Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers
Abstract
1. Introduction
1.1. Background and Rationale
1.2. Objectives and Scope
2. Overview of Cerebral Tumors and Pathophysiological Mechanisms
2.1. Classification and Epidemiology
2.2. Neuroanatomy and Tumor Location
2.3. Pathophysiological Mechanisms
3. Clinical Presentations: Psychiatric Syndromes and Symptoms
3.1. Mood and Anxiety Disorders
3.2. Psychotic Symptoms and Delirium
3.3. Cognitive and Behavioral Changes
3.4. Subtle Personality Changes
4. Diagnostic Challenges and Management
4.1. Diagnostic Challenges
4.2. Management Strategies
5. Prognosis, Outcomes, and Future Directions
5.1. Prognosis and Quality of Life: The Impact of Psychiatric Symptoms on Survival and Treatment Adherence
5.2. Outcomes over Time: The Evolution of Psychiatric Symptoms Across the Tumor Trajectory
5.3. Future Directions and Research Gaps: The Next Frontiers in Neuro-Oncology Psychiatry
6. Conclusions
6.1. Summary of Current Evidence
6.2. Diagnostic Advances and Transformation
6.3. Prognostic Importance and Mechanisms
6.4. Therapeutic Possibilities and Dynamic Nature of Presentation
6.5. Research Gaps and Opportunities
6.6. Vision for the Discipline
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javadi, S.A.H.S.; Rezaei, B. Brain tumors and indications for brain imaging in patients with psychiatric manifestations: A case report. Middle East Curr. Psychiatry 2021, 28, 56. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Shen, Z.; Wang, L.; Niu, X.; Wei, Y.; Sun, S.; Zhao, J. Mechanisms of neural infiltration-mediated tumor metabolic reprogramming impacting immunotherapy efficacy in non-small cell lung cancer. J. Exp. Clin. Cancer Res. CR 2024, 43, 284. [Google Scholar] [CrossRef] [PubMed]
- Allgood, J.E.; Roe, A.; Sparks, B.B.; Castillo, M.; Cruz, A.; Brooks, A.E.; Brooks, B.D. The Correlation of Sleep Disturbance and Location of Glioma Tumors: A Narrative Review. J. Clin. Med. 2023, 12, 4058. [Google Scholar] [CrossRef]
- Onciul, R.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Bratu, B.-G.; Costin, H.P.; Dumitrascu, D.-I.; Serban, M.; Ciurea, A.V. Deciphering Glioblastoma: Fundamental and Novel Insights into the Biology and Therapeutic Strategies of Gliomas. Curr. Issues Mol. Biol. 2024, 46, 2402–2443. [Google Scholar] [CrossRef] [PubMed]
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Rutkowski, K.; Gola, M.; Godlewski, J.; Starzyńska, A.; Marvaso, G.; Mastroleo, F.; Giulia Vincini, M.; Porazzi, A.; Zaffaroni, M.; Jereczek-Fossa, B.A. Understanding the role of nerves in head and neck cancers—A review. Oncol. Rev. 2025, 18, 1514004. [Google Scholar] [CrossRef]
- Barrash, J.; Bruss, J.; Anderson, S.W.; Kuceyeski, A.; Manzel, K.; Tranel, D.; Boes, A.D. Lesions in different prefrontal sectors are associated with different types of acquired personality disturbances. Cortex J. Devoted Study Nerv. Syst. Behav. 2022, 147, 169–184. [Google Scholar] [CrossRef]
- Vinti, V.; Dell’Isola, G.B.; Tascini, G.; Mencaroni, E.; Cara, G.D.; Striano, P.; Verrotti, A. Temporal Lobe Epilepsy and Psychiatric Comorbidity. Front. Neurol. 2021, 12, 775781. [Google Scholar] [CrossRef]
- Ruffini, G.; Castaldo, F.; Lopez-Sola, E.; Sanchez-Todo, R.; Vohryzek, J. The Algorithmic Agent Perspective and Computational Neuropsychiatry: From Etiology to Advanced Therapy in Major Depressive Disorder. Entropy 2024, 26, 953. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Vento, F.; Di Liegro, I. Role of Extracellular Vesicles in the Progression of Brain Tumors. Biology 2024, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Covache-Busuioc, R.-A.; Serban, M.; Ciurea, A.V.; Enyedi, M. Revolutionizing Neuroimmunology: Unraveling Immune Dynamics and Therapeutic Innovations in CNS Disorders. Int. J. Mol. Sci. 2024, 25, 13614. [Google Scholar] [CrossRef]
- Biegański, M.; Szeliga, M. Disrupted glutamate homeostasis as a target for glioma therapy. Pharmacol. Rep. PR 2024, 76, 1305–1317. [Google Scholar] [CrossRef]
- Çelik, Y. The Impact of Microbiota and Associated Blood Tryptophan Metabolites on Pain Perception in Patients Undergoing Lumbar Disc Herniation Surgery. Clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06502223 (accessed on 21 February 2025).
- Melanis, K.; Stefanou, M.-I.; Kitsos, D.K.; Athanasaki, A.; Theodorou, A.; Koropouli, E.; Keramida, A.; Dimitriadou, E.M.; Tzanetakos, D.; Andreadou, E.; et al. Paraneoplastic Neurological Syndromes as Initial Presentation of Tumors: An Eight-Year Single-Center Experience. J. Clin. Med. 2024, 13, 824. [Google Scholar] [CrossRef]
- Sharma, A.; Das, A.K.; Jain, A.; Purohit, D.K.; Solanki, R.K.; Gupta, A. Study of Association of Various Psychiatric Disorders in Brain Tumors. Asian J. Neurosurg. 2022, 17, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, B.B.; Jensen, J.S.; Grønhøj, C.; Wessel, I.; von Buchwald, C. Impact of delay in diagnosis and treatment-initiation on disease stage and survival in oral cavity cancer: A systematic review. Acta Oncol. Stockh. Swed. 2021, 60, 1083–1090. [Google Scholar] [CrossRef]
- Duan, H.; Ren, J.; Wei, S.; Yang, Z.; Li, C.; Wang, Z.; Li, M.; Wei, Z.; Liu, Y.; Wang, X.; et al. Integrated analyses of multi-omic data derived from paired primary lung cancer and brain metastasis reveal the metabolic vulnerability as a novel therapeutic target. Genome Med. 2024, 16, 138. [Google Scholar] [CrossRef]
- Smith, H.; Ahrendsen, J.T. What’s new in neuropathology 2024: CNS WHO 5th edition updates. J. Pathol. Transl. Med. 2024, 58, 346–349. [Google Scholar] [CrossRef]
- Moin, A.; Rizvi, S.M.D.; Hussain, T.; Gowda, D.V.; Subaiea, G.M.; Elsayed, M.M.A.; Ansari, M.; Alanazi, A.S.; Yadav, H. Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review. Life 2021, 11, 421. [Google Scholar] [CrossRef]
- Guo, X.; Gu, L.; Li, Y.; Zheng, Z.; Chen, W.; Wang, Y.; Wang, Y.; Xing, H.; Shi, Y.; Liu, D.; et al. Histological and molecular glioblastoma, IDH-wildtype: A real-world landscape using the 2021 WHO classification of central nervous system tumors. Front. Oncol. 2023, 13, 1200815. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, D.; Hutarew, G.; Zellinger, B.; Alinger-Scharinger, B.; Schlicker, H.U.; Schwartz, C.; Sotlar, K.; Kraus, T.F.J. EGFR Amplification Is a Phenomenon of IDH Wildtype and TERT Mutated High-Grade Glioma: An Integrated Analysis Using Fluorescence In Situ Hybridization and DNA Methylome Profiling. Biomedicines 2022, 10, 794. [Google Scholar] [CrossRef]
- Newhouse, A.; Kritzer, M.D.; Eryilmaz, H.; Praschan, N.; Camprodon, J.A.; Fricchione, G.; Chemali, Z. Neurocircuitry Hypothesis and Clinical Experience in Treating Neuropsychiatric Symptoms of Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2. J. Acad. Consult.-Liaison Psychiatry 2022, 63, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Azamat, S.; Buz-Yalug, B.; Dindar, S.S.; Yilmaz Tan, K.; Ozcan, A.; Can, O.; Ersen Danyeli, A.; Pamir, M.N.; Dincer, A.; Ozduman, K.; et al. Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas. Diagnostics 2024, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Wu, X.; Wang, Q. Research status and prospects of pituitary adenomas in conjunction with neurological and psychiatric disorders and the tumor microenvironment. Front. Neurosci. 2024, 18, 1294417. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, R.; Zhang, D.; Wang, Z.; Gao, L.; Yao, Y.; Deng, K.; Bao, X.; Feng, M.; Xu, Z.; et al. Hyperprolactinemia and Hypopituitarism in Acromegaly and Effect of Pituitary Surgery: Long-Term Follow-up on 529 Patients. Front. Endocrinol. 2021, 12, 807054. [Google Scholar] [CrossRef]
- Bonert, M.; Schittenhelm, J.; Begum, H.; Lu, J.-Q.; Swaminath, A.; Juergens, R.A.; Berzins, A.; Cutz, J.-C.; Naqvi, A.H. Neuroanatomical location of lung cancer brain metastases in 234 patients with a focus on cancer subtyping and biomarkers. PLoS ONE 2024, 19, e0314205. [Google Scholar] [CrossRef]
- Zimmer, A.S.; Van Swearingen, A.E.D.; Anders, C.K. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Rep. 2020, 5, e1274. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Fatahian, R.; Mansouri, K.; Dokaneheifard, S.; Shiri, M.H.; Hemmati, M.; Mohammadi, M. The global prevalence of primary central nervous system tumors: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 39. [Google Scholar] [CrossRef]
- Kim, K.M.; Lew, R.J.; Higashihara, T.J.; Yamashita, S.; Pang, M.; Stafford, M.; Goo, C.; Teehera, K.B.; Luu, K.; Ho, R.; et al. Differences in tumor size, clinical, demographic, and socioeconomic profiles of central nervous system tumors among a racially diverse cohort: A retrospective case-control study. Surg. Neurol. Int. 2024, 15, 459. [Google Scholar] [CrossRef]
- Jovčevska, I. Next Generation Sequencing and Machine Learning Technologies Are Painting the Epigenetic Portrait of Glioblastoma. Front. Oncol. 2020, 10, 798. [Google Scholar] [CrossRef]
- Onciul, R.; Tataru, C.-I.; Dumitru, A.V.; Crivoi, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Toader, C. Artificial Intelligence and Neuroscience: Transformative Synergies in Brain Research and Clinical Applications. J. Clin. Med. 2025, 14, 550. [Google Scholar] [CrossRef]
- Al Dahhan, N.Z.; Cox, E.; Nieman, B.J.; Mabbott, D.J. Cross-translational models of late-onset cognitive sequelae and their treatment in pediatric brain tumor survivors. Neuron 2022, 110, 2215–2241. [Google Scholar] [CrossRef] [PubMed]
- Billot, A.; Thiebaut de Schotten, M.; Parrish, T.B.; Thompson, C.K.; Rapp, B.; Caplan, D.; Kiran, S. Structural disconnections associated with language impairments in chronic post-stroke aphasia using disconnectome maps. Cortex 2022, 155, 90–106. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Joyce, M.K.P.; Uchendu, S.; Arnsten, A.F.T. Stress and Inflammation Target Dorsolateral Prefrontal Cortex Function: Neural Mechanisms Underlying Weakened Cognitive Control. Biol. Psychiatry 2025, 97, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, X.; Cheng, F.; Wang, S.; Li, C.; Zhou, D.; Zhang, W. Dorsolateral prefrontal cortex dysfunction caused by a go/no-go task in children with attention-deficit hyperactivity disorder: A functional near-infrared spectroscopy study. Front. Neurosci. 2023, 17, 1145485. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.R.; Luccarelli, J.; Praschan, N.; Fusunyan, M.; Fricchione, G.L. Molecular and Immunological Origins of Catatonia. Schizophr. Res. 2024, 263, 169–177. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.-G.; Li, J.; Zhang, Y.; Lv, Q.; Zeljic, K.; Gong, H.; Wei, H.; Liu, W.; Sun, B.; et al. Anterior limb of the internal capsule tractography: Relationship with capsulotomy outcomes in obsessive-compulsive disorder. J. Neurol. Neurosurg. Psychiatry 2021, 92, 637–644. [Google Scholar] [CrossRef]
- Zeltser, A.; Ochneva, A.; Riabinina, D.; Zakurazhnaya, V.; Tsurina, A.; Golubeva, E.; Berdalin, A.; Andreyuk, D.; Leonteva, E.; Kostyuk, G.; et al. EEG Techniques with Brain Activity Localization, Specifically LORETA, and Its Applicability in Monitoring Schizophrenia. J. Clin. Med. 2024, 13, 5108. [Google Scholar] [CrossRef]
- Abbate, C.; Trimarchi, P.D.; Fumagalli, G.G.; Gallucci, A.; Tomasini, E.; Fracchia, S.; Rebecchi, I.; Morello, E.; Fontanella, A.; Parisi, P.M.R.; et al. Diencephalic versus Hippocampal Amnesia in Alzheimer’s Disease: The Possible Confabulation-Misidentification Phenotype. J. Alzheimers Dis. 2023, 91, 363–388. [Google Scholar] [CrossRef]
- Long, Q.; Li, W.; Zhang, W.; Han, B.; Chen, Q.; Shen, L.; Liu, X. Electrical stimulation mapping in the medial prefrontal cortex induced auditory hallucinations of episodic memory: A case report. Front. Hum. Neurosci. 2022, 16, 815232. [Google Scholar] [CrossRef]
- Larroza, A.; Moratal, D.; D’ocón Alcañiz, V.; Arana, E.; por la Alzheimer’s Disease Neuroimaging Initiative. Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease. Neurol. Barc. Spain 2014, 29, 11–20. [Google Scholar] [CrossRef]
- Saviuk, M.; Sleptsova, E.; Redkin, T.; Turubanova, V. Unexplained Causes of Glioma-Associated Epilepsies: A Review of Theories and an Area for Research. Cancers 2023, 15, 5539. [Google Scholar] [CrossRef]
- Johnstone, B.; Cohen, D.; Dennison, A. The integration of sensations and mental experiences into a unified experience: A neuropsychological model for the “sense of self”. Neuropsychologia 2021, 159, 107939. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Hartwigsen, G.; Reid, A.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Left inferior parietal lobe engagement in social cognition and language. Neurosci. Biobehav. Rev. 2016, 68, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Peragallo, J.H. Visual function in children with primary brain tumors. Curr. Opin. Neurol. 2019, 32, 75–81. [Google Scholar] [CrossRef]
- Cantone, M.; Lanza, G.; Bella, R.; Pennisi, G.; Santalucia, P.; Bramanti, P.; Pennisi, M. Fear and disgust: Case report of two uncommon emotional disturbances evoked by visual disperceptions after a right temporal-insular stroke. BMC Neurol. 2019, 19, 193. [Google Scholar] [CrossRef]
- Tisserand, A.; Philippi, N.; Botzung, A.; Blanc, F. Me, Myself and My Insula: An Oasis in the Forefront of Self-Consciousness. Biology 2023, 12, 599. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Murphy, C.A.; Yudintsev, G.; Shinagawa, Y.; Banks, M.I.; Llano, D.A. Corticothalamic gating of population auditory thalamocortical transmission in mouse. eLife 2021, 10, e56645. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, P.; Gu, G.; Li, T.; Jiang, Z.; Wu, Z.; Wang, L.; Zhang, J.; Duan, Y.; Liu, Y.; et al. Brainstem tumors may increase the impairment of behavioral emotional cognition in children. J. Neurooncol. 2022, 160, 423–432. [Google Scholar] [CrossRef]
- Raikar, A.S.; Andrew, J.; Dessai, P.P.; Prabhu, S.M.; Jathar, S.; Prabhu, A.; Naik, M.B.; Raikar, G.V.S. Neuromorphic computing for modeling neurological and psychiatric disorders: Implications for drug development. Artif. Intell. Rev. 2024, 57, 318. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Ghadimi, K.; Abbas, I.; Karandish, A.; Crisman, C.; Eskandar, E.N.; Kobets, A.J. Cognitive Decline in Glioblastoma (GB) Patients with Different Treatment Modalities and Insights on Untreated Cases. Curr. Oncol. 2025, 32, 152. [Google Scholar] [CrossRef] [PubMed]
- Maschio, M.; Perversi, F.; Maialetti, A. Brain tumor-related epilepsy: An overview on neuropsychological, behavioral, and quality of life issues and assessment methodology. Front. Neurol. 2024, 15, 1480900. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Akeju, O.; Loggia, M.L.; Catana, C.; Pavone, K.J.; Vazquez, R.; Rhee, J.; Contreras Ramirez, V.; Chonde, D.B.; Izquierdo-Garcia, D.; Arabasz, G.; et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife 2014, 3, e04499. [Google Scholar] [CrossRef]
- Grimi, A.; Bono, B.C.; Lazzarin, S.M.; Marcheselli, S.; Pessina, F.; Riva, M. Gliomagenesis, Epileptogenesis, and Remodeling of Neural Circuits: Relevance for Novel Treatment Strategies in Low- and High-Grade Gliomas. Int. J. Mol. Sci. 2024, 25, 8953. [Google Scholar] [CrossRef]
- Dey, S.; Doddamani, R.S.; Banerjee Dixit, A.; Tripathi, M.; Sharma, M.C.; Chandra, P.S.; Banerjee, J. Altered Spontaneous Glutamatergic and GABAergic Activity in the Peritumoral Cortex of Low-Grade Gliomas Presenting with History of Seizures. Front. Neurosci. 2021, 15, 689769. [Google Scholar] [CrossRef]
- Alkhayat, D.; Khawaji, Z.Y.; Sunyur, A.M.; Sanyour, O.A.; Badawi, A.S. Overview of Paraneoplastic Autoantibody-Mediated Cognitive Impairment and Behavioral Changes: A Narrative Review. Cureus 2024, 16, e51787. [Google Scholar] [CrossRef]
- Fukushi, A.; Kim, H.-D.; Chang, Y.-C.; Kim, C.-H. Revisited Metabolic Control and Reprogramming Cancers by Means of the Warburg Effect in Tumor Cells. Int. J. Mol. Sci. 2022, 23, 10037. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 13302. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, X.; Lv, W.; Lai, H.-Y.; Shen, T. Interplay between the glymphatic system and neurotoxic proteins in Parkinson’s disease and related disorders: Current knowledge and future directions. Neural Regen. Res. 2023, 19, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Di Benedetto, S.; Müller, V. From Homeostasis to Neuroinflammation: Insights into Cellular and Molecular Interactions and Network Dynamics. Cells 2025, 14, 54. [Google Scholar] [CrossRef]
- Malta, T.M.; Sabedot, T.S.; Morosini, N.S.; Datta, I.; Garofano, L.; Vallentgoed, W.; Varn, F.S.; Aldape, K.; D’Angelo, F.; Bakas, S.; et al. The Epigenetic Evolution of Glioma Is Determined by the IDH1 Mutation Status and Treatment Regimen. Cancer Res. 2024, 84, 741–756. [Google Scholar] [CrossRef]
- van Kessel, E.; Berendsen, S.; Baumfalk, A.E.; Venugopal, H.; Krijnen, E.A.; Spliet, W.G.M.; van Hecke, W.; Giuliani, F.; Seute, T.; van Zandvoort, M.J.E.; et al. Tumor-related molecular determinants of neurocognitive deficits in patients with diffuse glioma. Neuro-Oncol. 2022, 24, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.R.; Sullaway, C.M.; Wefel, J.S. Depressive symptoms and executive function in relation to survival in patients with glioblastoma. J. Neurooncol. 2019, 142, 183–191. [Google Scholar] [CrossRef]
- Mastall, M.; Roth, P.; Bink, A.; Fischer Maranta, A.; Läubli, H.; Hottinger, A.F.; Hundsberger, T.; Migliorini, D.; Ochsenbein, A.; Seystahl, K.; et al. A phase Ib/II randomized, open-label drug repurposing trial of glutamate signaling inhibitors in combination with chemoradiotherapy in patients with newly diagnosed glioblastoma: The GLUGLIO trial protocol. BMC Cancer 2024, 24, 82. [Google Scholar] [CrossRef]
- Pasquini, L.; Peck, K.K.; Tao, A.; Del Ferraro, G.; Correa, D.D.; Jenabi, M.; Kobylarz, E.; Zhang, Z.; Brennan, C.; Tabar, V.; et al. Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization. Cancers 2023, 15, 836. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Z.; Cao, B.; Shi, Q.; Zhang, Z.; Fan, X.; Tang, Y.; Cheng, Z.; Wang, X.; Jing, S.; et al. Structural alterations of the salience network in patients with insular glioma. Brain Behav. 2023, 13, e2969. [Google Scholar] [CrossRef]
- Gong, F.; Jin, L.; Song, Q.; Yang, Z.; Chen, H.; Wu, J. Surgical techniques and function outcome for cingulate gyrus glioma, how we do it. Front. Oncol. 2022, 12, 986387. [Google Scholar] [CrossRef]
- Han, S.; Li, X.-X.; Wei, S.; Zhao, D.; Ding, J.; Xu, Y.; Yu, C.; Chen, Z.; Zhou, D.-S.; Yuan, T.-F. Orbitofrontal cortex-hippocampus potentiation mediates relief for depression: A randomized double-blind trial and TMS-EEG study. Cell Rep. Med. 2023, 4, 101060. [Google Scholar] [CrossRef]
- Rasgado-Toledo, J.; Issa-Garcia, V.; Alcalá-Lozano, R.; Garza-Villarreal, E.A.; González-Escamilla, G. Cortical and subcortical microstructure integrity changes after repetitive transcranial magnetic stimulation therapy in cocaine use disorder and relates to clinical outcomes. Addict. Biol. 2024, 29, e13381. [Google Scholar] [CrossRef] [PubMed]
- Beele, P.; Boelders, S.M.; Rutten, G.-J.M.; de Baene, W.; Gehring, K. Preoperative executive functioning impairments in patients with a meningioma: Does a frontal location matter? Brain Imaging Behav. 2024, 18, 989–1000. [Google Scholar] [CrossRef]
- Bokhari, S.A.; Elnoor, M.; Mansour, A.A.; Mustafa, K.; Osman, A.; Alhassan, A. Neuropsychiatric Manifestations of a Frontal Lobe Meningioma: A Case Report. Cureus 2024, 16, e68101. [Google Scholar] [CrossRef]
- Fawzi, H.A. Effects of Combined Metformin and Cabergoline in Comparison with Metformin Only Therapy on Ovarian and Hormonal Activities in Iraqi Patients with PCOS. Clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT05981742 (accessed on 21 February 2025).
- Auriemma, R.S.; Pirchio, R.; Pivonello, C.; Garifalos, F.; Colao, A.; Pivonello, R. Approach to the Patient with Prolactinoma. J. Clin. Endocrinol. Metab. 2023, 108, 2400–2423. [Google Scholar] [CrossRef]
- Shi, Z.; Cong, E.; Wu, Y.; Mei, X.; Wang, Y.; Peng, D. Case report: Treatment of psychiatric symptoms for an acromegalic patient with pituitary adenoma. Front. Psychiatry 2022, 13, 1068836. [Google Scholar] [CrossRef]
- Sigman, M.; Drury, K. Treatment Complexities of a Young Woman Suffering Psychosis and Pituitary Adenoma. Case Rep. Psychiatry 2011, 2011, 246820. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, C.-H.; Li, Y.-Z.; Zhou, J.-S.; Wang, S.-X.; Liu, M.-D.; Qiu, Z.-H.; Deng, C.; Ma, F.; Xia, C.-F.; et al. Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer. Nat. Med. 2024, 30, 1680–1688. [Google Scholar] [CrossRef]
- Araceli, T.; Fischl, A.; Haj, A.; Doenitz, C.; Stoerr, E.-M.; Hillberg, A.; Vogelhuber, M.; Rosengarth, K.; Riemenschneider, M.J.; Hau, P.; et al. Psycho-oncological burden in patients with brain metastases undergoing neurological surgery. Front. Oncol. 2024, 14, 1463467. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Pereira, J.; Garcia, A.R.; Figueira, I.; Malhó, R.; Brito, M.A. Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption. Int. J. Mol. Sci. 2021, 22, 7057. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.A.; Shamji, J.F.; Clem, M.A.; Perez, R.; Palka, J.M.; Stavinoha, P.L. Parent ratings of executive functioning in pediatric survivors of medulloblastoma and pilocytic astrocytoma. Appl. Neuropsychol. Child 2024, 13, 52–61. [Google Scholar] [CrossRef]
- Kautiainen, R.J.; Fox, M.E.; King, T.Z. The Neurological Predictor Scale Predicts Adaptive Functioning via Executive Dysfunction in Young Adult Survivors of Childhood Brain Tumor. J. Int. Neuropsychol. Soc. 2021, 27, 1–11. [Google Scholar] [CrossRef]
- Merck Sharp&Dohme LLC. A Three-Period Study to Evaluate the Effects of Modafinil on the Single-Dose Pharmacokinetics of MK-6552 in Healthy Male Participants. Clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06665230 (accessed on 21 February 2025).
- Clinical Academic Center (2CA-Braga). MODAFIMS: An Open-Label, Single-Center Clinical Trial to Evaluate Predictors of Response to MODAFinil in the Treatment of Cognitive Deficits in Patients with Multiple Sclerosis. Clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06592352 (accessed on 21 February 2025).
- Annigeri, B.; Priyankha, K.; Raman, R. Bilateral Thalamic Astrocytoma Presenting as Depression and Cognitive Impairment. Ann. Indian Psychiatry 2024, 8, 348–350. [Google Scholar] [CrossRef]
- Drexler, R.; Khatri, R.; Sauvigny, T.; Mohme, M.; Maire, C.L.; Ryba, A.; Zghaibeh, Y.; Dührsen, L.; Salviano-Silva, A.; Lamszus, K.; et al. A prognostic neural epigenetic signature in high-grade glioma. Nat. Med. 2024, 30, 1622–1635. [Google Scholar] [CrossRef]
- Rahme, G.J.; Javed, N.M.; Puorro, K.L.; Xin, S.; Hovestadt, V.; Johnstone, S.E.; Bernstein, B.E. Modeling epigenetic lesions that cause gliomas. Cell 2023, 186, 3674–3685.e14. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra Vega, S.; Olsson Bontell, T.; Kling, T.; Jakola, A.S.; Carén, H. Longitudinal DNA methylation analysis of adult-type IDH-mutant gliomas. Acta Neuropathol. Commun. 2023, 11, 23. [Google Scholar] [CrossRef]
- Yang, H. A Single-Center, Single-Arm Phase II Trial of Rituximab-Methotrexate-Temozolomide-Thiotepa (RMTT) Regimen as First-Line Therapy for Primary Central Nervous System Diffuse Large B-Cell Lymphomas(PCNS DLBCL). Clinicaltrials.gov. 2025. Available online: https://clinicaltrials.gov/study/NCT06832267 (accessed on 21 February 2025).
- Rita, C.G.; Nieto Gañan, I.; Jimenez Escrig, A.; Carrasco Sayalero, Á. Anti-N-Methyl-D-Aspartate Encephalitis as Paraneoplastic Manifestation of Germ-Cells Tumours: A Cases Report and Literature Review. Case Rep. Immunol. 2019, 2019, 4762937. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Jabbour, R.; Haydar, A.; Jaafar, F.; El Ayoubi, N.; Nawfal, O.; Beydoun, A. Case report: Rapid recovery after intrathecal rituximab administration in refractory anti-NMDA receptor encephalitis: Report of two cases. Front. Immunol. 2024, 15, 1369587. [Google Scholar] [CrossRef]
- Abilkaiyrkyzy, A.; Laamarti, F.; Hamdi, M.; Saddik, A.E. Dialogue System for Early Mental Illness Detection: Toward a Digital Twin Solution. IEEE Access 2024, 12, 2007–2024. [Google Scholar] [CrossRef]
- Garriga, R.; Mas, J.; Abraha, S.; Nolan, J.; Harrison, O.; Tadros, G.; Matic, A. Machine learning model to predict mental health crises from electronic health records. Nat. Med. 2022, 28, 1240–1248. [Google Scholar] [CrossRef]
- Rauch, P.; Stefanits, H.; Aichholzer, M.; Serra, C.; Vorhauer, D.; Wagner, H.; Böhm, P.; Hartl, S.; Manakov, I.; Sonnberger, M.; et al. Deep learning-assisted radiomics facilitates multimodal prognostication for personalized treatment strategies in low-grade glioma. Sci. Rep. 2023, 13, 9494. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Emmanuel, O.A.; Onifade, I.A.; Olotu, E.; Otorkpa, O.J.; Mehmood, Q.; Abdulai, S.I.; Jamiu, A.; Osinuga, A.; Oko, C.I.; et al. The role of machine learning in discovering biomarkers and predicting treatment strategies for neurodegenerative diseases: A narrative review. NeuroMarkers 2025, 2, 100034. [Google Scholar] [CrossRef]
- Liu, Y.; Sundman, M.H.; Ugonna, C.; Chen, Y.-C.A.; Green, J.M.; Haaheim, L.G.; Siu, H.M.; Chou, Y. Reproducible routes: Reliably navigating the connectome to enrich personalized brain stimulation strategies. Front. Hum. Neurosci. 2024, 18, 1477049. [Google Scholar] [CrossRef]
- Cătălina, G.R.; Gheorman, V.; Gheorman, V.; Forțofoiu, M.-C. The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare 2025, 13, 837. [Google Scholar] [CrossRef]
- Bond, K.M.; Curtin, L.; Ranjbar, S.; Afshari, A.E.; Hu, L.S.; Rubin, J.B.; Swanson, K.R. An image-based modeling framework for predicting spatiotemporal brain cancer biology within individual patients. Front. Oncol. 2023, 13, 1185738. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Lamers, F.; Jansen, R.; Berk, M.; Khandaker, G.M.; Picker, L.D.; Milaneschi, Y. Immuno-metabolic depression: From concept to implementation. Lancet Reg. Health-Eur. 2025, 48, 101166. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, F.; Zhang, L.; Hu, K.; Liu, S.; Zhong, S.; Yang, J.; Zeng, X.; Peng, X. Depression and Quality of Life in Patients with Gliomas: A Narrative Review. J. Clin. Med. 2022, 11, 4811. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Herr, K.; Jeon, H.J.; Kato, T.; Ng, C.H.; Yang, Y.K.; Zhang, L. A Delphi consensus on clinical features, diagnosis and treatment of major depressive disorder patients with anhedonia amongst psychiatrists in the Asia-Pacific. Front. Psychiatry 2024, 15, 1338063. [Google Scholar] [CrossRef]
- Grewal, E.P.; Richardson, L.G.K.; Sun, J.; Ramapriyan, R.; Martinez-Lage, M.; Miller, J.J.; Carter, B.S.; Cahill, D.P.; Curry, W.T.; Choi, B.D. Mutant IDH Modulates Suppressive Myeloid Populations in Malignant Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 4068–4076. [Google Scholar] [CrossRef]
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro-Oncol. 2023, 25, 4–25. [Google Scholar] [CrossRef]
- Akanuma, H.; Iizuka, T.; Abe, D.; Yoshida, K.; Matsuda, N.; Sugimoto, K.; Hashimoto, Y.; Kanai, K. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with small cell lung cancer and cytotoxic T-cell-mediated pathology: Case report. Front. Immunol. 2022, 13, 952868. [Google Scholar] [CrossRef]
- Nakhal, M.M.; Yassin, L.K.; Alyaqoubi, R.; Saeed, S.; Alderei, A.; Alhammadi, A.; Alshehhi, M.; Almehairbi, A.; Al Houqani, S.; BaniYas, S.; et al. The Microbiota–Gut–Brain Axis and Neurological Disorders: A Comprehensive Review. Life 2024, 14, 1234. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, K.; Zhu, W.; Li, H.; Zhu, W. Abnormal Cerebral Blood Flow and Functional Connectivity Strength in Subjects With White Matter Hyperintensities. Front. Neurol. 2021, 12, 752762. [Google Scholar] [CrossRef] [PubMed]
- Șovrea, A.S.; Boșca, A.B.; Dronca, E.; Constantin, A.-M.; Crintea, A.; Suflețel, R.; Ștefan, R.A.; Ștefan, P.A.; Onofrei, M.M.; Tschall, C.; et al. Non-Drug and Non-Invasive Therapeutic Options in Alzheimer’s Disease. Biomedicines 2025, 13, 84. [Google Scholar] [CrossRef]
- Kang, X.; Ge, Y.; Zhang, X.; Yang, T.; Xia, Y.; Wang, Y.; Li, J.; Chen, W.; Zhang, K.; Xiao, Z.; et al. Brain tumor and mood disorders: A retrospective analysis of anxiety and depression in patients with primary and metastatic brain tumors. Neurosurg. Rev. 2024, 48, 10. [Google Scholar] [CrossRef]
- McAfee, D.; Moyer, M.; Queen, J.; Mortazavi, A.; Boddeti, U.; Bachani, M.; Zaghloul, K.; Ksendzovsky, A. Differential metabolic alterations in IDH1 mutant vs. wildtype glioma cells promote epileptogenesis through distinctive mechanisms. Front. Cell. Neurosci. 2023, 17, 1288918. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Betts, M.J.; Hämmerer, D.; Düzel, E.; Mather, M.; Roiser, J.P.; Schneider, A.; Spottke, A.; Rostamzadeh, A.; Schott, B.H.; et al. Locus coeruleus signal intensity and emotion regulation in agitation in Alzheimer’s disease. Brain Commun. 2025, 7, fcae457. [Google Scholar] [CrossRef]
- Lin, J.; Liu, W.; Guan, J.; Cui, J.; Shi, R.; Wang, L.; Chen, D.; Liu, Y. Latest updates on the serotonergic system in depression and anxiety. Front. Synaptic Neurosci. 2023, 15, 1124112. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, L.; Alnajjar, O.; Bashar, H.; Abuarab, S.F.; Al-Adwan, R.; Chellappan, D.K.; Barakat, M. Corticosteroid-Induced Psychiatric Disorders: Mechanisms, Outcomes, and Clinical Implications. Diseases 2024, 12, 300. [Google Scholar] [CrossRef]
- Kessing, L.V. Why is lithium [not] the drug of choice for bipolar disorder? A controversy between science and clinical practice. Int. J. Bipolar Disord. 2024, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.; Wankhede, N.; Pawar, R.; Ballal, S.; Kumawat, R.; Goswami, M.; Khalid, M.; Taksande, B.; Upaganlawar, A.; Umekar, M.; et al. AI-driven innovations in Alzheimer’s disease: Integrating early diagnosis, personalized treatment, and prognostic modelling. Ageing Res. Rev. 2024, 101, 102497. [Google Scholar] [CrossRef]
- Toader, C.; Rădoi, P.M.; Ilie, M.-M.; Covache-Busuioc, R.-A.; Buica, V.; Glavan, L.-A.; Serban, M.; Corlatescu, A.D.; Crivoi, C.; Gorgan, R.M. Clinical Presentation, Treatment Outcomes, and Demographic Trends in Vestibular Schwannomas: A 135-Case Retrospective Study. J. Clin. Med. 2025, 14, 482. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, W. Optimization of antidepressant treatment by pharmacogenomics: A case report. BMC Psychiatry 2025, 25, 34. [Google Scholar] [CrossRef]
- Ghandour, F.; Squassina, A.; Karaky, R.; Diab-Assaf, M.; Fadda, P.; Pisanu, C. Presenting Psychiatric and Neurological Symptoms and Signs of Brain Tumors Before Diagnosis: A Systematic Review. Brain Sci. 2021, 11, 301. [Google Scholar] [CrossRef]
- Krix, S.; Wilczynski, E.; Falgàs, N.; Sánchez-Valle, R.; Yoles, E.; Nevo, U.; Baruch, K.; Fröhlich, H. Towards early diagnosis of Alzheimer’s disease: Advances in immune-related blood biomarkers and computational approaches. Front. Immunol. 2024, 15, 1343900. [Google Scholar] [CrossRef]
- Dutschke, L.L.; Steinau, S.; Wiest, R.; Walther, S. Brain Tumor-Associated Psychosis and Spirituality—A Case Report. Front. Psychiatry 2017, 8, 237. [Google Scholar] [CrossRef]
- Asmussen, L.; Frey, B.M.; Frontzkowski, L.K.; Wróbel, P.P.; Grigutsch, L.S.; Choe, C.; Bönstrup, M.; Cheng, B.; Thomalla, G.; Quandt, F.; et al. Dopaminergic mesolimbic structural reserve is positively linked to better outcome after severe stroke. Brain Commun. 2024, 6, fcae122, Correction in Brain Commun. 2024, 6, 191. [Google Scholar] [CrossRef]
- Lennox, B.; Yeeles, K.; Jones, P.B.; Zandi, M.; Joyce, E.; Yu, L.-M.; Tomei, G.; Pollard, R.; Vincent, S.-A.; Shimazaki, M.; et al. Intravenous immunoglobulin and rituximab versus placebo treatment of antibody-associated psychosis: Study protocol of a randomised phase IIa double-blinded placebo-controlled trial (SINAPPS2). Trials 2019, 20, 331. [Google Scholar] [CrossRef]
- Nicosia, N.; Giovenzana, M.; Misztak, P.; Mingardi, J.; Musazzi, L. Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders. Int. J. Mol. Sci. 2024, 25, 6521. [Google Scholar] [CrossRef]
- Rawani, N.S.; Chan, A.W.; Dursun, S.M.; Baker, G.B. The Underlying Neurobiological Mechanisms of Psychosis: Focus on Neurotransmission Dysregulation, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction. Antioxidants 2024, 13, 709. [Google Scholar] [CrossRef]
- Câmara-Pestana, P.; Magalhães, A.D.; Mendes, T.; Levy, P.; Coentre, R. Anti-NMDA Receptor Encephalitis Associated with an Ovarian Teratoma Presenting as First-episode Psychosis: A Case Report. J. Psychiatr. Pract. 2022, 28, 84–88. [Google Scholar] [CrossRef]
- Moura, M.; Silva-dos-Santos, A.; Afonso, J.; Talina, M. First-episode psychosis in a 15 year-old female with clinical presentation of anti-NMDA receptor encephalitis: A case report and review of the literature. BMC Res. Notes 2016, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Pacella, V.; Ricciardi, G.K.; Bonadiman, S.; Verzini, E.; Faraoni, F.; Scandola, M.; Moro, V. The Role of White Matter Disconnection in the Symptoms Relating to the Anarchic Hand Syndrome: A Single Case Study. Brain Sci. 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Ayyıldız, N.; Beyer, F.; Üstün, S.; Kale, E.H.; Mançe Çalışır, Ö.; Uran, P.; Öner, Ö.; Olkun, S.; Anwander, A.; Witte, A.V.; et al. Changes in the superior longitudinal fasciculus and anterior thalamic radiation in the left brain are associated with developmental dyscalculia. Front. Hum. Neurosci. 2023, 17, 1147352. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Luo, R.-Y.; Wang, M.-T.; Yuan, C.-Y.; Sun, Y.-Y.; Jing, J.-Y. Mechanisms underlying delirium in patients with critical illness. Front. Aging Neurosci. 2024, 16, 1446523. [Google Scholar] [CrossRef]
- Hayes, A.R.; Grossman, A.B. Distinguishing Cushing’s disease from the ectopic ACTH syndrome: Needles in a haystack or hiding in plain sight? J. Neuroendocrinol. 2022, 34, e13137. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood–brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Vazquez, E.A. Engineering and Technological Advancements in Repetitive Transcranial Magnetic Stimulation (rTMS): A Five-Year Review. Brain Sci. 2024, 14, 1092. [Google Scholar] [CrossRef]
- Humanis Saglık, A.S. An Open-Label, Balanced, Randomized, Two Treatment, Two Sequence, Two Period, Two Way Cross-over, Single Dose Bioequivalence Study of Perampanel Tablets 12 mg of Humanis Sağlık A.Ş., Turkey and Fycompa 12 mg Film-Coated Tablets of Eisai GmbH Edmund-Rumpler-Straße 3 60549 Frankfurt am Main Germany in Normal, Healthy, Adult, Human Subjects Under Fasting Condition. Clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06450223 (accessed on 21 February 2025).
- Chan, A. Repurposing Riluzole for Augmenting Brain-Derived Neuropathic Factor (BDNF) Levels and Cognitive Function in Breast Cancer Patients Experiencing Cancer-Related Cognitive Impairment: An Interventional Pilot Clinical Trial. Clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06580002 (accessed on 21 February 2025).
- Srivastav, A.; D’Souza, P.R.; Pai, K.; Shetty, S.B.; Aneja, S.; Ramesh, P.S. Neurological Conundrums: A Case Series on Brain Tumors Masquerading as Psychiatric Disorders. Indian J. Psychol. Med. 2025, 02537176251324471. [Google Scholar] [CrossRef]
- Pines, A.R.; Frandsen, S.B.; Drew, W.; Meyer, G.M.; Howard, C.; Palm, S.T.; Schaper, F.L.W.V.J.; Lin, C.; Butenko, K.; Ferguson, M.A.; et al. Mapping Lesions That Cause Psychosis to a Human Brain Circuit and Proposed Stimulation Target. JAMA Psychiatry 2025, 84, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, D.P.R.; Lino, F.; Ferrarese, D.; Belella, D.; Della Pepa, G.M.; Doglietto, F. Brain Tumor at Diagnosis: From Cognition and Behavior to Quality of Life. Diagnostics 2023, 13, 541. [Google Scholar] [CrossRef]
- Dubbelman, M.A.; Hendriksen, H.M.A.; Harrison, J.E.; Vijverberg, E.G.B.; Prins, N.D.; Kroeze, L.A.; Ottenhoff, L.; Van Leeuwenstijn, M.M.S.S.A.; Verberk, I.M.W.; Teunissen, C.E.; et al. Cognitive and Functional Change Over Time in Cognitively Healthy Individuals According to Alzheimer Disease Biomarker-Defined Subgroups. Neurology 2024, 102, e207978. [Google Scholar] [CrossRef] [PubMed]
- Grogans, S.E.; Bliss-Moreau, E.; Buss, K.A.; Clark, L.A.; Fox, A.S.; Keltner, D.; Cowen, A.S.; Kim, J.J.; Kragel, P.A.; MacLeod, C.; et al. The nature and neurobiology of fear and anxiety: State of the science and opportunities for accelerating discovery. Neurosci. Biobehav. Rev. 2023, 151, 105237. [Google Scholar] [CrossRef]
- Sansone, G.; Pini, L.; Salvalaggio, A.; Gaiola, M.; Volpin, F.; Baro, V.; Padovan, M.; Anglani, M.; Facchini, S.; Chioffi, F.; et al. Patterns of gray and white matter functional networks involvement in glioblastoma patients: Indirect mapping from clinical MRI scans. Front. Neurol. 2023, 14, 1175576. [Google Scholar] [CrossRef]
- Huey, E.D. A Critical Review of Behavioral and Emotional Disinhibition. J. Nerv. Ment. Dis. 2020, 208, 344–351. [Google Scholar] [CrossRef]
- Sun, S.; Yu, H.; Yu, R.; Wang, S. Functional connectivity between the amygdala and prefrontal cortex underlies processing of emotion ambiguity. Transl. Psychiatry 2023, 13, 334. [Google Scholar] [CrossRef]
- Hertrich, I.; Dietrich, S.; Blum, C.; Ackermann, H. The Role of the Dorsolateral Prefrontal Cortex for Speech and Language Processing. Front. Hum. Neurosci. 2021, 15, 645209. [Google Scholar] [CrossRef] [PubMed]
- De Baene, W.; Jansma, M.J.; Schouwenaars, I.T.; Rutten, G.-J.M.; Sitskoorn, M.M. Task-evoked reconfiguration of the fronto-parietal network is associated with cognitive performance in brain tumor patients. Brain Imaging Behav. 2020, 14, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.C.; Fahed, M.; Manning, K.J.; Wang, L. The neurobiology of apathy in depression and neurocognitive impairment in older adults: A review of epidemiological, clinical, neuropsychological and biological research. Transl. Psychiatry 2022, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Neacsiu, A.D.; Szymkiewicz, V.; Galla, J.T.; Li, B.; Kulkarni, Y.; Spector, C.W. The neurobiology of misophonia and implications for novel, neuroscience-driven interventions. Front. Neurosci. 2022, 16, 893903. [Google Scholar] [CrossRef]
- Di Tella, S.; De Marco, M.; Baglio, F.; Silveri, M.C.; Venneri, A. Resting-state functional connectivity is modulated by cognitive reserve in early Parkinson’s disease. Front. Psychol. 2023, 14, 1207988. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Lopriore, P.; Pace, A.P.; Latino, R.R.; Assogna, M.; Mancuso, M.; Gragnaniello, D.; Granieri, E.; Pugliatti, M.; et al. Frontotemporal Dementia, Where Do We Stand? A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11732. [Google Scholar] [CrossRef]
- Noll, K.R.; Ziu, M.; Weinberg, J.S.; Wefel, J.S. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J. Neurooncol. 2016, 128, 323–331. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Etter, G.; Carmichael, J.E.; Williams, S. Linking temporal coordination of hippocampal activity to memory function. Front. Cell. Neurosci. 2023, 17, 1233849. [Google Scholar] [CrossRef]
- Forbes, E.; Hassien, A.; Tan, R.J.; Wang, D.; Lega, B. Modulation of hippocampal theta oscillations via deep brain stimulation of the parietal cortex depends on cognitive state. Cortex J. Devoted Study Nerv. Syst. Behav. 2024, 175, 28–40. [Google Scholar] [CrossRef]
- Schurz, M.; Berenz, J.-P.; Maerz, J.; Perla, R.; Buchheim, A.; Labek, K. Brain Activation for Social Cognition and Emotion Processing Tasks in Borderline Personality Disorder: A Meta-Analysis of Neuroimaging Studies. Brain Sci. 2024, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Sabharwal, A.; Kotov, R.; Szekely, A.; Parsey, R.; Barch, D.M.; Mohanty, A. Disconnection Between Amygdala and Medial Prefrontal Cortex in Psychotic Disorders. Schizophr. Bull. 2016, 42, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Nardini, D.; Melchiorre, S.; Ciprietti, C.; Polito, G.; Punzi, M.; Dono, F.; Santilli, M.; Thomas, A.; Sensi, S.L.; et al. Predicting conversion in cognitively normal and mild cognitive impairment individuals with machine learning: Is the CSF status still relevant? Alzheimers Dement. J. Alzheimers Assoc. 2025, 21, e14398. [Google Scholar] [CrossRef]
- Khatri, D.K.; Kadbhane, A.; Patel, M.; Nene, S.; Atmakuri, S.; Srivastava, S.; Singh, S.B. Gauging the role and impact of drug interactions and repurposing in neurodegenerative disorders. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100022. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef]
- Fatemizadeh, M.; Riahi, E.; Hassanzadeh, G.; Torkaman-Boutorabi, A.; Radfar, F.; Farahmandfar, M. Deep brain stimulation of the anterior cingulate cortex reduces opioid addiction in preclinical studies. Sci. Rep. 2025, 15, 2065. [Google Scholar] [CrossRef]
- Fountzilas, E.; Pearce, T.; Baysal, M.A.; Chakraborty, A.; Tsimberidou, A.M. Convergence of evolving artificial intelligence and machine learning techniques in precision oncology. NPJ Digit. Med. 2025, 8, 75. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Schwarz, K.; Schmitz, F. Synapse Dysfunctions in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Walz, A.; Restaino, A.C.; Amit, M.; Barclay, S.M.; Vichaya, E.G.; Spanos, W.C.; Dantzer, R.; Talbot, S.; Vermeer, P.D. Tumor-infiltrating nerves functionally alter brain circuits and modulate behavior in a mouse model of head-and-neck cancer. eLife 2024, 13, RP97916. [Google Scholar] [CrossRef]
- Franczak, Ł.; Podwalski, P.; Wysocki, P.; Dawidowski, B.; Jędrzejewski, A.; Jabłoński, M.; Samochowiec, J. Impulsivity in ADHD and Borderline Personality Disorder: A Systematic Review of Gray and White Matter Variations. J. Clin. Med. 2024, 13, 6906. [Google Scholar] [CrossRef]
- Xie, M.; Qin, H.; Liu, L.; Wu, J.; Zhao, Z.; Zhao, Y.; Fang, Y.; Yu, X.; Su, C. GABA regulates metabolic reprogramming to mediate the development of brain metastasis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2025, 44, 61. [Google Scholar] [CrossRef] [PubMed]
- Anziano, M.; Mouthon, M.; Thoeny, H.; Sperber, C.; Spierer, L. Mental flexibility depends on a largely distributed white matter network: Causal evidence from connectome-based lesion-symptom mapping. Cortex 2023, 165, 38–56. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Rudie, J.D.; Weiss, D.A.; Saluja, R.; Rauschecker, A.M.; Wang, J.; Sugrue, L.; Bakas, S.; Colby, J.B. Multi-Disease Segmentation of Gliomas and White Matter Hyperintensities in the BraTS Data Using a 3D Convolutional Neural Network. Front. Comput. Neurosci. 2019, 13, 84. [Google Scholar] [CrossRef]
- Vollmuth, P.; Foltyn, M.; Huang, R.Y.; Galldiks, N.; Petersen, J.; Isensee, F.; van den Bent, M.J.; Barkhof, F.; Park, J.E.; Park, Y.W.; et al. Artificial intelligence (AI)-based decision support improves reproducibility of tumor response assessment in neuro-oncology: An international multi-reader study. Neuro-Oncol. 2023, 25, 533–543. [Google Scholar] [CrossRef]
- Chauveau, F.; Winkeler, A.; Chalon, S.; Boutin, H.; Becker, G. PET imaging of neuroinflammation: Any credible alternatives to TSPO yet? Mol. Psychiatry 2025, 30, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Dahl, K.; Dudley-Jones, R.; Schiöth, H.B. A neuroinflammatory compulsivity model of anorexia nervosa (NICAN). Neurosci. Biobehav. Rev. 2024, 159, 105580. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, N.; Sherry, C.; Zimmerman, J.; Park, H.; Lewis, C.; Donnenberg, A.; Zaidi, A.H.; Fan, Y.; Xiao, K.; Bartlett, D.; et al. Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis. J. Transl. Med. 2024, 22, 402. [Google Scholar] [CrossRef]
- Wang, S.; Yin, F.; Guo, Z.; Li, R.; Sun, W.; Wang, Y.; Geng, Y.; Sun, C.; Sun, D. Association between gut microbiota and glioblastoma: A Mendelian randomization study. Front. Genet. 2024, 14, 1308263. [Google Scholar] [CrossRef]
- Osuch, B.; Misztal, T.; Pałatyńska, K.; Tomaszewska-Zaremba, D. Implications of Kynurenine Pathway Metabolism for the Immune System, Hypothalamic–Pituitary–Adrenal Axis, and Neurotransmission in Alcohol Use Disorder. Int. J. Mol. Sci. 2024, 25, 4845. [Google Scholar] [CrossRef]
- Goudarzi, N.; Taheri, Z.; Nezhad Salari, A.M.; Kazemzadeh, K.; Tafakhori, A. Recognition and classification of facial expression using artificial intelligence as a key of early detection in neurological disorders. Rev. Neurosci. 2025, 36, 479–495. [Google Scholar] [CrossRef]
- Corominas-Teruel, X.; Bracco, M.; Fibla, M.; Segundo, R.M.S.; Villalobos-Llaó, M.; Gallea, C.; Beranger, B.; Toba, M.; Valero-Cabré, A.; Colomina, M.T. High-density transcranial direct current stimulation to improve upper limb motor function following stroke: Study protocol for a double-blind randomized clinical trial targeting prefrontal and/or cerebellar cognitive contributions to voluntary motion. Trials 2023, 24, 783. [Google Scholar] [CrossRef]
- Samman, R.R.; Timraz, J.H.; Al-Nakhli, A.M.; Haidar, S.; Muhammad, Q.; Thalib, H.I.; Mousa, A.H.; Kharoub, M.S. The Impact of Brain Tumors on Emotional and Behavioral Functioning. Cureus 2024, 16, e75315. [Google Scholar] [CrossRef]
- Taslim, S.; Shadmani, S.; Saleem, A.R.; Kumar, A.; Brahma, F.; Blank, N.; Bashir, M.A.; Ansari, D.; Kumari, K.; Tanveer, M.; et al. Neuropsychiatric Disorders: Bridging the Gap Between Neurology and Psychiatry. Cureus 2024, 16, e51655. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, W.; Deng, J.; Shi, Y.; Nie, W.; Luo, S.; Zhang, H. Exploring dysconnectivity of the large-scale neurocognitive network across psychiatric disorders using spatiotemporal constrained nonnegative matrix factorization method. Cereb. Cortex 2022, 32, 4576–4591. [Google Scholar] [CrossRef]
- Parker, N.; Cheng, W.; Hindley, G.F.L.; Parekh, P.; Shadrin, A.A.; Maximov, I.I.; Smeland, O.B.; Djurovic, S.; Dale, A.M.; Westlye, L.T.; et al. Psychiatric disorders and brain white matter exhibit genetic overlap implicating developmental and neural cell biology. Mol. Psychiatry 2023, 28, 4924–4932. [Google Scholar] [CrossRef] [PubMed]
- Justiz-Vaillant, A.A.; Gopaul, D.; Soodeen, S.; Arozarena-Fundora, R.; Barbosa, O.A.; Unakal, C.; Thompson, R.; Pandit, B.; Umakanthan, S.; Akpaka, P.E. Neuropsychiatric Systemic Lupus Erythematosus: Molecules Involved in Its Imunopathogenesis, Clinical Features, and Treatment. Molecules 2024, 29, 747. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Waisberg, E.; Ong, J.; Paladugu, P.; Amiri, D.; Saintyl, J.; Yelamanchi, J.; Nahouraii, R.; Jagadeesan, R.; Tavakkoli, A. Artificial Intelligence-Based Methodologies for Early Diagnostic Precision and Personalized Therapeutic Strategies in Neuro-Ophthalmic and Neurodegenerative Pathologies. Brain Sci. 2024, 14, 1266. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Boele, F.W.; Rooney, A.G.; Grant, R.; Klein, M. Psychiatric symptoms in glioma patients: From diagnosis to management. Neuropsychiatr. Dis. Treat. 2015, 11, 1413–1420. [Google Scholar] [CrossRef]
- Pilarska, A.; Pieczyńska, A.; Hojan, K. Neuropsychological monitoring of cognitive function and ICF–based mental components in patients with malignant brain tumours. Front. Psychol. 2023, 14, 1033185. [Google Scholar] [CrossRef]
- Nishiura, S.; Miyawaki, D.; Hirai, K.; Sukigara, A.; Kakishita, Y.; Inoue, K. Association between hallucinations and sensory processing difficulties in children and adolescents. Front. Psychiatry 2024, 15, 1472328. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.N.; Jackson, G.D. The Piriform Cortex and Human Focal Epilepsy. Front. Neurol. 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.; Russo, R.; Schimperna, F.; D’Apolito, G.; Panfili, M.; Grimaldi, A.; Perna, A.; Ferranti, A.M.; Varcasia, G.; Giordano, C.; et al. Magnetic Resonance Imaging of Primary Adult Brain Tumors: State of the Art and Future Perspectives. Biomedicines 2023, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Aarabi, M.H. Functional and structural lesion network mapping in neurological and psychiatric disorders: A systematic review. Front. Neurol. 2023, 14, 1100067. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rossi, F.; Benes Lima, L.; King, C. Frontotemporal disorders: The expansive panoply of syndromes and spectrum of etiologies. Front. Neurol. 2024, 14, 1305071. [Google Scholar] [CrossRef]
- Gonçalves, S.d.A.B.; Caramelli, P.; Mariano, L.I.; Guimarães, H.C.; Gambogi, L.B.; Resende, E.d.P.F.; Teixeira, A.L.; de Souza, L.C. Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. 2020, 1737, 146799. [Google Scholar] [CrossRef]
- Ninatti, G.; Pini, C.; Gelardi, F.; Sollini, M.; Chiti, A. The Role of PET Imaging in the Differential Diagnosis Between Radiation Necrosis and Recurrent Disease in Irradiated Adult-Type Diffuse Gliomas: A Systematic Review. Cancers 2023, 15, 364. [Google Scholar] [CrossRef]
- Hussain, D.; Abbas, N.; Khan, J. Recent Breakthroughs in PET-CT Multimodality Imaging: Innovations and Clinical Impact. Bioengineering 2024, 11, 1213. [Google Scholar] [CrossRef]
- Karami Fath, M.; Azami, J.; Masoudi, A.; Mosaddeghi Heris, R.; Rahmani, E.; Alavi, F.; Alagheband Bahrami, A.; Payandeh, Z.; Khalesi, B.; Dadkhah, M.; et al. Exosome-based strategies for diagnosis and therapy of glioma cancer. Cancer Cell Int. 2022, 22, 262. [Google Scholar] [CrossRef]
- Weng, S.; Lai, Q.-L.; Wang, J.; Zhuang, L.; Cheng, L.; Mo, Y.; Liu, L.; Zhao, Z.; Zhang, Y.; Qiao, S. The Role of Exosomes as Mediators of Neuroinflammation in the Pathogenesis and Treatment of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 899944. [Google Scholar] [CrossRef]
- Luarte, A.; Nardocci, G.; Chakraborty, A.; Batiz, L.F.; Pino-Lagos, K.; Wyneken, Ú. Astrocyte-derived extracellular vesicles in stress-associated mood disorders. Does the immune system get astrocytic? Pharmacol. Res. 2023, 194, 106833. [Google Scholar] [CrossRef] [PubMed]
- Dalboni da Rocha, J.L.; Lai, J.; Pandey, P.; Myat, P.S.M.; Loschinskey, Z.; Bag, A.K.; Sitaram, R. Artificial Intelligence for Neuroimaging in Pediatric Cancer. Cancers 2025, 17, 622. [Google Scholar] [CrossRef]
- Özge, A.; Domaç, F.M.; Tekin, N.; Sünbül, E.A.; Öksüz, N.; Atalar, A.Ç.; Çallı, S.Y.; Fidan, Y.S.; Evlice, A.; Beştepe, E.E.; et al. One Patient, Three Providers: A Multidisciplinary Approach to Managing Common Neuropsychiatric Cases. J. Clin. Med. 2023, 12, 5754. [Google Scholar] [CrossRef]
- Moroşan, G.-C.; Moroşan, A.-C.; Ionescu, C.; Sava, A. Neuropsychiatric symptoms as early indicators of brain tumors. Arch. Clin. Cases 2024, 11, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Banik, A.; Saurabh, S.; Maulik, M.; Khatri, S.N. Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders. J. Neurosci. 2022, 42, 1888–1907. [Google Scholar] [CrossRef]
- Majeed, J.; Sabbagh, M.N.; Kang, M.H.; Lawrence, J.J.; Pruitt, K.; Bacus, S.; Reyna, E.; Brown, M.; Decourt, B. Cancer drugs with high repositioning potential for Alzheimer’s disease. Expert Opin. Emerg. Drugs 2023, 28, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Berk, M.; Bloch, G.; Briquet-Laugier, V.; Brouillon, C.; Cuthbert, B.N.; Dantzer, R.; Davis, M.C.; De Picker, L.J.; Drevets, W.C.; et al. Advancing precision psychiatry and targeted treatments: Insights from immunopsychiatry. Brain. Behav. Immun. 2025, 125, 319–329. [Google Scholar] [CrossRef]
- Knabbe, J.; Kowalski, T.; Seliger, C. Pharmacological treatment of depression in patients with brain tumors. Int. J. Cancer 2024, 155, 1533–1543. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Ciccarelli, M.; De Simone, G.; Mazza, B.; Barone, A.; Vellucci, L. Canonical and Non-Canonical Antipsychotics’ Dopamine-Related Mechanisms of Present and Next Generation Molecules: A Systematic Review on Translational Highlights for Treatment Response and Treatment-Resistant Schizophrenia. Int. J. Mol. Sci. 2023, 24, 5945. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Laszlovszky, I.; Krámos, B.; Visegrády, A.; Bobok, A.; Lévay, G.; Lendvai, B.; Román, V. Neuronal Dopamine D3 Receptors: Translational Implications for Preclinical Research and CNS Disorders. Biomolecules 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, A.; Saguin, E.; Balcerac, A.; Mouchabac, S.; Ferreri, F.; Gaillard, R.; Colas, M.-D.; Delacour, H.; Bourla, A. Pharmacogenetic Guidelines for Psychotropic Drugs: Optimizing Prescriptions in Clinical Practice. Pharmaceutics 2023, 15, 2540. [Google Scholar] [CrossRef]
- Jiao, W.; Lin, J.; Deng, Y.; Ji, Y.; Liang, C.; Wei, S.; Jing, X.; Yan, F. The immunological perspective of major depressive disorder: Unveiling the interactions between central and peripheral immune mechanisms. J. Neuroinflamm. 2025, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Basak, S.; Chowdhury, P.; Bhowmik, P.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Targeting Cytokine-Mediated Inflammation in Brain Disorders: Developing New Treatment Strategies. Pharmaceuticals 2025, 18, 104. [Google Scholar] [CrossRef]
- Wang, Q.T. Effect of Oral Minocycline on Acute Stroke Outcome: A Randomized Open Label Prospective Study. Clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT06107725 (accessed on 21 February 2025).
- Karmakar, S.; Lal, G. Role of Serotonergic System in Regulating Brain Tumor-Associated Neuroinflammatory Responses. Methods Mol. Biol. 2024, 2761, 181–207. [Google Scholar] [CrossRef]
- Zoon, T.J.C.; van Rooijen, G.; Contarino, M.F.; van der Gaag, S.; Zutt, R.; van Asseldonk, J.T.; van den Munckhof, P.; Schuurman, P.R.; Denys, D.A.J.P.; de Bie, R.M.A. A multicenter double-blind randomized crossover study comparing the impact of dorsal subthalamic nucleus deep brain stimulation versus standard care on apathy in Parkinson’s disease: A study protocol. Trials 2024, 25, 104. [Google Scholar] [CrossRef]
- van Dellen, E. Precision psychiatry: Predicting predictability. Psychol. Med. 2024, 54, 1500–1509. [Google Scholar] [CrossRef]
- Chmiel, J.; Stępień-Słodkowska, M.; Ramik-Mażewska, I. Efficacy of Transcranial Direct Current Stimulation (tDCS) on Neuropsychiatric Symptoms in Substance Use Disorder (SUD)—A Review and Insights into Possible Mechanisms of Action. J. Clin. Med. 2025, 14, 1337. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The social determinants of mental health and disorder: Evidence, prevention and recommendations. World Psychiatry 2024, 23, 58–90. [Google Scholar] [CrossRef]
- Okpete, U.E.; Byeon, H. Challenges and prospects in bridging precision medicine and artificial intelligence in genomic psychiatric treatment. World J. Psychiatry 2024, 14, 1148–1164. [Google Scholar] [CrossRef]
- Taylor, S.M.; Tumin, D.; Tiu, L.C.; Patel, P.S.; Honaker, M.D. Predictors and Outcomes of Mental Health Conditions Among Patients with Colorectal Cancer. J. Gastrointest. Cancer 2024, 56, 20. [Google Scholar] [CrossRef]
- Perry, L.M.; Kleber, K.T.; Rajasekar, G.; Nuño, M.; Bold, R.J. The Impact of Pre-Existing Psychiatric Disorders on Outcomes After Pancreatic Cancer Surgery. Pancreas 2022, 51, 1376–1380. [Google Scholar] [CrossRef]
- Habimana, S.; Biracyaza, E.; Mpunga, T.; Nsabimana, E.; Kayitesi, F.; Nzamwita, P.; Jansen, S. Prevalence and associated factors of depression and anxiety among patients with cancer seeking treatment at the Butaro Cancer Center of Excellence in Rwanda. Front. Public Health 2023, 11, 972360. [Google Scholar] [CrossRef]
- Balan, I.; Boero, G.; Chéry, S.L.; McFarland, M.H.; Lopez, A.G.; Morrow, A.L. Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life 2024, 14, 582. [Google Scholar] [CrossRef]
- Sic, A.; Cvetkovic, K.; Manchanda, E.; Knezevic, N.N. Neurobiological Implications of Chronic Stress and Metabolic Dysregulation in Inflammatory Bowel Diseases. Diseases 2024, 12, 220. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Z.; Meng, Y.; Geng, L.; Lian, H.; Shi, Z.; Zhuang, Z.; Cai, W.; He, M. Neural mechanisms underlying cognitive impairment in depression and cognitive benefits of exercise intervention. Behav. Brain Res. 2025, 476, 115218. [Google Scholar] [CrossRef] [PubMed]
- Baydili, İ.; Tasci, B.; Tasci, G. Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses. Diagnostics 2025, 15, 434. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Aljboor, G.S.R.; Costin, H.P.; Corlatescu, A.D.; Glavan, L.-A.; Gorgan, R.M. Cerebellar Cavernoma Resection: Case Report with Long-Term Follow-Up. J. Clin. Med. 2024, 13, 7525. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Mondragon-Soto, M.G.; Altawalbeh, G.; Baumgart, L.; Gempt, J.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Aftahy, A.K. Enhancing outcomes: Neurosurgical resection in brain metastasis patients with poor Karnofsky performance score—A comprehensive survival analysis. Front. Oncol. 2024, 13, 1343500. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Raid, S.; Palm, J.; Rübe, C. Radiation-Induced Brain Injury: Age Dependency of Neurocognitive Dysfunction Following Radiotherapy. Cancers 2023, 15, 2999. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060, Correction in Lancet Neurol. 2022, 21, e10. [Google Scholar] [CrossRef]
- Chiu, F.-Y.; Yen, Y. Imaging biomarkers for clinical applications in neuro-oncology: Current status and future perspectives. Biomark. Res. 2023, 11, 35. [Google Scholar] [CrossRef]
- Crooms, R.C.; Nnemnbeng, J.F.; Taylor, J.W.; Goldstein, N.E.; Gorbenko, K.; Vickrey, B.G. Clinician perspectives on integrating neuro-oncology and palliative care for patients with high-grade glioma. Neuro-Oncol. Pract. 2024, 11, 404–412. [Google Scholar] [CrossRef]
- Sabeghi, P.; Zarand, P.; Zargham, S.; Golestany, B.; Shariat, A.; Chang, M.; Yang, E.; Rajagopalan, P.; Phung, D.C.; Gholamrezanezhad, A. Advances in Neuro-Oncological Imaging: An Update on Diagnostic Approach to Brain Tumors. Cancers 2024, 16, 576. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: State of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14, 1161. [Google Scholar] [CrossRef]
- Mark, I.; Poole, N.; Agrawal, N. Integration of neuroscience into psychiatric training and practice: Suggestions for implementation. BJPsych Bull. 2025, 49, 278–284. [Google Scholar] [CrossRef]
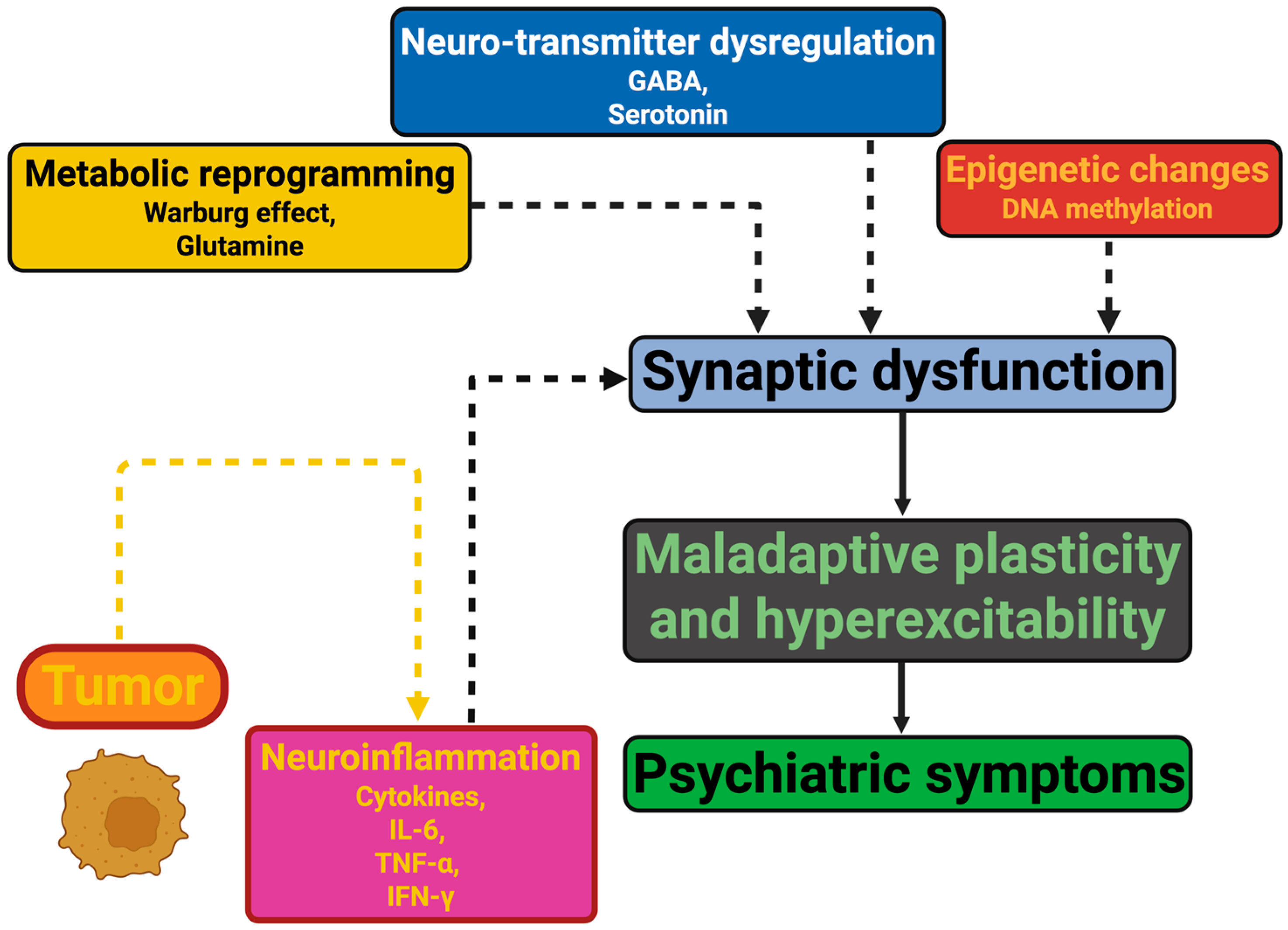
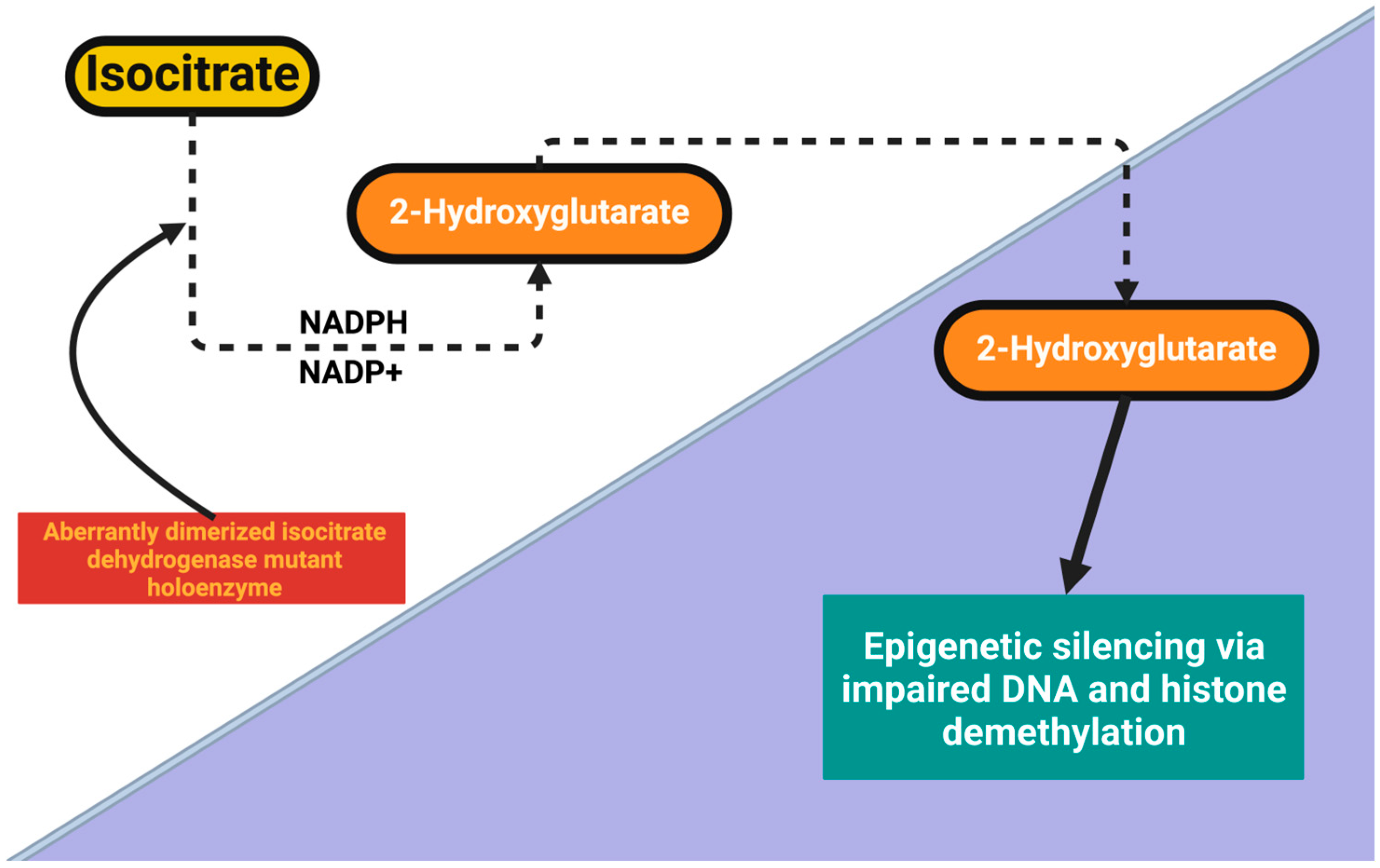
| Tumor Type | Psychiatric Manifestations | Pathophysiological Insights | Key Findings and Clinical Implications | References |
|---|---|---|---|---|
| Glioblastoma (GBM) | Depression, executive dysfunction, emotional blunting | Glioma-induced glutamate excess disrupts fronto-striatal circuits | High glutamate levels in peritumoral regions correlate with depressive symptoms; NMDA receptor antagonists (memantine) show promise in symptom reduction. | [66,67,68] |
| Low-Grade Gliomas | Personality changes, apathy, cognitive rigidity | Disruption of fronto-limbic pathways and anterior cingulate hypometabolism | Functional MRI (fMRI) reveals decreased ACC activation; dopaminergic modulation with pramipexole improves motivation and goal-directed behavior. | [69,70,71] |
| Meningioma (Frontal and Orbitofrontal) | Disinhibition, impulsivity, hypersexuality, compulsive behaviors | Orbitofrontal cortex compression leads to impaired impulse control and social cognition | High-resolution tractography demonstrates disconnection of the uncinate fasciculus in symptomatic patients; neuromodulation (TMS) targeting the DLPFC improves inhibitory control. | [72,73,74,75] |
| Pituitary Macroadenomas | Psychosis, mood instability, cognitive slowing | Endocrine dysfunction (hyperprolactinemia, hypercortisolism) induces dopaminergic dysregulation | Dopamine agonists (cabergoline) reverse psychotic symptoms in prolactinomas, but cognitive impairments persist in Cushing’s patients’ post-surgery. | [76,77,78,79] |
| Brain Metastases (Lung, Breast, Melanoma) | Anxiety, panic attacks, depressive symptoms, cognitive deficits | Multifocal disruption of limbic circuits, BBB permeability alterations, paraneoplastic autoantibodies | Higher systemic IL-6 and TNF-α levels correlate with severe affective disturbances; checkpoint inhibitors exacerbate neuropsychiatric dysfunction in 20% of cases. | [80,81,82] |
| Medulloblastoma (Pediatric Tumors) | Executive dysfunction, emotional dysregulation, ADHD-like symptoms | Cerebellar-prefrontal network disruption impairs cognitive flexibility | Post-treatment ADHD-like symptoms linked to cerebellar dysfunction; targeted cognitive rehabilitation improves attention and executive function. | [83,84] |
| Thalamic Gliomas | Apathy, psychomotor slowing, altered consciousness states | Thalamic relay dysfunction impairs arousal and motivation circuits | PET imaging demonstrates severe metabolic hypoactivity in anterior thalamus correlating with anhedonia; stimulant-based interventions (modafinil) improve alertness. | [85,86,87] |
| Glioblastoma (IDH-Wildtype vs. IDH-Mutant) | Cognitive impairment, depression, emotional blunting | IDH-mutant gliomas exhibit preserved neurocognitive function compared to IDH-wildtype | Epigenetic changes in IDH-mutant gliomas lead to reduced psychiatric burden; DNA methylation therapies show promise for cognitive preservation. | [88,89,90] |
| Anti-NMDA Receptor Paraneoplastic Encephalitis (Ovarian Teratomas, Small-Cell Lung Cancer) | Severe psychosis, aggression, catatonia, autonomic instability | Autoimmune attack on NMDA receptors disrupts excitatory neurotransmission | Plasma exchange and immunotherapy (rituximab) rapidly resolve psychiatric symptoms; early diagnosis via CSF biomarkers is crucial. | [91,92,93] |
| Multiple Tumor Types | Predictive modeling of psychiatric deterioration | Machine learning applied to multimodal imaging, speech, and inflammatory markers | AI model predicts psychiatric symptom emergence 3–6 months before clinical manifestation with 85% accuracy; potential for preemptive psychiatric intervention. | [94,95,96] |
| Modality/Intervention | Primary Target or Principle | Clinical Readiness | Advantages | Limitations/Considerations |
|---|---|---|---|---|
| MR Venography (MRV) | Visualization of venous sinus patency and flow | Widely available | Non-invasive, high spatial resolution | May miss dynamic changes; contrast use in some protocols |
| Intracranial Pressure Monitoring | Continuous measurement of ICP waveform and amplitude | Routine in select neurosurgical settings | Direct physiological data | Invasive, risk of infection |
| Diffusion Tensor Imaging (DTI) | Assessment of white matter integrity and AQP4-related changes | Research to early clinical adoption | Detects microstructural changes | Requires advanced analysis; interpretation variability |
| CSF Cytokine Panel | Measurement of inflammatory mediators | Research use | Provides immunological insight | Limited availability; unclear clinical thresholds |
| Venous Sinus Stenting | Restoration of venous outflow | Specialized centers | Rapid ICP reduction in selected cases | Procedural risk; not universally applicable |
| CRISPR-Mediated AQP4 Repair | Gene editing to restore perivascular localization | Preclinical | Targeted, potentially disease-modifying | Long-term safety unknown; ethical considerations |
| Aquaporin Modulators | Pharmacologic modulation of AQP4 activity | Early clinical/experimental | Non-invasive, adaptable | Off-target effects; variable patient response |
| Anti-inflammatory Biologics | Modulation of microglial and cytokine activity | Limited clinical evidence | Addresses inflammatory component | Cost, immuno-suppression risk |
| Pathophysiological Node | Molecular Mechanisms | Diagnostic Signatures | Therapeutic Strategies | Evidence Level |
|---|---|---|---|---|
| Venous outflow impairment | Jugular/venous sinus congestion, ↑ central venous pressure | MRV, jugular Doppler, ICP waveform | Endovascular stenting, CSF shunting | Clinical series |
| AQP4 depolarization | Loss of perivascular localization, altered astrocytic endfeet | Immunohistochemistry, DTI | CRISPR-mediated repair, gene therapy | Preclinical |
| Glymphatic slowdown | Reduced CSF–ISF exchange, protein aggregation | Contrast MRI, PET tracers | Sleep optimization, aquaporin modulators | Mixed |
| Neuroinflammation | Microglial activation, cytokine release | PET (TSPO), CSF cytokine panel | Anti-inflammatory biologics | Early clinical |
| Global access gap | Limited imaging, surgical resources | Health system mapping | Low-cost diagnostics, task-shifting | Policy/implementation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers. Int. J. Mol. Sci. 2025, 26, 8114. https://doi.org/10.3390/ijms26178114
Șerban M, Toader C, Covache-Busuioc R-A. Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers. International Journal of Molecular Sciences. 2025; 26(17):8114. https://doi.org/10.3390/ijms26178114
Chicago/Turabian StyleȘerban, Matei, Corneliu Toader, and Răzvan-Adrian Covache-Busuioc. 2025. "Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers" International Journal of Molecular Sciences 26, no. 17: 8114. https://doi.org/10.3390/ijms26178114
APA StyleȘerban, M., Toader, C., & Covache-Busuioc, R.-A. (2025). Brain Tumors, AI and Psychiatry: Predicting Tumor-Associated Psychiatric Syndromes with Machine Learning and Biomarkers. International Journal of Molecular Sciences, 26(17), 8114. https://doi.org/10.3390/ijms26178114





