NFATc1 Abrogation in B Cells Ameliorates Contact Hypersensitivity Responses
Abstract
1. Introduction
2. Results
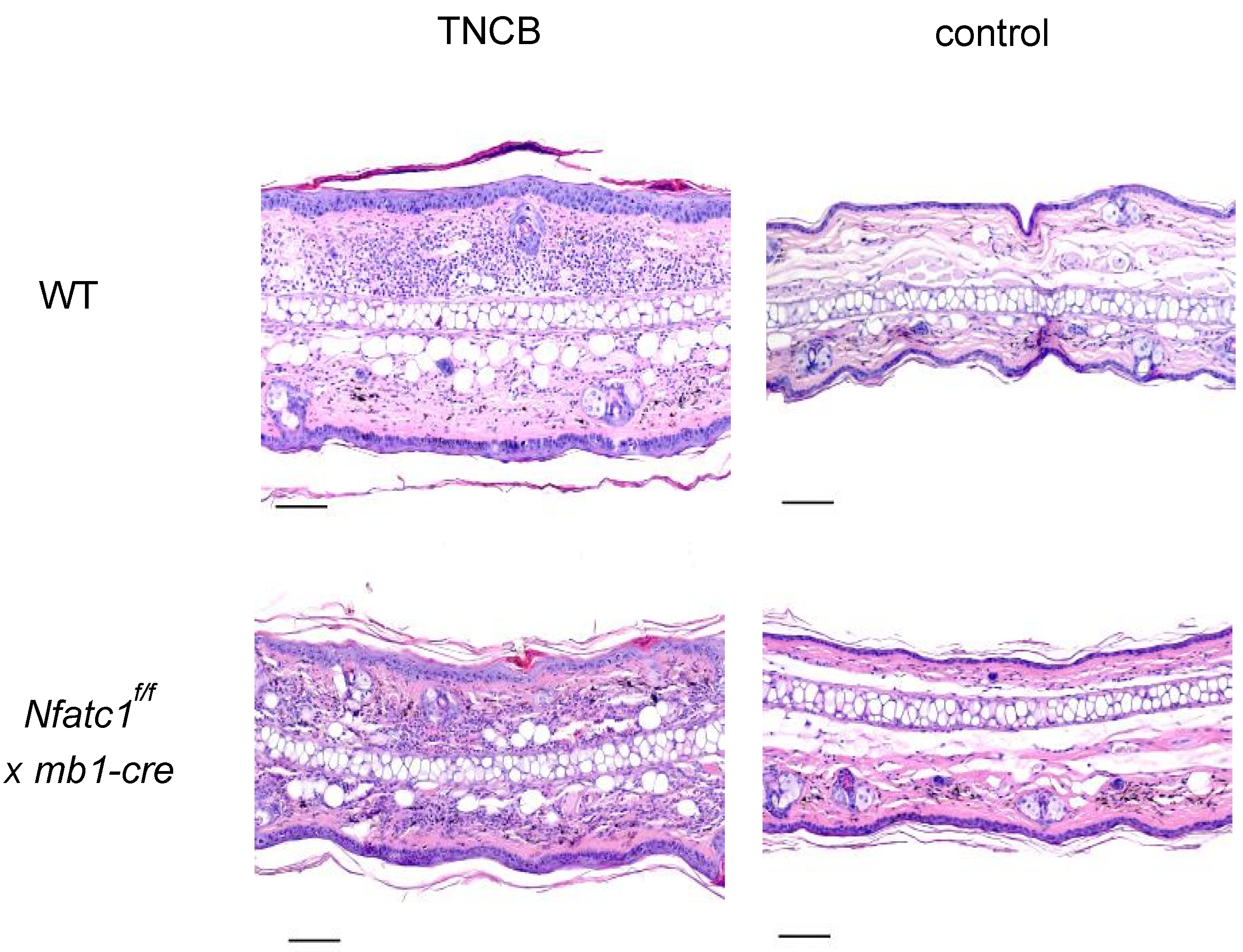
2.1. Contact Hypersensitivity Induction by Trinitrochlorobenzene (TNCB)
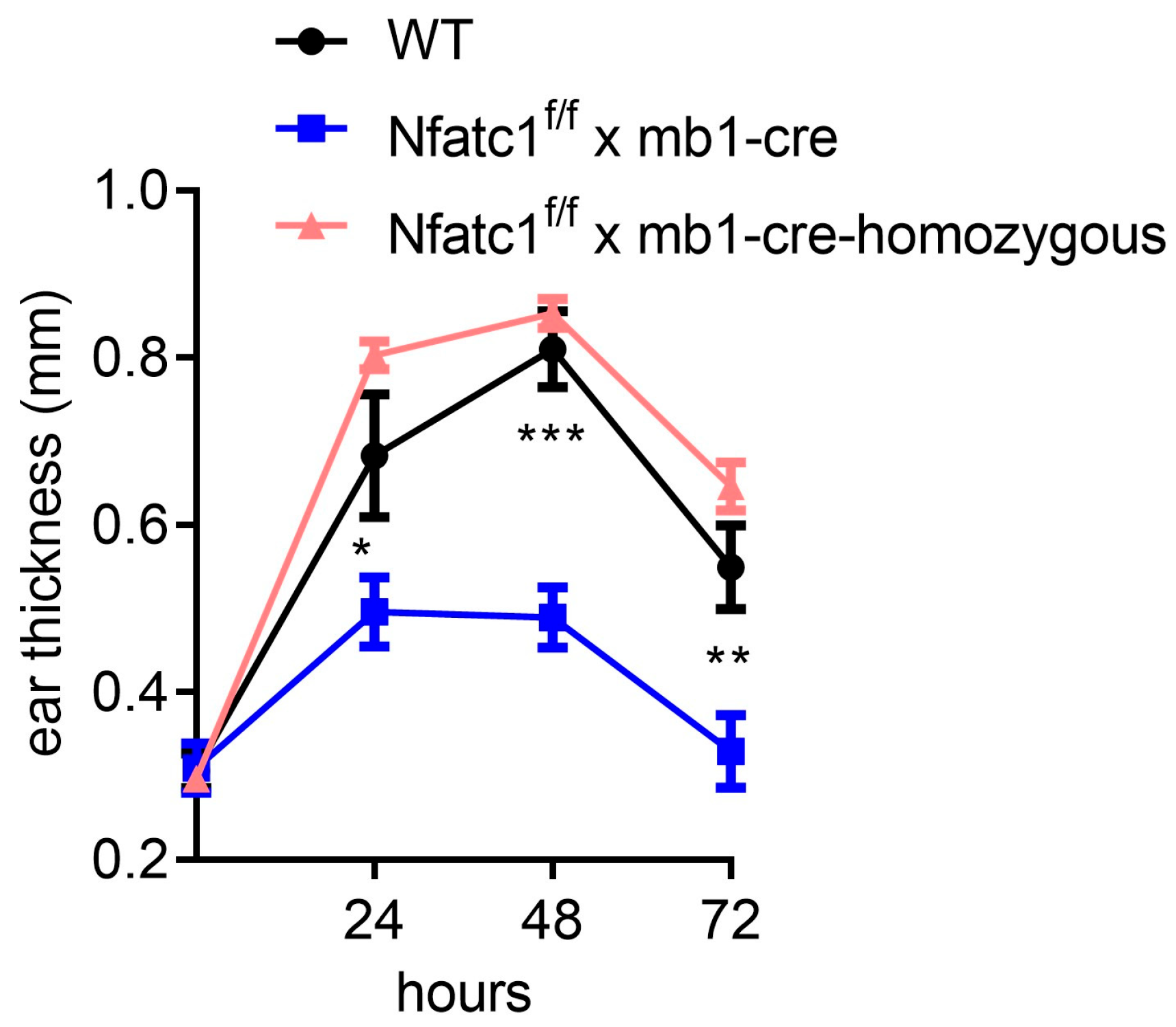
2.2. Mice with NFATc1 Deficiency in B Cells Exhibit Reduced CHS Responses
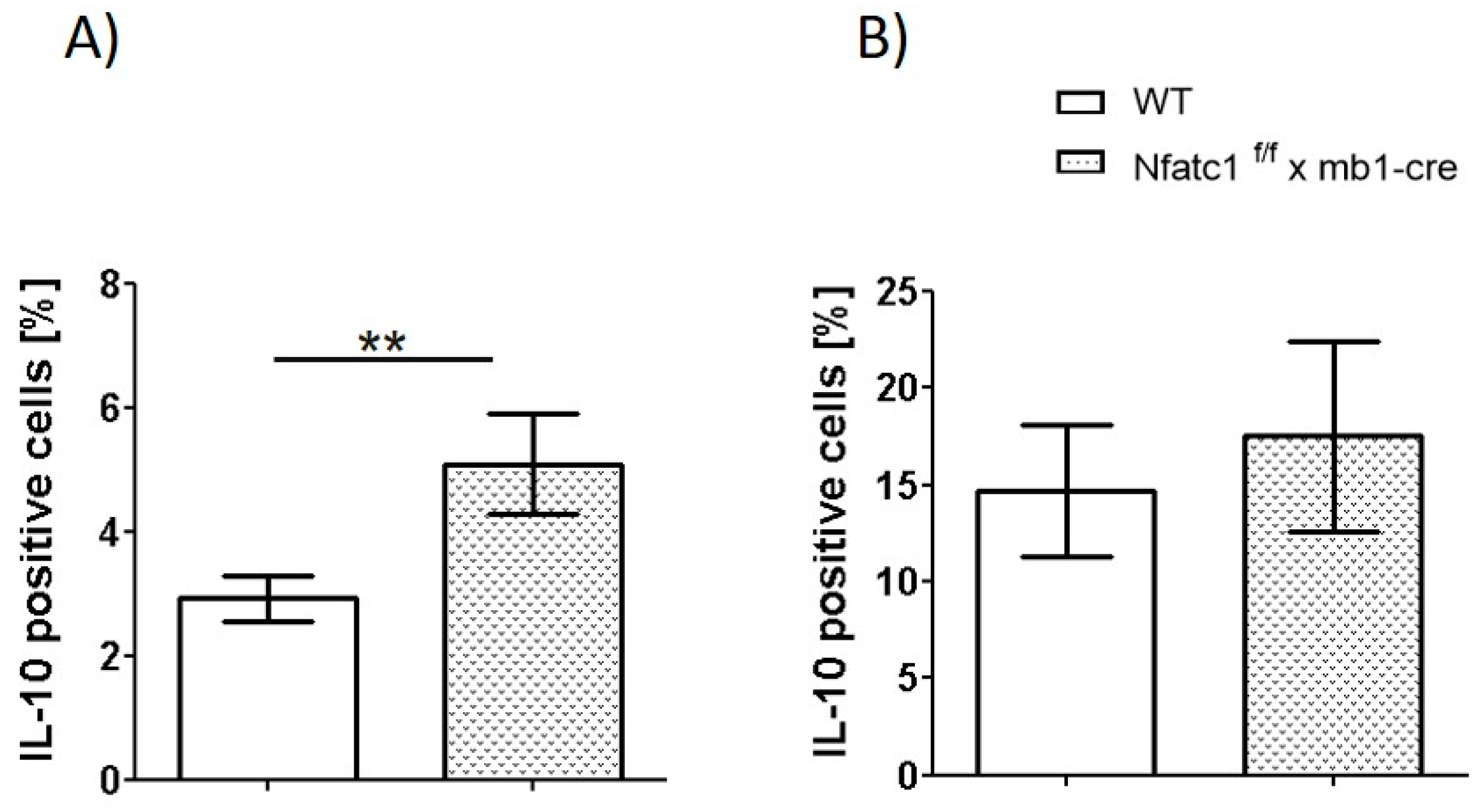
2.3. IL-10-Producing Regulatory B Cells Are Enhanced in Nfat1f/f x mb1-Cre Mice
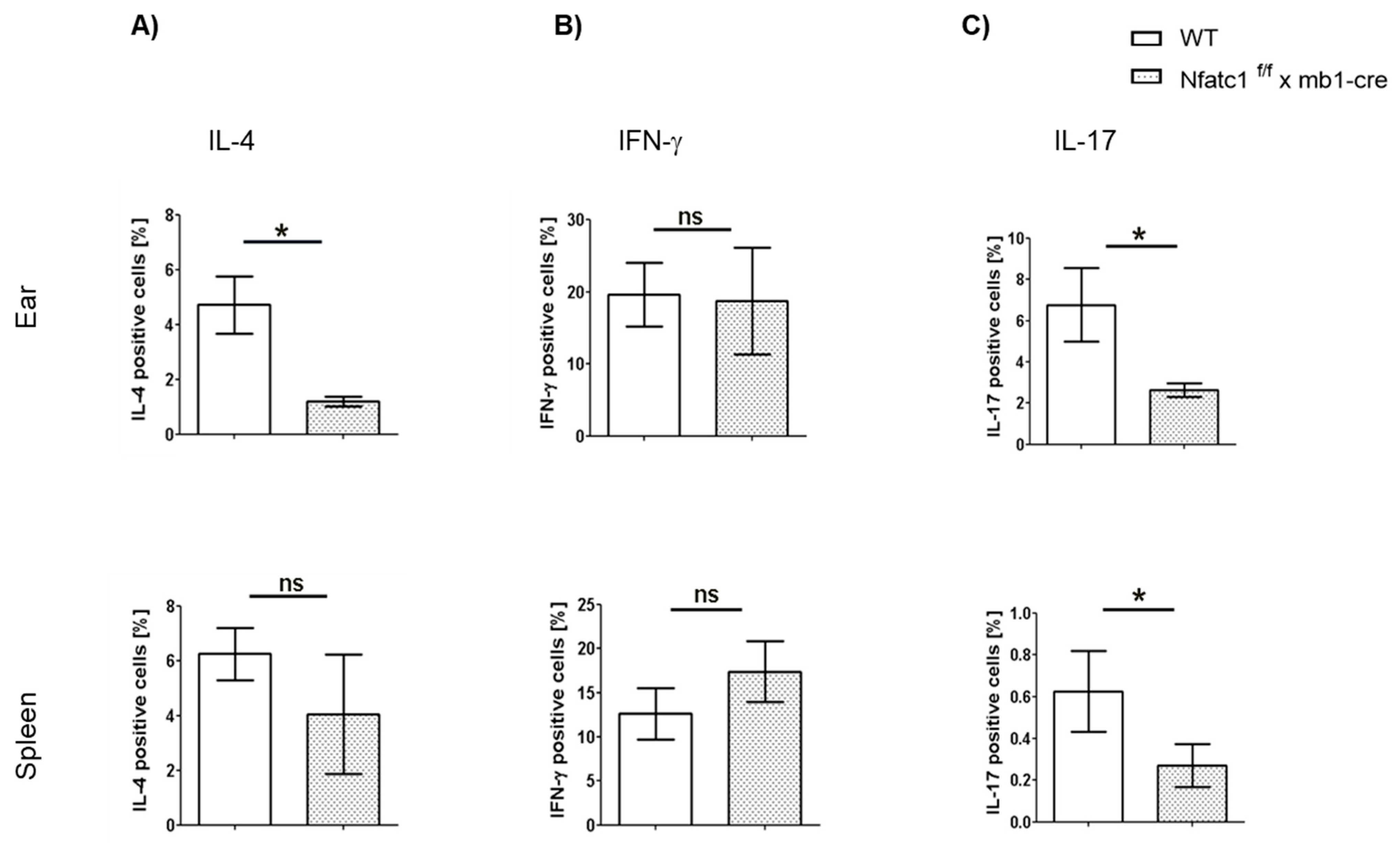
2.4. Influence of NFATc1 Deficiency in B Cells on Cytokine Production by T Cells
3. Discussion
4. Material and Methods
4.1. Mice, Isolation, and Culture of Cells
4.2. Flow Cytometry
4.3. Histological and Immunofluorescence Staining
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassau, S.; Fonacier, L. Allergic contact dermatitis. Med. Clin. N. Am. 2020, 104, 61–76. [Google Scholar] [CrossRef]
- Ljubojevic Hadzavdic, S.; Pustisek, N.; Zuzul, K.; Svigir, A. Contact allergy: An update. G. Ital. Di Dermatol. E Venereol. 2018, 153, 419–428. [Google Scholar] [CrossRef]
- Jakasa, I.; Thyssen, J.P.; Kezic, S. The role of skin barrier in occupational contact dermatitis. Exp. Dermatol. 2018, 27, 909–914. [Google Scholar] [CrossRef]
- Andersen, K.E. Occupational issues of allergic contact dermatitis. Int. Arch. Occup. Environ. Health 2003, 76, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Kalboussi, H.; Kacem, I.; Aroui, H.; El Maalel, O.; Maoua, M.; Brahem, A.; El Guedri, S.; Chatti, S.; Ghariani, N.; Mrizak, N. Impact of allergic contact dermatitis on the quality of life and work productivity. Dermatol. Res. Pract. 2019, 2019, 3797536. [Google Scholar] [CrossRef]
- Vocanson, M.; Hennino, A.; Rozieres, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Kader, H.; Kerstan, A.; Hetta, H.F.; Serfling, E.; Goebeler, M.; Muhammad, K. Intricate relationship between adaptive and innate immune system in allergic contact dermatitis. Yale J. Biol. Med. 2020, 93, 699–709. [Google Scholar]
- Scheinman, P.L.; Vocanson, M.; Thyssen, J.P.; Johansen, J.D.; Nixon, R.L.; Dear, K.; Botto, N.C.; Morot, J.; Goldminz, A.M. Contact dermatitis. Nat. Rev. Dis. Primers 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; DiIulio, N.A.; Fairchild, R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon gamma-producing (tc1) effector CD8+ T cells and interleukin (il) 4/Il-10-producing (th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996, 183, 1001–1012. [Google Scholar] [CrossRef]
- Schmidt, M.; Raghavan, B.; Muller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef]
- Adam, C.; Wohlfarth, J.; Haussmann, M.; Sennefelder, H.; Rodin, A.; Maler, M.; Martin, S.F.; Goebeler, M.; Schmidt, M. Allergy-inducing chromium compounds trigger potent innate immune stimulation via ros-dependent inflammasome activation. J. Investig. Dermatol. 2017, 137, 367–376. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R.; Weber, F.C.; Jakob, T.; Freudenberg, M.A.; Schmidt, M.; Goebeler, M. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy 2011, 66, 1152–1163. [Google Scholar] [CrossRef]
- Akiba, H.; Kehren, J.; Ducluzeau, M.T.; Krasteva, M.; Horand, F.; Kaiserlian, D.; Kaneko, F.; Nicolas, J.F. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ t cytotoxic 1 cells inducing keratinocyte apoptosis. J. Immunol. 2002, 168, 3079–3087. [Google Scholar] [CrossRef]
- Chuvpilo, S.; Jankevics, E.; Tyrsin, D.; Akimzhanov, A.; Moroz, D.; Jha, M.K.; Schulze-Luehrmann, J.; Santner-Nanan, B.; Feoktistova, E.; Konig, T.; et al. Autoregulation of nfatc1/a expression facilitates effector T cells to escape from rapid apoptosis. Immunity 2002, 16, 881–895. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Deb, J.; Patra, A.K.; Thuy Pham, D.A.; Chen, W.; Vaeth, M.; Berberich-Siebelt, F.; Klein-Hessling, S.; Lamperti, E.D.; Reifenberg, K.; et al. Nfatc1 affects mouse splenic B cell function by controlling the calcineurin--nfat signaling network. J. Exp. Med. 2011, 208, 823–839. [Google Scholar] [CrossRef]
- Alrefai, H.; Muhammad, K.; Rudolf, R.; Pham, D.A.; Klein-Hessling, S.; Patra, A.K.; Avots, A.; Bukur, V.; Sahin, U.; Tenzer, S.; et al. Nfatc1 supports imiquimod-induced skin inflammation by suppressing il-10 synthesis in B cells. Nat. Commun. 2016, 7, 11724. [Google Scholar] [CrossRef] [PubMed]
- Mowad, C.M.; Anderson, B.; Scheinman, P.; Pootongkam, S.; Nedorost, S.; Brod, B. Allergic contact dermatitis: Patient diagnosis and evaluation. J. Am. Acad. Dermatol. 2016, 74, 1029–1040. [Google Scholar] [CrossRef]
- Watanabe, R.; Fujimoto, M.; Ishiura, N.; Kuwano, Y.; Nakashima, H.; Yazawa, N.; Okochi, H.; Sato, S.; Tedder, T.F.; Tamaki, K. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am. J. Pathol. 2007, 171, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Hobeika, E.; Thiemann, S.; Storch, B.; Jumaa, H.; Nielsen, P.J.; Pelanda, R.; Reth, M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13789–13794. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Grabbe, S.; Schwarz, T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 1998, 19, 37–44. [Google Scholar] [CrossRef]
- Salonga, R.B.; Hisaka, S.; Nose, M. Effect of the hot water extract of Artocarpus camansi leaves on 2, 4, 6-trinitrochlorobenzene (tncb)-induced contact hypersensitivity in mice. Biol. Pharm. Bull. 2014, 37, 493–497. [Google Scholar] [CrossRef][Green Version]
- Azeem, M.; Helal, M.; Klein-Hessling, S.; Serfling, E.; Goebeler, M.; Muhammad, K.; Kerstan, A. Nfatc1 fosters allergic contact dermatitis responses by enhancing the induction of il-17-producing CD8 cells. J. Investig. Dermatol. 2025, 145, 1995–2006.e5. [Google Scholar] [CrossRef]
- Mauri, C.; Menon, M. The expanding family of regulatory B cells. Int. Immunol. 2015, 27, 479–486. [Google Scholar] [CrossRef]
- Noh, G.; Lee, J.H. Regulatory B cells and allergic diseases. Allergy Asthma Immunol. Res. 2011, 3, 168–177. [Google Scholar] [CrossRef]
- Lee, D.; Jo, M.G.; Min, K.Y.; Choi, M.Y.; Kim, Y.M.; Kim, H.S.; Choi, W.S. il-10(+) regulatory B cells mitigate atopic dermatitis by suppressing eosinophil activation. Sci. Rep. 2024, 14, 18164. [Google Scholar] [CrossRef]
- Kader, H.A.; Sabih Ur Rehman, S.; Saraswathiamma, D.; Shiek, S.S.; Bencomo-Hernandez, A.A.; Moorakkan, U.R.; Haider, M.T.; Munawar, N.; Iratni, R.; Serfling, E.; et al. Nfatc1 deficiency in B cells ameliorates atopic dermatitis. Sci. Rep. 2025, 15, 25170. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M. Role of th17 cells in skin inflammation of allergic contact dermatits. Clin. Dev. Immunol. 2013, 2013, 261037. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Seike, M.; Hagiwara, T.; Sato, A.; Ohtsu, H. Close relationship between t helper (th)17 and th2 response in murine allergic contact dermatitis. Clin. Exp. Dermatol. 2014, 39, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Kamata, M.; Ishiura, N.; Shibata, S.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Tedder, T.F.; Sato, S. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J. Leukoc. Biol. 2013, 94, 563–573. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The regulatory B cell-mediated peripheral tolerance maintained by mast cell il-5 suppresses oxazolone-induced contact hypersensitivity. Sci. Adv. 2019, 5, eaav8152. [Google Scholar] [CrossRef] [PubMed]
- Fjelbye, J.; Antvorskov, J.C.; Buschard, K.; Issazadeh-Navikas, S.; Engkilde, K. CD1d knockout mice exhibit aggravated contact hypersensitivity responses due to reduced interleukin-10 production predominantly by regulatory B cells. Exp. Dermatol. 2015, 24, 853–856. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Matsushita, T.; Yanaba, K.; Bouaziz, J.D.; Fujimoto, M.; Tedder, T.F. Regulatory B cells inhibit eae initiation in mice while other B cells promote disease progression. J. Clin. Investig. 2008, 118, 3420–3430. [Google Scholar] [CrossRef]
- Radomir, L.; Kramer, M.P.; Perpinial, M.; Schottlender, N.; Rabani, S.; David, K.; Wiener, A.; Lewinsky, H.; Becker-Herman, S.; Aharoni, R.; et al. The survival and function of il-10-producing regulatory B cells are negatively controlled by slamf5. Nat. Commun. 2021, 12, 1893. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef]
- Baur, J.; Otto, C.; Steger, U.; Klein-Hessling, S.; Muhammad, K.; Pusch, T.; Murti, K.; Wismer, R.; Germer, C.T.; Klein, I.; et al. The transcription factor nfatc1 supports the rejection of heterotopic heart allografts. Front. Immunol. 2018, 9, 1338. [Google Scholar] [CrossRef]
- Martin, S.F. Induction of contact hypersensitivity in the mouse model. Methods Mol. Biol. 2013, 961, 325–335. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Dube, P.; Griffith, T.S. Regulation of contact hypersensitivity by interleukin 10. J. Exp. Med. 1994, 179, 1597–1604. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grän, F.; Azeem, M.; Serfling, E.; Goebeler, M.; Kerstan, A.; Muhammad, K. NFATc1 Abrogation in B Cells Ameliorates Contact Hypersensitivity Responses. Int. J. Mol. Sci. 2025, 26, 8125. https://doi.org/10.3390/ijms26178125
Grän F, Azeem M, Serfling E, Goebeler M, Kerstan A, Muhammad K. NFATc1 Abrogation in B Cells Ameliorates Contact Hypersensitivity Responses. International Journal of Molecular Sciences. 2025; 26(17):8125. https://doi.org/10.3390/ijms26178125
Chicago/Turabian StyleGrän, Franziska, Muhammad Azeem, Edgar Serfling, Matthias Goebeler, Andreas Kerstan, and Khalid Muhammad. 2025. "NFATc1 Abrogation in B Cells Ameliorates Contact Hypersensitivity Responses" International Journal of Molecular Sciences 26, no. 17: 8125. https://doi.org/10.3390/ijms26178125
APA StyleGrän, F., Azeem, M., Serfling, E., Goebeler, M., Kerstan, A., & Muhammad, K. (2025). NFATc1 Abrogation in B Cells Ameliorates Contact Hypersensitivity Responses. International Journal of Molecular Sciences, 26(17), 8125. https://doi.org/10.3390/ijms26178125






