Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study
Abstract
1. Introduction
2. Results
2.1. Weight and Toxicity Analysis
2.2. Macroscopic Analysis
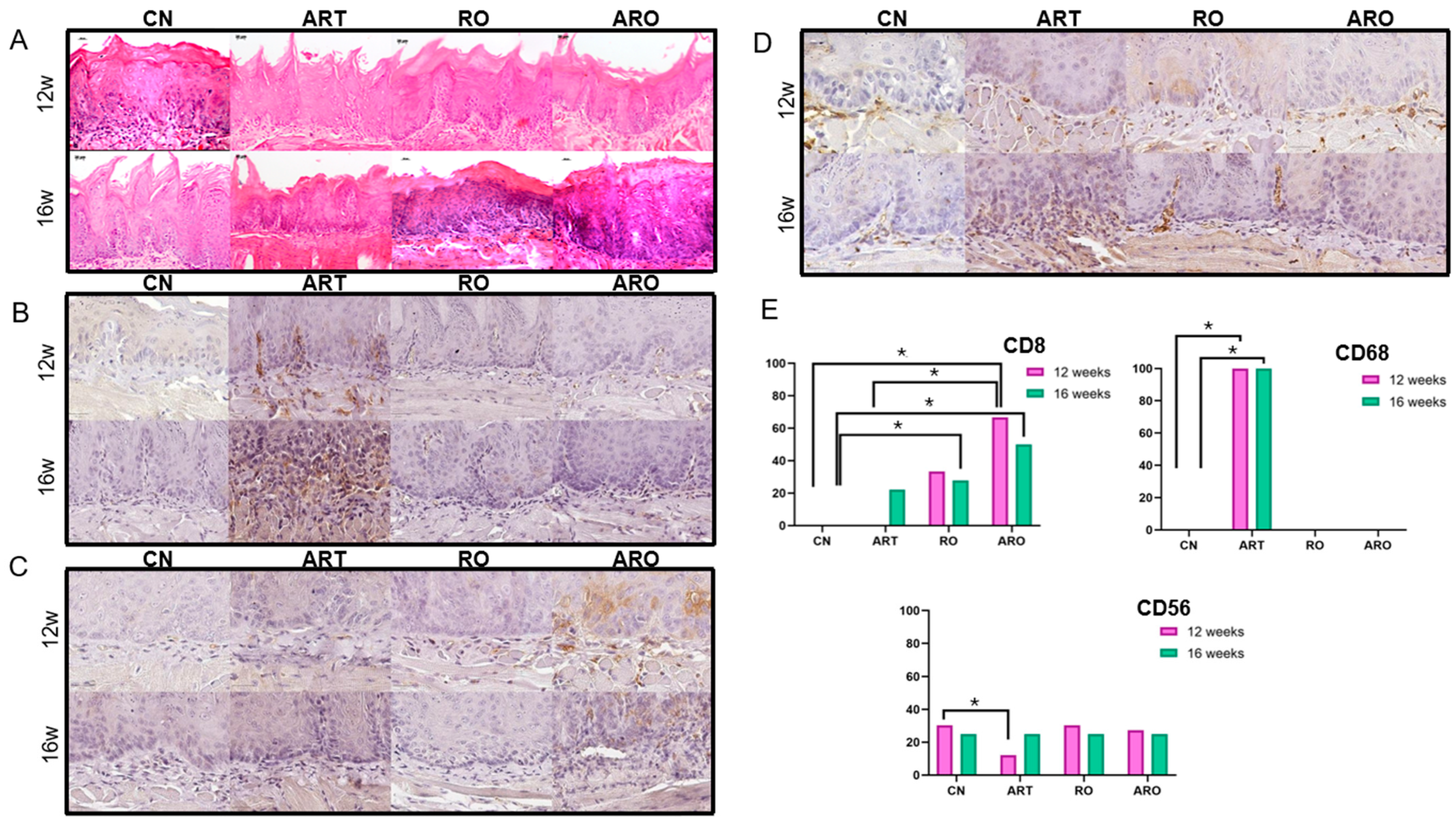
2.3. Histological Analysis
2.4. Immunohistochemistry
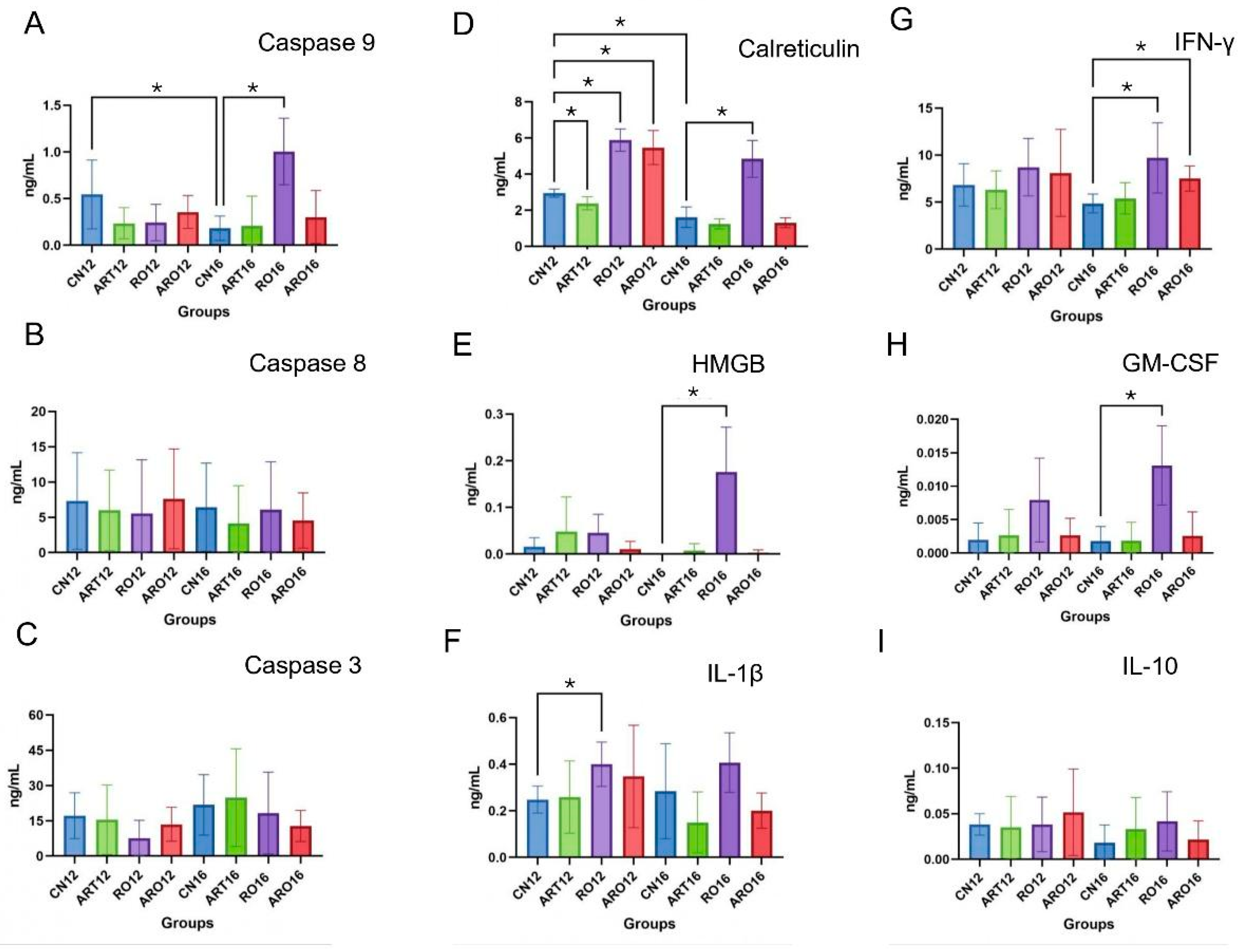
2.5. ELISA
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Guidelines
4.2. Animals and Housing Conditions
4.3. Oral Leukoplakia Induction with 4-NQO
4.4. Experimental Treatments
4.5. Histological Procedures
4.6. Immunohistochemistry Protocol
4.7. ELISA Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- Petti, S. Pooled Estimate of World Leukoplakia Prevalence: A Systematic Review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Zhang, C.; Li, B.; Zeng, X.; Hu, X.S.; Hua, H. The Global Prevalence of Oral Leukoplakia: A Systematic Review and Meta-Analysis from 1996 to 2022. BMC Oral Health 2023, 23, 645. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Barros, L.A.; Ramos-García, P.; González-Moles, M.Á.; Aguirre-Urizar, J.M.; Warnakulasuriya, S. Malignant Transformation of Oral Leukoplakia: Systematic Review and Comprehensive Meta-Analysis. Oral Dis. 2025, 31, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Urizar, J.M.; Lafuente-Ibáñez de Mendoza, I.; Warnakulasuriya, S. Malignant Transformation of Oral Leukoplakia: Systematic Review and Meta-Analysis of the Last 5 Years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef]
- van der Waal, I. Potentially Malignant Disorders of the Oral and Oropharyngeal Mucosa; Terminology, Classification and Present Concepts of Management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Palma, V.d.M.; Koerich Laureano, N.; Frank, L.A.; Rados, P.V.; Visioli, F. Chemoprevention in Oral Leukoplakia: Challenges and Current Landscape. Front. Oral Health 2023, 4, 1191347. [Google Scholar] [CrossRef]
- Zeng, Z.-W.; Chen, D.; Chen, L.; He, B.; Li, Y. A Comprehensive Overview of Artemisinin and Its Derivatives as Anticancer Agents. Eur. J. Med. Chem. 2023, 247, 115000. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chan, B.C.L.; Qiu, M.H.; Leung, P.C.; Wong, C.K. Artemisinin and Its Derivatives as Potential Anticancer Agents. Molecules 2024, 29, 3886. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin Enhances the Anti-Tumor Immune Response in 4T1 Breast Cancer Cells In Vitro and In Vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef]
- Khanal, P. Antimalarial and Anticancer Properties of Artesunate and Other Artemisinins: Current Development. Monatsh. Chem. 2021, 152, 387–400. [Google Scholar] [CrossRef]
- Letis, A.S.; Seo, E.J.; Nikolaropoulos, S.S.; Efferth, T.; Giannis, A.; Fousteris, M.A. Synthesis and Cytotoxic Activity of New Artemisinin Hybrid Molecules against Human Leukemia Cells. Bioorganic Med. Chem. 2017, 25, 3357–3367. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an Anticancer Drug: Recent Advances in Target Profiling and Mechanisms of Action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front. Pharmacol. 2020, 11, 529881. [Google Scholar] [CrossRef]
- Bobinait, R.; Viškelis, P.; Venskutonis, P.R. Variation of Total Phenolics, Anthocyanins, Ellagic Acid and Radical Scavenging Capacity in Various Raspberry (Rubus spp.) Cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, T.J.; Ryan, N.M.; Bruschweiler-Li, L.; Wang, C.; Bernier, M.C.; Somogyi, A.; Yan, P.S.; Cooperstone, J.L.; Mo, X.; Brüschweiler, R.P.; et al. Metabolic Regulation of Glycolysis and AMP Activated Protein Kinase Pathways during Black Raspberry-Mediated Oral Cancer Chemoprevention. Metabolites 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Su, H.; Guo, Z.; Li, H.; Jiang, Z.; Cao, Y.; Li, C. Rubus occidentalis and Its Bioactive Compounds against Cancer: From Molecular Mechanisms to Translational Advances. Phytomedicine 2024, 126, 155029. [Google Scholar] [CrossRef]
- Ryan, N.M.; Lamenza, F.F.; Upadhaya, P.; Pracha, H.; Springer, A.; Swingler, M.; Siddiqui, A.; Oghumu, S. Black Raspberry Extract Inhibits Regulatory T-Cell Activity in a Murine Model of Head and Neck Squamous Cell Carcinoma Chemoprevention. Front. Immunol. 2022, 13, 932742. [Google Scholar] [CrossRef]
- Haller, J.; Abedi, N.; Hafedi, A.; Shehab, O.; Wietecha, M.S. Spatial Transcriptomics Unravel the Tissue Complexity of Oral Pathogenesis. J. Dent. Res. 2024, 103, 1331–1339. [Google Scholar] [CrossRef]
- Gates, J.C.; Abouyared, M.; Shnayder, Y.; Farwell, D.G.; Day, A.; Alawi, F.; Moore, M.; Holcomb, A.J.; Birkeland, A.; Epstein, J. Clinical Management Update of Oral Leukoplakia: A Review From the American Head and Neck Society Cancer Prevention Service. Head Neck 2025, 47, 733–741. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, W.Y.; Chang, C.W.; Lin, M.C.; Wang, C.P.; Lou, P.J.; Chen, T.C. Chemoprevention of Oral Cancer: A Review and Future Perspectives. Head Neck 2023, 45, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Hasina, R.; Wroblewski, K.; Mankame, T.P.; Doçi, C.L.; Lingen, M.W. Dual Inhibition of Vascular Endothelial Growth Factor Receptor and Epidermal Growth Factor Receptor Is an Effective Chemopreventive Strategy in the Mouse 4-NQO Model of Oral Carcinogenesis. Cancer Prev. Res. 2010, 3, 1493–1502. [Google Scholar] [CrossRef]
- Hasina, R.; Martin, L.E.; Kasza, K.; Jones, C.L.; Jalil, A.; Lingen, M.W. ABT-510 Is an Effective Chemopreventive Agent in the Mouse 4-Nitroquinoline 1-Oxide Model of Oral Carcinogenesis. Cancer Prev. Res. 2009, 2, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. From Ancient Herb to Modern Drug: Artemisia Annua and Artemisinin for Cancer Therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Giusti, M.M. Contribution of Berry Anthocyanins to Their Chemopreventive Properties. In Berries and Cancer Prevention; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–40. [Google Scholar] [CrossRef]
- Han, C.; Ding, H.; Casto, B.; Stoner, G.D.; D’Ambrosio, S.M. Inhibition of the Growth of Premalignant and Malignant Human Oral Cell Lines by Extracts and Components of Black Raspberries. Nutr. Cancer 2005, 51, 207–217. [Google Scholar] [CrossRef]
- Oghumu, S.; Casto, B.C.; Ahn-Jarvis, J.; Weghorst, L.C.; Maloney, J.; Geuy, P.; Horvath, K.Z.; Bollinger, C.E.; Warner, B.M.; Summersgill, K.F.; et al. Inhibition of Pro-Inflammatory and Anti-Apoptotic Biomarkers during Experimental Oral Cancer Chemoprevention by Dietary Black Raspberries. Front. Immunol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhao, S.; Xu, Y.; Huang, Y.; Liu, S.; Su, P.; Wang, C.; Li, Y.; Li, H.; et al. Single-Cell RNA Sequencing Highlights the Immunosuppression of IDO1+ Macrophages in the Malignant Transformation of Oral Leukoplakia. Theranostics 2024, 14, 4787. [Google Scholar] [CrossRef]
- Soopanit, T.; Laokulrath, N.; Chayopasakul, V.; Pongsapich, W. Prognostic Value and Clinicopathological Status of PD-L1 Expression and CD8+ TILs in Oral Squamous Cell Cancer Patients with or without Traditional Risk Factors. Head Neck 2023, 45, 1017–1025. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Q.; Jiang, N.; Zhang, Y.; Su, Z.; Lv, L.; Sang, X.; Chen, R.; Feng, Y.; Chen, Q. Dihydroartemisinin Regulates Immune Cell Heterogeneity by Triggering a Cascade Reaction of CDK and MAPK Phosphorylation. Signal Transduct. Target. Ther. 2022, 7, 222. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, J.; Fang, J.; Chen, P.; Zhou, J.; Wang, H.; Sun, Z.; Chen, Y.; Yin, L. Dihydroartemisinin Remodels Tumor Micro-Environment and Improves Cancer Immunotherapy through Inhibiting Cyclin-Dependent Kinases. Int. Immunopharmacol. 2024, 139, 112637. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef]
- John, S.; Joseph, A.P.; Pillai, V.B.R.; Ramani, P.; P, J.; Ramalingam, K. Evaluation of Cytotoxic T Lymphocytes and Natural Killer Cell Distribution in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: An Immunohistochemical Study. Cureus 2024, 16, e56323. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, H.; Yao, H.; Lu, L.; He, G.; Wu, M.; Zheng, C.; Li, Y.; Chen, S.; Li, L.; et al. Artemisinin Derivatives Inhibit Non-Small Cell Lung Cancer Cells Through Induction of ROS-Dependent Apoptosis/Ferroptosis. J. Cancer 2021, 12, 4075. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Howard, L.; Lee, S.O. Chemical Composition of Volatile Extracts from Black Raspberries, Blueberries, and Blackberries and Their Antiproliferative Effect on A549 Non-Small-Cell Lung Cancer Cells. Life 2022, 12, 2056. [Google Scholar] [CrossRef]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and Cancer. Cell Res. 2020, 31, 5. [Google Scholar] [CrossRef]
- Nedungadi, D.; Ryan, N.; Anderson, K.; Lamenza, F.F.; Jordanides, P.P.; Swingler, M.J.; Rakotondraibe, L.; Riedl, K.M.; Iwenofu, H.; Oghumu, S. Modulation of the Oral Glucocorticoid System during Black Raspberry Mediated Oral Cancer Chemoprevention. Carcinogenesis 2022, 43, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chen, F.; Zhang, X.; Clinton, S.K.; Tang, X.; Sun, Z.; Chen, T. Suppression of Oxidative Stress and NFκB/MAPK Signaling by Lyophilized Black Raspberries for Esophageal Cancer Prevention in Rats. Nutrients 2017, 9, 413. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Crippa, M.P.; Manfredi, A.A.; Mezzapelle, R.; Rovere Querini, P.; Venereau, E. High-Mobility Group Box 1 Protein Orchestrates Responses to Tissue Damage via Inflammation, Innate and Adaptive Immunity, and Tissue Repair. Immunol. Rev. 2017, 280, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.J.; Malta, I.S.; de Almeida Lança, M.L.; de Vasconcellos, L.M.R.; Adorno-Farias, D.; Jara, J.A.; Kaminagakura, E. Effects of Artemisinin and Cisplatin on the Malignant Progression of Oral Leukoplakia. In Vitro and in Vivo Study. J. Cancer Res. Clin. Oncol. 2024, 150, 390. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Vincent-Chong, V.K.; DeJong, H.; Attwood, K.; Hershberger, P.A.; Seshadri, M. Preclinical Prevention Trial of Calcitriol: Impact of Stage of Intervention and Duration of Treatment on Oral Carcinogenesis. Neoplasia 2019, 21, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, O.L.; Smoliga, J.M. Translating Dosages from Animal Models to Human Clinical Trials—Revisiting Body Surface Area Scaling. FASEB J. 2015, 29, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus Guidelines for the Detection of Immunogenic Cell Death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Kresty, L.A.; Mallery, S.R.; Stoner, G.D. Black Raspberries in Cancer Clinical Trials: Past, Present and Future. J. Berry Res. 2016, 6, 251–261. [Google Scholar] [CrossRef]
- Fernandes, E.E.; Lança, M.L.d.A.; de Souza, Y.A.; El-Achkar, V.N.; Costa, V.; Carlos, R.; Ribeiro-Silva, A.; Sichero, L.; Villa, L.L.; León, J.E.; et al. Impact of HPV Types and Dendritic Cells on Recurrent Respiratory Papillomatosis’ Aggressiveness. Diseases 2025, 13, 43. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lança, M.L.d.A.; Conceição, N.S.C.d.; Malta, I.S.; Meneses, D.O.; Almeida, L.Y.; Kaminagakura, E. Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study. Int. J. Mol. Sci. 2025, 26, 8120. https://doi.org/10.3390/ijms26178120
Lança MLdA, Conceição NSCd, Malta IS, Meneses DO, Almeida LY, Kaminagakura E. Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study. International Journal of Molecular Sciences. 2025; 26(17):8120. https://doi.org/10.3390/ijms26178120
Chicago/Turabian StyleLança, Maria Leticia de Almeida, Nathan Steven Cezar da Conceição, Isabella Souza Malta, Daniela Oliveira Meneses, Luciana Yamamoto Almeida, and Estela Kaminagakura. 2025. "Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study" International Journal of Molecular Sciences 26, no. 17: 8120. https://doi.org/10.3390/ijms26178120
APA StyleLança, M. L. d. A., Conceição, N. S. C. d., Malta, I. S., Meneses, D. O., Almeida, L. Y., & Kaminagakura, E. (2025). Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study. International Journal of Molecular Sciences, 26(17), 8120. https://doi.org/10.3390/ijms26178120






