The Significance of the Wnt/β-Catenin Pathway and Related Proteins in Gastrointestinal Malignancies
Abstract
1. Introduction
2. Methods
3. Wnt/β-Catenin Pathway: General Information
3.1. Elements of the Wnt/β-Catenin Signaling Pathway and Their Roles
3.2. The Role of Wnt/β-Catenin Signaling Pathway in Carcinogenesis
3.3. Wnt Pathway-Targeted Therapy in Gastrointestinal Cancers
4. The Role of the Wnt/β-Catenin Pathway and Its Components as Potential Biomarkers in Gastrointestinal Cancers
4.1. Gastric Cancer
4.1.1. Ligands, Receptors, and Co-Receptors
4.1.2. Intracellular Components
4.1.3. Wnt Pathway-Targeted Therapy in Gastric Cancer (GC)
4.2. Colorectal Cancer
4.2.1. Ligands, Receptors, and Co-Receptors
4.2.2. Intracellular Components
4.2.3. Wnt Pathway-Targeted Therapy in Colorectal Cancer (CRC)
4.3. Esophageal Cancer
4.3.1. Ligands, Receptors, and Co-Receptors
4.3.2. Intracellular Components
4.3.3. Wnt Pathway-Targeted Therapy in Esophageal Cancer (EC)
4.4. Liver Cancer
4.4.1. Ligands, Receptors, and Co-Receptors
4.4.2. Intracellular Components
4.4.3. Wnt Pathway-Targeted Therapy in Liver Cancer
4.5. Pancreatic Cancer
4.5.1. Ligands, Receptors, and Co-Receptors
4.5.2. Intracellular Components
4.5.3. Wnt Pathway-Targeted Therapy in Pancreatic Cancer (PC)
5. Perspectives and Clinical Implications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, Version 1.0; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.fr/today (accessed on 1 February 2024).
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- GBD Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Colorectal Cancer; Continuous Update Project Expert Report 2018; WCRF/AICR: London, UK, 2018. [Google Scholar]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G. The global burden of cancer attributable to infections in 2018. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. Lancet 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- McColl, K.E.L. What is causing the rising incidence of esophageal adenocarcinoma in the West and will it also happen in the East? J. Gastroenterol. 2019, 54, 669–673. [Google Scholar] [CrossRef]
- Sheikh, M.; Poustchi, H.; Pourshams, A.; Etemadi, A.; Islami, F.; Khoshnia, M.; Gharavi, A.; Hashemian, M.; Roshandel, G.; Khademi, H.; et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the Golestan Cohort Study. Gastroenterology 2019, 156, 1416–1427. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer; Continuous Update Project Report 2012; WCRF/AICR: London, UK, 2012. [Google Scholar]
- Song, M.; Giovannucci, E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among White adults in the United States. JAMA Oncol. 2016, 2, 1154–1161. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Schlesinger-Raab, A.; Werner, J.; Friess, H.; Hölzel, D.; Engel, J. Age and outcome in gastrointestinal cancers: A popula-tion-based evaluation of oesophageal, gastric and colorectal cancer. Visc. Med. 2017, 33, 245–253. [Google Scholar] [CrossRef]
- Widlak, M.M.; Thomas, C.L.; Thomas, M.G.; Tomkins, C.; Smith, S.; O’Connell, N.; Wurie, S.; Burns, L.; Harmston, C.; Evans, C.; et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment. Pharmacol. Ther. 2017, 45, 354–363. [Google Scholar] [CrossRef]
- Niehrs, C. The complex world of Wnt receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Wu, X.; Que, H.; Li, Q.; Wei, X. Wnt/β-catenin mediated signaling pathways in cancer: Recent advances, and applications in cancer therapy. Mol. Cancer 2025, 24, 171. [Google Scholar] [CrossRef]
- Perugorria, M.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Mutze, K.; Baarsma, H.A.; Bracke, K.R.; Alsafadi, H.A.; Lehmann, M.; Costa, R.; Stornaiuolo, M.; Novellino, E.; Brusselle, G.G.; et al. Reduced frizzled receptor 4 expression prevents Wnt/β-catenin-driven alveolar lung repair in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017, 196, 172–185. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Cruciat, C.; Niehrs, C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013, 5, a015081. [Google Scholar] [CrossRef]
- Ring, L.; Neth, P.; Weber, C.; Steffens, S.; Faussner, A. β-Catenin-dependent pathway activation by both promiscuous “canonical” Wnt3a-, and specific “noncanonical” Wnt4- and Wnt5a-FZD receptor combinations with strong differences in LRP5 and LRP6 dependency. Cell Signal. 2014, 26, 260–267. [Google Scholar] [CrossRef]
- Foord, S.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.-P.; Davenport, A.P.; Spedding, M.P.; Harmar, A.J. International Union of Pharmacology. XLVI. G. protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef]
- Forget, M.A.; Turcotte, S.; Beauseigle, D.; Godin-Ethier, J.; Pelletier, S.; Martin, J.; Tanguay, S.; Lapointe, R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br. J. Cancer. 2007, 96, 646–653. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Z.; Yu, Y.; Chu, H.Y.; Yu, S.; Yao, S.; Zhang, G.; Zhang, B.T. Drug Discovery of DKK1 Inhibitors. Front Pharmacol. 2022, 13, 847387. [Google Scholar] [CrossRef]
- Tan, S.; Yi, P.; Wang, H.; Xia, L.; Han, Y.; Wang, H.; Zeng, B.; Tang, L.; Pan, Q.; Tian, Y.; et al. RAC1 Involves in the Radioresistance by Mediating Epithelial-Mesenchymal Transition in Lung Cancer. Front. Oncol. 2020, 10, 649. [Google Scholar]
- Poggi, L.; Casarosa, S.; Carl, M. An Eye on the Wnt Inhibitory Factor Wif1. Front Cell Dev Biol. 2018, 6, 167. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, H.; Yang, B.; Li, L.; Shi, Q.; Jiang, C.; Liu, H. WIF-1 gene inhibition and Wnt signal transduction pathway activation in NSCLC tumorigenesis. Oncol. Lett. 2017, 13, 1183–1188. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Wnt signaling in cancer: From biomarkers to targeted therapies and clinical translation. Mol. Cancer 2025, 24, 107. [Google Scholar] [CrossRef]
- Gammons, M.; Renko, M.; Johnson, C.; Rutherford, T.; Bienz, M. Wnt signalosome assembly by DEP domain swapping of dishevelled. Mol. Cell 2016, 64, 92–104. [Google Scholar] [CrossRef]
- Niehrs, C.; Shen, J. Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 2010, 67, 2551–2562. [Google Scholar] [CrossRef]
- Jiang, X.; Charlat, O.; Zamponi, R.; Yang, Y.; Cong, F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol. Cell 2015, 58, 522–533. [Google Scholar] [CrossRef]
- Bilic, J.; Huang, Y.; Davidson, G.; Zimmermann, T.; Cruciat, C.R.; Bienz, M.; Niehrs, C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007, 316, 1619–1622. [Google Scholar] [CrossRef]
- Gao, C.; Chen, Y. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef]
- Saito-Diaz, K.; Benchabane, H.; Tiwari, A.; Tian, A.; Li, B.; Thompson, J.J.; Hyde, A.S.; Sawyer, L.M.; Jodoin, J.N.; Santos, E.; et al. APC inhibits ligand-independent Wnt signaling by the clathrin endocytic pathway. Dev. Cell 2018, 44, 566–581.e8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Simon, N.; Steffen, U.; Andes, F.T.; Scholtysek, C.; Müller, D.I.H.; Weidner, D.; Andreev, D.; Kleyer, A.; Culemann, S.; et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci. Transl. Med. 2020, 12, eaay4447. [Google Scholar] [CrossRef]
- Schatoff, E.; Goswami, S.; Zafra, M.P.; Foronda, M.; Shusterman, M.; Leach, B.I.; Katti, A.; Diaz, B.J.; Dow, L.E. Distinct colorectal cancer-associated APC mutations dictate response to tankyrase inhibition. Cancer Discov. 2019, 9, 1358–1371. [Google Scholar] [CrossRef]
- Abitbol, S.; Dahmani, R.; Coulouarn, C.; Ragazzon, B.; Mlecnik, B.; Senni, N.; Savall, M.; Bossard, P.; Sohier, P.; Drouet, V.; et al. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J. Hepatol. 2018, 68, 1203–1213. [Google Scholar] [CrossRef]
- Yardy, G. Mutations in the AXIN1 gene in advanced prostate cancer. Eur. Urol. 2009, 56, 486–494. [Google Scholar] [CrossRef]
- Guezguez, B.; Almakadi, M.; Benoit, Y.D.; Shapovalova, Z.; Rahmig, S.; Fiebig-Comyn, A.; Casado, F.L.; Tanasijevic, B.; Bresolin, S.; Masetti, R.; et al. GSK3 deficiencies in hematopoietic stem cells initiate pre-neoplastic state that is predictive of clinical outcomes of human acute leukemia. Cancer Cell 2016, 29, 61–74. [Google Scholar] [CrossRef]
- Steinhausen, E.; Lefering, R.; Tjardes, T.; Neugebauer, E.A.; Bouillon, B.; Rixen, D. A risk-adapted approach is beneficial in the management of bilateral femoral shaft fractures in multiple trauma patients: An analysis based on the trauma registry of the German Trauma Society. J. Trauma. Acute Care Surg. 2014, 76, 1288–1293. [Google Scholar] [CrossRef]
- Hankey, W.; Frankel, W.L.; Groden, J. Functions of the APC tumor suppressor protein dependent and independent of canonical Wnt signaling: Implications for therapeutic targeting. Cancer Metastasis Rev. 2018, 37, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Thomas, D.; Emmons, A.; Giordano, T.; Kleer, C. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin. Cancer Res. 2008, 14, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.; Groden, J. Biology of the adenomatous polyposis coli tumor suppressor. J. Clin. Oncol. 2000, 18, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457. [Google Scholar] [CrossRef]
- Yan, R.; Fan, X.; Xiao, Z.; Liu, H.; Huang, X.; Liu, J.; Zhang, S.; Yao, J.; An, G.; Ge, Y.; et al. Inhibition of DCLK1 sensitizes resistant lung adenocarcinomas to EGFR-TKI through suppression of Wnt/β-catenin activity and cancer stemness. Cancer Lett. 2022, 531, 83–97. [Google Scholar] [CrossRef]
- Wend, P.; Wend, K.; Krum, S.A.; Miranda-Carboni, G.A. The role of Wnt10B in physiology and disease. Acta Physiol. 2012, 204, 34–51. [Google Scholar] [CrossRef]
- Lindsey, S.; Langhans, S.A. Crosstalk of Oncogenic Signaling Pathways during Epithelial-Mesenchymal Transition. Front. Oncol. 2014, 4, 358. [Google Scholar] [CrossRef]
- Fodde, R.; Brabletz, T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007, 19, 150–158. [Google Scholar] [CrossRef]
- Wang, S.T.; Yuen, J.; Xu, S.P.; Lee, H.H.; Yan, S.T.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef]
- Atlasi, Y.; Looijenga, L.; Fodde, R. Cancer stem cells, pluripotency, and cellular heterogeneity: A Wnter perspective. Curr. Top. Dev. Biol. 2014, 107, 373–404. [Google Scholar]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jun, Y.; Kim, J.; Nam, J. Roles of Wnt Target Genes in the Journey of Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1604. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Karasawa, H.; Funayama, R.; Shirota, M.; Suzuki, T.; Maeda, S.; Suzuki, H.; Yamamura, A.; Naitoh, T.; Nakayama, K.; et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019, 8, 6370–6382. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Yeung, V.; Cattaruzza, F.; Hussein, R.; Yen, W.; Murriel, C.; Evans, J.W.; O’Young, G.; Brunner, A.L.; Wang, M.; et al. RSPO3 antagonism inhibits growth and tumorigenicity in colorectal tumors harboring common Wnt pathway mutations. Sci. Rep. 2017, 7, 15270. [Google Scholar] [CrossRef]
- Muñoz-Galván, S.; Lucena-Cacace, A.; Perez, M.; Otero-Albiol, D.; Gomez-Cambronero, J.; Carnero, A. Tumor cell-secreted PLD increases tumor stemness by senescence-mediated communication with microenvironment. Oncogene 2019, 38, 1309–1323. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Wang, J.; Si, T.; Xing, W. Tumor associated macrophages derived TGF-β promotes colorectal cancer progression through HIF1-TRIB3 signaling. Cancer Sci. 2021, 112, 4198–4207. [Google Scholar] [CrossRef]
- Patel, S.; Alam, A.; Pant, R.; Chattopadhyay, S. Wnt Signaling and Its Significancer Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019, 10, 2872. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Ma, J.; Sun, X.; Wang, Y.; Yan, J.; Yan, J.; Yu, Q.; Diao, J.; Yang, C.; et al. Fibrinogen improves liver function via promoting cell aggregation and fibronectin assembly in hepatic spheroids. Biomaterials 2022, 280, 121266. [Google Scholar] [CrossRef]
- Dholakia, J.; Scalise, C.; Katre, A.; Goldsberry, W.; Meza-Perez, S.; Randall, T.; Norian, L.A.; Novak, L.; Arend, R.C. Sequential modulation of the Wnt/β-catenin signaling pathway enhances tumor-intrinsic MHC I expression and tumor clearance. Gynecol. Oncol. 2022, 164, 170–180. [Google Scholar] [CrossRef]
- Begenik, H.; Kemik, A.S.; Emre, H.; Dulger, A.C.; Demirkiran, D.; Ebinc, S.; Kemik, O. The Association between Serum Dickkopf-1 Levels and Esophageal Squamous Cell Carcinoma. Hum. Exp. Toxicol. 2014, 33, 785–788. [Google Scholar] [CrossRef]
- Han, S.X.; Zhou, X.; Sui, X.; He, C.C.; Cai, M.J.; Ma, J.L.; Zhang, Y.Y.; Zhou, C.Y.; Ma, C.X.; Varela-Ramirez, A.; et al. Serum Dickkopf-1 Is a Novel Serological Biomarker for the Diagnosis and Prognosis of Pancreatic Cancer. Oncotarget 2015, 6, 19907–19917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Li, Y.F.; Deng, Z.Q.; and Cao, J.Q. Prognostic Significance of Dickkopf-1 in Gastric Cancer Survival: A Meta-Analysis. Genet. Test. Mol. Biomarkers 2016, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, B.; Qi, L.; Li, Y.; Zhao, X.; Zhang, D.; Zhang, D.; Zhang, Y. Dickkopf-1 Expression Is Down-Regulated during the Colorectal Adenoma-Carcinoma Sequence and Correlates with Reduced Microvessel Density and VEGF Expression. Histopathology 2015, 67, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kwon, Y.J.; Seo, W.Y.; Kim, U.; Ahn, S.; Choi, S.M.; Bang, H.T.; Kim, K.; Kim, J.S. Tankyrase-selective inhibitor STP1002 shows preclinical antitumour efficacy without on-target toxicity in the gastrointestinal tract. Eur. J. Cancer 2022, 173, 41–51. [Google Scholar] [CrossRef]
- Yu, W.-K.; Xu, Z.-Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.-D.; Qin, J.-J. rgeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef]
- Dvory-Sobol, H.; Sagiv, E.; Liberman, E.; Kazanov, D.; Arber, N. Suppression of gastric cancer cell growth by targeting the beta-catenin/T-cell factor pathway. Cancer 2007, 109, 188–197. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Ranjan, A.; Kim, S.H.; Srivastava, S.K. Inhibition of beta-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear beta-catenin/TCF-1 complex: Critical role of STAT-3. Oncotarget 2015, 6, 11561–11574. [Google Scholar] [CrossRef]
- Peshin, S.; Bashir, F.; Kodali, N.A.; Dharia, A.; Zaiter, S.; Singal, S.; Moka, N. Immunotherapy in GI Cancers: Lessons from Key Trials and Future Clinical Applications. Antibodies 2025, 14, 58. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Li, M.; Zhang, Y.; Zhang, X.; Zhang, X.; Xu, Y.; Wei, W.; Wang, J.; Xu, X.; et al. C644-0303, a small-molecule inhibitor of the Wnt/β-catenin pathway, suppresses colorectal cancer growth. Cancer Sci. 2021, 112, 4722–4735. [Google Scholar] [CrossRef]
- Kim, M.J.; Huang, Y.; Park, J. Targeting Wnt Signaling for Gastrointestinal Cancer Therapy: Present and Evolving Views. Cancers 2020, 12, 3638. [Google Scholar] [CrossRef]
- Nayak, A.; Streiff, H.; Gonzalez, I.; Adekoya, O.O.; Silva, I.; Shenoy, A.K. Wnt Pathway-Targeted Therapy in Gastrointestinal Cancers: Integrating Benchside Insights with Bedside Applications. Cells 2025, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Byun, T.; Karimi, M.; Marsh, J.L.; Milovanovic, T.; Lin, F.; Holcombe, R.F. Expression of secreted Wnt antagonists in gastro-intestinal tissues: Potential role in stem cell homeostasis. J. Clin. Pathol. 2005, 58, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Ishimoto, T.; Oshima, H.; Oshima, M.; Kai, K.; Torii, R.; Masuko, T.; Baba, H.; Saya, H.; Nagano, O. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010, 101, 673–678. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol. Med. Rep. 2012, 5, 1191–1196. [Google Scholar]
- Mao, J.; Fan, S.; Ma, W.; Fan, P.; Wang, B.; Zhang, J.; Wang, H.; Tang, B.; Zhang, Q.; Yu, X.; et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014, 5, e1039. [Google Scholar] [CrossRef]
- Torres, V.I.; Godoy, J.A.; Inestrosa, N.C. Modulating Wnt signaling at the root: Porcupine and Wnt acylation. Pharmacol. Ther. 2019, 198, 34–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Dong, X. Wnt2 contributes to the progression of gastric cancer by promoting cell migration and invasion. Oncol. Lett. 2018, 16, 2857–2864. [Google Scholar] [CrossRef]
- Wang, H.S.; Nie, X.; Wu, R.B.; Yuan, H.W.; Ma, Y.H.; Liu, X.L.; Zhang, J.Y.; Deng, X.L.; Na, Q.; Jin, H.Y.; et al. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Onco. Targets Ther. 2016, 9, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Regel, I.; Lian, F.; Friedrich, T.; Hitkova, I.; Hofheinz, R.D.; Ströbel, P.; Langer, R.; Keller, G.; Röcken, C.; et al. Wnt6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene 2013, 32, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Chung, J.W.; Yang, J.Y. Wnt5A correlates with clinicopathological characteristics in gastric cancer: A meta-analysis. Cell. Physiol. Biochem. 2017, 41, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kurayoshi, M.; Oue, N.; Yamamoto, H.; Kishida, M.; Inoue, A.; Asahara, T.; Yasui, W.; Kikuchi, A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006, 66, 10439–10448. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, L.; Zhu, Y.; Ke, X.; Wang, Q.; Min, H. Regulation of proliferation and epithelial-to-mesenchymal transition (EMT) of gastric cancer by ZEB1 via modulating Wnt5A and related mechanisms. Med. Sci. Monit. 2019, 25, 1663–1670. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Mai, W.; Xiao, Y.; You, X.; Qin, L. FZD8 indicates a poor prognosis and promotes gastric cancer invasion and metastasis via β-catenin signaling pathway. Ann. Clin. Lab. Sci. 2020, 50, 13–23. [Google Scholar]
- Flanagan, D.J.; Barker, N.; Costanzo, N.S.D.; Mason, E.A.; Gurney, A.; Meniel, V.S.; Koushyar, S.; Austin, C.R.; Ernst, M.; Pearson, H.B.; et al. Frizzled-7 Is Required for Wnt Signaling in Gastric Tumors with and Without Apc Mutations. Cancer Res. 2019, 79, 970–981. [Google Scholar] [CrossRef]
- Dong, D.; Na, L.; Zhou, K.; Wang, Z.; Sun, Y.; Zheng, Q.; Zheng, Q.; Gao, J.; Zhao, C.; Wang, W. FZD5 prevents epithelial-mesenchymal transition in gastric cancer. Cell Commun. Signal. 2021, 19, 21. [Google Scholar] [CrossRef]
- Yan, J.; Liu, T.; Zhou, X.; Dang, Y.; Yin, C.; Zhang, G. FZD6, targeted by miR-21, represses gastric cancer cell proliferation and migration via activating non-canonical Wnt pathway. Am. J. Transl. Res. 2016, 8, 2354–2364. [Google Scholar]
- Scavo, M.P.; Rizzi, F.; Depalo, N.; Armentano, R.; Coletta, S.; Serino, G.; Fanizza, E.; Pesole, P.L.; Cervellera, A.; Carella, N.; et al. Exosome released FZD10 increases Ki-67 expression via phospho-ERK1/2 in colorectal and gastric cancer. Front. Oncol. 2021, 11, 730093. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Wei, X.; Li, L.; Xue, T.; Fan, L.; Fan, L.; Ma, H.; Xia, Y.; Wang, Y.D.; et al. LRP5 promotes gastric cancer via activating canonical Wnt/β-catenin and glycolysis pathways. Am. J. Pathol. 2022, 192, 503–517. [Google Scholar] [CrossRef]
- Gong, Y.; Bourhis, E.; Chiu, C.; Stawicki, S.; DeAlmeida, V.I.; Liu, B.Y.; Phamluong, K.; Cao, T.C.; Carano, R.A.; Ernst, J.A.; et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS ONE 2010, 5, e12682. [Google Scholar] [CrossRef]
- Zheng, J.; Li, C.; Zhu, Z.; Yang, F.; Wang, X.; Jiang, P.; Yan, F. A 5′-tRNA derived fragment named tiRNA-Val-CAC-001 works as a suppressor in gastric cancer. Cancer Manag. Res. 2022, 14, 2323–2337. [Google Scholar] [CrossRef]
- Li, L.F.; Wei, Z.J.; Sun, H.; Jiang, B. Abnormal β-catenin immunohistochemical expression as a prognostic factor in gastric cancer: A meta-analysis. World J. Gastroenterol. 2014, 20, 12313–12321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ye, G.; Peng, J.; He, B.; Xu, S.; Fan, W.; Fan, W.; Wang, W. Expression of Wnt3, β-catenin and MMP-7 in gastric cancer and pre-cancerous lesions and their correlations with Helicobacter pylori infection. Zhong Nan Da Xue Bao Yi Xue Ban 2021, 46, 575–582. [Google Scholar]
- Deng, S.; Zhang, X.; Qin, Y.; Chen, W.; Fan, H.; Feng, X.; Wang, J.; Yan, R.; Zhao, Y.; Cheng, Y.; et al. miRNA-192 and -215 activate Wnt/β-catenin signaling pathway in gastric cancer via APC. J. Cell Physiol. 2020, 235, 6218–6229. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Kim, J.H.; Lee, H.E.; Lee, H.S.; Cho, S.J.; Kim, W.H.; Lee, B.L. GSK-3β is a prognostic biomarker in human gastric carcinoma. Cancer Res. 2008, 68, 4093. [Google Scholar]
- Chu, H.Y.; Chen, Z.; Wang, L.; Zhang, Z.K.; Tan, X.; Liu, S.; Zhang, B.T.; Lu, A.; Yu, Y.; Zhang, G. Dickkopf-1: A Promising Target for Cancer Immunotherapy. Front. Immunol. 2021, 12, 658097. [Google Scholar] [CrossRef]
- Wang, J.; Lu, R.; Fu, X.; Dan, Z.; Zhang, Y.G.; Chang, X.; Liu, Q.; Xia, Y.; Liu, X.; Sun, J. Novel regulatory roles of Wnt1 in infection-associated colorectal cancer. Neoplasia 2018, 20, 499–509. [Google Scholar] [CrossRef]
- Jung, Y.S.; Jun, S.; Lee, S.H.; Sharma, A.; Park, J.I. Wnt2 complements Wnt/β-catenin signaling in colorectal cancer. Oncotarget 2015, 6, 37257–37268. [Google Scholar] [CrossRef]
- Qi, L.; Sun, B.; Liu, Z.; Cheng, R.; Li, Y.; Zhao, X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J. Exp. Clin. Cancer Res. 2014, 33, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Q.; Shang, R.; Yao, L.; Wu, L.; Zhang, M.; Zhang, L.; Xu, M.; Lu, Z.; Zhou, J.; et al. Wnt4 secreted by tumor tissues promotes tumor progression in colorectal cancer by activation of the Wnt/β-catenin signalling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Sun, B.; Liu, Z.; Zhao, X.; Qi, L.; Li, Y.; Gu, Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J. Cell Physiol. 2014, 229, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Yoo, H.I.; Kim, I.; Park, J.; Kim, Y.S. FZD6 expression is negatively regulated by miR-199a-5p in human colorectal cancer. BMB Rep. 2015, 48, 360–366. [Google Scholar] [CrossRef]
- Vincan, E.; Flanagan, D.J.; Pouliot, N.; Brabletz, T.; Spaderna, S. Variable FZD7 expression in colorectal cancers indicates regulation by the tumour microenvironment. Dev. Dyn. 2010, 239, 311–317. [Google Scholar] [CrossRef]
- Yao, Q.; An, Y.; Hou, W.; Cao, Y.N.; Yao, M.F.; Ma, N.N.; Hou, L.; Zhang, H.; Liu, H.J.; Zhang, B. LRP6 promotes invasion and metastasis of colorectal cancer through cytoskeleton dynamics. Oncotarget 2017, 8, 109632–109645, Erratum in Oncotarget 2020, 11, 3102. [Google Scholar] [CrossRef]
- Bruun, J.; Kolberg, M.; Nesland, J.M.; Svindland, A.; Nesbakken, A.; Lothe, R.A. Prognostic significance of β-catenin, E-cadherin, and SOX9 in colorectal cancer: Results from a large population-representative series. Front. Oncol. 2014, 4, 118. [Google Scholar] [CrossRef]
- Smith, J.J.; Deane, N.G.; Dhawan, P.; Beauchamp, R.D. Regulation of metastasis in colorectal adenocarcinoma: A collision between development and tumor biology. Surgery 2008, 144, 353–366. [Google Scholar] [CrossRef]
- Veloudis, G.; Pappas, A.; Gourgiotis, S.; Falidas, E.; Dimitriou, N.; Karavokiros, I.; Aggelou, A.; Komborozos, V.; Petraki, C.; Menounos, P.; et al. Assessing the clinical utility of Wnt pathway markers in colorectal cancer. J. BUON 2017, 22, 431–436. [Google Scholar]
- Kim, S.; Jeong, S. Mutation hotspots in the β-catenin gene: Lessons from the human cancer genome databases. Mol. Cells 2019, 42, 8–16. [Google Scholar]
- Parker, T.M.; Neufeld, K.L. APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes. Sci. Rep. 2020, 10, 2957. [Google Scholar] [CrossRef]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Domoto, T.; Pyko, I.V.; Furuta, T.; Miyashita, K.; Uehara, M.; Shimasaki, T.; Nakada, M.; Minamoto, T. Glycogen synthase kinase-3β is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016, 107, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Kretz, A.L.; Lemke, J.; Fauler, M.; Werner, J.U.; Paschke, S.; Leithäuser, F.; Henne-Bruns, D.; Hillenbrand, A.; Knippschild, U. CK1α overexpression correlates with poor survival in colorectal cancer. BMC Cancer 2018, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, Q.; Mao, Y.; Jiang, X.; Chen, X.; Liu, P.; Lv, B.; Huang, T.; Yang, J.; Cheng, Y.; et al. Wnt2-mediated FZD2 stabilization regulates esophageal cancer metastasis via STAT3 signaling. Front. Oncol. 2020, 10, 1168. [Google Scholar] [CrossRef]
- Oguma, J.; Ozawa, S.; Kazuno, A.; Nitta, M.; Ninomiya, Y.; Kajiwara, H. Wnt3a expression is associated with poor prognosis of esophageal squamous cell carcinoma. Oncol. Lett. 2018, 15, 3100–3108. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, Z.; Pan, M.; Xu, L.; Feng, J.; Zhang, Y.; Shao, C.; Guo, K.; Duan, H.; Zhang, Y.; et al. Wnt5A promotes the metastasis of esophageal squamous cell carcinoma by activating the HDAC7/SNAIL signaling pathway. Cell Death Dis. 2022, 13, 480. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, G.; Fang, Y.; Qiu, M.; Lin, J.; Sun, J.; Yang, D. Increased Wnt6 expression in tumor cells predicts unfavorable survival in esophageal squamous cell carcinoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 11421–11427. [Google Scholar]
- Cao, T.T.; Xiang, D.; Liu, B.L.; Huang, T.X.; Tan, B.B.; Zeng, C.M.; Wang, Z.Y.; Ming, X.Y.; Zhang, L.Y.; Jin, G.; et al. FZD7 is a novel prognostic marker and promotes tumor metastasis via Wnt and EMT signaling pathways in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 65957–65968. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.L.; Zhang, C.Y.; Ma, Y.F.; Zhao, R.; Wang, Y.Y. The prognostic role of FZD6 in esophageal squamous cell carcinoma patients. Clin. Transl. Oncol. 2020, 22, 1172–1179. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, X.; Jiang, Y.; Wei, Z.; Wang, P.; Chen, F.; Li, X.; Sun, C.; Zhao, H.; Zeng, X.; et al. LRP6 is identified as a potential prognostic marker for oral squamous cell carcinoma via MALDI-IMS. Cell Death Dis. 2017, 8, e3035. [Google Scholar] [CrossRef] [PubMed]
- Li, A.F.; Hsu, P.K.; Tzao, C.; Wang, Y.C.; Hung, I.C.; Huang, M.H.; Hsu, H.S. Reduced axin protein expression is associated with a poor prognosis in patients with squamous cell carcinoma of esophagus. Ann. Surg. Oncol. 2009, 16, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Kudo, J.; Nishiwaki, T.; Haruki, N.; Ishiguro, H.; Shibata, Y.; Terashita, Y.; Sugiura, H.; Shinoda, N.; Kimura, M.; Kuwabara, Y.; et al. Aberrant nuclear localization of beta-catenin without genetic alterations in beta-catenin or Axin genes in esophageal cancer. World J. Surg. Oncol. 2007, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Fukuchi, M.; Miyazaki, T.; Masuda, N.; Kato, H.; Kuwano, H. Reduced expression of Axin correlates with tumour progression of oesophageal squamous cell carcinoma. Br. J. Cancer 2003, 88, 1734–1739. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, X.Y.; Liu, K.P.; Li, S.M. Expression and significance of adenomatous polyposis coli, beta-catenin, E-cadherin and cyclin D1 in esophageal squamous cell carcinoma assessed by tissue microarray. Ai Zheng 2009, 28, 38–41. [Google Scholar]
- Fang, J.; Jasperson, K. Germline APC Mutation and Familial Barrett Esophagus: Causal or Coincidence? Gastroenterol. Hepatol. 2011, 7, 342–344. [Google Scholar]
- Wlodarczyk, J.; Rudnicka-Sosin, L.; Kużdżał, J. Expression of β-catenin and its correlation with metastatic progression of esophagogastric junction adenocarcinoma. Pol. J. Pathol. 2015, 66, 414–419. [Google Scholar] [CrossRef]
- Ramirez, J.G.; Smit, D.J.; Viol, F.; Schrader, J.; Ghadban, T.; Pantel, K.; Izbicki, J.R.; Reeh, M. High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients. Cancers 2021, 13, 4980. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Donnelly, L.; Elghobashi-Meinhardt, N.; Long, T.; Zhou, R.W.; Sun, Y.; Wang, B.; Li, X. Mechanisms and inhibition of Porcupine-mediated Wnt acylation. Nature 2022, 607, 816–822. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Woodcock, S.A.; Phillips, C.; Eagle, C.; Sansom, O.J. Targeting ligand-dependent Wnt pathway dysregulation in gastrointestinal cancers through porcupine inhibition. Pharmacol. Ther. 2022, 238, 108179. [Google Scholar] [CrossRef]
- Spitzner, M.; Emons, G.; Schütz, K.B.; Wolff, H.A.; Rieken, S.; Ghadimi, B.M.; Schneider, G.; Grade, M. Inhibition of Wnt/β-Catenin Signaling Sensitizes Esophageal Cancer Cells to Chemoradiotherapy. Int. J. Mol. Sci. 2021, 22, 10301. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 study of single-agent Wnt974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Yao, M.; Cai, Y.; Gu, J.J.; Yang, X.L.; Wang, L.; Yao, D.F. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Uen, Y.H.; Tian, Y.F.; Sun, C.S.; Sheu, M.J.; Kuo, H.T.; Koay, L.B.; Lin, C.Y.; Tzeng, C.C.; Cheng, C.J.; et al. Wnt-1 protein as a prognostic biomarker for hepatitis B-related and hepatitis C-related hepatocellular carcinoma after surgery. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1562–1569. [Google Scholar] [CrossRef]
- Bengochea, A.; de Souza, M.M.; Lefrancois, L.; Le Roux, E.; Galy, O.; Chemin, I.; Kim, M.; Wands, J.R.; Trepo, C.; Hainaut, P.; et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br. J. Cancer 2008, 99, 143–150. [Google Scholar] [CrossRef]
- Desert, R.; Mebarki, S.; Desille, M.; Sicard, M.; Lavergne, E.; Renaud, S.; Bergeat, D.; Sulpice, L.; Perret, C.; Turlin, B.; et al. “Fibrous nests” in human hepatocellular carcinoma express a Wnt-induced gene signature associated with poor clinical outcome. Int. J. Biochem. Cell Biol. 2016, 81, 195–207. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.C.; Tsedensodnom, O.; Hartley, R.; Lim, Y.S.; Yu, E.; Merle, P.; Wands, J.R. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J. Hepatol. 2008, 48, 780–791. [Google Scholar] [CrossRef]
- Tung, E.K.-K.; Wong, B.Y.-C.; Yau, T.-O.; Ng, I.O.-L. Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion. PLoS ONE 2012, 7, e36565. [Google Scholar] [CrossRef]
- Tien, L.T.; Ito, M.; Nakao, M.; Niino, D.; Serik, M.; Nakashima, M.; Wen, C.Y.; Yatsuhashi, H.; Ishibashi, H. Expression of beta-catenin in hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 2398–2401. [Google Scholar] [CrossRef]
- Monga, S.P. β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef]
- Waisberg, J.; Saba, G.T. Wnt/-β-catenin pathway signaling in human hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2631–2635. [Google Scholar] [CrossRef]
- Taniguchi, K.; Roberts, L.R.; Aderca, I.N.; Dong, X.; Qian, C.; Murphy, L.M.; Nagorney, D.M.; Burgart, L.J.; Roche, P.C.; Smith, D.I.; et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002, 21, 4863–4871. [Google Scholar] [CrossRef]
- Ban, K.C.; Singh, H.; Krishnan, R.; Seow, H.F. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003, 199, 201–208. [Google Scholar] [CrossRef]
- Gabata, R.; Harada, K.; Mizutani, Y.; Ouchi, H.; Yoshimura, K.; Sato, Y.; Kitao, A.; Kimura, K.; Kouji, H.; Miyashita, T.; et al. Anti-tumor Activity of the Small Molecule Inhibitor PRI-724 Against β-Catenin-activated Hepatocellular Carcinoma. Anticancer Res. 2020, 40, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Rainusso, N.; Lee, Y.-C.; Dawson, B.; Coarfa, C.; Han, R.; Larson, J.L.; Shuck, R.; Kurenbekova, L.; Yustein, J.T. Tegavivint and the β-Catenin/ALDH Axis in Chemotherapy-Resistant and Metastatic Osteosarcoma. J. Natl. Cancer Inst. 2019, 111, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Q.; He, C.; Li, F.; Sheng, H.; Shen, X.; Zhang, X.; Zhu, S.; Chen, H.; Chen, X.; et al. Activation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancer. Am. J. Cancer Res. 2014, 4, 537–544. [Google Scholar] [PubMed]
- Nan, J.N.; Kim, O.R.; Lee, M.A. β-Catenin expression is associated with cell invasiveness in pancreatic cancer. Korean J. Intern. Med. 2019, 34, 618–625. [Google Scholar] [CrossRef]
- Bo, H.; Zhang, S.; Gao, L.; Chen, Y.; Zhang, J.; Chang, X.; Zhu, M. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer 2013, 13, 496. [Google Scholar] [CrossRef]
- Wu, D.J.; Jiang, Y.S.; He, R.Z.; Tao, L.Y.; Yang, M.W.; Fu, X.L.; Yang, J.Y.; Zhu, K. High expression of Wnt7A predicts poor prognosis and promotes tumor metastasis in pancreatic ductal adenocarcinoma. Sci. Rep. 2018, 8, 15792. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Zhang, Y. Expression and prognostic impact of FZDs in pancreatic adenocarcinoma. BMC Gastroenterol. 2021, 21, 79. [Google Scholar] [CrossRef]
- Zeng, G.; Germinaro, M.; Micsenyi, A.; Monga, N.K.; Bell, A.; Sood, A.; Malhotra, V.; Sood, N.; Midda, V.; Monga, D.K.; et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia 2006, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, K.; Hagström, J.; Mustonen, H.; Juuti, A.; Nordling, S.; Kallio, P.; Alitalo, K.; Seppänen, H.; Haglund, C. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer 2016, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, T.; Ishigaki, Y.; Nakamura, Y.; Takata, T.; Nakaya, N.; Nakajima, H.; Sato, I.; Zhao, X.; Kitano, A.; Kawakami, K.; et al. Glycogen synthase kinase 3β inhibition sensitizes pancreatic cancer cells to gemcitabine. J. Gastroenterol. 2012, 47, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.L.; Akula, S.M.; Meher, A.K.; Steelman, L.S.; Gizak, A.; Duda, P.; Rakus, D.; Martelli, A.M.; Ratti, S.; Cocco, L.; et al. GSK-3β Can Regulate the Sensitivity of MIA-PaCa-2 Pancreatic and MCF-7 Breast Cancer Cells to Chemotherapeutic Drugs, Targeted Therapeutics and Nutraceuticals. Cells 2021, 10, 816. [Google Scholar] [CrossRef]
- Pecoraro, C.; Faggion, B.; Balboni, B.; Carbone, D.; Peters, G.J.; Diana, P.; Assaraf, Y.G.; Giovannetti, E. GSK3β as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist. Updates 2021, 58, 100779. [Google Scholar] [CrossRef]
- Ding, L.; Madamsetty, V.S.; Kiers, S.; Alekhina, O.; Ugolkov, A.; Dube, J.; Zhang, Y.; Zhang, J.-S.; Wang, E.; Dutta, S.K.; et al. Glycogen Synthase Kinase-3 Inhibition Sensitizes Pancreatic Cancer Cells to Chemotherapy by Abrogating the TopBP1/ATR-Mediated DNA Damage Response. Clin. Cancer Res. 2019, 25, 6452–6462. [Google Scholar] [CrossRef]
- Santoro, R.; Zanotto, M.; Simionato, F.; Zecchetto, C.; Merz, V.; Cavallini, C.; Piro, G.; Sabbadini, F.; Boschi, F.; Scarpa, A.; et al. Modulating TAK1 expression inhibits YAP and TAZ oncogenic functions in pancreatic cancer. Mol. Cancer Ther. 2020, 19, 247–257. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Giannakis, M.; Le, D.T.; Pishvaian, M.J.; Weinberg, B.A.; Papadopoulos, K.P.; Shen, L.; Gong, J.; Li, J.; Strickler, J.H.; Zhou, A.; et al. Phase 1 study of Wnt pathway Porcupine inhibitor CGX1321 and phase 1b study of CGX1321 + pembrolizumab (pembro) in patients (pts) with advanced gastrointestinal (GI) tumors. J. Clin. Oncol. 2023, 41, 3514. [Google Scholar] [CrossRef]
- Sferrazza, G.; Corti, M.; Brusotti, G.; Pierimarchi, P.; Temporini, C.; Serafino, A.; Calleri, E. Nature-derived compounds modulating Wnt/β-catenin pathway: A preventive and therapeutic opportunity in neoplastic diseases. Acta Pharm. Sin. B 2020, 10, 1814–1834. [Google Scholar] [CrossRef]
- Yan, Y.; Kumar, A.B.; Finnes, H.; Markovic, S.N.; Park, S.; Dronca, R.S.; Don, H. Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front. Immunol. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, J.; Deek, M.P.; Eskander, M.F.; Gulhati, P.; Kennedy, T.; Shah, M.M.; Grandhi, M.S.; Berim, L.; Spencer, K.R.; et al. Combination of Immunotherapy and Radiation Therapy in Gastrointestinal Cancers: An Appraisal of the Current Literature and Ongoing Research. Curr. Oncol. 2023, 30, 6432–6446. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2021, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Murkute, S.L.; Bhowmik, S.; Prasad, C.P.; Mohapatra, P. Belling the “cat”: Wnt/β-catenin signaling and its significance in future cancer therapies. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer. 2024, 1879, 189195. [Google Scholar] [CrossRef]
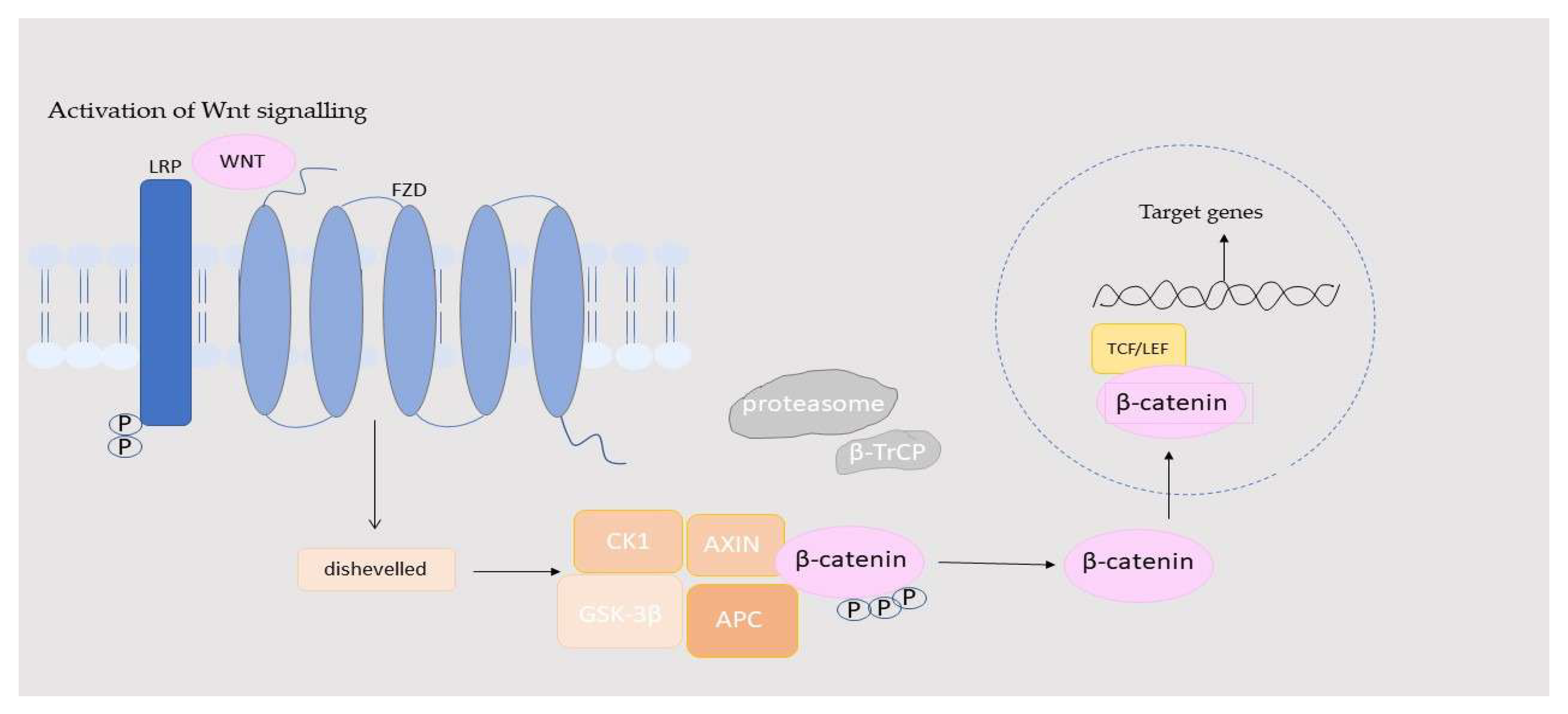
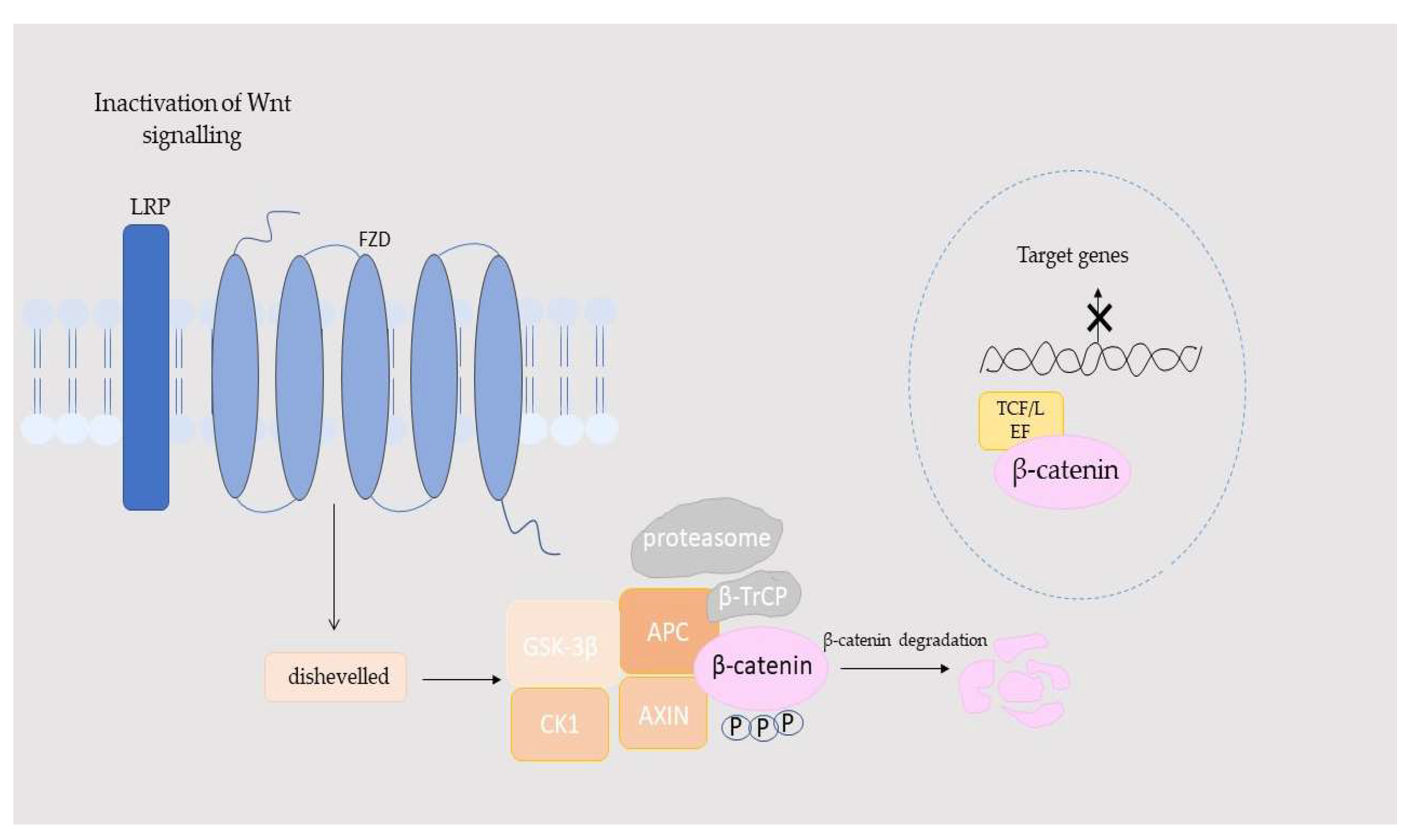
| Component | Location | Function in the Wnt/β-Catenin Pathway | Activation/Inhibition Role | References |
|---|---|---|---|---|
| DVL | Cytoplasm/membrane | Transmits Wnt signal; recruits AXIN and GSK-3β | Activator | [31,33,34,35] |
| APC | Cytoplasm | Facilitates β-catenin degradation, preventing pathway activation | Inhibitor | [36] |
| LRP5/6 | Plasma membrane | Co-receptor for Wnt; phosphorylation leads to β-catenin stabilization | Activator | [32,34,37] |
| RSPO | Extracellular space | Enhances Wnt signaling by inhibiting RNF43/ZNRF3-mediated degradation of FZD receptors | Activator—amplifies Wnt response | [33,38] |
| Wnt (ligands) | Extracellular space | Initiates signaling by binding to FZD and LRP5/6 receptors | Activator | [38] |
| FZD | Plasma membrane | Wnt receptor; transduces signal to DVL | Activator | [38] |
| Axin | Cytoplasm/membrane | Scaffolding protein; assembles β-catenin destruction complex | Inhibitor | [38] |
| GSK-3β | Cytoplasm | Phosphorylates β-catenin, marking it for degradation | Inhibitor | [38] |
| CK-1α | Cytoplasm | Phosphorylates β-catenin and LRP6 | Dual role—supports inhibition (β-catenin phosphorylation) and activation (LRP6 phosphorylation) | [34,37] |
| β-catenin | Cytoplasm/nucleus | Transcriptional co-activator; activates Wnt target genes | Activator | [38] |
| β-TrCP | Cytoplasm | Ubiquitin ligase marking β-catenin for degradation | Inhibitor | [38] |
| TCF/LEF | Nucleus | Transcription factors activated by β-catenin | Activator | [38] |
| Groucho/TLE | Nucleus | Repressor of TCF/LEF; inhibits Wnt target gene expression in the absence of β-catenin | Inhibitor—prevents unintended activation | [38] |
| PORCN | Endoplasmic reticulum/golgi | Enzyme required for Wnt ligand secretion and lipid modification | Activator—essential for Wnt secretion | [38] |
| GI Cancer | Pathway Component | Biomarker Relevance | Clinical Significance | References |
|---|---|---|---|---|
| Gastric Cancer | Wnt2 | Prognostic | Overexpression was linked to advanced stage and lymph node metastasis | [83] |
| Wnt3 | Therapeutic target | Silencing was shown to reduce proliferation, promote apoptosis | [84] | |
| Wnt5A | Prognostic | Expression was correlated with invasion, metastasis, and poor survival | [86,87,88] | |
| FZD7 | Prognostic/predictive | High expression was linked to metastasis and immune response | [90] | |
| LRP5/LRP6 | Prognostic/therapeutic target | High expression was associated with advanced stage and cancer stemness | [94,95] | |
| β-catenin | Prognostic | High expression was correlated with poor differentiation and metastasis | [97,98] | |
| APC | Prognostic | Expression was downregulated via miRNAs and linked to increased proliferation | [99] | |
| GSK3β | Prognostic | Higher expression was observed in early stages and associated with better survival | [100] | |
| Colorectal Cancer | Wnt2/Wnt3A/Wnt4 | Diagnostic/prognostic | Expression was associated with EMT, invasion, and angiogenesis | [103,104,105] |
| Wnt5A | Prognostic | Higher expression was correlated with better prognosis | [106] | |
| FZD6/FZD7 | Prognostic | Elevated expression was found in tumor core and linked to Wnt activation | [107,108] | |
| p-LRP6 | Prognostic | Expression correlated with advanced stage and shorter DFS | [109] | |
| β-catenin | Prognostic | Nuclear β-catenin staining in tumor cells has been associated with poor prognosis; The presence of B-catenin mutations could help to identify individuals at higher risk of developing CRC and guide personalized treatment strategies | [110,111,112,113,114] | |
| APC | Diagnostic/prognostic | The presence of APC mutations could help to identify individuals at higher risk of developing CRC and guide personalized treatment strategies | [113,114] | |
| GSK3β/CK1 | Prognostic/therapeutic target | Elevated levels were linked to chemoresistance and poor survival | [116,117] | |
| Esophageal Cancer | Wnt2/Wnt3/Wnt5A/Wnt6 | Prognostic/therapeutic target | High expression was linked to EMT, metastasis, and shorter OS | [99,119,120,121] |
| FZD2/FZD6/FZD7 | Prognostic | Overexpression was correlated with invasion and poor PFS | [119,123,124] | |
| LRP6 | Prognostic/therapeutic | Expression promoted cell proliferation and was associated with poor differentiation | [125] | |
| Axin | Prognostic | Low expression was linked to invasion and lymph node metastasis | [128,136] | |
| β-catenin | Diagnostic/prognostic | The presence of B-catenin mutations could help to identify individuals at higher risk of developing EC; β-catenin mutations have been associated with tumor aggressiveness and metastasis, potentially aiding in predicting disease progression | [30,130,131] | |
| Liver Cancer (HCC) | Wnt1/Wnt3A/Wnt5A/Wnt10B | Prognostic | Overexpression was correlated with poor survival and recurrence | [137,138,139] |
| FZD2/FZD7 | Prognostic/therapeutic target | Expression was linked to EMT and early tumorigenesis | [140,141] | |
| LRP6 | Prognostic/therapeutic target | Upregulation was associated with drug resistance and poor prognosis | [142] | |
| β-catenin | Prognostic | Nuclear localization was linked to poorly differentiated HCC | [143] | |
| CTNNB1 mutations | Diagnostic | Mutations were found in ~20–40% of HCC cases, especially those related to HCV | [144,145] | |
| Axin1/2/GSK3β/APC | Prognostic | Mutations or suppression were found to drive pathway activation | [146,147] | |
| Pancreatic Cancer (PDAC/PAAD) | Wnt2/Wnt3A/Wnt5A/Wnt7A | Prognostic/therapeutic target | Expression was linked to metastasis and poor prognosis | [128,150,151,152,153] |
| FZD1–FZD10 | Prognostic/therapeutic target | Overexpression was correlated with disease progression and clinical stage | [154] | |
| β-catenin | Prognostic | High expression was linked to better survival in some contexts | [155] | |
| PROX1 + β-catenin | Prognostic | Combined expression was indicative of improved survival | [156] | |
| GSK3β/Axin/APC | Functional regulators | Impaired β-catenin degradation was linked to its accumulation | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowicz, A.; Łukaszewicz-Zając, M. The Significance of the Wnt/β-Catenin Pathway and Related Proteins in Gastrointestinal Malignancies. Int. J. Mol. Sci. 2025, 26, 8130. https://doi.org/10.3390/ijms26178130
Romanowicz A, Łukaszewicz-Zając M. The Significance of the Wnt/β-Catenin Pathway and Related Proteins in Gastrointestinal Malignancies. International Journal of Molecular Sciences. 2025; 26(17):8130. https://doi.org/10.3390/ijms26178130
Chicago/Turabian StyleRomanowicz, Adrianna, and Marta Łukaszewicz-Zając. 2025. "The Significance of the Wnt/β-Catenin Pathway and Related Proteins in Gastrointestinal Malignancies" International Journal of Molecular Sciences 26, no. 17: 8130. https://doi.org/10.3390/ijms26178130
APA StyleRomanowicz, A., & Łukaszewicz-Zając, M. (2025). The Significance of the Wnt/β-Catenin Pathway and Related Proteins in Gastrointestinal Malignancies. International Journal of Molecular Sciences, 26(17), 8130. https://doi.org/10.3390/ijms26178130







