacogenetic Guidelines" International Journal of Molecular Sciences 26, no. 17: 8136. https://doi.org/10.3390/ijms26178136
APA StyleGil-Rodriguez, A., Recarey-Rama, S., Fernández Montes, A., Rodríguez-Viyuela, A., Barros, F., Carracedo, A., & Maroñas, O. (2025). A Lack of Complete Linkage Disequilibrium Between c.1236G>A and c.1129-5923C>G HapB3 Variants of DPYD: A Call to Revise European Pharmacogenetic Guidelines. International Journal of Molecular Sciences, 26(17), 8136. https://doi.org/10.3390/ijms26178136






